Engineering of TiO2 or ZnO—Graphene Oxide Nanoheterojunctions for Hybrid Solar Cells Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Devices Preparation
2.2.1. Organic Layer Deposition
2.2.2. Inorganic Layer Deposition
2.2.3. Aluminum Electrode Deposition
2.3. Characterization Techniques
3. Results and Discussion
3.1. Build-Up of PAH and GO LbL Films
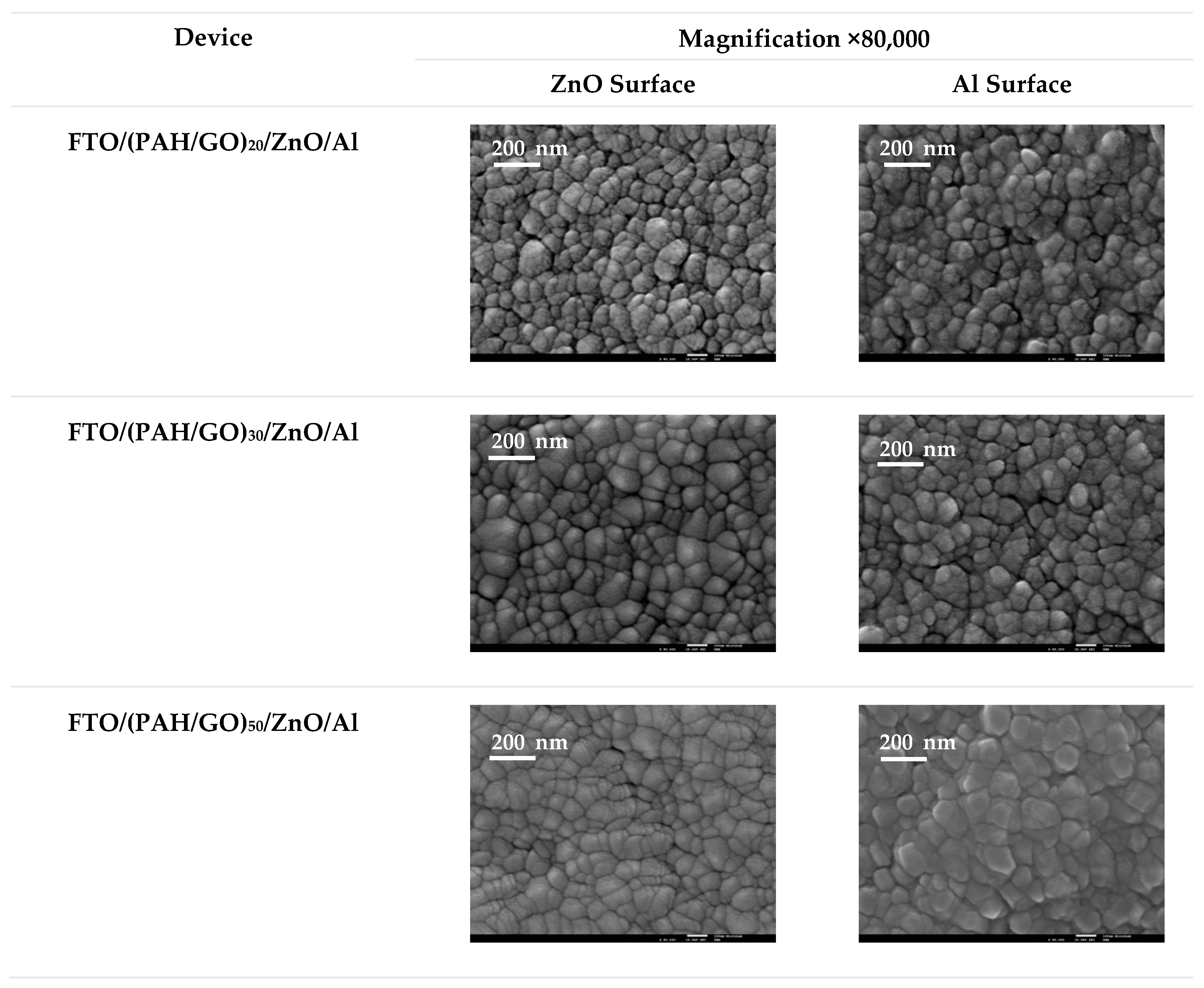
3.2. Morphological Characterization
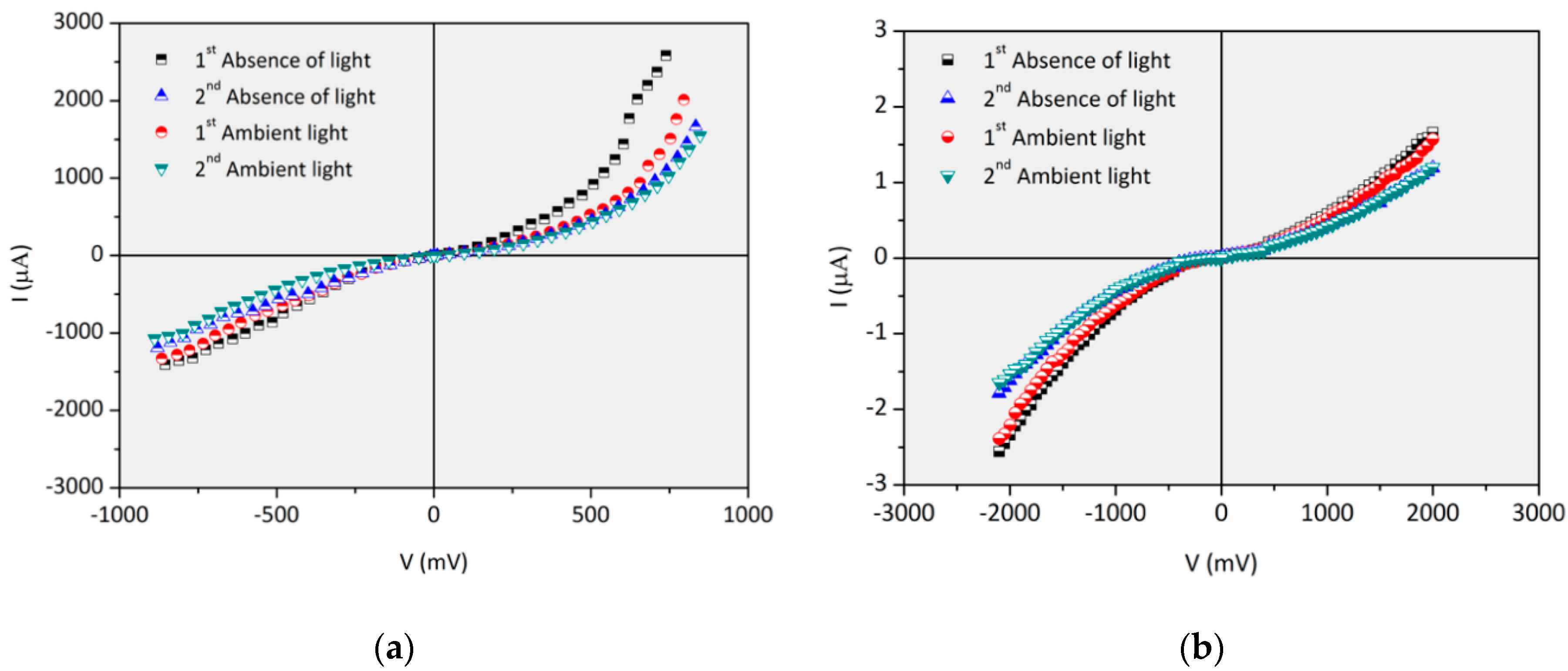
3.3. Influence of the Number of Bilayers on the Electric Properties of FTO/(PAH/GO)x/TiO2/Al and FTO/(PAH/GO)x/ZnO/Al (x = 20 and 30)
3.4. Comparison of the Electric Properties of FTO/(PAH/GO)50/TiO2/Al and FTO/(PAH/GO)50/ZnO/Al
3.5. Electric Characterization of (PAH/GO)20 onto IDE Gold Electrodes Glass Substrates
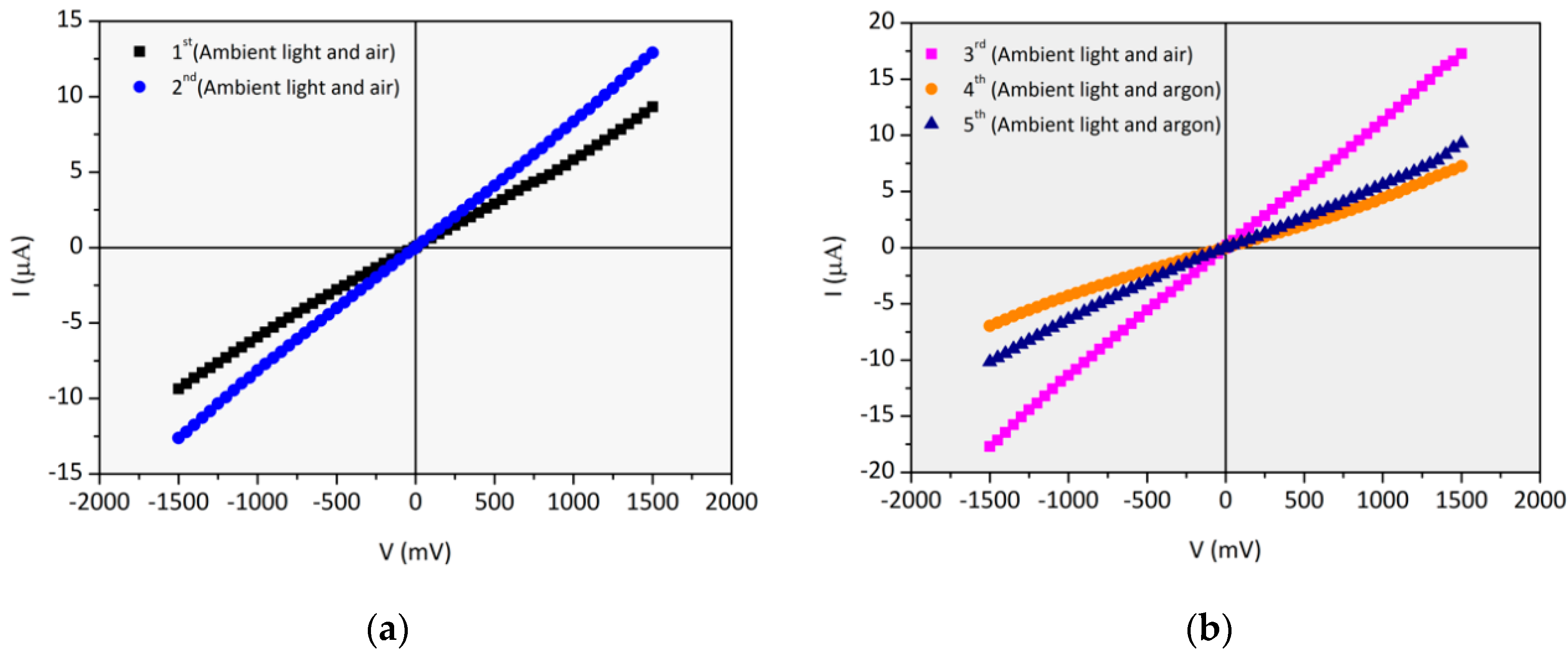
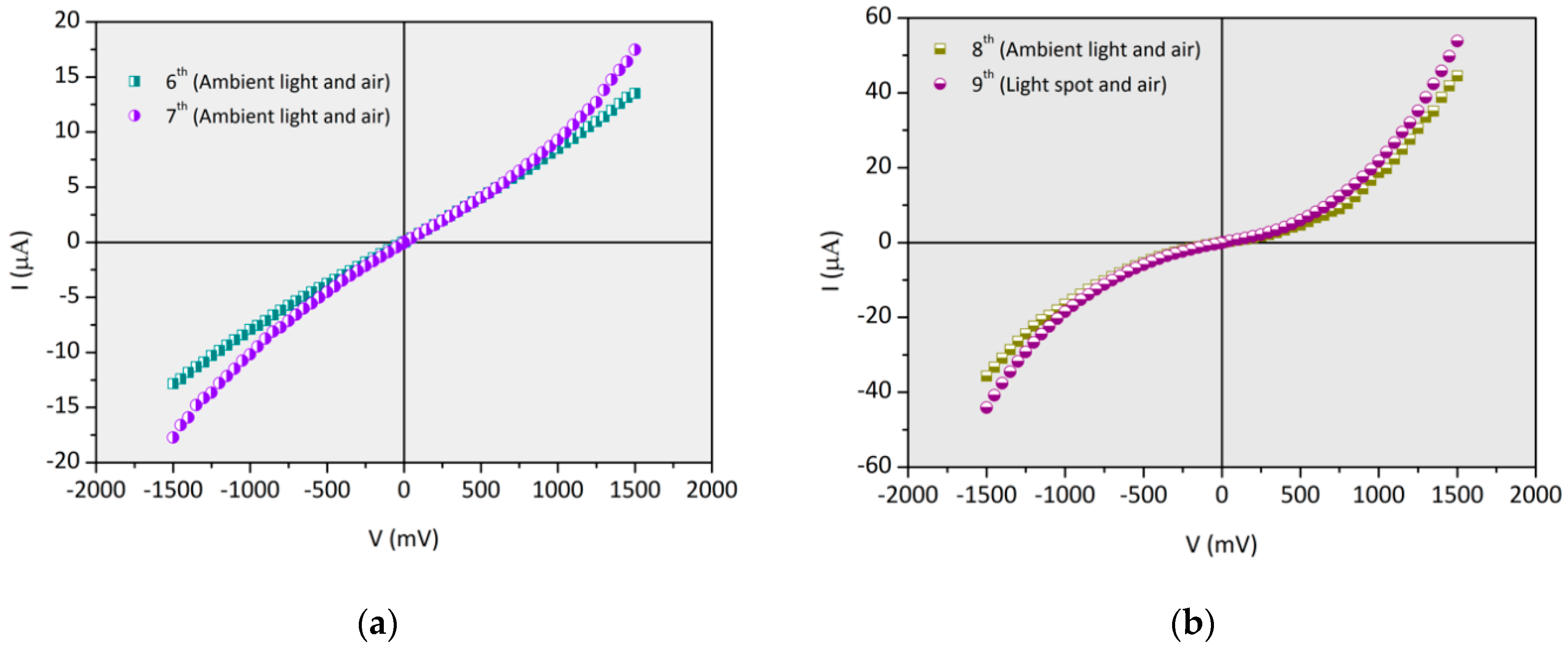
3.5.1. (PAH/GO)20 onto IDE Gold Electrodes Glass Substrates: I–V Curves
3.5.2. (PAH/GO)20 onto IDE Gold Electrodes Glass Substrates: Impedance Spectra
- Dry environment
- Humid environment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Energy Outlook. 2020. Available online: http://www.eia.doe.gov/oiaf/ieo/index.html (accessed on 1 February 2021).
- The Intergovernmental Panel on Climate Change. Climate Change and Land. 2019. Available online: http://www.ipcc.ch (accessed on 1 February 2021).
- Green, M.A.; Hishikawa, Y.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Yoshita, M.; Ho-Baillie, A.W.Y. Solar cell efficiency tables (Version 53). Prog. Photovolt. Res. Appl. 2019, 23, 3–12. [Google Scholar] [CrossRef]
- Alaaeddin, M.H.; Sapuan, S.M.; Zuhri, M.Y.M.; Zainudin, E.S.; L-Oqla, F.M.A. Photovoltaic applications: Status and manufacturing prospects. Renew. Sustain. Energy Rev. 2019, 102, 318–332. [Google Scholar] [CrossRef]
- Brown, G.F.; Wu, J. Third generation photovoltaics. Laser Photonics Rev. 2009, 3, 394–405. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed Balance Limit of Efficiency of p-n Junction Solar Cells. J. Appl. Phys. 1961, 32, 510. [Google Scholar] [CrossRef]
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar-cell based on dye- sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Hao, S.; Li, P.; Lin, J.; Huang, M.; Fang, L.; Huang, Y. Progress on the electrolytes for dye-sensitized solar cells. Pure Appl. Chem. 2008, 80, 2241–2258. [Google Scholar] [CrossRef]
- Chung, I.; Lee, B.; He, J.; Chang, R.P.; Kanatzidis, M.G. All-solid-state dye-sensitized solar cells with high efficiency. Nature 2012, 485, 486–489. [Google Scholar] [CrossRef]
- Hyuk, I.S.; Sun, L.C.; Ah, C.J.; Hui, L.Y.; Nilkamal, M.; Jung, K.H.; Nazeeruddin, M.K.; Michael, G.; Il, S.S. Toward interaction of sensitizer and functional moieties in hole-transporting materials for efficient semiconductor-sensitized solar cells. Nano Lett. 2011, 11, 4789–4793. [Google Scholar]
- Chang, J.A.; Im, S.H.; Lee, Y.H.; Kim, H.J.; Lim, C.S.; Heo, J.H.; Seok, S.I. Panchromatic photon-harvesting by hole-conducting materials in inorganic–organic heterojunction sensitized-solar cell through the formation of nanostructured electron channels. Nano Lett. 2012, 12, 1863–1867. [Google Scholar] [CrossRef]
- Im, S.H.; Kim, H.-J.; Kim, S.W.; Kim, S.-W.; Seok, S.I. All solid state multiply layered PbS colloidal quantum-dot-sensitized photovoltaic cells. Energy Environ. Sci. 2011, 4, 4181–4186. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G.; Lin, J.-Y. The Applications of Polymers in Solar Cells: A Review. Polymers 2019, 11, 143. [Google Scholar] [CrossRef]
- Luceño-Sánchez, J.A.; Díez-Pascual, A.M.; Capilla, R.P. Materials for photovoltaics: State of art and recent developments. Int. J. Mol. Sci. 2019, 20, 976. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.; Uddin, A. Organic-inorganic hybrid solar cells: A comparative review. Sol. Energy Mater. Sol. Cells 2012, 107, 87–111. [Google Scholar] [CrossRef]
- Narayan, M.; Singh, J. A theoretical study on the operation principle of hybrid solar cells. Electronics 2015, 4, 303–310. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, M.; Wang, X.; Yang, F.; Meng, X. Recent progress in organic-inorganic hybrid solar cells. J. Mater. Chem. A 2013, 1, 8694–8709. [Google Scholar] [CrossRef]
- Roland, S.; Neubert, S.; Albrecht, S.; Stannowski, B.; Seger, M.; Facchetti, A.; Schlatmann, R.; Rech, B.; Neher, D. Hybrid Organic/Inorganic Thin-Film Multijunction Solar Cells Exceeding 11% Power Conversion Efficiency. Adv. Mater. 2015, 27, 1262–1267. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Han, C.; Jiang, B.; Iocozzia, J.; He, C.; Shi, D.; Jiang, T.; Lin, Z. Graphene-based materials with tailored nanostructures for energy conversion and storage. Mater. Sci. Eng. R Rep. 2016, 102, 1–72. [Google Scholar] [CrossRef]
- Hu, C.; Song, L.; Zhang, Z.; Chen, N.; Feng, Z.; Qu, L. Tailored graphene systems for unconventional applications in energy conversion and storage devices. Energy Environ. Sci. 2015, 8, 31–54. [Google Scholar] [CrossRef]
- Zhao, L.; Lin, Z. Crafting Semiconductor Organic—Inorganic Nanocomposites via Placing Conjugated Polymers in Intimate Contact with Nanocrystals for Hybrid Solar Cells. Adv. Mater. 2012, 24, 4353–4368. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.S.; Scully, R.; McGehee, M.D. Effects of molecular interface modification in hybrid organic-inorganic photovoltaic cells. J. Appl. Phys. 2007, 101, 114503. [Google Scholar] [CrossRef]
- Kruger, R.A.; Gordon, T.J.; Baumgartner, T.; Sutherland, T.C. End-Group Functionalization of Poly(3-hexylthiophene) as an Efficient Route to Photosensitize Nanocrystalline TiO2 Films for Photovoltaic Applications. ACS Appl. Mater. Interfaces 2011, 3, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, Q.; Gomes, P.J.; Ribeiro, P.A.; Jones, N.C.; Hoffmann, S.V.; Mason, N.J.; Oliveira, O.N., Jr.; Raposo, M. Determination of Degree of Ionization of Poly(allylamine hydrochloride) (PAH) and Poly[1-[4-(3-carboxy-4 hydroxyphenylazo) benzene sulfonamido]-1,2-ethanediyl, sodium salt] (PAZO) in Layer-by-Layer Films using Vacuum Photoabsorption Spectroscopy. Langmuir 2013, 29, 448–455. [Google Scholar] [CrossRef]
- Ferreira, Q.; Gomes, P.J.; Raposo, M.; Giacometti, J.A.; Oliveira Jr, O.N.; Ribeiro, P.A. Influence of Ionic Interactions on the Photoinduced Birefringence of Poly [1-[4-(3-Carboxy-4 Hydroxyphenylazo) Benzene Sulfonamido]-1,2-Ethanediyl, Sodium Salt] Films. J. Nanosci. Nanotechnol. 2007, 7, 2659–2666. [Google Scholar] [CrossRef]
- Ferreira, Q.; Gomes, P.J.; Maneira MJ, P.; Ribeiro, P.A.; Raposo, M. Mechanisms of Adsorption of an Azo- polyelectrolyte onto Layer-by-Layer Films. Sens. Actuators B Chem. 2007, 126, 311–317. [Google Scholar] [CrossRef]
- Sério, S.; Melo Jorge, M.E.; Maneira, M.J.P.; Nunes, Y. Influence of O2 Partial Pressure on the Growth of Nanostructured Anatase Phase TiO2 Thin Films Prepared by DC Reactive Magnetron Sputtering. Mater. Chem. Phys. 2011, 126, 73–81. [Google Scholar] [CrossRef]
- Siopa, D.; Sério, S.; Jorge, M.E.M.; Viana, A.S.; Gomes, A. ZnO seed layers prepared by DC reactive magnetron sputtering to be applied as electrodeposition substrates. J. Electrochem. Soc. 2016, 163, H697–H704. [Google Scholar] [CrossRef]
- Das, S.; Pandey, D.; Thomas, J.; Roy, T. The role of graphene and other 2D materials in solar photovoltaics. Adv. Mater. 2019, 31, 1802722. [Google Scholar] [CrossRef]
- Carreira, D.; Ribeiro, P.; Raposo, M.; Sério, S. Development of Hybrid Solar Cells based on TiO2 or ZnO-Graphene Oxide Heterojunctions. In Proceedings of the 8th International Conference on Photonics, Optics and Laser Technology—Volume 1: PHOTOPTICS, Valletta, Malta, 27–29 February 2020; pp. 185–191, ISBN 978-989-758-401-5. [Google Scholar]
- Krogman, K.C.; Zacharia, N.S.; Schroeder, S.; Hammond, P.T. Automated process for improved uniformity and versatility of layer-by-layer deposition. Langmuir 2007, 23, 3137–3141. [Google Scholar] [CrossRef]
- Magro, C.; Mateus, E.P.; Paz-Garcia, J.M.; Sério, S.; Raposo, M.; Ribeiro, A.B. Electronic Tongue Coupled to an Electrochemical Flow Reactor for Emerging Organic Contaminants Real Time Monitoring. Sensors 2019, 19, 5349. [Google Scholar] [CrossRef] [PubMed]
- Magalhães-Mota, G.; Farinha, P.; Sério, S.; Ribeiro, P.A.; Raposo, M. Photochromic Materials Towards Energy Harvesting. In Optics, Photonics and Laser Technology. Springer Series in Optical Sciences; Ribeiro, P., Raposo, M., Eds.; Springer Publisher: Cham, Germany, 2018; Volume 218, pp. 223–241. [Google Scholar]
- Maria, R. Processos de adsorção em Filmes Automontados de Poli(o-metoxianilina): Evidência de Pontes de Hidrogénio além da Interação Iónica. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brasil, 1999. [Google Scholar]
- Topsakal, M.; Ciraci, S. Effects of Charging and Electric Field on Graphene Oxide. J. Phys. Chem. C 2013, 117, 5943–5952. [Google Scholar] [CrossRef]


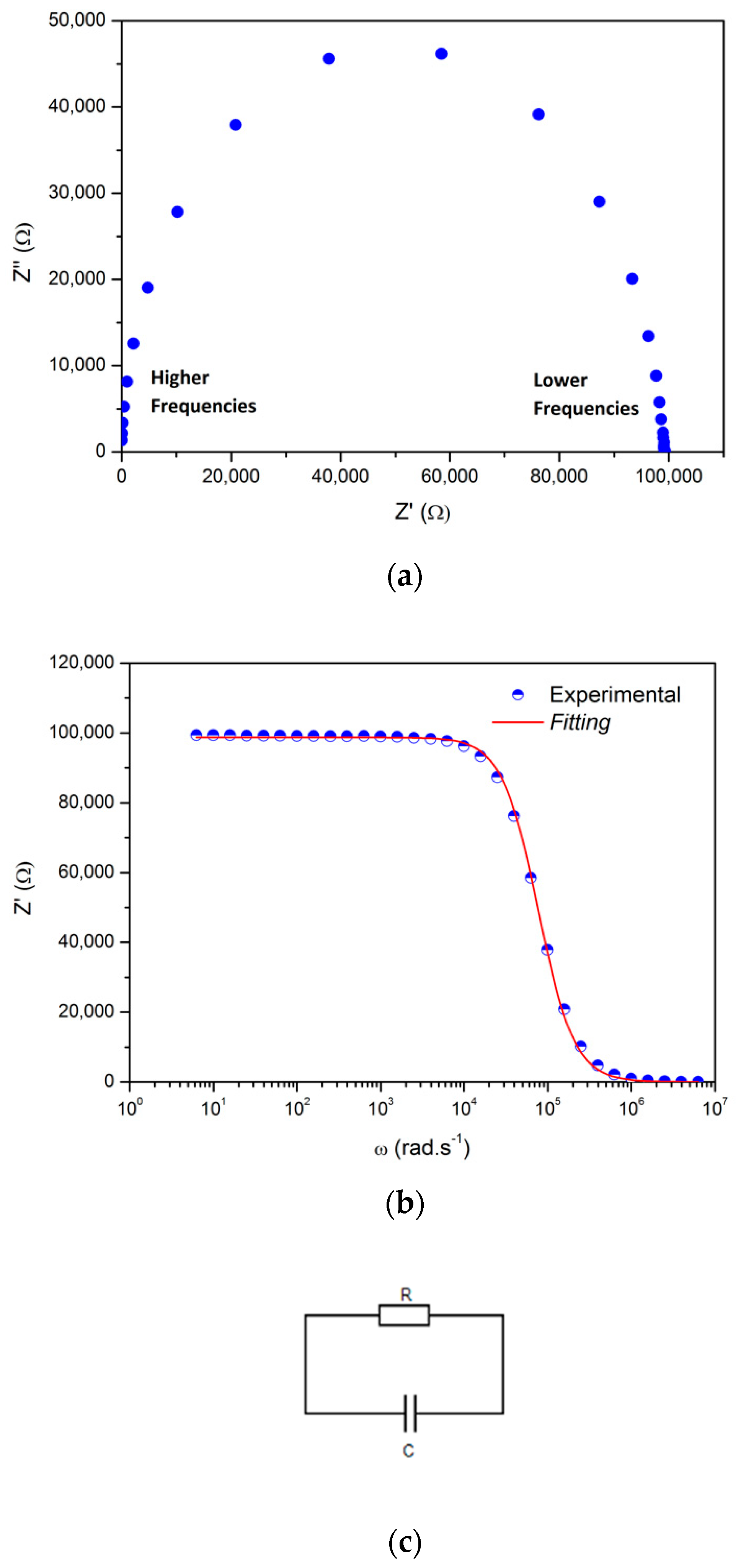

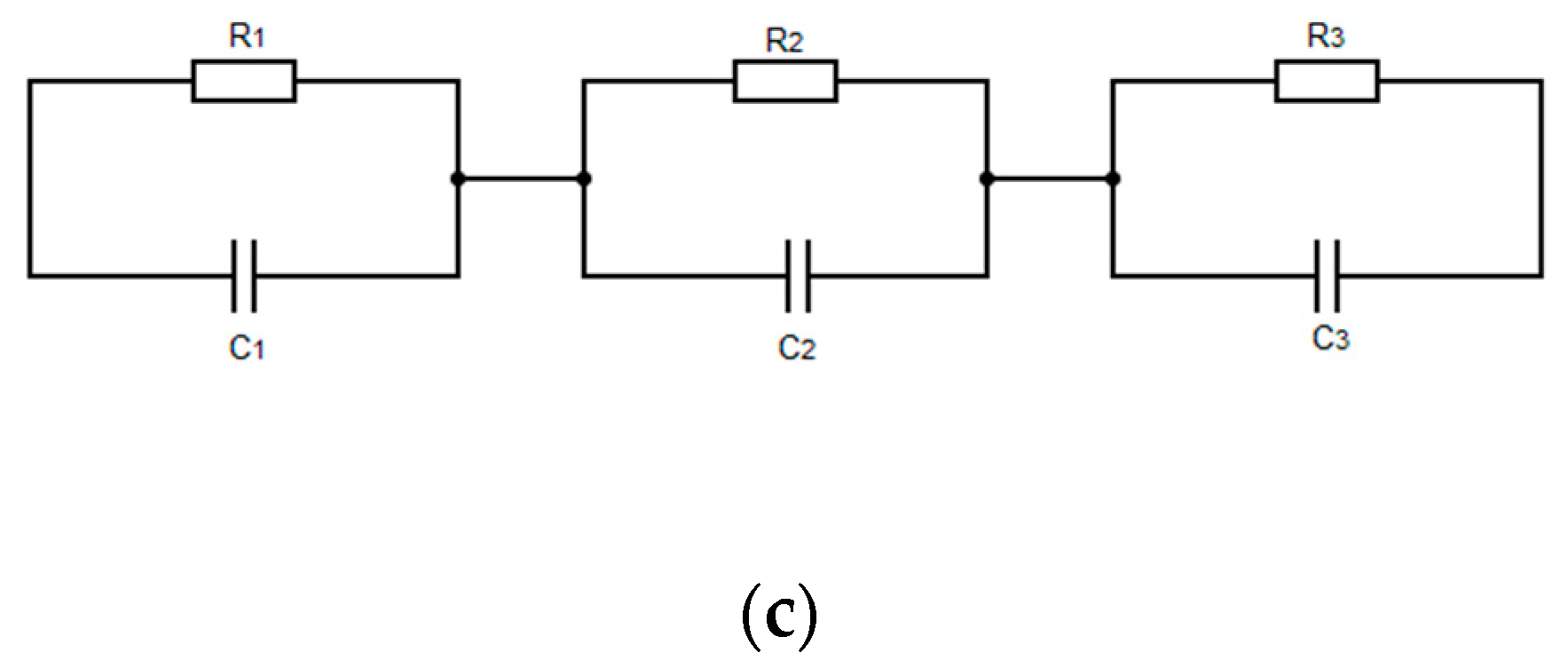
| Environmental Conditions | Equation | A (R1[Ω]) | B (R1C1[s]) | C (R2[Ω]) | D (R2C2[s]) | E (R3[Ω]) | F (R3C3[s]) |
|---|---|---|---|---|---|---|---|
| Dry (<10% RH) | (1) | 98 747 | 1.3 × 10−5 | --- | --- | --- | --- |
| Humid (50–60% RH) | (2) | 30,060 | 0.05457 | 11,642 | 6.2 × 10−4 | 17,513 | 3.5 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreira, D.; Ribeiro, P.A.; Raposo, M.; Sério, S. Engineering of TiO2 or ZnO—Graphene Oxide Nanoheterojunctions for Hybrid Solar Cells Devices. Photonics 2021, 8, 75. https://doi.org/10.3390/photonics8030075
Carreira D, Ribeiro PA, Raposo M, Sério S. Engineering of TiO2 or ZnO—Graphene Oxide Nanoheterojunctions for Hybrid Solar Cells Devices. Photonics. 2021; 8(3):75. https://doi.org/10.3390/photonics8030075
Chicago/Turabian StyleCarreira, Duarte, Paulo A. Ribeiro, Maria Raposo, and Susana Sério. 2021. "Engineering of TiO2 or ZnO—Graphene Oxide Nanoheterojunctions for Hybrid Solar Cells Devices" Photonics 8, no. 3: 75. https://doi.org/10.3390/photonics8030075
APA StyleCarreira, D., Ribeiro, P. A., Raposo, M., & Sério, S. (2021). Engineering of TiO2 or ZnO—Graphene Oxide Nanoheterojunctions for Hybrid Solar Cells Devices. Photonics, 8(3), 75. https://doi.org/10.3390/photonics8030075








