Abstract
We report on a novel strategy for constructing graphene oxide nanomaterials with strongly enhanced photothermal (PT) and photoacoustic (PA) performance in the near-infrared (NIR)-II biowindow by chemical reduction. Optical spectra clearly reveal that obvious enhancement of optical absorption is observed in the whole NIR wideband from the NIR-I to NIR-II region for chemically reduced graphene oxide (CR-G) nanomaterials, which is mainly arising from the restoration of the electronic conjugation within the graphene oxide sheets and therefore inducing a black-body re-introduction effect of typical graphite-like materials. We experimentally synthesized CR-G samples with different degrees of reduction to demonstrate the efficiency of the proposed strategy. Experimental results show that the PT performance of the CR-G samples is greatly improved owing to the absorption enhancement by chemical reduction in the NIR-II biowindow. Furthermore, both in vitro and in vivo PA imaging of the CR-G samples with different degrees of reduction are performed to demonstrate their enhanced NIR-II PA performances. This work provides a feasible guidance for the rational design of graphene oxide nanomaterials with great potential for PT and PA applications in the NIR-II biowindow by chemical reduction.
1. Introduction
Carbon is one of the most common elements in our ecosystem [1,2]. In the past decades, carbon-based nanomaterials have attracted large interests benefiting from their environmental and biological friendliness in both industrial and nanomedical applications [3,4,5]. Among them, as a new member of the sp2 nanocarbon family, the stable, free-standing graphene oxide in a form of single-atom 2D flat sheet has shown unique physicochemical properties such as outstanding effective surface areas, easy of functionalization and wide-band optical absorption in near infrared (NIR) region, which enables unprecedented potential to support its adoption into next-generation biomedicine nanotechnologies [6,7,8,9]. Specifically, benefiting from its good photosensitizing and photothermal (PT) characteristics, significant progress has been made by using the ultrathin graphene oxide for PT and photoacoustic (PA) imaging, biosensing, and cancer therapies [10,11,12]. However, in view the fact that the optical absorption capacity of the graphene oxide in the tissue-transparent biowindows still cannot meet the requirement of high-contrast imaging and therapy for deep-seated diseases, constructing graphene oxide with enhanced optical absorption is still a critical issue. Therefore, rational design and proper engineering of graphene oxide with enhanced absorption are of great potential for their wide applications in the fields of biomedical imaging and phototherapy. Lee et al. have proved that the optical absorption of the graphene oxide can be greatly enhanced by loading with gold nanorods, which however lost the stability of the nanocomposite [13]. Robinson proposed that the aromaticity and conjugation characteristics of graphene oxide sheets are partially restored through chemical reduction, and the optical absorption is improved. However, the choice of reducing agent is dangerous in experimental operations, and the gradual decrease of oxygen-containing groups will cause the reduced hydrophilicity of the graphene oxide flakes to be weakened, thereby may induce rapidly agglomerating [14,15]. Meanwhile, even though the NIR-II region has been proved to be a more competitive optical-window for bio-applications in deep-seated tissues, benefiting from the large allowable laser irradiation and less optical scattering [16,17], the previous works only focused on the absorption enhancement in the NIR-I (650–950 nm) region [18,19]. Developing strategy that can realize enhanced optical absorption enhancement in the NIR-II (980–1700 nm) region, and thereafter guarantee enhanced PA and PT applications in deep tissue is still of great significance.
Rationally tailoring and manipulating the performance of graphene oxide through beneficial chemical engineering to achieve improved functionalities is of great importance for promoting the developments and new potentials in bioimaging and phototherapy applications. In this work, we report on a strongly enhanced PT and PA performance of graphene oxide nanomaterials in NIR-II biowindow by chemical reduction. Optical spectra clearly reveal that through chemical transformation, obvious enhancement of optical absorption is observed in the whole NIR wideband region for the chemically-reduced graphene oxide (CR-G) nanomaterials, which is mainly arising from the restoration of the electronic conjugation within the graphene oxide sheets and therefore inducing a black-body re-introduction effect of typical graphite-like materials [20]. We chose the green reducing agent “ascorbic acid” and experimentally synthesized CR-G samples with different degrees of reduction to demonstrate the efficiency of the proposed strategy, in which the biggest advantages of the synthetic method are the simple experimental conditions, the low cost and ability to be mass-produced. In this way, like conjugated polymers, the electronic conjugation level of graphene oxide is chemically controllable, offering possibilities to tailor the optical and electrical properties of graphene oxide sheets, and enhanced PT/PA performance in NIR-II region then can be observed. Experimental results show that the PT performance of the CR-G samples is greatly improved owing to the absorption enhancement by chemical reduction. Furthermore, both in vitro and in vivo PA imaging of the CR-G samples with different degrees of reduction are performed to demonstrate their enhanced PA performances. This work provides a feasible guidance for the rational design of graphene oxide nanomaterials with enhanced PT and PA performance in NIR-II biowindow by chemical reduction.
2. Material and Methods
2.1. Synthesis of CR-G Nanomaterials
Graphene oxide is considered to the most potential nanomaterials that can rival or even surpass the performance of existed carbon-based nanomaterials [21]. The commonly used strategy for the synthesis of graphene oxide nanomaterials is based on the chemical transformation, where graphite is firstly transformed to graphite oxide and then exfoliated and dispersed in water in the form of single-layered sheets [22]. After that, choosing ascorbic acid as the reducing agent is considered to be one of the suitable choices for reducing graphite oxide, not only because it produces highly reduced graphite oxide nanosheets suspended in water or hydrogel at room temperature or mild temperature, but also because it produced an environmentally friendly by-product dehydroascorbic acid after the reaction [23,24]. Compared with other synthetic methods, the reduction method is characterized by its low cost, high yield, and mass production, which is expected to be one of the most effective ways to prepare graphene oxide on a large scale. More specially, reports have shown that this treatment is one of the mostly used strategies for triggering chemical reduction in 2D materials with different degrees of reduction by controlling the dosage of the reducing agent [25,26]. In our work, chemically reduced graphene oxide (CR-G) nanomaterials are constructed via reduction reaction by using the natural antioxidant ascorbic acid as reducing agent, as indicated in the Figure 1a. The ascorbic acid can remove carbon atoms connected to the oxygen-containing functional groups on the graphene oxide, which therefore forms vacancy defects at the original carbon atom positions. In our experiments, 10, 20, and 30 mg ascorbic acid were separately added to 40 mL with concentration of 0.5 mg/mL graphene oxide dispersion (XFNANO Tech. Co., Ltd., Nanjing, China) and treated with ultrasound for one hour to synthetize VD-G with different reduction degrees. The samples were then dialyzed with deionized water for 12 h. The three groups constructed CR-G water dispersions of graphene oxide with different defect degrees were obtained and indicated by the mass ratio of the ascorbic acid and graphene oxide as CR-G0.5, CR-G1.0, CR-G1.5, respectively, where the graphene oxide is considered as a non-reduced sample indicated as CR-G0. Our previous work has proved that the CR-G1.5 sample represents a complete reduction reaction of graphene oxide [27]. Through chemical reduction with the appearance of vacancy defects, the electronic structure is chemically tuned, where the electronic conjugation within the graphene oxide sheets is restored by the reduction [20]. In this way, the optical absorption of CR-G at the NIR-I and NIR-II regions then is significantly enhanced owing to the re-introduction of black body property of graphite-like materials [15,20,25], and enhanced PT/PA performance then can be observed in NIR-II region, providing a feasible guidance for the rational design of graphene oxide nanomaterials with enhanced PT/PA performance for imaging and therapy by chemical reduction in NIR-II biowindow (Figure 1b).
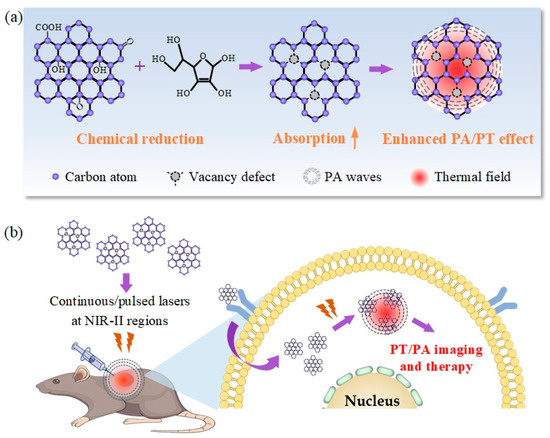
Figure 1.
(a) The schematic diagram of chemically constructing chemically reduced graphene oxide chemically-reduced graphene oxide (CR-G) with enhanced photothermal (PT) and photoacoustic (PA) performances. (b) The PT/PA applications of CR-G nanomaterials in NIR-II biowindow.
2.2. PT Effect of CR-G
PT experiments on the constructed CR-G samples with different degrees of reduction are performed by irradiating an Eppendorf (EP) tube containing four CR-G solution. A 980 nm continuous laser was used as the heating source and a thermal imager (TVS-200EX, NEC Avio Infrared Technologies Co. Ltd., Japan) was used to realtime monitor the temperature. The laser power is controlled to be about 100 mW, and the laser spot size is about 5 mm. The signals and the thermal images were collected by using thermal camera. Four CR-G samples were irradiated with a diode laser emitting 980 nm to study their PT performances.
2.3. PA Imaging In Vitro and Vivo
During the experiments, The suspensions of the four samples were injected into a transparent non-absorbent rubber tube with a diameter of 1 mm for photoacoustic imaging. The concentration of the four samples is set to be 0.5 mg/mL, a pulsed laser (DS20HE-1064D/R, Photonics Industries Co., Ltd., New York, NY, USA) with wavelength of 1064 nm and repetition rate of 5 kHz is used as the excitation source. The PA signals are received by the ultrasonic transducer (custom hollow ultrasonic transducer with central aperture of 6 mm, central frequency of 10 MHz, and bandwidth of 95%) and passed into the amplifier (LNA-650, RF BAY, Gaithersburg, MD, USA), and finally collected by the digital acquisition card (M2p.5960, Großhansdorf, Germany). Furthermore, to demonstrate the beneficial PA bio-applications by using CR-G samples, in vivo B-scan PA imaging of the CR-G samples injected on the back of mouse models is designed and performed. The specific experimental procedure is to inject 100 μL (0.1 mg/mL)nanoparticles into the mouse’s right limb 3 mm deep subcutaneously after anesthetizing the mouse, then the injection site of the mouse’s right limb is imaged by the same PA system.
3. Results
3.1. Characterization of the CR-G Nanomaterials
The constructed CR-G samples are firstly characterized by the scanning electron microscopy (SEM) (ZEISS Gemini 500), as shown in the Figure 2a. Results show that with the mass of ascorbic acid increases, the produced CR-G samples become porously micro-structured with obvious microscopic pores, indicating an enhanced reduction of the graphene oxide. This result is consistent with the results observed in the literature [28]. In addition, we also provide high-resolution transmission electron microscopy (HR-TEM) (FEI Talos F200X) of CR-G1.5. Figure 2b demonstrates that the existence of vacancy defects can be clearly observed through the HR-TEM image in the absence of one or two adjacent carbon atoms, which also shows that the reduction reaction is indeed proceeding. Similar phenomenon is observed by the gas adsorption/desorption study (Brunauer–Emmett–Teller (BET) nitrogen sorption–desorption measurement, Micromeritics ASAP 2460, America) to quantitatively characterize the porosity of the constructed CR-G samples with different reduction degrees, where the measured specific surface area (SBET) and the pore volume of the CR-G samples were obtained. As shown in the Figure 2d, the SBET increases from 14.1 m2/g (CR-G0) to 42.6 m2/g (CR-G1.5), and the pore volume increases from 0.03 cm3/g (CR-G0) to 0.1 cm3/g (CR-G1.5). These results indicate that the porosity grows with the reduction degree increases. Furthermore, in order to investigate the degree of the reduction, Raman spectroscopy (Renishaw, in Via, 532 nm) was used to test the three constructed CR-G samples. Results in Figure 2c show that two characteristic peaks, D peak at 1330–1340 cm−1 that representing the breathing vibration mode of the six-membered ring [29], and G peak at 1580–1600 cm−1 that representing sp2 hybridization of carbon atoms [30], are observed. The intensity ratio ID/IG usually reflects the change in the conjugation state of GO electrons during the reduction process in which ID and IG indicate the intensity of D peak and G peak, respectively [31]. It indicated that the ratio of D/G band gradually increases as the reduction reaction proceeds, which also observed a similar phenomenon in the Raman spectra of reduced graphite oxide [15], further it confirmed the progress of the reduction reaction. It is demonstrated that with the mass of the ascorbic acid increases, the ID/IG becomes larger, indicating that the reduction degree of the CR-G samples increases. Corresponding conclusion is drawn by the electron paramagnetic resonance (EPR, frequency of 9.85 G, German Bruker EMXplus-10/12) and experimental results are shown in the Figure 2e, where the calculated characteristic factor g = hν/βH. It is known that g = 2.0023, indicating the existence of free electron [32,33]. The g of three groups of CR-G graphene oxide is about 2.0024–2.0027 with various reduction degrees. This demonstrates the presence of unpaired electron in the graphene oxide produced by the reduction reaction. Here, h, ν, β, and H are the Planck constant, the frequency, the Bohr magneton of the electron, and the magnetic field, respectively. These results demonstrate that the CR-G samples have been successfully constructed with different degree of reduction.
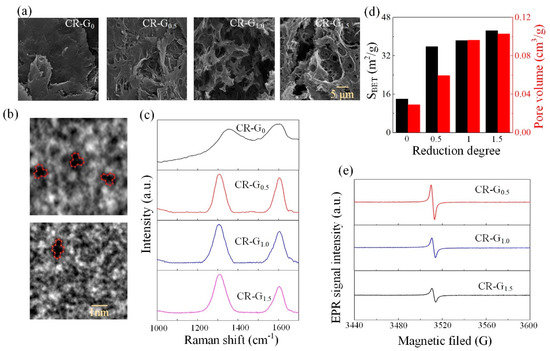
Figure 2.
Characterization of the constructed chemically reduced graphene oxide (CR-G). (a) The scanning electron microscopy (SEM) of CR-G nanomaterials with different reduction degrees. (b) HR-TEM images of CR-G1.5 graphene oxide with single-vacancy and double-vacancy defect. (c) The Raman spectra of CR-G nanomaterials with different reduction degrees. (d) The specific surface area (SBET) and the pore volume of the CR-G nanomaterials with different reduction degrees. (e) The electron paramagnetic resonance (EPR) spectrum of CR-G nanomaterials with different reduction degrees.
3.2. The Optical Absorption Properties of the CR-G Nanomaterials
The optical absorption properties of the constructed CR-G samples with different degrees of reduction were then characterized by the UV-visible spectrophotometer (Lambda 35, Perkin-Elmer, Waltham, MA, USA). Four groups of graphene oxide aqueous dispersion were placed in a quartz cuvette, and then the UV-visible absorption spectra was shown in the Figure 3a, with the degree of reduction increases, the optical absorption exhibits obvious increase both in the NIR-I and NIR-II biowindows, indicating that the electronic structure of the graphene oxide is chemically tuned and the black body property of graphite-like materials is successfully re-introduced by chemical reduction. Then, the absorption values of the whole NIR-I and NIR-II biowindows are averaged and normalized, respectively, to obtain Figure 3b. The optical absorption enhancement by chemical reduction for the CR-G samples with different degrees in both NIR-I and NIR-II biowindows are quantitatively analyzed in the Figure 3b, where a maximum of about 2.7 times enhancement is observed for the CR-G1.5 samples, providing good promise for enhanced PT/PA performance of graphene oxide nanomaterials in imaging and therapeutic applications by chemical reduction in the NIR-II biowindow.
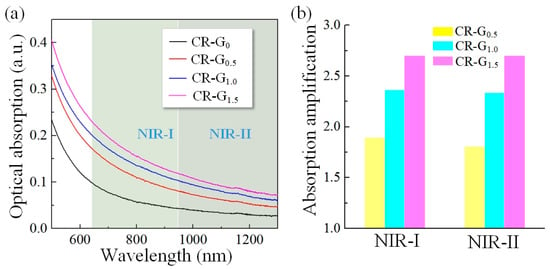
Figure 3.
Optical characterization of the chemically-reduced graphene oxide (CR-G). (a) The measured optical absorption spectra of CR-G nanomaterials with different degrees of reduction in the near infrared (NIR)-I and NIR-II biowindows. (b) Quantitative analysis of the optical absorption enhancement by chemical reduction for the CR-G nanomaterials with different degrees of reduction in both NIR-I and NIR-II biowindows.
3.3. PT and PA Applications
Then, we compared the temperature increases of the four CR-G samples with different reduction degrees, and results were presented in the Figure 4a. After about 5 min laser irradiation, the temperature of the samples become stable, where the largest temperature increase of the CR-G1.5 samples compared with others was observed owing to the enhanced optical absorption benefiting from chemical reduction. The corresponding thermal images of the CR-G with different degrees of reduction under different irradiation time are shown in the Figure 4b, in which similar conclusion can be drawn. The experiments demonstrate enhanced PT performance of the CR-G samples, prefiguring great potential for the wide applications of CR-G nanomaterials in the PT field.
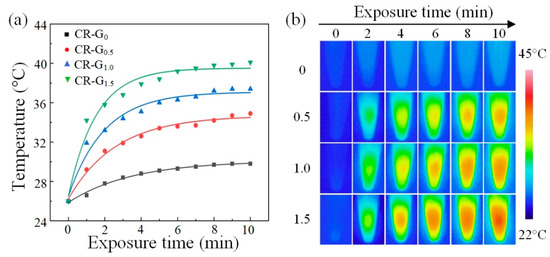
Figure 4.
Photothermal characterization of the chemically-reduced graphene oxide (CR-G). (a) Temperature of the CR-G nanomaterials with different degrees of reduction as a function of 980 nm laser exposure time. (b) The thermal images of the CR-G nanomaterials with different degrees of reduction as a function of exposure time.
To demonstrate the enhanced PA effect of graphene oxide by chemical reduction in NIR-II biowindow, in vitro and in vivo PA experiments on the four CR-G samples with different degrees of reduction are performed. As shown in the Figure 5a, in vitro PA imaging results indicate that the PA signal intensities of the CR-G samples increase with the different reduction degrees in NIR-II region, where the bottom figure shows the PA signal amplitude profile corresponding to the yellow dotted line. The obtained PA signal amplitudes of the CR-G samples are compared with their optical absorption in the Figure 5b based on the Figure 3 and Figure 5a, where the PA signal amplitudes correspond well with their optical absorption, indicating that the enhanced PA performance of the CR-G samples is arising from the enhanced optical absorption benefiting from the chemical reduction. The left part of Figure 5c shows the diagram of the B-scan PA imaging, and the right part is the obtained PA images of mouse models injected with CR-G samples with different reduction degrees, overlaying with corresponding B-scan ultrasound images. Figure 5d is the statistical analysis of the obtained PA signal amplitudes with the reduction degree for the Figure 5c. Results show that enhanced PA performance of the CR-G samples is observed with the increase of the reduction degree, indicating the great potential for the PA applications of graphene oxide nanomaterials by chemical reduction in the NIR-II biowindow.
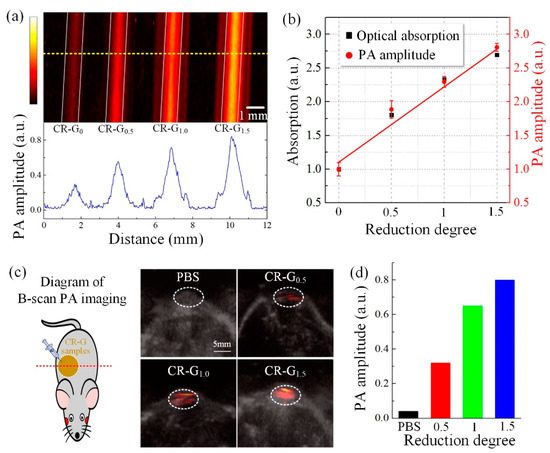
Figure 5.
Photoacoustic (PA) characterization of the chemically-reduced graphene oxide (CR-G). (a) The in vitro PA imaging and signal amplitude profile of CR-G nanomaterials with different reduction degrees. The comparison of the optical absorption and the PA signal amplitudes of CR-G nanomaterials with different reduction degrees in NIR-II region. (b) The statistical analysis of the obtained PA signal amplitudes with the reduction degrees (a). (c) B-scan in vivo PA imaging overlaid ultrasound imaging for the mouse model injected with CR-G nanomaterials with different reduction degrees in NIR-II region. (d) The statistical analysis of the obtained PA signal amplitudes with the reduction degrees (c).
The stability of the CR-G samples in physiological environment is a critical concern that related to their wide applications in the biomedical filed [34]. Here, in order to demonstrate their biological stability by time, PA experiments on the CR-G samples with different degrees of reduction are performed in NIR-II region. Before experiments, the CR-G samples were dispersed in normal saline to mimic the physiological fluids. Each prepared normal saline immersed CR-G sample is 8 mL that contains 4 mg graphene oxide. As shown in the pictures of the normal saline immersed CR-G samples in Figure 6, the colors of the samples keep nearly unchanged within 24 h. Moreover, the PA signal amplitudes of the three samples remained largely stable within 24 h, with the signal amplitude decrease less than 8%. The result demonstrates that the constructed CR-G samples can be existed in physiological fluids for a certain period with good stability.
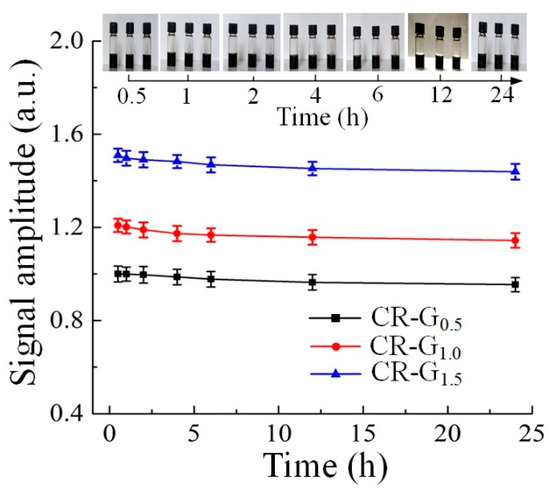
Figure 6.
The stability of the chemically-reduced graphene oxide (CR-G) nanomaterials with different degrees of reduction in normal saline.
4. Discussion
At present, with regard to the biological application of reduced graphene oxide, researchers have achieved enhanced photoacoustic and photothermal performance by coupling other photosensitizers on reduced graphene oxide, for the excellent photothermal performance of Alzheimer’s photothermal therapy, and drug-loaded graphene nanomaterials for chemical photothermal therapy [9,13]. Here, we explored the enhancement of the photothermal and photoacoustic properties of graphene oxide through chemical reduction methods in the NIR-II biowindow. During the chemical reduction process, the electronic structure is chemically tuned, where the electronic conjugation within the graphene oxide sheets is restored by the reduction. The electronic conjugation level of graphene oxide is chemically controllable, enhanced PT/PA performance then can be observed in the NIR-II biowindow. However, in this article, the PA imaging demonstration of this strategy only performs at subcutaneous injection in mice, while the tumor targeting recognition therapeutic effects of the nanomaterials and the related PT therapy on tumor remain to be studied and explored in further studies.
5. Conclusions
In conclusion, we reported a novel strategy for constructing graphene oxide nanomaterials with strongly enhanced PT and PA performances in the NIR-II biowindow by chemical reduction. Through chemical transformation, obvious enhancement of optical absorption was observed in the whole NIR wideband region for the CR-G nanomaterials. We experimentally synthesized CR-G samples with different degrees of reduction to demonstrate the efficiency of the proposed strategy. Experimental results demonstrated that both the PT and PA performances of the CR-G samples is greatly improved by chemical reduction in NIR-II region. Our study provides a feasible guidance for the rational design of graphene oxide nanomaterials with great potential for PT and PA applications by chemical reduction in NIR-II region.
Author Contributions
Conceptualization and precise instruction, Y.S.; writing—original draft preparation, X.S.; experimental validation, L.L., D.C. and W.F.; All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the National Natural Science Foundation of China (Nos. 61627827, 61805085), Guangdong Basic and Applied Basic Research Foundation (2021A1515011874), the Science and Technology Planning Project of Guangdong Province, China (Nos. 2015B020233016, and 2018A030310519), the Guangzhou Science and technology plan project (No. 201904010321), the Science and Technology Program of Guangzhou (No. 2019050001).
Institutional Review Board Statement
The present study was performed following the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council). This study was approved by the Institutional Animal Care and Use Committee of our university (South China Normal University, Guangzhou, China). The approval number is SCNU-BIP-2021-048 (Date of approval: 26 Octember 2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shaver, G.R.; Billings, W.D.; Chapin, F.S.; Giblin, A.E.; Nadelhoffer, K.J.; Oechel, W.C.; Rastetter, E.B. Global change and the carbon balance of arctic ecosystems. BioScience 1992, 42, 433–441. [Google Scholar] [CrossRef]
- Neff, J.C.; Asner, G.P. Dissolved organic carbon in terrestrial ecosystems: Synthesis and a model. Ecosystems 2001, 4, 29–48. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kaner, R.B. Graphene-based materials. Science 2008, 320, 1170–1171. [Google Scholar] [CrossRef]
- Wang, X.; Witte, R.S.; Xin, H. Thermoacoustic and photoacoustic characterizations of few-layer graphene by pulsed excitations. Appl. Phys. Lett. 2016, 108, 143104. [Google Scholar]
- Kostarelos, K. Translating graphene and 2D materials into medicine. Nat. Rev. Mater. 2016, 1, 16084. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Graphene nanomesh promises extremely efficient in vivo photothermal therapy. Small 2013, 9, 3593–3601. [Google Scholar] [CrossRef] [PubMed]
- Markovic, Z.M.; Harhaji-Trajkovic, L.M.; Todorovic-Markovic, B.M.; Kepić, D.P.; Arsikin, K.M.; Jovanović, S.P.; Trajkovic, V.S. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, X.; Ren, J.; Qu, K.; Qu, X. Using graphene oxide high near-infrared absorbance for photothermal treatment of Alzheimer’s disease. Adv. Mater. 2012, 24, 1722–1728. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.T.; Liu, Z. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Wu, M.C.; Deokar, A.R.; Liao, J.H.; Shih, P.Y.; Ling, Y.C. Graphene-based photothermal agent for rapid and effective killing of bacteria. ACS Nano 2013, 7, 1281–1290. [Google Scholar] [CrossRef]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.Y. Gold Nanorod/Reduced Graphene Oxide Composite Nanocarriers for Near-Infrared-Induced Cancer Therapy and Photoacoustic Imaging. Acs Appl. Nano Mater. 2021, 4, 11849–11860. [Google Scholar] [CrossRef]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Piner, R.D.; Chen, X. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly (sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Sun, A.; Guo, H.; Gan, Q. Evaluation of visible NIR-I and NIR-II light penetration for photoacoustic imaging in rat organs. Opt. Express 2020, 28, 9002–9013. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Pu, K. Molecular fluorescence and photoacoustic imaging in the second near-infrared optical window using organic contrast agents. Adv. Biosyst. 2018, 2, 1700262. [Google Scholar] [CrossRef]
- Sheng, Z.; Song, L.; Zheng, J. Protein-assisted fabrication of nano-reduced graphene oxide for combined in vivo photoacoustic imaging and photothermal therapy. Biomaterials 2013, 34, 5236–5243. [Google Scholar] [CrossRef]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.I. Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Kuang, B.; Song, W.; Ning, M.; Li, J.; Zhao, Z.; Guo, D.; Jin, H. Chemical reduction dependent dielectric properties and dielectric loss mechanism of reduced graphene oxide. Carbon 2018, 127, 209–217. [Google Scholar] [CrossRef]
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chhowalla, M.; Shenoy, V.B. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2010, 2, 581–587. [Google Scholar] [CrossRef]
- Fang, W.; Shi, Y.; Xing, D. Vacancy-defect-dipole amplifies the thermoacoustic conversion efficiency of carbon nanoprobes. Nano Res. 2020, 13, 2413–2419. [Google Scholar] [CrossRef]
- Yousefi, N.; Wong, K.K.W.; Hosseinidoust, Z. Hierarchically porous, ultra-strong reduced graphene oxide-cellulose nanocrystal sponges for exceptional adsorption of water contaminants. Nanoscale 2018, 10, 7171–7184. [Google Scholar] [CrossRef] [Green Version]
- Cançado, L.G.; Jorio, A.; Ferreira, E.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Ferrari, A.C. Quantifying defects in graphene via Raman spectroscopy at different excitation energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the nature of defects in graphene by Raman spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castner, T.G.; Känzig, W. The electronic structure of V-centers. J. Phys. Chem. Solids 1957, 3, 178–195. [Google Scholar] [CrossRef]
- Wei, H.; Yin, X.; Li, X.; Li, M.; Dang, X.; Zhang, L.; Cheng, L. Controllable synthesis of defective carbon nanotubes/Sc2Si2O7 ceramic with adjustable dielectric properties for broadband high-performance microwave absorption. Carbon 2019, 147, 276–283. [Google Scholar] [CrossRef]
- Pinheiro, V.B.; Holliger, P. Towards XNA nanotechnology: New materials from synthetic genetic polymers. Trends Biotechnol. 2014, 32, 321–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).