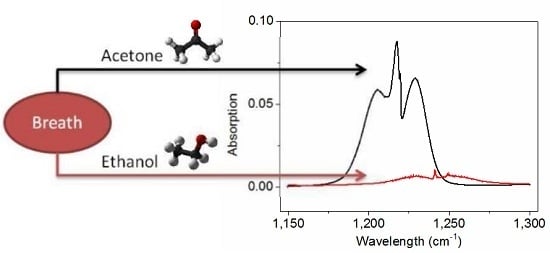
Influence of Ethanol on Breath Acetone Measurements Using an External Cavity Quantum Cascade Laser
Abstract
:1. Introduction
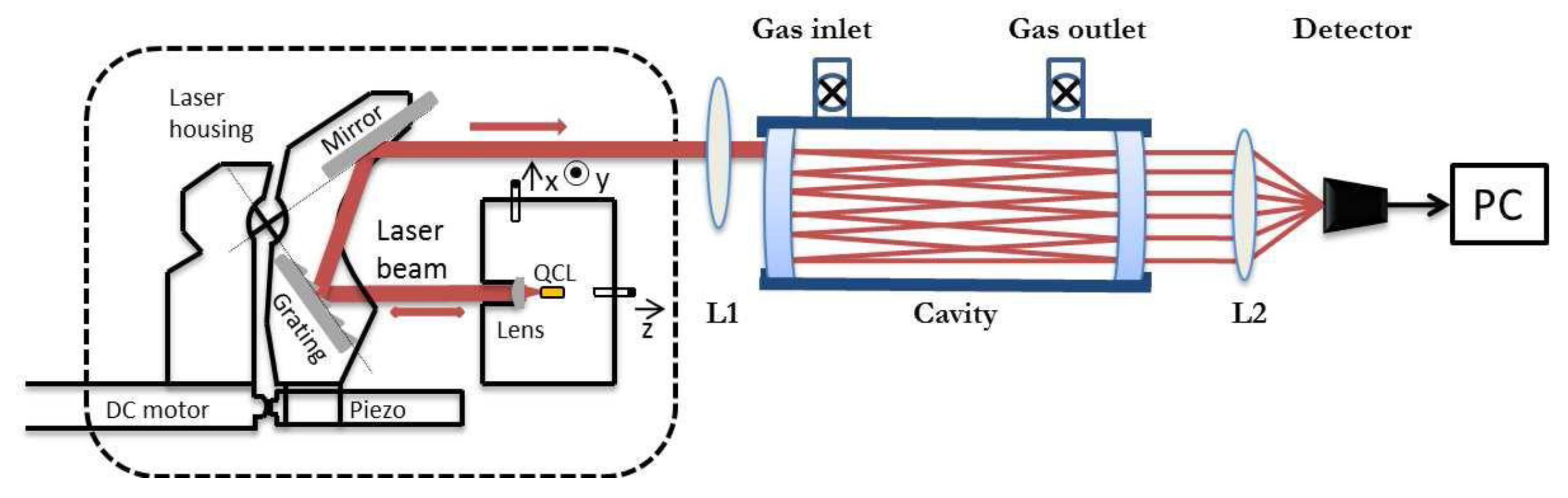
2. Experimental Setup
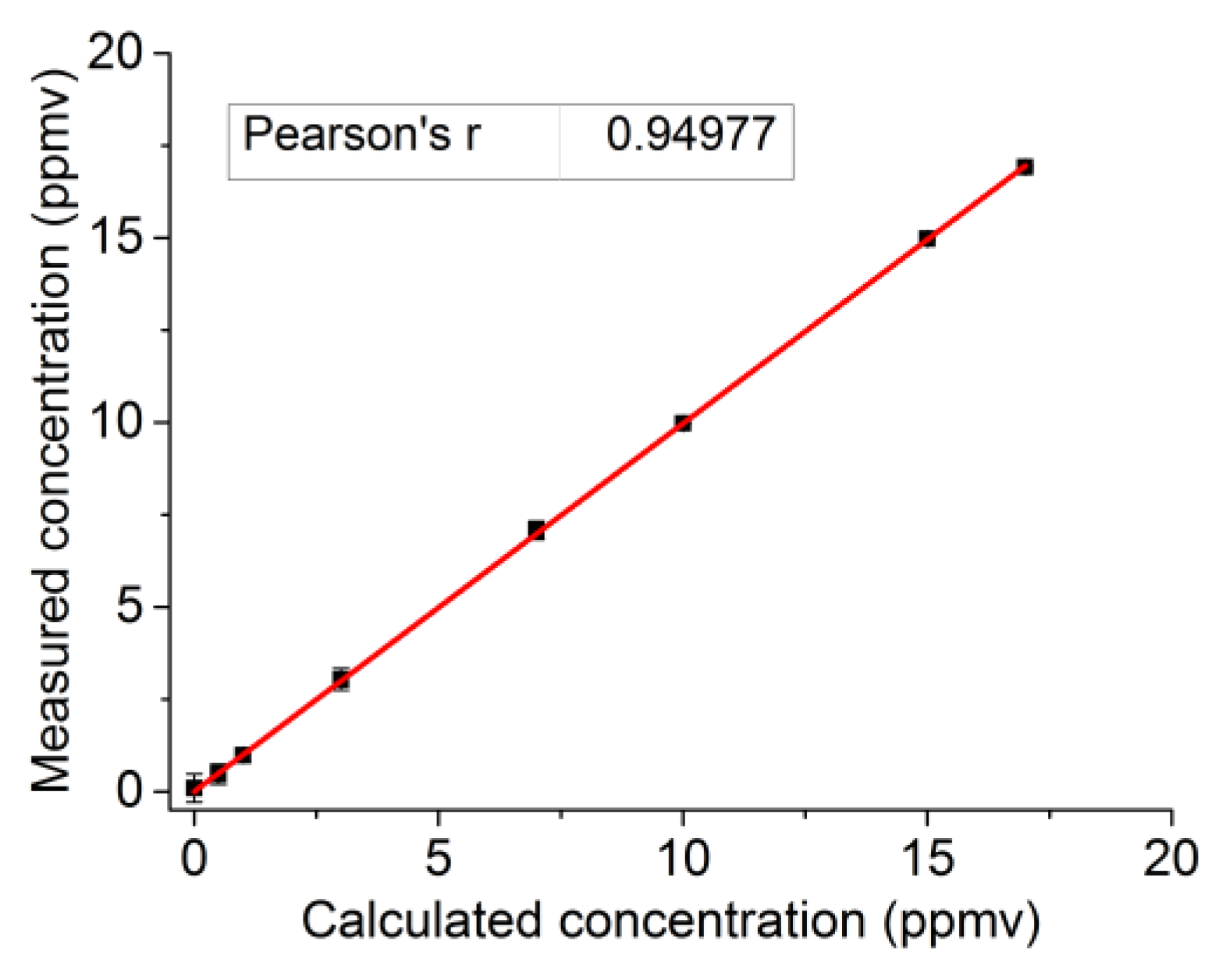
3. Calibration and Detection Limit
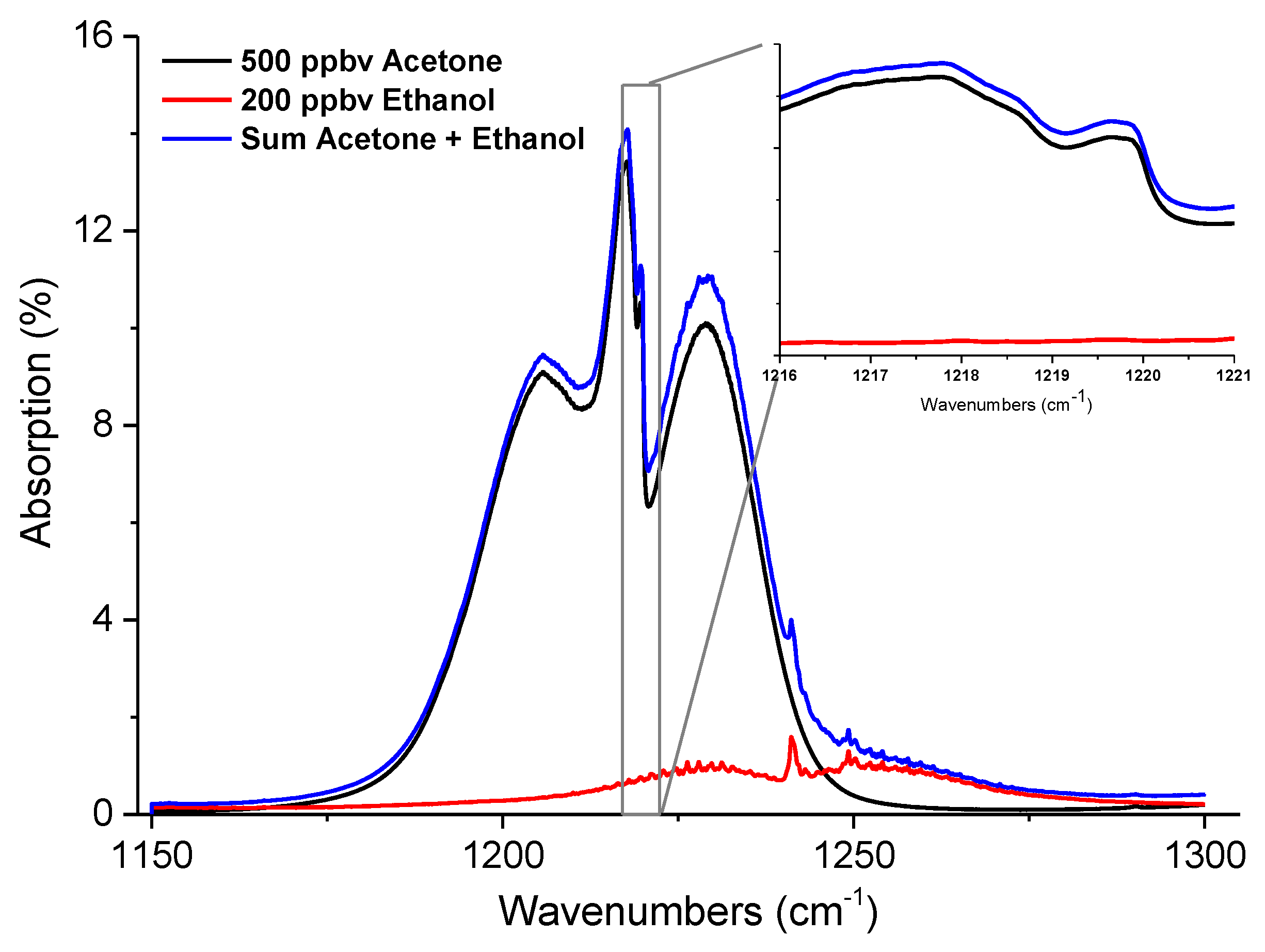
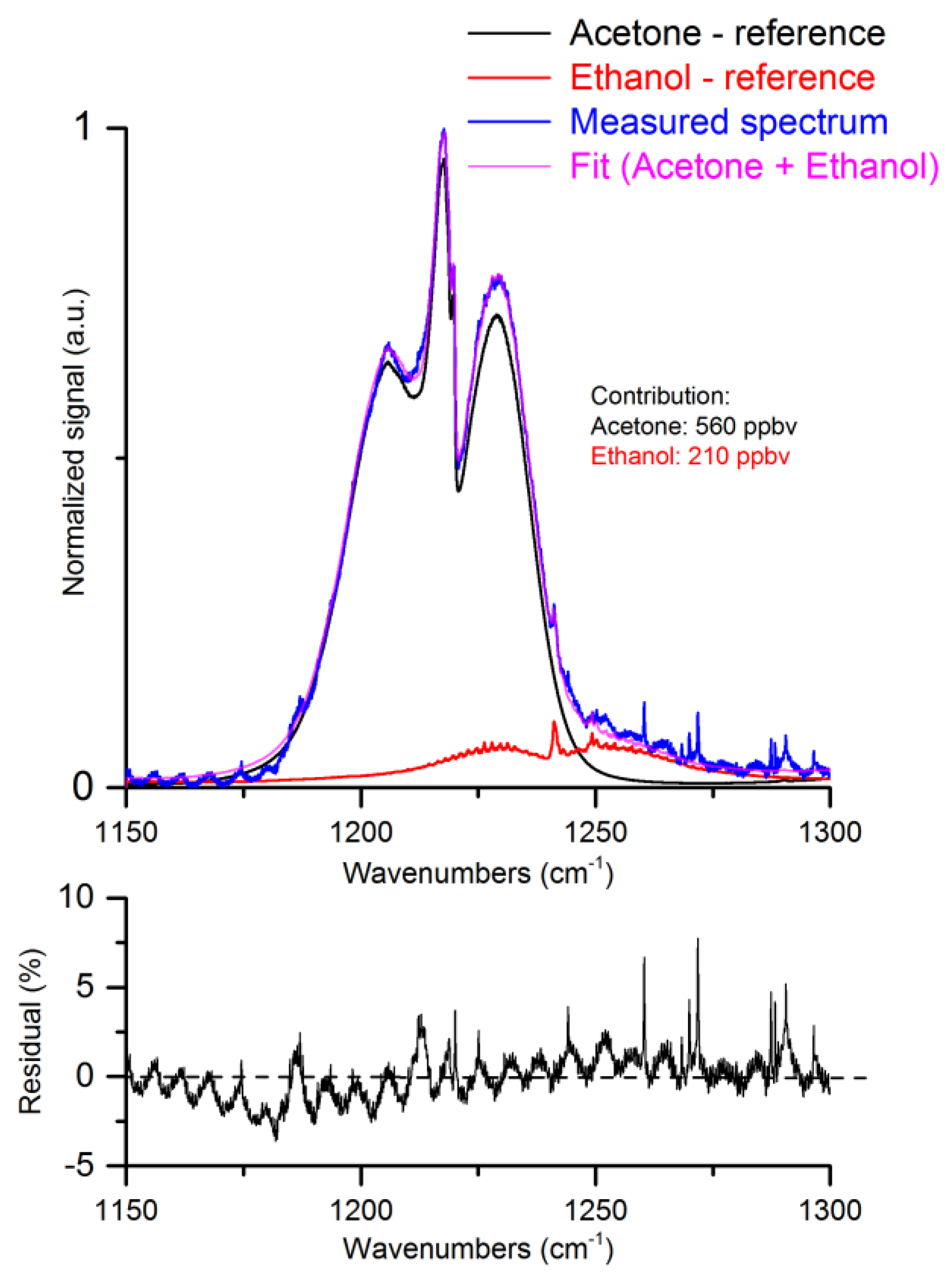
4. Ethanol Influence on Detection of Exhaled Acetone
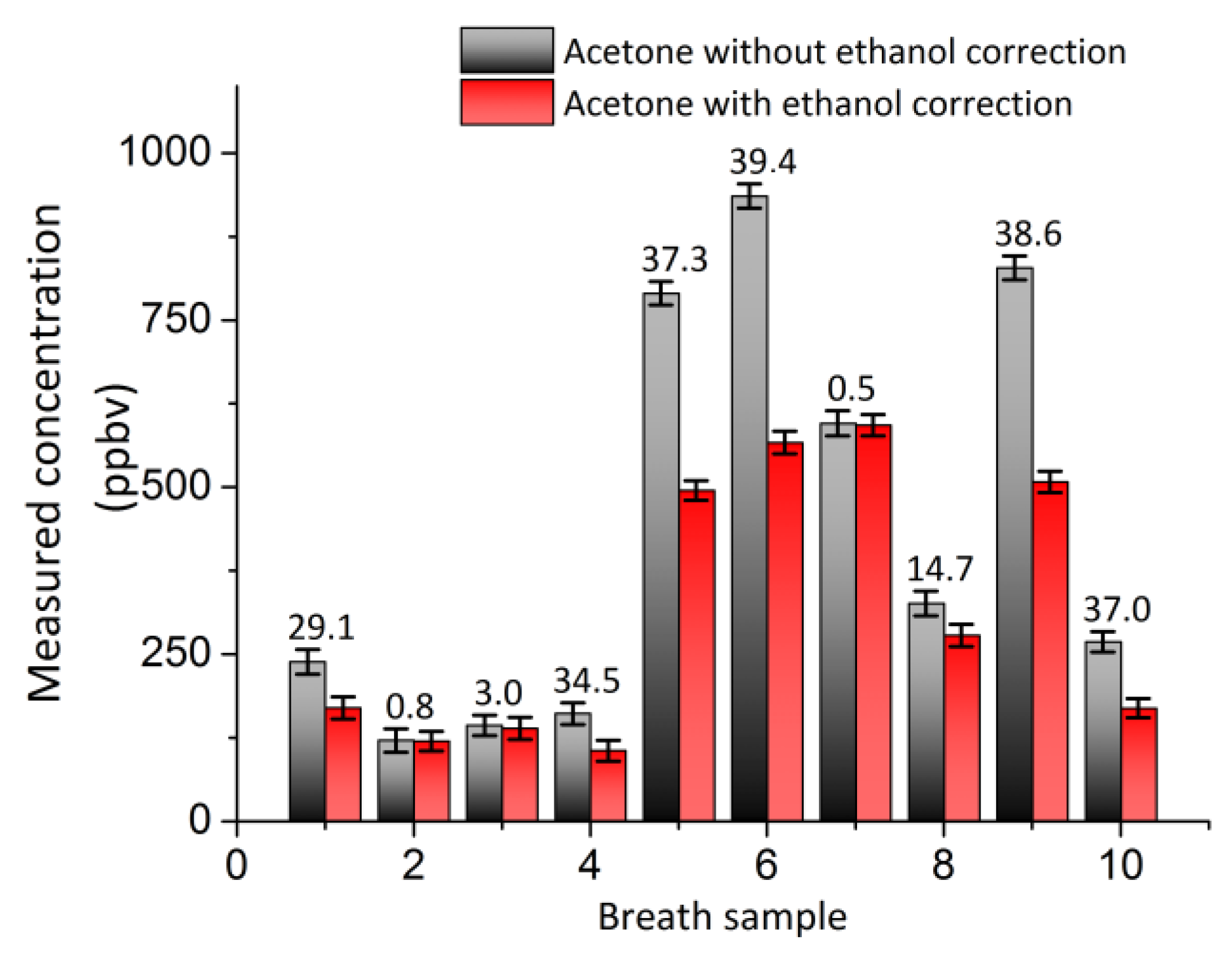
5. Interference Free Acetone Determination
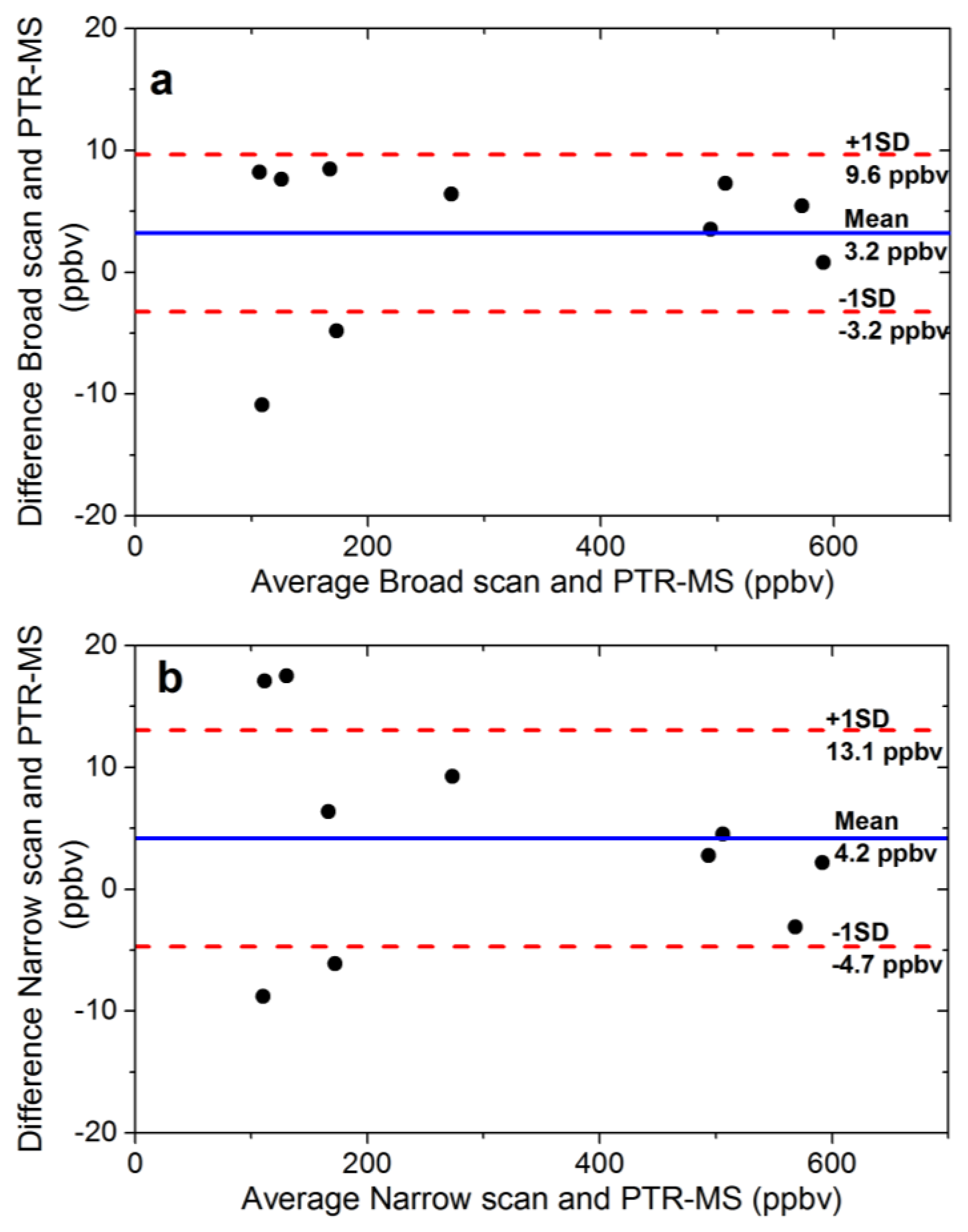
6. Validation of Ethanol-Corrected Breath Acetone Measurements with PTR-MS
7. Narrowband Detection of Exhaled Acetone
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amann, A.; Miekisch, W.; Schubert, J.; Buszewski, B.; Ligor, T.; Jezierski, T.; Pleil, J.; Risby, T. Analysis of exhaled breath for disease detection. Annu. Rev. Anal. Chem. 2014, 7, 455–482. [Google Scholar] [CrossRef] [PubMed]
- Risby, T.H.; Tittel, F.K. Current status of midinfrared quantum and interband cascade lasers for clinical breath analysis. Opt. Eng. 2010, 49, 111123. [Google Scholar]
- Samudrala, D.; Lammers, G.; Mandon, J.; Blanchet, L.; Schreuder, T.H.A.; Hopman, M.T.; Harren, F.J.M.; Tappy, L.; Cristescu, S.M. Breath acetone to monitor life style interventions in field conditions: An exploratory study. Obesity 2014, 22, 980–983. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Spanel, P.; Fryer, A.A.; Hanna, F.; Ferns, G.A.A. Can volatile compounds in exhaled breath be used to monitor control in diabetes mellitus? J. Breath Res. 2011, 5, 022001. [Google Scholar] [CrossRef] [PubMed]
- Musa-Veloso, K.; Likhodii, S.S.; Cunnane, S.C. Breath acetone is a reliable indicator of ketosis in adults consuming ketogenic meals. Am. J. Clin. Nutr. 2002, 76, 65–70. [Google Scholar] [PubMed]
- Smith, D.; Spanel, P.; Davies, S. Trace gases in breath of healthy volunteers when fasting and after a protein-calorie meal: A preliminary study. J. Appl. Physiol. 1999, 87, 1584–1588. [Google Scholar] [PubMed]
- King, J.; Kupferthaler, A.; Unterkofler, K.; Koc, H.; Teschl, S.; Teschl, G.; Miekisch, W.; Schubert, J.; Hinterhuber, H.; Amann, A. Isoprene and acetone concentration profiles during exercise on an ergometer. J. Breath Res. 2009, 3, 027006. [Google Scholar] [CrossRef] [PubMed]
- Mieth, M.; Schubert, J.K.; Groeger, T.; Sabel, B.; Kischkel, S.; Fuchs, P.; Hein, D.; Zimmermann, R.; Miekisch, W. Automated needle trap heart-cut GC/MS and needle trap comprehensive two-dimensional GC/TOF-MS for breath gas analysis in the clinical environment. Anal. Chem. 2010, 82, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Spanel, P.; Smith, D. A longitudinal study of ammonia, acetone and propanol in the exhaled breath of 30 subjects using selected ion flow tube mass spectrometry, SIFT-MS. Physiol. Meas. 2006, 27, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Pizzini, A.; Arendacká, B.; Zerlauth, K.; Filipiak, W.; Schmid, A.; Dzien, A.; Neuner, S.; Lechleitner, M.; Scholl-Bürgi, S.; et al. Breath acetone—aspects of normal physiology related to age and gender as determined in a PTR-MS study. J. Breath Res. 2009, 3, 027003. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Spanel, P.; Smith, D. A longitudinal study of ethanol and acetaldehyde in the exhaled breath of healthy volunteers using selected-ion flow-tube mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Arslanov, D.D.; Swinkels, K.; Cristescu, S.M.; Harren, F.J.M. Real-time, subsecond, multicomponent breath analysis by optical parametric oscillator based off-axis integrated cavity output spectroscopy. Opt. Express 2011, 19, 24078–24089. [Google Scholar] [CrossRef] [PubMed]
- Maulini, R.; Mohan, A.; Giovannini, M.; Faist, J.; Gini, E. External cavity quantum-cascade laser tunable from 8.2 to 10.4 m using a gain element with a heterogeneous cascade. Appl. Phys. Lett. 2006, 88, 201113. [Google Scholar] [CrossRef]
- Peng, C.; Luo, G.P.; Le, H.Q. Broadband, continuous, and fine-tune properties of external-cavity thermoelectric-stabilized mid-infrared quantum-cascade lasers. Appl. Opt. 2003, 42, 4877–4882. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, G.; Lewicki, R.; Curl, R.F.; Tittel, F.K.; Diehl, L.; Capasso, F.; Troccoli, M.; Hofler, G.; Bour, D.; Corzine, S.; et al. Widely tunable mode-hop free external cavity quantum cascade lasers for high resolution spectroscopy and chemical sensing. Appl. Phys. B Lasers Opt. 2008, 92, 305–311. [Google Scholar] [CrossRef]
- Curl, R.F.; Capasso, F.; Gmachl, C.; Kosterev, A.A.; McManus, B.; Lewicki, R.; Pusharsky, M.; Wysocki, G.; Tittel, F.K. Quantum cascade lasers in chemical physics. Chem. Phys. Lett. 2010, 487, 1–18. [Google Scholar] [CrossRef]
- Centeno, R.; Marchenko, D.; Mandon, J.; Cristescu, S.M.; Wulterkens, G.; Harren, F.J.M. High power, widely tunable, mode-hop free, continuous wave external cavity quantum cascade laser for multi-species trace gas detection. Appl. Phys. Lett. 2014, 105, 261907. [Google Scholar] [CrossRef]
- Ciaffoni, L.; Hancock, G.; Harrison, J.J.; van Helden, J.-P.H.; Langley, C.E.; Peverall, R.; Ritchie, G.A.D.; Wood, S. Demonstration of a mid-infrared cavity enhanced absorption spectrometer for breath acetone detection. Anal. Chem. 2013, 85, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Hancock, G.; Langley, C.E.; Peverall, R.; Ritchie, G.A.D.; Taylor, D. Laser-based method and sample handling protocol for measuring breath acetone. Anal. Chem. 2014, 86, 5838–5843. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, G.; Curl, R.F.; Tittel, F.K.; Maulini, R.; Bulliard, J.M.; Faist, J. Widely tunable mode-hop free external cavity quantum cascade laser for high resolution spectroscopic applications. Appl. Phys. B Lasers Opt. 2005, 81, 769–777. [Google Scholar] [CrossRef]
- Marchenko, D.; Mandon, J.; Cristescu, S.M.; Merkus, P.J.F.M.; Harren, F.J.M. Quantum cascade laser-based sensor for detection of exhaled and biogenic nitric oxide. Appl. Phys. B Lasers Opt. 2013, 111, 359–365. [Google Scholar] [CrossRef]
- Parameswaran, K.R.; Rosen, D.I.; Allen, M.G.; Ganz, A.M.; Risby, T.H. Off-axis integrated cavity output spectroscopy with a mid-infrared interband cascade laser for real-time breath ethane measurements. Appl. Opt. 2009, 48, B73–B79. [Google Scholar] [CrossRef] [PubMed]
- Lewicki, R.; Wysocki, G.; Kosterev, A.A.; Tittel, F.K. Qepas based detection of broadband absorbing molecules using a widely tunable, cw quantum cascade laser at 8.4 μm. Opt. Express 2007, 15, 7357–7366. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C.; Taubman, M.S.; Bernacki, B.E.; Cannon, B.D.; Stahl, R.D.; Schiffern, J.T.; Myers, T.L. Real-time trace gas sensing of fluorocarbons using a swept-wavelength external cavity quantum cascade laser. Analyst 2014, 139, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.S.; Paul, J.B.; Gupta, J.B.; O’Keefe, A. Sensitive absorption measurements in the near-infrared region using off-axis integrated-cavity-output spectroscopy. Appl. Phys. B Lasers Opt. 2002, 75, 261–265. [Google Scholar] [CrossRef]
- Moyer, E.J.; Sayres, D.S.; Engel, G.S.; Clair, J.M.S.; Keutsch, F.N.; Allen, N.T.; Kroll, J.H.; Anderson, J.G. Design considerations in high-sensitivity off-axis integrated cavity output spectroscopy. Appl. Phys. B Lasers Opt. 2008, 92, 467–474. [Google Scholar] [CrossRef]
- Paul, J.B.; Lapson, L.; Anderson, J.G. Ultrasensitive absorption spectroscopy with a high-finesse optical cavity and off-axis alignment. Appl. Opt. 2001, 40, 4904–4910. [Google Scholar] [CrossRef] [PubMed]
- Centeno, R.; Mandon, J.; Cristescu, S.M.; Harren, F.J.M. Three mirror off axis integrated cavity output spectroscopy for the detection of ethylene using a quantum cascade laser. Sens. Actuators B Chem. 2014, 203, 311–319. [Google Scholar] [CrossRef]
- Mandon, J.; Hogman, M.; Merkus, J.F.M.; van Amsterdam, J.; Harren, F.J.M.; Cristescu, S.M. Exhaled nitric oxide monitoring by quantum cascade laser: Comparison with chemiluminescent and electrochemical sensors. J. Biomed. Opt. 2012, 17, 017003. [Google Scholar] [CrossRef] [PubMed]
- Crespo, E.; Cristescu, S.M.; de Ronde, H.; Kuijper, S.; Kolk, A.H.J.; Anthony, R.M.; Harren, F.J.M. Proton transfer reaction mass spectrometry detects rapid changes in volatile metabolite emission by mycobacterium smegmatis after the addition of specific antimicrobial agents. J. Microbiol. Meth. 2011, 86, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Boamfa, E.I.; Steeghs, M.M.L.; Cristescu, S.M.; Harren, F.J.M. Trace gas detection from fermentation processes in apples; an intercomparison study between proton-transfer-reaction mass spectrometry and laser photoacoustics. Int. J. Mass Spectrom. 2004, 239, 193–201. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Centeno, R.; Mandon, J.; Harren, F.J.M.; Cristescu, S.M. Influence of Ethanol on Breath Acetone Measurements Using an External Cavity Quantum Cascade Laser. Photonics 2016, 3, 22. https://doi.org/10.3390/photonics3020022
Centeno R, Mandon J, Harren FJM, Cristescu SM. Influence of Ethanol on Breath Acetone Measurements Using an External Cavity Quantum Cascade Laser. Photonics. 2016; 3(2):22. https://doi.org/10.3390/photonics3020022
Chicago/Turabian StyleCenteno, Raymund, Julien Mandon, Frans J. M. Harren, and Simona M. Cristescu. 2016. "Influence of Ethanol on Breath Acetone Measurements Using an External Cavity Quantum Cascade Laser" Photonics 3, no. 2: 22. https://doi.org/10.3390/photonics3020022
APA StyleCenteno, R., Mandon, J., Harren, F. J. M., & Cristescu, S. M. (2016). Influence of Ethanol on Breath Acetone Measurements Using an External Cavity Quantum Cascade Laser. Photonics, 3(2), 22. https://doi.org/10.3390/photonics3020022