1. Introduction
Diabetic retinopathy (DR) is currently the leading cause of blindness among the working-age population in most of the developed world. Over the past several decades, the incidence and prevalence of DR have been rapidly rising worldwide. For decades, panretinal photocoagulation (PRP) has been regarded to be the standard treatment approach to high-risk proliferative diabetic retinopathy (PDR), even with the emergence of pharmacological treatments [
1,
2].
PRP involves applying laser burns to the peripheral retina, causing coagulation necrosis of the retinal tissue. The mechanism of action of PRP is still an area of active investigation, but it is hypothesized that PRP can preserve visual function by destroying a fraction of highly metabolically active photoreceptor cells, thus, reducing retinal oxygen demand [
3]. PRP has evolved from a conventional argon-type laser towards modern, semi-automated, pattern scanning laser (PASCAL
®) photocoagulation. Conventional PRP application is associated with several side effects, such as permanent retinal scarring, as well as decreased peripheral, color, and night vision. With the advancement and improvement of PRP procedures, the side effects and associated pain have been reduced, but complications such as exudative detachment of the retina or choroid continue to occur occasionally [
4].
Since the 1990s, optical coherence tomography (OCT) has been widely used in retina clinics. OCT can provide high-resolution cross-sectional images of the retina and choroid. OCT imaging before and after PRP provides direct visualization of retinal and choroidal structures over wide areas to identify any abnormalities. The measurement of choroidal thickness can also be achieved through OCT images, and is a valuable tool that can help to evaluate the effect of retinal laser treatment [
5,
6].
Here, we report a series of three eyes with self-limited choroidal detachment evaluated by OCT after PRP using either a green solid-state laser or PASCAL®.
2. Materials and Methods
Study participants were acquired from the University of Michigan Kellogg Eye Center Grand Blanc, a tertiary care academic medical center, in accordance with the University of Michigan Institutional Review Board Committee approval (HUM00180995, PI: Y.M. Paulus). The study adhered to the tenets of the Declaration of Helsinki. Consent to publish this case series has been obtained from all the patients in writing. Retinal laser panretinal photocoagulation was performed with a PASCAL photocoagulator (OptiMedica, Santa Clara, CA, USA) or green diode-pumped solid-state laser (532 nm wavelength, GYC-1000, Nidek Co., Ltd., Aichi, Japan) with a Mainster PRP 165 Laser Lens (Ocular Instruments Inc., Bellevue, WA, USA).
2.1. OCT Scanning
Patients underwent OCT examinations before PRP (Patient 1), immediately after, and 1 month after PRP treatment at the PRP-treated area (Patients 1, 2, and 3). The OCT examinations were performed under pupillary dilation with tropicamide 1% ophthalmic and phenylephrine 2.5% ophthalmic. The choroidal OCT images were obtained with the HD 5-raster imaging mode of the Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA, USA). The choroidal thickness was measured manually utilizing the built-in analysis software (Zeiss Forum Version 3.2, Jena, Germany), defined as the vertical distance from the outer boundary of the highly reflective retinal pigment epithelium (RPE) layer to the choroid-sclera interface. After PRP treatment, the detached choroid accounted for most of the significant increase in choroidal thickness. For each OCT image (B-scans), the choroidal thickness was measured and averaged at the three thickest points per scan and averaged per time point per patient. The OCT photos were acquired in the retina area immediately beyond the temporal arcades in regions that had received PRP therapy.
2.2. Statistical Analyses
The choroidal thickness values are expressed as the mean ± standard deviation (SD). A Mann–Whitney U test was performed to compare choroidal thickness prior and post PRP (Patient 1), immediately after PRP and 1 month after PRP (Patients 1, 2, 3). All p-values were one-tailed and obtained using the SPSS software (Version 10.1.0, SPSS, Chicago, IL, USA). The Benjamini–Hochberg procedure was used to adjust the p-value to control the false discovery rate for multiple comparisons; p-values < 0.05 were considered to indicate statistically significant differences.
3. Results
3.1. Case 1
The patient is a 70-year-old Caucasian male with type II insulin-dependent diabetes mellitus, diagnosed 31 years prior to presentation, on insulin for 15 years. He also had diabetic neuropathy, hypertension, mitral valve regurgitation, lower back pain, cataract extraction and intraocular lens insertion on both eyes, and a choroidal nevus on the left eye. His last hemoglobin A1c (HbA1c), 3 months prior to laser, measured 11.0% (97 mmol/mol) and his fasting blood glucose was 111 mg/dL that morning. He had previously been noted to have severe non-proliferative diabetic retinopathy with diabetic macular edema of both eyes. Visual acuity measured 20/25 on the right eye and 20/20 on the left eye, and intraocular pressure was 12 on both eyes. The right eye had been treated with focal laser 5 years previously along with intravitreal injections of Lucentis (ranibizumab) 6 times, last treatment 1 month previous, along with bevacizumab (Avastin), Triesence, and Kenalog, last treatment 6 years previous. The left eye that had not received any previous treatment presented with neovascularization of the disc (NVD) and preretinal hemorrhage on routine follow-up. He underwent panretinal photocoagulation on the left eye using a PASCAL photocoagulator (OptiMedica, Santa Clara, CA, USA) with a Mainster165 lens, using 30 ms pulse duration, 200 um aerial spot size, 300 mW power, with a 1900 laser spots applied in the periphery 360 degrees. Following PRP, the NVD and preretinal hemorrhage resolved and the visual acuity remained 20/20.
3.2. Case 2
The patient is a 61-year-old African American male with type II diabetes for 18 years, previously on insulin that he self-discontinued. He had Stage 5 kidney disease, congestive heart failure, hypertension, hyperlipidemia, and cataracts of both eyes. His HbA1c, 6 months prior, measured 5.0% (31 mmol/mol), and his fasting blood glucose was 98 mg/dL, last week, when he last measured it.
His visual acuity was 20/20 OD and 20/20-2 OS with IOP of 16 OD and 13 OS. An examination demonstrated vitreous hemorrhage and neovascularization elsewhere (NVE) of both eyes and NVD OS. He underwent bilateral injections of Avastin (bevacizumab) along with PRP 1 week later of the left eye followed by the right eye. The left eye underwent PRP using a green diode-pumped solid-state laser (532 nm wavelength, GYC-1000, Nidek Co., Ltd., Aichi, Japan) with a Mainster165 lens, using 200 ms pulse duration, 200 um aerial spot size, 190–240 mW power, with 597 laser spots applied in the periphery 360 degrees on the initial treatment that was staged in multiple sessions due to patient discomfort. One month later, additional PRP was performed with 512 spots using 100 ms pulse duration, 200 um spot size, and 320 mW power. Following laser, the NVD and preretinal hemorrhage resolved and the visual acuity remained 20/20 in both eyes.
3.3. Case 3
The patient is a 58-year-old Caucasian man with type II diabetes mellitus (DM) for at least 11 years with a history of poor control and a HbA1c, 8 months prior, measuring 14.1% (131 mmol/mol) that improved to 8.1% (65 mmol/mol) recently. He also had hypertension and a diabetic foot ulcer. His visual acuity measured 20/50 OD and 20/30 OS with an IOP of 16 OD and 15 OS. He was noted to have NVD, NVE, and preretinal and vitreous hemorrhage OD, along with diabetic macular edema (DME). He underwent Avastin injection OD followed by PRP 1-week later OD. The right eye underwent PRP using a green solid-state laser (GYC-1000, Nidek Co., Ltd., Aichi, Japan) with a Mainster165 lens, using 200 ms pulse duration, 200 um aerial spot size, 330 mW power, with 392 laser spots applied in the periphery limited due to the patient having pain and vitreous hemorrhage. His vision improved to 20/25 OD, although he was lost to follow-up for completion of the PRP.
3.4. Quantification of OCT Measurements
The demographic data and clinical characteristics of the three patients are summarized in
Table 1. The mean choroidal thickness of every patient and the subsequent changes are summarized in
Table 2 and
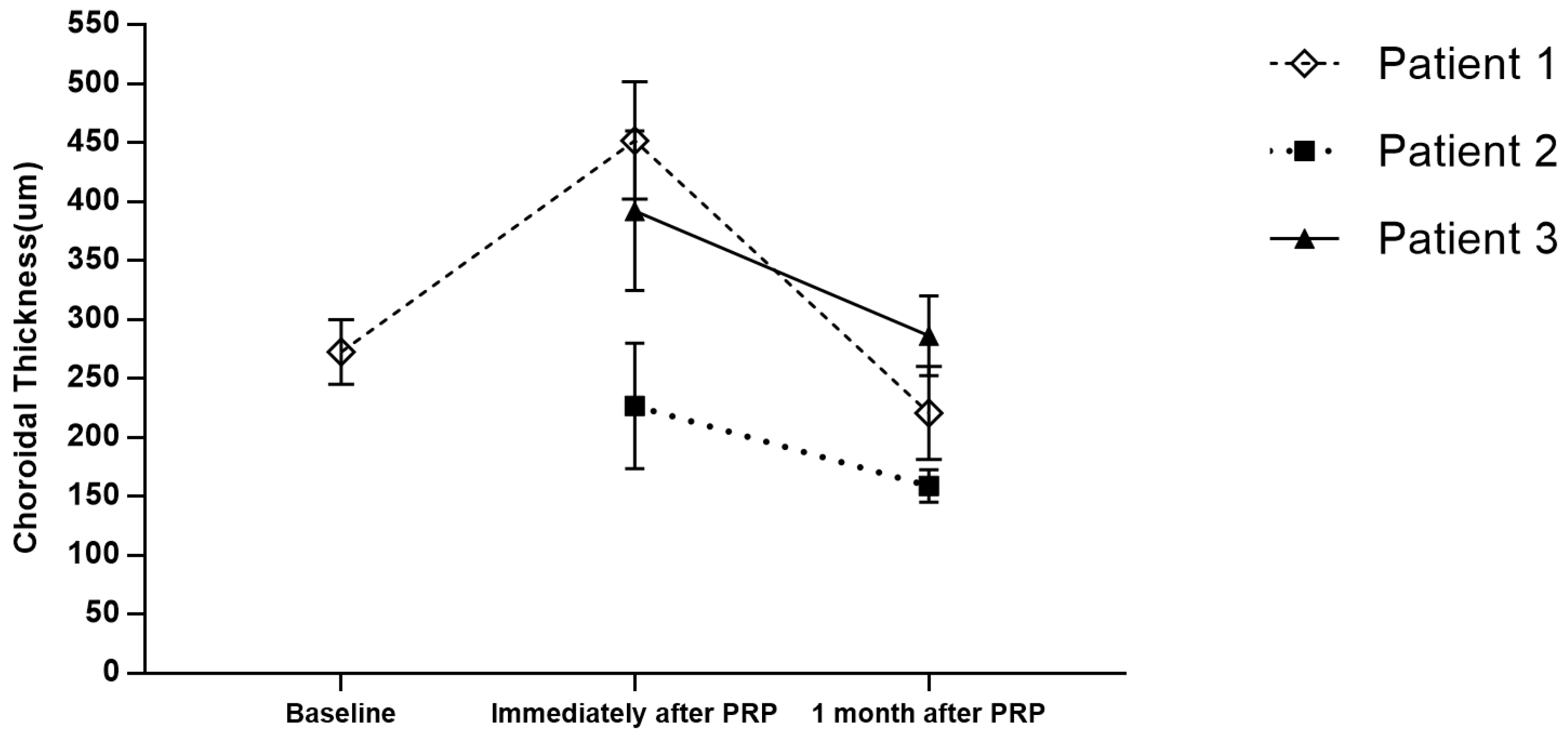
Figure 1.
Figure 2 shows representative OCT image acquired from Patient 1.
Patients 2 and 3 both underwent conventional green solid-state laser PRP and were observed with choroidal detachment at the PRP-treated areas immediately after treatment. At 1-month follow-up, the choroidal detachment was resolved spontaneously without any interventions.
Patient 1 was treated with PRP using a PASCAL
® photocoagulator; the mean choroidal thickness was 272.50 ± 27.58 μm at baseline and 451.83 ± 49.83 μm immediately after PRP (
p < 0.001), showing a significant increase in the choroidal thickness, which indicates detachment of the choroidal layer [
7]. One month later, the choroidal detachment spontaneously resolved and the choroidal thickness decreased to a mean of 220.57 ± 39.48 μm (*
p < 0.001). In Patient 2, the mean choroidal thickness immediately after PRP was 226.60 ± 53.26 μm, and 159.00 ± 13.74 μm at 1 month follow-up (*
p = 0.022). In Patient 3, the mean choroidal thickness immediately after PRP was 379 ± 52.25 μm, and 311.50 ± 16.62 μm at 1 month follow-up (*
p = 0.014). The corresponding Benjamini–Hochberg adjusted *
p-values are all <0.05.
Choroidal detachment occurred with both green solid-state laser PRP as well as PASCAL® PRP treatment. The OCT images demonstrated that the self-limitation of choroidal detachment resolved by one month in all patients.
4. Discussion
Choroidal detachment is a rare but recognized complication following both conventional green solid-state laser and PASCAL
® PRP for patients with proliferative diabetic retinopathy [
8,
9]. As compared with PASCAL
® PRP, choroid-related complications are found more commonly following conventional-type PRP, likely because the longer pulse duration results in increased heat diffusion to a larger surrounding region [
10,
11,
12,
13]. In 1997, a prospective case series by Yuki et al. [
14] reported that ciliochoroidal effusions/detachments were observed on ultrasound biomicroscopy (UBM) in 90% of eyes three days following a full treatment session of green solid-state laser PRP. All cases resolved completely within one week.
However, further investigation found that these so-called ciliochoroidal detachments were not true detachments but a swelling and splitting of outer layers of the ciliary body and the choroid due to fluid accumulation [
14,
15]. Some studies have suggested that the tendency of choroidal detachment following argon laser PRP could be decreased if PRP was performed in multiple sessions instead of a single session [
16,
17].
PASCAL
® was developed in 2006 to rapidly apply numerous spots in a defined pattern to reduce treatment time, increase patient comfort, and to improve the accuracy of treatment. The predetermined patterns of 4–56 burns can be rapidly applied in less than one second with shortened pulse durations, which makes a big difference in conventional laser parameters, thus, decreasing the incidence of side effects and complications [
18,
19,
20]. In a recent study by Velez-Montoya et al. [
21], two cases of choroidal detachment were noted in a series of 1301 patients who underwent PASCAL PRP. However, the reported incidence of choroidal detachment was probably underestimated since some of the cases were asymptomatic, subclinical, and spontaneously resolve.
The possible etiology of choroidal detachment after PRP can be hypothesized due to changes in the fluid dynamics in the eye. The administration of PRP leads to a disruption of choriocapillaris and transduction of fluid. Moreover, PRP-induced inflammation is associated with augmented blood-retinal barrier permeability [
14,
22,
23].
In our cases, all three patients presented a significant choroidal detachment immediately after PRP treatment but the choroidal detachments were temporary and resolved within one month. Two of them were treated with a green solid-state laser and one of them was treated with a PASCAL
®. Although PASCAL
® takes advantage of reduced collateral injury and improved safety, patients are still at risk of developing complications such as choroidal detachment and different levels of vision change [
24]. OCT examinations play a critical role in diagnosing and evaluating this complication in patients. This non-invasive examination is important in patients with blurred vision after the treatment.
5. Conclusions
In summary, this case series demonstrates that choroidal detachment likely occurs more frequently than currently appreciated after PRP treatments in patients with PDR. These cases were all subclinical, self-limited, choroidal detachments where the patients were asymptomatic, and this was noted on OCT imaging. More cases are needed to investigate the correlation between laser settings and the incidence of choroidal detachment so that clinicians can take measures to prevent certain complications. This finding could explain why some patients report transient blurry vision following PRP which typically spontaneously resolves by the time of follow-up with the retina specialist.
Author Contributions
Conceptualization, X.X., Q.L. and Y.M.P.; methodology, Y.M.P.; validation, X.X., Q.L. and Y.M.P.; formal analysis, X.X., Q.L. and Y.M.P.; investigation, X.X., Q.L. and Y.M.P.; resources, X.X., Q.L. and Y.M.P.; data curation, X.X. and Y.M.P.; writing—original draft preparation, X.X.; writing—review and editing, X.X., Q.L. and Y.M.P.; supervision, Q.L. and Y.M.P.; project administration, Q.L. and Y.M.P.; funding acquisition, Q.L. and Y.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the National Eye Institute (grant numbers 1K08EY027458 and 1R41EY031219), the Fight for Sight International Retinal Research Foundation (grant number FFSGIA16002), an Alcon Research Institute Young Investigator Grant, unrestricted departmental support from Research to Prevent Blindness, generous support of the Helmut F. Stern Career Development Professorship in Ophthalmology and Visual Sciences (YMP), and the University of Michigan Department of Ophthalmology and Visual Sciences.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the University of Michigan (HUM00180995 “Retinal Laser Therapy Evaluation”, PI: Yannis Paulus, on 23 April 2020) as not regulated.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Limited de-identified data will be provided on reasonable request with the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Paulus, Y.Y.; Gariano, R.R. Diabetic retinopathy: A growing concern in an aging population. Geriatrics 2009, 64, 16–20. [Google Scholar]
- Writing Committee for the Diabetic Retinopathy Clinical Research Network; Gross, J.J.; Glassman, A.A.; Jampol, L.L.; Inusah, S.; Aiello, L.L.; Antoszyk, A.A.; Baker, C.C.; Berger, B.B.; Bressler, N.N.; et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMM 2015, 314, 2137–2146. [Google Scholar] [CrossRef]
- Deschler, E.E.; Sun, J.J.; Silva, P.P. Side-effects and complications of laser treatment in diabetic retinal disease. Semin. Ophthalmol. 2014, 29, 290–300. [Google Scholar] [CrossRef]
- Chappelow, A.A.; Tan, K.; Waheed, N.N.; Kaiser, P.P. Panretinal photocoagulation for proliferative diabetic retinopathy: Pattern scan laser versus argon laser. Am. J. Ophthalmol. 2012, 153, 137–142. [Google Scholar] [CrossRef]
- Okamoto, M.; Matsuura, T.; Ogata, N. Effects of panretinal photocoagulation on choroidal thickness and choroidal blood flow in patients with severe nonproliferative diabetic retinopathy. Retina 2016, 36, 805–811. [Google Scholar] [CrossRef]
- Kim, J.J.; Lee, D.D.; Joe, S.S.; Kim, J.J.; Yoon, Y.Y. Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3378–3384. [Google Scholar] [CrossRef]
- Diep, M.M.; Madigan, M.M. Choroidal detachments: What do optometrists need to know? Clin. Exp. Optom. 2019, 102, 116–125. [Google Scholar] [CrossRef]
- Huamonte, F.F.; Peyman, G.G.; Goldberg, M.M.; Locketz, A. Immediate fundus complications after retinal scatter photocoagulation. I. Clinical picture and pathogenesis. Ophthalmic Surg. 1976, 7, 88–99. [Google Scholar] [CrossRef]
- Liang, J.J.; Huamonte, F.F. Reduction of immediate complications after panretinal photocoagulation. Retina 1984, 4, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.S.; Husain, D. Panretinal photocoagulation a review of complications. Semin. Ophthalmol. 2018, 33, 83–88. [Google Scholar] [CrossRef]
- Paulus, Y.Y.; Kaur, K.; Egbert, P.P.; Blumenkranz, M.M.; Moshfeghi, D.D. Human Histopathology of PASCAL Laser Burns. Eye 2013, 27, 995–996. [Google Scholar] [CrossRef]
- Sramek, C.; Paulus, Y.Y.; Nomoto, H.; Huie, P.; Brown, J.; Palanker, D. Dynamics of retinal photocoagulation and rupture. J. Biomed. Opt. 2009, 4, 034007. [Google Scholar] [CrossRef] [PubMed]
- Paulus, Y.M.; Palanker, D.; Blumenkranz, M.S. Short-Pulse Laser Treatment: Redefining Retinal Therapy. Retin. Physician 2010, 7, 54–59. [Google Scholar]
- Yuki, T.; Kimura, Y.; Nanbu, S.; Kishi, S.; Shimizu, K. Ciliary body and choroidal detachment after laser photocoagulation for diabetic retinopathy. a high-frequency ultrasound study. Ophthalmology 1997, 104, 1259–1264. [Google Scholar] [CrossRef]
- Weiter, J.J.; Brockhurst, R.R.; Tolentino, F. Uveal effusion following pan-retinal photocoagulation. Ann. Ophthalmol. 1979, 11, 1723–1727. [Google Scholar] [PubMed]
- Doft, B.B.; Blankenship, G.G. Single versus multiple treatment sessions of argon laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology 1982, 89, 772–779. [Google Scholar] [CrossRef]
- Muqit, M.M.; Marcellino, G.G.; Henson, D.D.; Young, L.L.; Patton, N.; Charles, S.S.; Turner, G.G.; Stanga, P.P. Single-session vs multiple-session patten scanning laser panretinal photocoagulation in proliferative diabetic retinopathy: The Manchester Pascal Study. Arch. Ophthalmol. 2010, 128, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Palanker, D. Evolution of concepts and technologies in ophthalmic laser therapy. Annu. Rev. Vis. Sci. 2016, 2, 295–319. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Blumenkranz, M.M.; Paulus, Y.Y.; Wiltberger, M.M.; Andersen, D.D.; Huie, P.; Palanker, D. Effect of Pulse Duration on Size and Character of the Lesion in Retinal Photocoagulation. Arch. Ophthalmol. 2008, 126, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Blumenkranz, M.M.; Yellachich, D.; Andersen, D.D.; Wiltberger, M.M.; Mordaunt, D.; Marcellino, G.G.; Palanker, D. Semiautomated patterned scanning laser for retinal photocoagulation. Retina 2006, 26, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Velez, M.M.; Fromow, G.G.; Marcellino, G.G.; Quiroz, M.M.; Morales, C.C. Pattern scan laser photocoagulation: Safety and complications, experience after 1301 consecutive cases. Br. J. Ophthalmol. 2010, 94, 720. [Google Scholar] [CrossRef]
- Paulus, Y.Y.; Kuo, C.C.; Morohoshi, K.; Nugent, A.; Zheng, L.L.; Nomoto, H.; Blumenkranz, M.M.; Palanker, D.; Ono, S.S. Serum inflammatory markers after rupture retinal laser injury in mice. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 362. [Google Scholar] [CrossRef] [PubMed]
- Natesh, S.; Ranganath, A.; Harsha, K.; Yadav, N.N.; Bhujang, B.B. Choroidal detachment after pascal photocoagulation. Can. J. Ophthalmol. 2011, 46, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Seymenoğlu, R.R.; Ulusoy, M.M.; Başer, E.E. Safety and efficacy of panretinal photocoagulation in patients with high-risk proliferative diabetic retinopathy using pattern scan laser versus conventional YAG laser. Kaohsiung J. Med. Sci. 2016, 32, 22–26. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).