Cobalt and Carbon Complex as Counter Electrodes in Dye-Sensitized Solar Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, C.F.; Chen, P.H.; Hsieh, T.H.; Han, H.C.; Chiu, K.Y. Cobalt derivatives as counter electrodes in dye sensitized solar cells. In Proceedings of the 2015 22nd International Workshop on Active-Matrix Flatpanel Displays and Devices (AM-FPD), Kyoto, Japan, 1–4 July 2015. [Google Scholar]
- Popov, A.I.; Geske, D.H. Studies on the Chemistry of Halogen and of Polyhalides. XIII. Voltammetry of Iodine Species in Acetonitrile. J. Am. Chem. Soc. 1958, 80, 1340–1352. [Google Scholar] [CrossRef]
- Imperiyka, M.; Ahmad, A.; Hanifah, S.A.; Bella, F. A UV-prepared linear polymer electrolyte membrane for dye-sensitized solar cells. J. Phys. B 2014, 450, 151–154. [Google Scholar] [CrossRef]
- Sacco, A.; Bella, F.; Pierre, S.D.L.; Cstellino, M.; Bianco, S.; Bongiovanni, R.; Pirri, C.F. Electrodes/Electrolyte Interfaces in the Presence of a Surface-Modified Photopolymer Electrolyte: Application in Dye-Sensitized Solar Cells. Chem. Phys. Chem. 2015, 16, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, N.; Bonomo, M.; Fagiolari, L.; Barbero, N.; Gerbaldi, C.; Bella, F.; Barolo, C. Recent advances in eco-friendly and cost-effective materials towards sustainable dye-sensitized solar cells. Green Chem. 2020, 22, 7168–7218. [Google Scholar] [CrossRef]
- Galliano, S.; Bella, F.; Bonomo, M.; Viscardi, G.; Gerbaldi, C.; Boschloo, G.; Barolo, C. Hydrogel Electrolytes Based on Xanthan Gum: Green Route towards Stable Dye-Sensitized Solar Cells. Nanomaterials 2020, 10, 1585. [Google Scholar] [CrossRef]
- Devadiga, D.; Selvakumar, M.; Shetty, P.; Mahesha, M.G.; Devadiga, M.G.; Devadiga, D.; Ahipa, T.N.; Kumar, S.S. Novel photosensitizer for dye-sensitized solar cell based on ionic liquid–doped blend polymer electrolyte. J. Solid State Electrochem. 2021, 25, 1461–1478. [Google Scholar] [CrossRef]
- Wu, Z.S.; Zhang, J.; Guo, W.J.; Liu, Y.D.; Wan, D.Y.; Long, J.X.; Cheng, Z.J. Effects of side substituents in bithiophene spacer on the performance of dye-sensitized solar cells with cobalt electrolyte. Sol. Energy 2021, 218, 503–511. [Google Scholar] [CrossRef]
- Fang, X.M.; Ma, T.L.; Guan, G.Q.; Akiyama, M.; Kida, T.; Abe, E. Effect of the thickness of the Pt film coated on a counter electrode on the performance of a dye-sensitized solar cell. J. Am. Chem. Soc. 2004, 570, 257–263. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 335, 737–740. [Google Scholar] [CrossRef]
- Yue, G.T.; Wu, J.H.; Xiao, Y.M.; Lin, J.M.; Huang, M.L. Lowcost poly(3,4-ethylenedioxythiophene):polystyrenesulfonate/carbon black counter electrode for dye-sensitized solar cells. Electrochim. Acta. 2012, 67, 113–118. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, X.Z.; Li, K.X.; Li, D.M.; Luo, Y.H.; Li, H.; Song, W.B.; Chen, L.Q.; Meng, Q.B. Application of carbon materials as counter electrodes of dye-sensitized solar cells. Electrochem. Commun. 2007, 9, 596–598. [Google Scholar] [CrossRef]
- Suzuki, K.; Yamaguchi, M.; Kumagai, M.; Yanagida, S. Application of Carbon Nanotubes to Counter Electrodes of Dye-sensitized Solar Cells. Chem. Lett. 2003, 32, 28–29. [Google Scholar] [CrossRef]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H.J. Carbon Nanotubes in Biology and Medicine: In vitro and in vivo Detection, Imaging and Drug Delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef]
- Li, P.J.; Wu, J.H.; Lin, J.M.; Huang, M.L.; Huang, Y.F.; Li, Q.H. High-performance and low platinum loading Pt/Carbon black counter electrode for dye-sensitized solar cells. Sol. Energy J. 2009, 83, 845–849. [Google Scholar] [CrossRef]
- Deng, M.H.; Zhang, Q.X.; Huang, S.Q.; Li, D.M.; Luo, Y.H.; Shen, Q.; Toyoda, T.; Meng, Q. Low-Cost Flexible Nano-Sulfide/Carbon Composite Counter Electrode for Quantum-Dot-Sensitized Solar Cell. Nanoscale Res. Lett. 2010, 5, 986–990. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, Z.Y.; Liu, N.; Wu, D.; Tao, W.U.; Xu, F.; Jiang, K. Graphene–CdS composite, synthesis and enhanced photocatalytic activity. Appl. Surf. Sci. 2012, 258, 2473–2478. [Google Scholar] [CrossRef]
- Yue, G.T.; Wu, J.H.; Xiao, Y.M.; Lina, J.M.; Huanga, M.L.; Lan, Z.; Fana, L. Functionalized graphene/poly(3,4-ethylenedioxythiophene):polystyrenesulfonate as counter electrode catalyst for dye-sensitized solar cells. Energy J. 2013, 54, 315–321. [Google Scholar] [CrossRef]
- Lin, C.F.; Chou, Y.C.; Haung, J.F.; Chen, P.H.; Han, H.C.; Chiu, K.Y.; Su, Y.O. Dye sensitized solar cells with carbon black as counter electrodes. Jpn. J. Appl. Phys. 2016, 55, 03CE01-4. [Google Scholar] [CrossRef]
- Vijaya, S.; Landi, G.; Wu, J.J.; Anandan, S. MoS2 nanosheets based counter electrodes: An alternative for Pt-free dye-sensitized solar cells. Electrochim. Acta 2019, 294, 134–141. [Google Scholar] [CrossRef]
- Jiang, Q.S.; Chen, R.; Guo, X.; Zhu, Y.; Cheng, W.; Li, W.; Yang, X.; Chen, H.C. Potentiostatic deposition of cobalt nickel selenides on FTO for advanced dye-sensitized solar cells. Sol. Energy 2019, 179, 59–66. [Google Scholar] [CrossRef]
- Yeh, M.H.; Lin, L.U.; Lee, C.P.; Wei, H.Y. A composite catalytic film of PEDOT:PSS/TiN–NPs on a flexible counter-electrode substrate for a dye-sensitized solar cell. J. Mater. Chem. 2011, 21, 19021–19029. [Google Scholar] [CrossRef]
- Li, C.T.; Lee, C.P.; Li, Y.Y.; Yeh, M.H.; Ho, K.C. SrTiCo, Og NPs/PEDOT-PSS counter electrodes for high efficiency dye: Sensitized solar cells. J. Mater. Chem. 2013, 1, 14888–14896. [Google Scholar] [CrossRef]
- Wei, W.; Wang, H.; Hu, Y.H. A review on PEDOT-based counter electrodes for dye-sensitized solar cells. Int. J. Energy Res. 2014, 38, 1099–1111. [Google Scholar] [CrossRef]
- Jing, M.; Sing, Q.; Zhang, F.; Wu, M. Improvement on the catalytic activity of the flexible PEDOT counter electrode in dye-sensitized solar cells. Mater. Res. Bull. 2018, 100, 213–219. [Google Scholar]
- Bella, F.; Porcarelli, L.; Mantione, D.; Gerbaldi, C.; Barolo, C.; Grätzel, M.; Mecerreyes, D. A water-based and metal-free dye solar cell exceeding 7% efficiency using a cationic poly(3,4-ethylenedioxythiophene) derivative. Chem. Sci. 2020, 11, 1485–1493. [Google Scholar] [CrossRef]
- Eliaz, N.; Venkatakrishna, K.; Hegde, A.C. Electroplating and characterization of Zn–Ni, Zn–Co and Zn–Ni–Co alloys. Surf. Coat. Technol. 2010, 205, 1969–1978. [Google Scholar] [CrossRef]
- Lin, J.Y.; Liao, J.H.; Chou, S.W. Cathodic electrodeposition of highly porous cobalt sulfide counter electrodes for dye-sensitized solar cells. Electrochim. Acta 2011, 56, 8818–8826. [Google Scholar] [CrossRef]
- Chae, S.Y.; Hwang, Y.J.; Choi, J.H.; Joo, O.S. Cobalt sulfide thin films for counter electrodes of dye-sensitized solar cells with cobalt complex based electrolytes. Electrochim. Acta. 2013, 114, 745–749. [Google Scholar] [CrossRef]
- Rao, S.S.; Gopi, C.V.V.M.; Kim, S.K.; Son, M.K.; Jeong, M.S.; Savariraj, A.D.; Prabakar, K.; Kim, H.J. Cobalt sulfide thin film as an efficient counter electrode for dye-sensitized solar cells. Electrochim. Acta 2014, 133, 174–179. [Google Scholar]
- Banerjee, A.; Upadhyay, K.K.; Bhatnagar, S.; Tathavadekar, M.; Bansode, U.; Agarkar, S.; Ogale, S.B. Nickel cobalt sulfide nanoneedle array as an effective alternative to Pt as a counter electrode in dye sensitized solar cells. RSC Adv. 2014, 4, 8289–8294. [Google Scholar] [CrossRef]
- Duan, X.L.; Gao, Z.Y.; Changa, J.L.; Wu, D.P.; Ma, P.F.; Hea, J.J.; Xu, F.; Gao, S.; Jiang, K. CoS2–graphene composite as efficient catalytic counter electrode for dye-sensitized solar cell. Electrochim. Acta 2013, 114, 173–179. [Google Scholar] [CrossRef]
- Kung, C.W.; Chen, H.W.; Lin, C.Y.; Huang, K.C.; Vittal, R.; Ho, K.C. CoS Acicular Nanorod Arrays for the Counter Electrode of an Efficient Dye-Sensitized Solar Cell. ACS Nano 2012, 6, 7016–7025. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Li, C.T.; Chien, H.T.; Salunkhe, R.R.; Suzuki, N.; Yamauchi, Y.; Ho, K.C. Platinum-Free Counter Electrode Comprised of Metal-Organic-Framework (MOF)-Derived Cobalt Sulfide Nanoparticles for Efficient Dye-Sensitized Solar Cells (DSSCs). Sci. Rep. 2014, 4, 6983. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, C.W.; Punnoose, D.; Gopi, C.V.V.M.; Kim, S.K.; Prabakar, K.; SrinivasaRao, S. Nickel doped cobalt sulfide as a high performance counter electrode for dye-sensitized solar cells. Appl. Surf. Sci. 2015, 328, 78–85. [Google Scholar] [CrossRef]
- Miao, X.H.; Pan, K.; Wang, G.F.; Liao, Y.P.; Wang, L.; Zhou, W.; Jiang, B.; Pan, Q.; Tian, G. Well-Dispersed CoS Nanoparticles on a Functionalized Graphene Nanosheet Surface: A Counter Electrode of Dye-Sensitized Solar Cells. Chem. Eur. J. 2014, 20, 474–482. [Google Scholar] [CrossRef]
- Swami, S.K.; Chaturvedi, N.; Kumar, A.; Kapoor, R.; Dutta, V.; Frey, J.; Moehl, T.; Gratzel, M.; Mathew, S.; Nazeeruddin, M.K. Investigation of electrodeposited cobalt sulphide counter electrodes and their application in next-generation dye sensitized solar cells featuring organic dyes and cobalt-based redox electrolytes. J. Power Sources 2015, 275, 80–89. [Google Scholar] [CrossRef]
- Mba, M.; D’Acunzo, M.; Salice, P.; Carofiglio, T.; Maggini, M.; Bignozzi, C.A. Sensitization of Nanocrystalline TiO2 with Multibranched Organic Dyes and Co(III)/(II) Mediators: Strategies to Improve Charge Collection Efficiency. J. Phys. Chem. C 2013, 117, 19885–19896. [Google Scholar] [CrossRef]
- Huo, J.; Zheng, M.; Tu, Y.; Wu, J.H.; Hu, L.H.; Dai, S.Y. A high performance cobalt sulfide counter electrode for dye-sensitized solar cells. Electrochim. Acta 2015, 159, 166–173. [Google Scholar] [CrossRef]
- Wang, M.K.; Anghel, A.M.; Marsan, B.; Ha, N.L.C.; Pootrakulchote, N.; Zakeeruddin, S.M.; Grätzel, M. CoS Supersedes Pt as Efficient Electrocatalyst for Triiodide Reduction in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2009, 131, 15976–15977. [Google Scholar] [CrossRef]
- Sharma, J.K.; Srivastavaa, P.; Singh, G.; Akhtarb, S.M.; Ameenc, S. Green synthesis of Co3O4 nanoparticles and their applications in thermal decomposition of ammonium perchlorate and dye-sensitized solar cells. Mater. Sci. Eng. B 2015, 193, 181–188. [Google Scholar] [CrossRef]
- He, B.L.; Tang, Q.W.; Meng, X.; Yu, L.M. Poly(vinylidene fluoride)–implanted cobalt–platinum alloy counter electrodes for dye–sensitized solar cells. Electrochim. Acta 2014, 147, 209–215. [Google Scholar] [CrossRef]
- Motlak, M.; Barakat, N.A.M.; Akhtar, M.S.; Hamza, A.H.; Kim, B.S.; Kim, C.S.; Khalil, A.K.; Almajid, A.A. High performance of NiCo nanoparticles-doped carbon nanofibers as counter electrode for dye-sensitized solar cells. Electrochim. Acta 2015, 160, 1–6. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, Q.; Yang, J.; Chu, W.; Li, W.; Li, X.; Hou, Y.; Hou, J. Regulation of Microstructure and Composition of Cobalt Selenide Counter Electrode by Electrochemical Atomic Layer Deposition for High Performance Dye-Sensitized Solar Cells. Electrochim. Acta 2016, 220, 169–175. [Google Scholar] [CrossRef]
- Huang, Y.J.; Lee, C.P.; Pang, H.W.; Li, C.T.; Fan, M.S.; Vittal, R.; Ho, K.C. Microemulsion-controlled synthesis of CoSe2/CoSeO3 composite crystals for electrocatalysis in dye-sensitized solar cells. Mater. Today Energy 2017, 6, 189–197. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, J.; Duan, Y.; Yuan, H.; Tang, Q. 9.07%-Efficiency dye-sensitized solar cell from Pt-free RuCoSe ternary alloy counter electrode. Mater. Lett. 2018, 218, 76–79. [Google Scholar] [CrossRef]
- Murugadoss, V.; Panneerselvam, P.; Yan, C.; Guo, Z.; Angaiah, S. A simple one-step hydrothermal synthesis of cobaltnickel selenide/graphene nanohybrid as an advanced platinum free counter electrode for dye sensitized solar cell. Electrochim. Acta 2019, 312, 157–167. [Google Scholar] [CrossRef]
- Pang, B.; Lin, S.; Shi, Y.; Wang, Y.; Chen, Y.; Ma, S.; Feng, J.; Zhang, C.; Yu, L.; Dong, L. Synthesis of CoFe2O4/graphene composite as a novel counter electrode for high performance dye-sensitized solar cells. Electrochim. Acta 2019, 297, 70–76. [Google Scholar] [CrossRef]
- Acciari, G.; Adamo, G.; Ala, G.; Busacca, A.; Caruso, M.; Giglia, G.; Imburgia, A.; Livreri, P.; Miceli, R.; Parisi, A.; et al. Experimental Investigation on the Performances of Innovative PV Vertical Structures. Photonics 2019, 6, 86. [Google Scholar] [CrossRef]


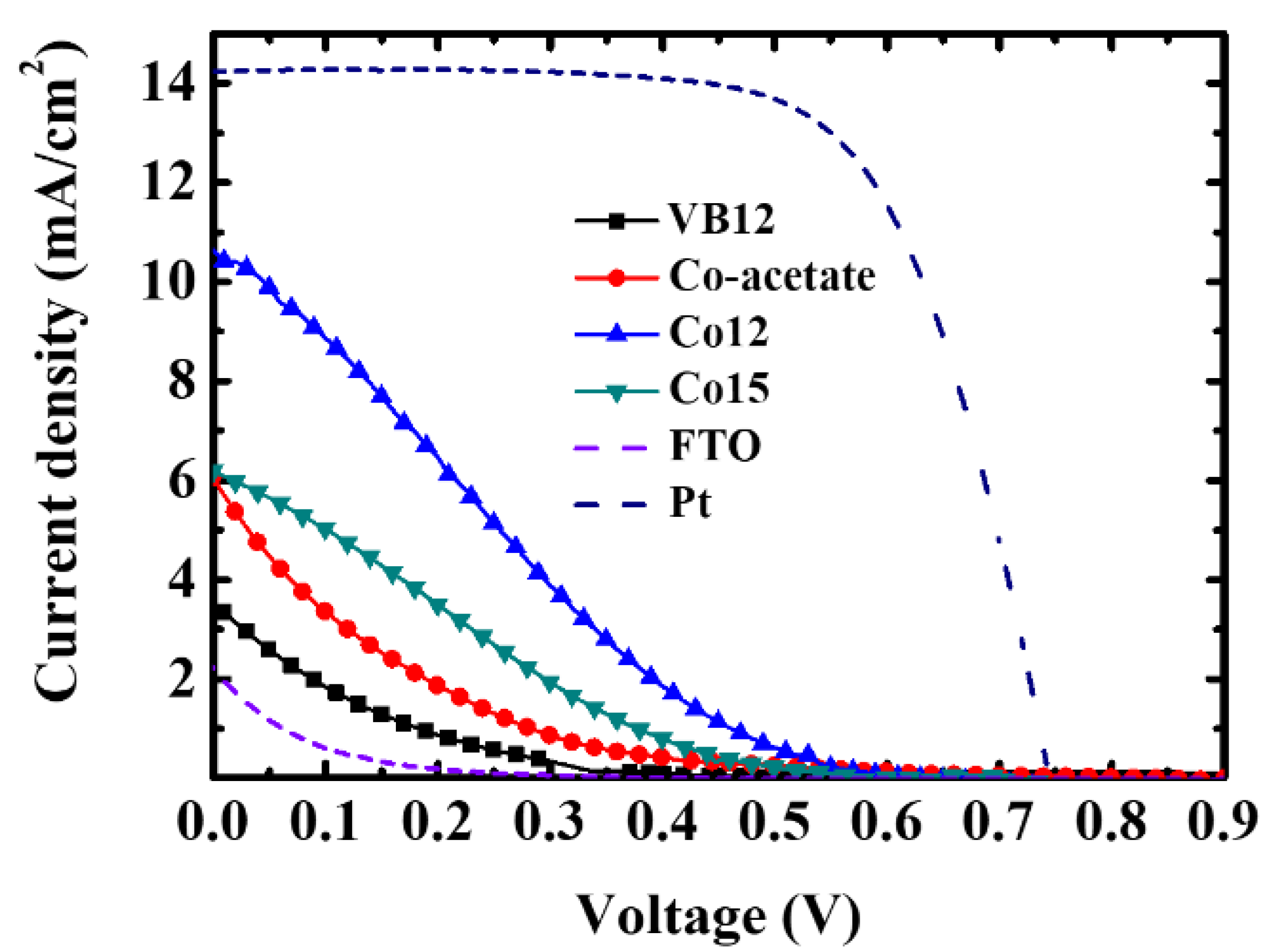

| CEs | JSC (mA/cm2) | VOC (V) | FF | η (%) |
|---|---|---|---|---|
| VB12 | 3.34 (0.59) | 0.71 (0.01) | 8.17 (1.08) | 0.19 (0.01) |
| Cobalt(II) acetate | 5.71 (0.22) | 0.85 (0.01) | 8.08 (1.02) | 0.38 (0.02) |
| Co12 | 10.59 (0.57) | 0.62 (0.01) | 19.84 (1.01) | 1.25 (0.11) |
| Co15 | 6.08 (0.21) | 0.63 (0.01) | 18.37 (1.23) | 0.70 (0.27) |
| Pt | 14.24 (0.29) | 0.75 (0.02) | 67.12 (0.26) | 7.16 (0.09) |
| FTO | 2.21 (0.28) | 0.44 (0.01) | 6.73 (0.61) | 0.06 (0.01) |
| CEs | JSC (mA/cm2) | VOC (V) | FF | η (%) |
|---|---|---|---|---|
| Co12 | 10.59 (0.57) | 0.62 (0.01) | 19.84 (1.01) | 1.25 (0.11) |
| Co12+CB | 13.23 (0.14) | 0.65 (0.01) | 63.28 (2.08) | 5.42 (0.26) |
| Co15 | 6.08 (0.21) | 0.63 (0.01) | 18.37 (1.23) | 0.70 (0.27) |
| Co15+CB | 11.15 (0.33) | 0.74 (0.01) | 65.46 (1.73) | 5.81 (0.19) |
| CB (1.5 M) | 13.17 (0.08) | 0.77 (0.01) | 63.92 (1.05) | 6.38 (0.08) |
| CB (0.75 M) | 12.21 (0.09) | 0.75 (0.01) | 63.28 (1.13) | 5.76 (0.1) |
| Pt | 14.24 (0.29) | 0.75 (0.01) | 67.12 (0.26) | 7.16 (0.09) |
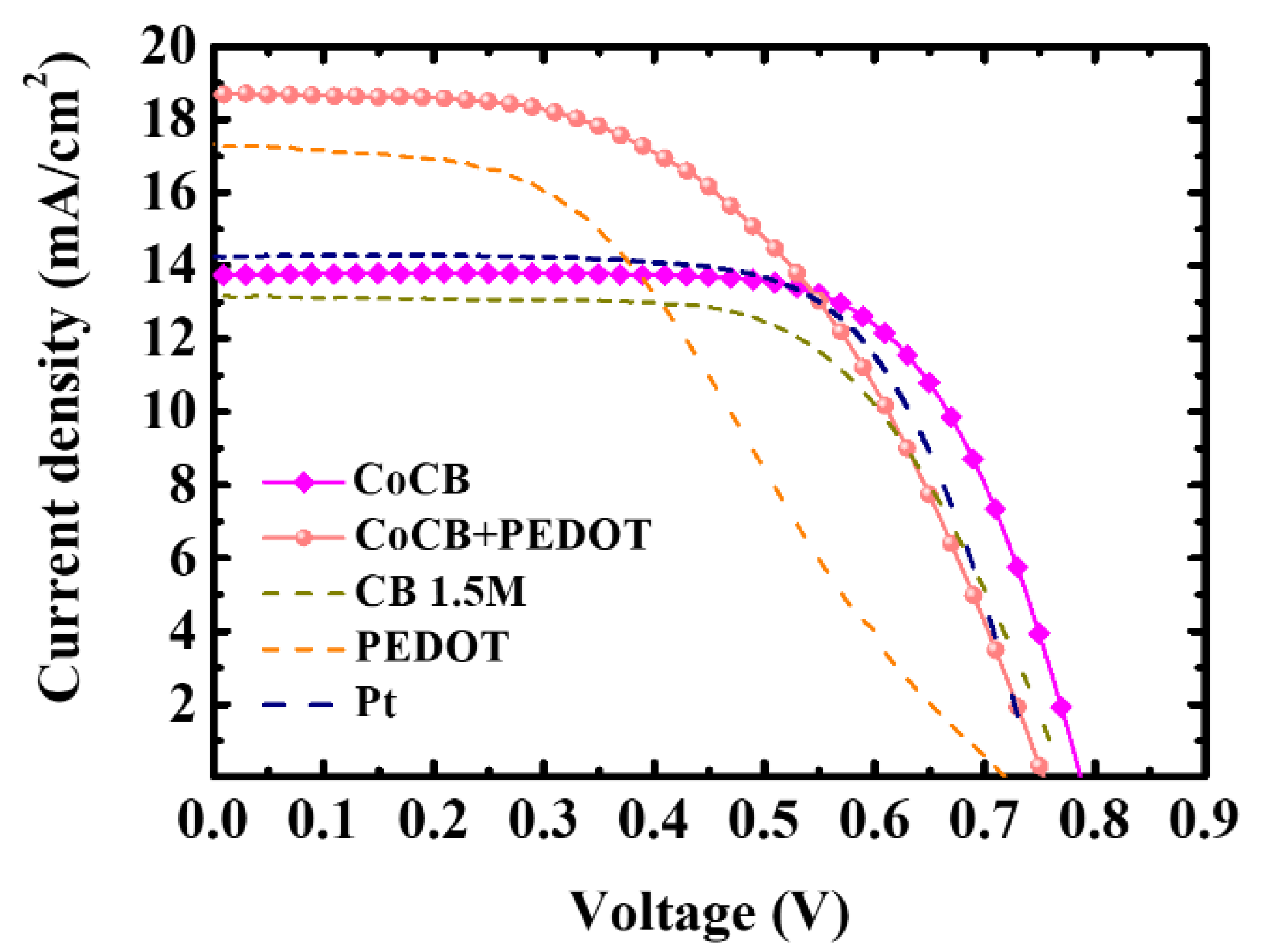
| CEs | JSC (mA/cm2) | VOC (V) | FF | η (%) |
|---|---|---|---|---|
| CoCB | 13.75 (0.29) | 0.78 (0.02) | 69.28 (0.45) | 7.44 (0.22) |
| CoCB + PEDOT:PSS | 18.65 (0.63) | 0.75 (0.02) | 51.31 (1.49) | 7.13 (0.18) |
| CB (1.5M) | 13.17 (0.08) | 0.77 (0.01) | 63.92 (1.05) | 6.38 (0.08) |
| PEDOT:PSS | 17.33 (0.42) | 0.72 (0.02) | 42.60 (2.13) | 5.32 (0.38) |
| Pt | 14.24 (0.29) | 0.75 (0.01) | 67.12 (0.26) | 7.16 0.09) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-F.; Hsieh, T.-H.; Chou, Y.-C.; Chen, P.-H.; Chen, C.-W.; Wu, C.-H. Cobalt and Carbon Complex as Counter Electrodes in Dye-Sensitized Solar Cells. Photonics 2021, 8, 166. https://doi.org/10.3390/photonics8050166
Lin C-F, Hsieh T-H, Chou Y-C, Chen P-H, Chen C-W, Wu C-H. Cobalt and Carbon Complex as Counter Electrodes in Dye-Sensitized Solar Cells. Photonics. 2021; 8(5):166. https://doi.org/10.3390/photonics8050166
Chicago/Turabian StyleLin, Chi-Feng, Ting-Hsuan Hsieh, Yu-Chen Chou, Pin-Hung Chen, Ci-Wun Chen, and Chun-Han Wu. 2021. "Cobalt and Carbon Complex as Counter Electrodes in Dye-Sensitized Solar Cells" Photonics 8, no. 5: 166. https://doi.org/10.3390/photonics8050166
APA StyleLin, C.-F., Hsieh, T.-H., Chou, Y.-C., Chen, P.-H., Chen, C.-W., & Wu, C.-H. (2021). Cobalt and Carbon Complex as Counter Electrodes in Dye-Sensitized Solar Cells. Photonics, 8(5), 166. https://doi.org/10.3390/photonics8050166




