Passivation Effect of CsPbI3 Quantum Dots on the Performance and Stability of Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
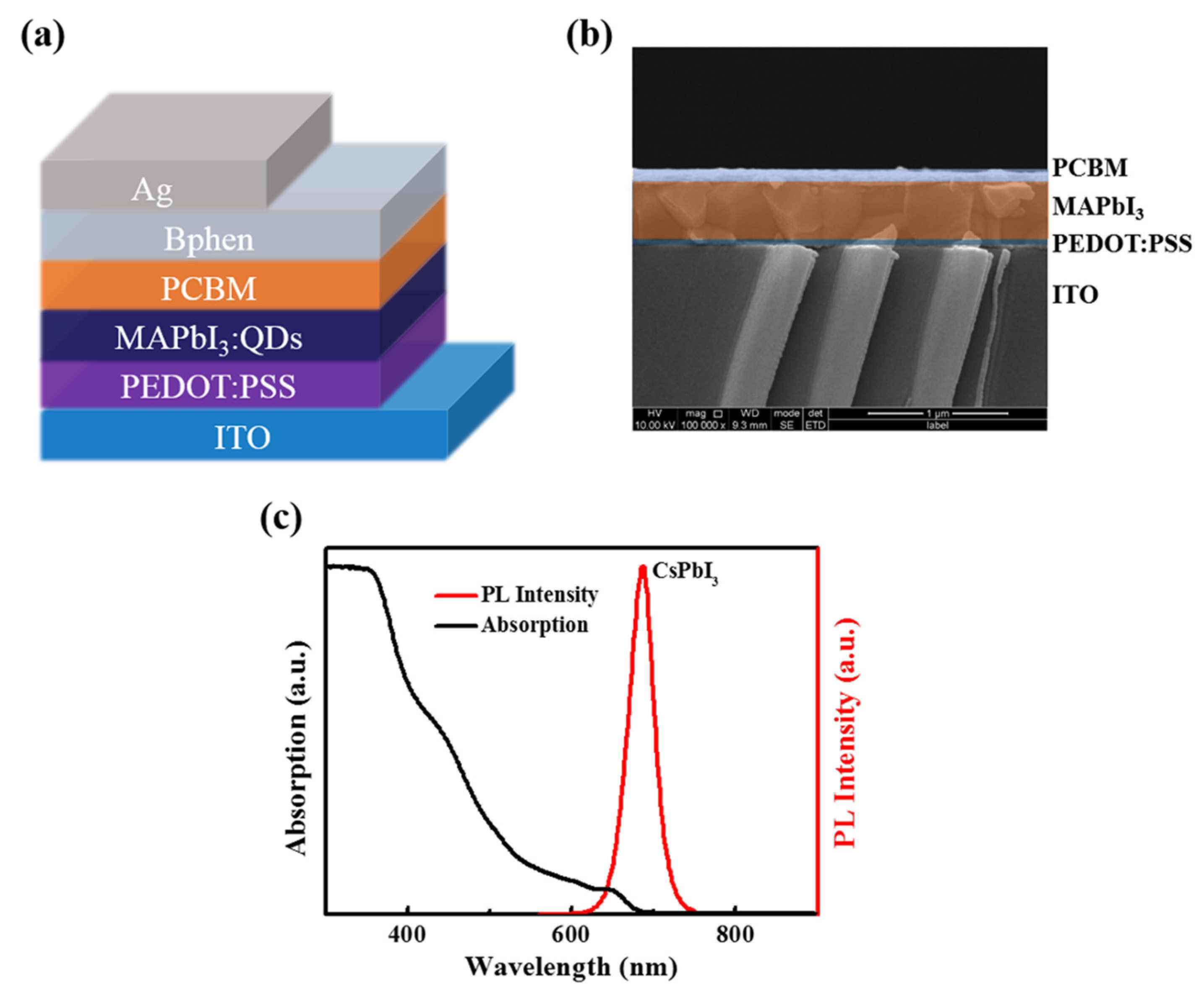
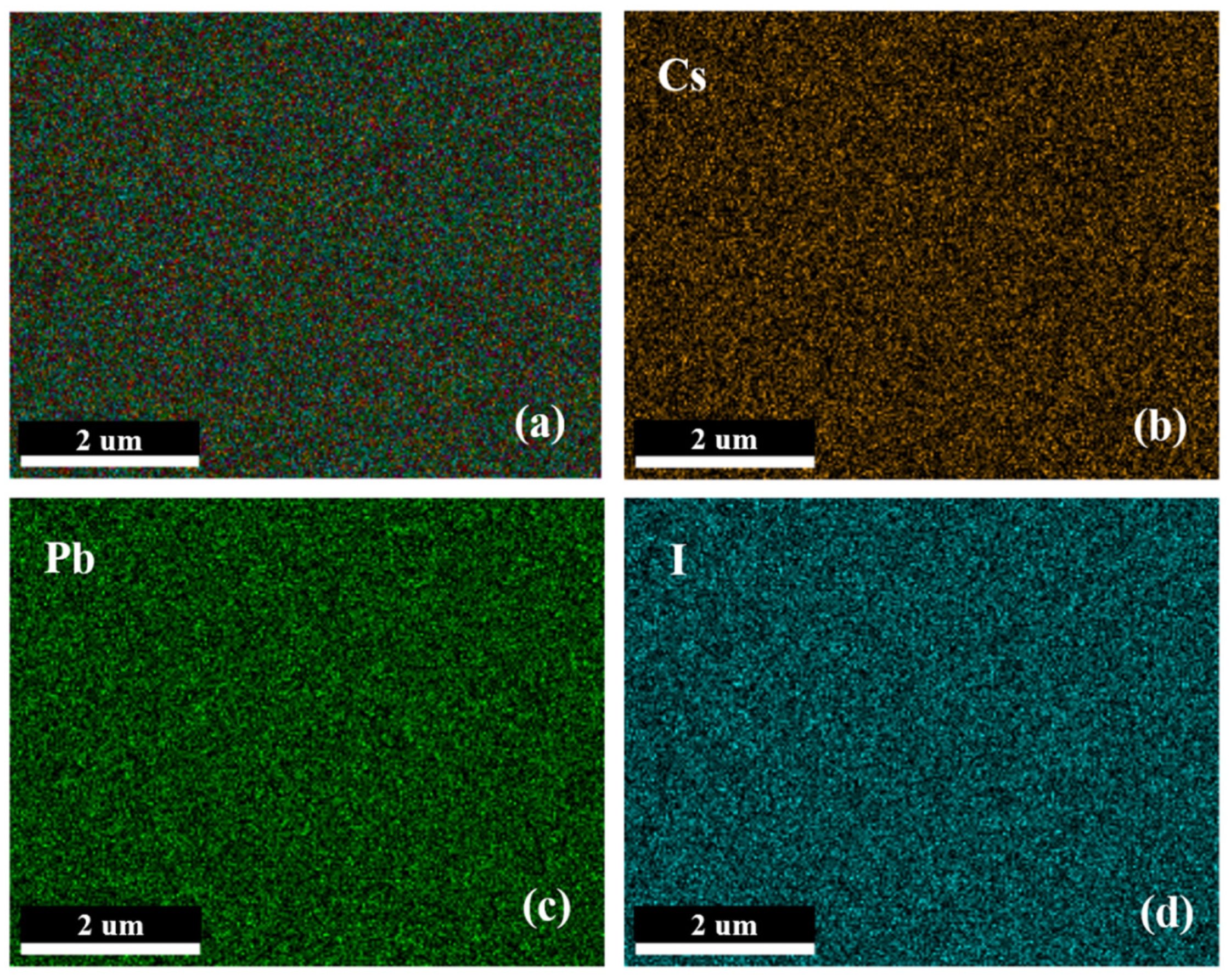
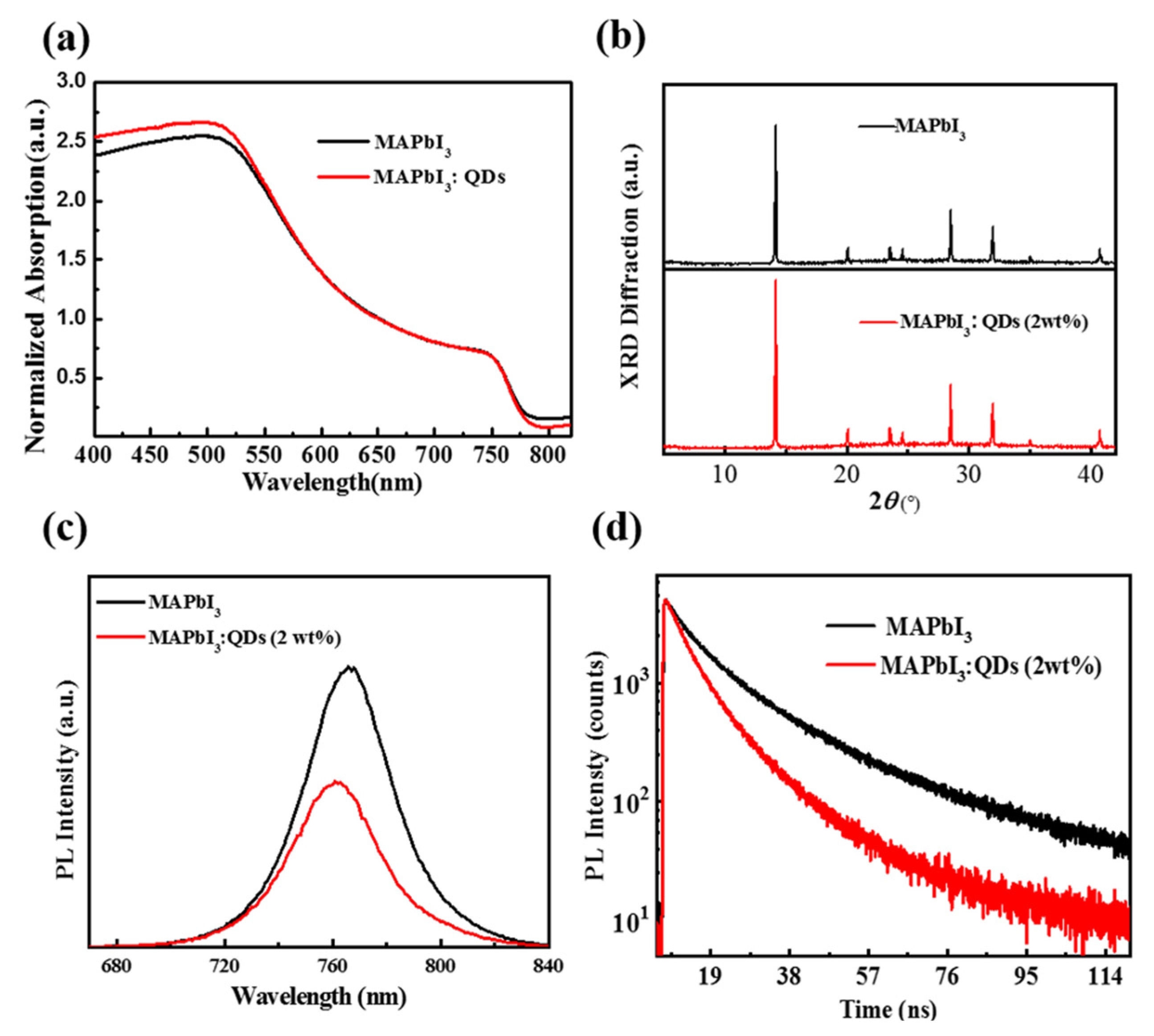
3.1. Characterization and Test
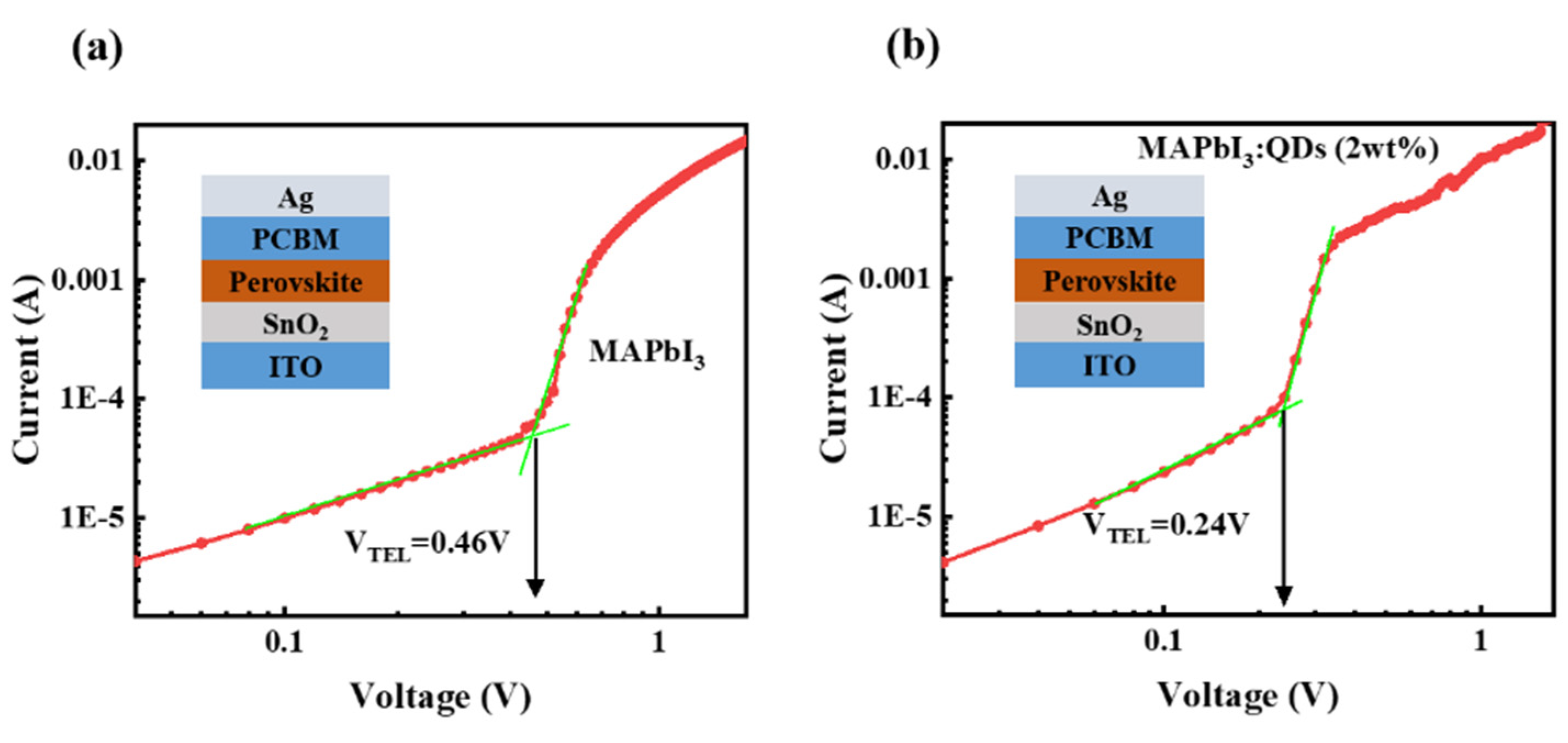
3.2. Performance of PSCs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Gao, P.; Gratzel, M.; Nazeeruddin, M.K. Organohalide lead perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 2448–2463. [Google Scholar] [CrossRef]
- Berhe, T.A.; Su, W.N.; Chen, C.H.; Pan, C.J.; Cheng, J.H.; Chen, H.M.; Tsai, M.C.; Chen, L.Y.; Hwang, B.J. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Jung, H.S.; Park, N.G. Perovskite solar cells: From materials to devices. Small 2015, 11, 10–25. [Google Scholar] [CrossRef]
- Correa-Baena, J.P.; Saliba, M.; Buonassisi, T.; Gratzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and challenges of perovskite solar cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.Z.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapor deposition. Nature 2013, 501, 395. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476. [Google Scholar] [CrossRef] [PubMed]
- Erkan, A.; Michele, D.B.; Stefaan, D.W. Defect and contact passivation for perovskite solar cells. Adv. Mater. 2019, 31, 190428. [Google Scholar]
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.K. Iodide management in formamidine-lead-halide-based perovskite layers for efficient solar cells. Science 2017, 356, 1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.S.; Yan, K.Y.; Zhu, H.Y.; Li, G.X.; Wang, H.; Zhu, H.P.; Hang, P.J.; Zhao, S.H.; Guo, W.Y.; Ye, D.Q. Identifying the functional groups effect on passivating perovskite solar cells. Sci. Bull. 2020, 65, 1726–1734. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, K. Additive engineering for efficient and stable perovskite solar cells. Adv. Energy Mater. 2020, 10, 1902579. [Google Scholar] [CrossRef]
- Li, X.; Ibrahim, D.M.; Yi, C.Y.; Luo, J.S.; Tschumi, M.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Han, H.W. Improved performance and stability of perovskite solar cells by crystal crosslinking with alkyl phosphonic acid ω-ammonium chlorides. Nat. Chem. 2015, 7, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Liu, H.; Zhang, Y.H.; Mohammed, O.F. Photoluminescence origin of zero-dimensional Cs4PbBr6 perovskite. ACS Energy Lett. 2020, 5, 87–89. [Google Scholar] [CrossRef]
- Im, J.H.; Lee, C.R.; Lee, J.W.; Park, S.W.; Park, N.G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093. [Google Scholar] [CrossRef] [Green Version]
- Song, J.Z.; Li, J.H.; Li, X.M.; Xu, L.M.; Dong, Y.H.; Zeng, H.B. Quantum dot light-emitting diodes based on inorganic perovskite cesium lead halides (CsPbX3). Adv. Mater. 2015, 27, 7162. [Google Scholar] [CrossRef]
- Pan, J.; Quan, L.N.; Zhao, Y.B.; Peng, W.; Murail, B.; Sarmah, S.P.; Yuan, M.J. Highly efficient perovskite-quantum-dot light-emitting diodes by surface engineering. Adv. Mater. 2016, 28, 8718–8725. [Google Scholar] [CrossRef]
- Cha, M.Y.; Da, P.M.; Wang, J.; Wang, J.; Wang, W.Y.; Chen, Z.H.; Xiu, F.X.; Zheng, G.F.; Wang, Z.S. Enhancing perovskite solar cell performance by interface engineering using CH3NH3PbBr0.9I2.1 quantum dots. J. Am. Chem. Soc. 2016, 138, 8581–8587. [Google Scholar] [CrossRef]
- Dong, C.; Han, X.X.; Zhao, Y.; Li, J.J.; Chang, L.; Zhao, W.N. A green anti-solvent process for high performance carbon-based CsPbI2Br all-inorganic perovskite solar cell. Sol. RRL 2018, 2, 1800139. [Google Scholar] [CrossRef]
- Shi, J.W.; Li, F.C.; Jin, Y.; Liu, C.; Ben, C.K.; Yuan, S.; Li, Y.Y.; Wang, Z.K.; Yuan, J.Y.; Ma, W.L. In Situ Ligand Bonding Management of CsPbI3 Perovskite Quantum Dots Enables High-Performance Photovoltaics and Red Light-Emitting Diodes. Angew. Chem. Int. Ed. 2020, 59, 22230–22237. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Saliba, M.; Yadav, P.; Holzhey, P.; Hagfeldt, A.; Zakeeruddin, S.M.; Grätzel, M. Synergistic crystal and interface engineering for efficient and stable perovskite photovoltaics. Adv. Energy Mater. 2018, 9, 1802646. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Hu, Q.; Liu, T.; Zhao, L.; Luo, D.; Wu, J.; Zhang, Y.; Zhang, W.; Liu, F.; Russell, T.P.; et al. Charge-carrier balance for highly efficient inverted planar heterojunction perovskite solar cells. Adv. Mater. 2016, 28, 10718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Huang, J.; Qin, R.; Yang, G.; Yu, J. Efficient visible-near-infrared hybrid perovskite: PbS quantum dot photodetectors fabricated using an antisolvent additive solution process. Adv. Opt. Mater. 2018, 6, 1800979. [Google Scholar] [CrossRef]
- Koster LJ, A.; Kemerink, M.; Wienk, M.M.; Maturová, K.; Janssen, R.A. Quantifying bimolecular recombination losses in organic bulk heterojunction solar cells. Adv. Mater. 2011, 23, 1670–1674. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.J.; Wang, Z.J.; Zhang, D.Y.; Qin, X.D.; Huang, J.; Yu, J.S. Energy Barrier Alignment for Efficient Perovskite Photodetectors Consisting of PEIE Combined with PCBM Electron Transport Layer. IEEE J. Photovolt. 2021, 11, 362–367. [Google Scholar] [CrossRef]
- Khadka, D.B.; Shirai, Y.; Yanagida, M.; Ryan, J.W.; Miyano, K. Exploring the effects of interfacial carrier transport layers on device performance and optoelectronic properties of planar perovskite solar cells. J. Mater. Chem. C 2017, 5, 8819. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Yadav, P.; Prochowicz, D.; Sponseller, M.; Osherov, A.; Bulovic, V.; Kong, J. Controllable Perovskite Crystallization via Antisolvent Technique Using Chloride Additives for Highly Efficient Planar Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1803587. [Google Scholar] [CrossRef]
- Chen, L.C.; Tien, C.H.; Tseng, Z.L.; Ruan, J.H. Enhanced Efficiency of MAPbI3 Perovskite Solar Cells with FAPbX3 Perovskite Quantum Dots. Nanomaterials 2019, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Ghahremanirad, E.; Olyaee, S.; Hedayati, M. The Influence of Embedded Plasmonic Nanostructures on the Optical Absorption of Perovskite Solar Cells. Photonics 2019, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Frolova, L.A.; Davlethanov, A.I.; Dremova, N.N.; Zhidkov, I.; Akbulatov, A.F.; Kurmaev, E.Z.; Aldoshin, S.M.; Stevenson, K.J.; Troshin, P.A. Efficient and Stable MAPbI3-Based Perovskite Solar Cells Using Polyvinyl carbazole Passivation. J. Phys. Chem. Lett. 2020, 11, 6772–6778. [Google Scholar] [CrossRef]


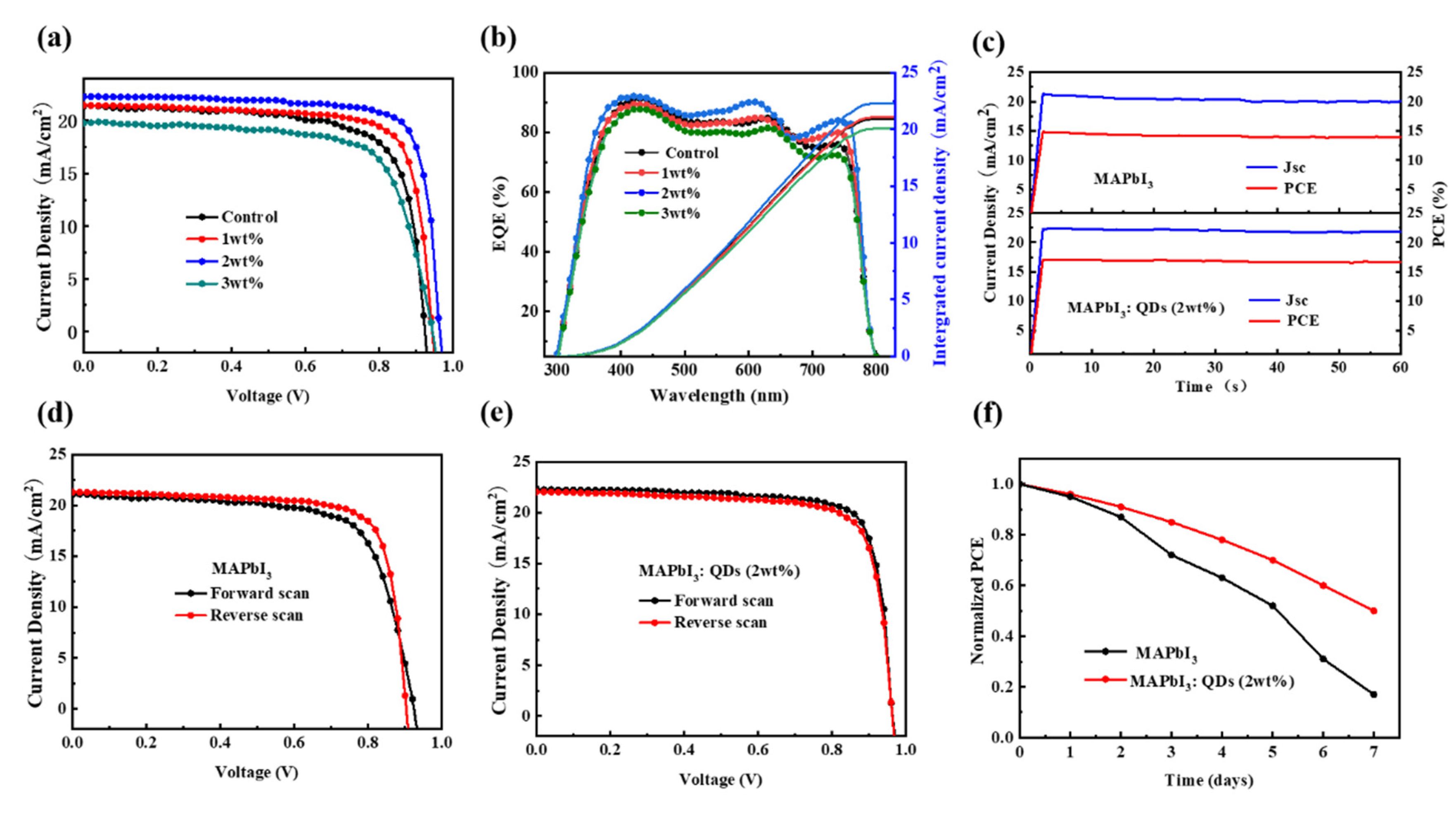
| Devices | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| Control | 21.32 ± 0.78 | 0.934 ± 0.005 | 74.59 ± 2.01 | 14.85 ± 0.53 |
| 1 wt% | 21.46 ± 0.58 | 0.942 ± 0.003 | 76.17 ± 2.07 | 15.39 ± 0.19 |
| 2 wt% | 22.27 ± 0.63 | 0.961 ± 0.003 | 79.63 ± 1.29 | 17.04 ± 0.33 |
| 3 wt% | 19.73 ± 0.46 | 0.953 ± 0.002 | 74.11 ± 2.52 | 13.93 ± 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Zhou, D.; Li, J.; Yu, J. Passivation Effect of CsPbI3 Quantum Dots on the Performance and Stability of Perovskite Solar Cells. Photonics 2022, 9, 3. https://doi.org/10.3390/photonics9010003
Yang G, Zhou D, Li J, Yu J. Passivation Effect of CsPbI3 Quantum Dots on the Performance and Stability of Perovskite Solar Cells. Photonics. 2022; 9(1):3. https://doi.org/10.3390/photonics9010003
Chicago/Turabian StyleYang, Genjie, Dianli Zhou, Jiawen Li, and Junsheng Yu. 2022. "Passivation Effect of CsPbI3 Quantum Dots on the Performance and Stability of Perovskite Solar Cells" Photonics 9, no. 1: 3. https://doi.org/10.3390/photonics9010003
APA StyleYang, G., Zhou, D., Li, J., & Yu, J. (2022). Passivation Effect of CsPbI3 Quantum Dots on the Performance and Stability of Perovskite Solar Cells. Photonics, 9(1), 3. https://doi.org/10.3390/photonics9010003





