Abstract
Recent developments in quantum cascade lasers have enabled the development of new sensors for in-situ applications that have so far only been possible with extractive systems. In this work, a sensor is presented using a unique Wavelength Modulation Spectroscopy approach to measure tetrafluoromethane, a strong greenhouse gas. The sensor was characterized in a laboratory environment indicating a long-term detection limit of 20 ppb·m and a short-term value of well below 10 ppb·m. To demonstrate the feasibility of the sensor in a real-world environment, it was installed at an Alcoa aluminum smelter. A co-located Fourier Transform Infrared Spectrometer allowed direct comparison measurements of both systems. General agreement between the two methods was observed, leading to the conclusion that the developed in-situ quantum cascade laser based sensor has the potential to continuously measure tetrafluoromethane at aluminum smelters.
1. Introduction
In recent years, the negative effect of so-called greenhouse gases like carbon dioxide (CO), methane (CH) and nitrous oxide (NO) on global climate has become evident. The impact of different gases can be directly compared by means of their global warming potentials (GWP); a numerical value describing the propensity to trap heat expressed as a multiple of the potential of carbon dioxide [1]. As depicted in Table 1, the perfluorocarbons (PFCs) tetrafluoromethane (CF) and hexafluoroethane (CF), have very high GWP values indicating significant contributions to the greenhouse effect despite low atmospheric concentrations.

Table 1.
Overview of atmospheric concentration levels, lifetimes and global warming potentials for a time period of 500 years [1].
The aluminum smelting process, also known as the Hall-Héroult process, is one of the largest anthropogenic contributors to atmospheric PFCs [2]. When the dissolved alumina content in the cryolitic bath falls below ~1.5%, a rapid rise in the operating voltage occurs and the carbon anodes begin to react with the fluoride-based electrolyte (NaAlF) forming PFCs. These transient events are referred to as anode effects (AEs) [3]. Recent reports indicate that in addition to AEs, PFC generation may also occur during normal potroom activities that result in either (1) a localized increase in anode current density or (2) a localized deficiency in alumina concentration; PFCs emitted during these periods not associated with AEs have been referred to as low-voltage PFCs (LV-PFCs) [4,5,6].
Fourier Transform Infrared (FTIR) spectrometers are commonly employed by the industry to measure PFCs in real time, providing an accurate snapshot of the emission profile during typical potroom operation. The technique relies on extractive sampling from the exhaust stream and subsequent conditioning of the sample gas prior to the measurement. The exhaust stream at aluminum smelters is rather hostile to FTIR spectrometers: the sample gases contain a high dust loading, chemically aggressive species (e.g., hydrogen fluoride, HF), and spectrally interfering compounds (e.g., water vapor, HO) [7]. As such, deployment of an FTIR spectrometer for continuous monitoring of PFCs would require significant upkeep and daily maintenance. Instead, the industry relies on short-term in-plant campaigns to spectroscopically determine levels of PFCs emitted during a representative sampling of AEs spanning typical production cycles. Combining PFC emission values with production and AE metrics yields plant-specific slope terms used to estimate the long-term relationship between PFC emissions and smelter process parameters [8].
In-plant measurements are also performed in order to ensure regulatory compliance, at a frequency prescribed by the ruling government (e.g., triennial for Quebecois smelters [9]) or upon significant changes to the process control algorithm impacting AE metrics.
Tunable laser absorption spectroscopy (TLAS) is nowadays considered to be a mature technology for in-situ measurements allowing to perform year-round continuous emission measurements (CEMS) with a higher accuracy and faster response time than extractive methods while requiring significantly lower maintenance.
The aluminum industry is using TLAS sensors for many years in potrooms and exhaust stacks to perform in-situ measurements of HF [10]. This success has led to the aluminum industry’s interest in evaluating a similar technology for in-situ CF offering the capability to continuously monitor PFC emissions.
2. In-Situ Tetrafluoromethane Sensor
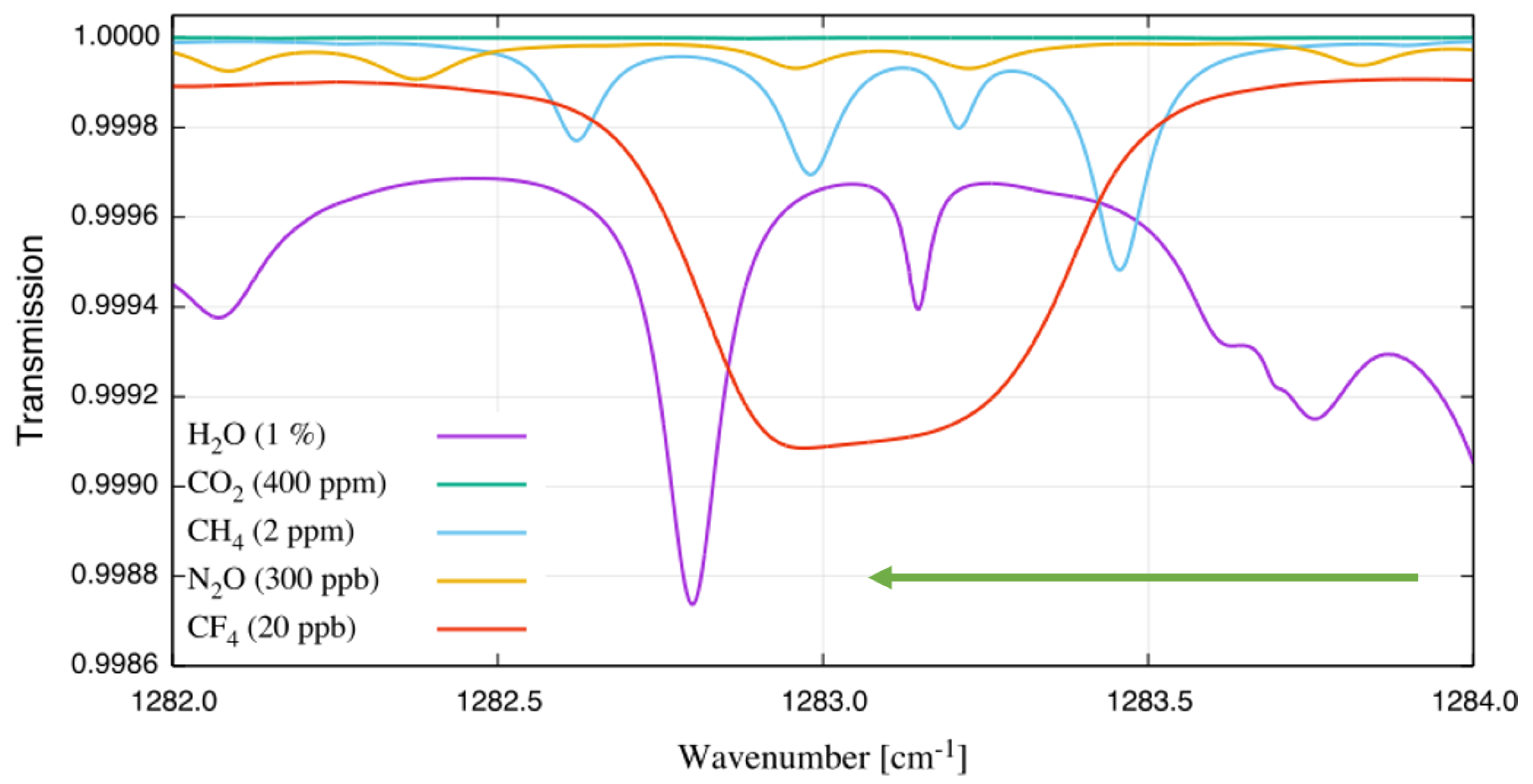
According to the HITRAN (high-resolution transmission molecular absorption database) [11] and PNNL (vapor phase infrared spectral library) [12] spectroscopic databases, CF does not absorb in the near-infrared but displays a strong absorption feature in the mid-infrared range located around 1283 cm. A simulated transmission spectrum of 20 ppb CF in ambient air using an optical path length (OPL) of 1 m and ambient pressure is shown in Figure 1. The simulated spectra were assembled using data available from the HITRAN and HITEMP (high-temperature molecular spectroscopic database) [13] databases. A gas temperature of 125 C was chosen since it is a typical value for emission monitoring applications. Under the described conditions, the absorption feature for CF is nearly 1 cm broad, containing more than 8000 absorption lines. Interference from HO, CH, and NO is significant, whereas CO does not absorb in this wavelength range.
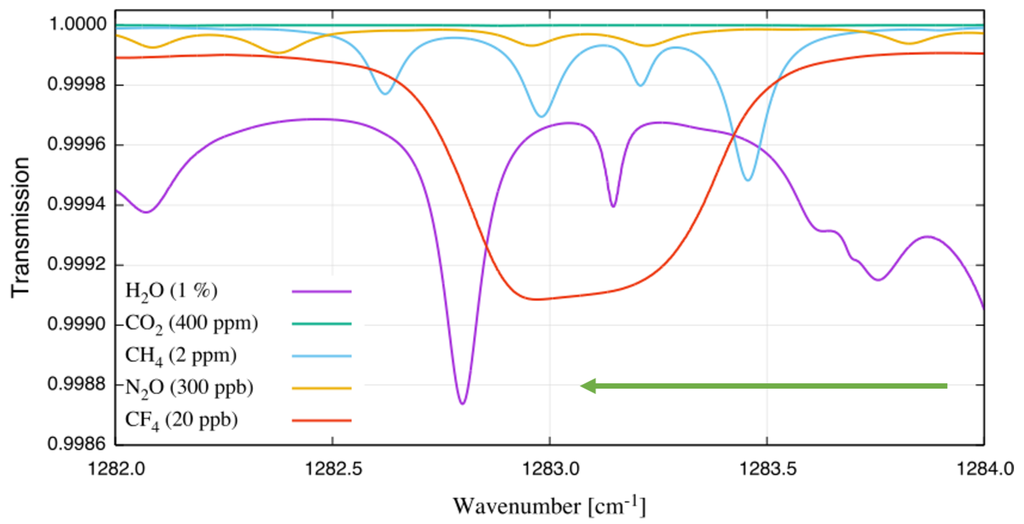
Figure 1.
Transmission simulation of ambient air and tetrafluoromethane using the HITRAN and HITEMP databases. The simulation is performed for an optical path length of 1 m, ambient pressure and a gas temperature of 125 C. The gas components and their concentrations are provided in the legend.
First reports of mid-infrared TLAS sensors for in-situ measurements of PFCs employed lead-salt lasers [14,15,16]. These sensors achieved detection limits in the low parts-per-billion (ppb) range with a few seconds acquisition time, the systems were employing multi-pass cells and extractive sampling. However, lead-salt lasers require cryogenic cooling and are in many cases unreliable; therefore they are not generally considered suitable light sources for sensors in permanent industrial installations.
Recent developments in quantum cascade laser (QCL) technology [17] have made compact and reliable mid-infrared laser sources available that are able to operate in continuous-wave (cw) mode at room temperature and even above. QCLs have proven to be feasible in several field applications; e.g., the measurement of nitric oxide (NO) in household waste incinerators [18] or atmospheric sensing of nitrous oxide and carbon monoxide (CO) [19].
The CF sensor presented in this work is based on the commercial LaserGas Q™ platform [20] and is an on-stack sensor consisting of two units: a transmitter and receiver. In the transmitter, a distributed feedback (DFB) QCL from Corning Incorporated (now Thorlabs Quantum Electronics, Inc., Dearborn Heights, MI, USA) in an optimized HHL-package [21] is used. The laser has an optical output power of about 35 mW at +40 C (driving conditions: 320 mA, ~9.5 V), a current tuning rate of 0.015 cm/mA and a temperature tuning rate of 0.09 cm/K. The beam is collimated and focused by antireflection (AR)-coated CaF-lenses. A mercury cadmium telluride (MCT) detector from Vigo System S.A. (PVI-2TE-8) is used in the receiver unit.
Optical detection of the sensor is based on the Wavelength Modulation Spectroscopy (WMS) technique previously described by, for example, Linnerud et al. [10] and Kluczynski et al. [22].
The emission optical frequency of the quantum cascade laser was adjusted to about 1283.9 cm by setting the operation temperature to +37.5 C while applying a direct current of approximately 290 mA. A current ramp of 58.5 mA is subsequently used to tune the optical frequency within a narrow range of about 0.9 cm. The tuning range is indicated by the green arrow in Figure 1. The current ramp, and thus the emission wavelength of the laser, is modulated to enable WMS-based detection. The laser beam is passing through the sample and a detector is measuring the transmitted optical signal on the receiver side. A hardware mixer extracts the second harmonic component within the preamplified detector signal. The second harmonic signal is sampled by an analogue-digital converter; 64 points of the scan are recorded. Adjustable low-pass filters embedded in the signal-processing software are used to further improve the signal-to-noise ratio.
To help reduce the strong interference from HO and other gases (Figure 1), only a small portion of the CF absorption feature is scanned. The wavelength region of the strongest curvature change at about 1283.3 cm is used to perform measurements with a modulation amplitude that is much smaller than the width of the absorption feature [23]. The measured trace is therefore closer to the true second derivative of the direct absorption signal. This is different to conventional WMS, where modulation amplitudes comparable to the width of the absorption line are applied [10,22]. Although the sensitivity of this unique approach is reduced (as will be shown in the noise analysis in Section 3), a much better selectivity can been achieved. This is especially useful to detect broad absorption features with sharp edges typical for the Q-branch of ro-vibrational spectra.
3. Laboratory Evaluation
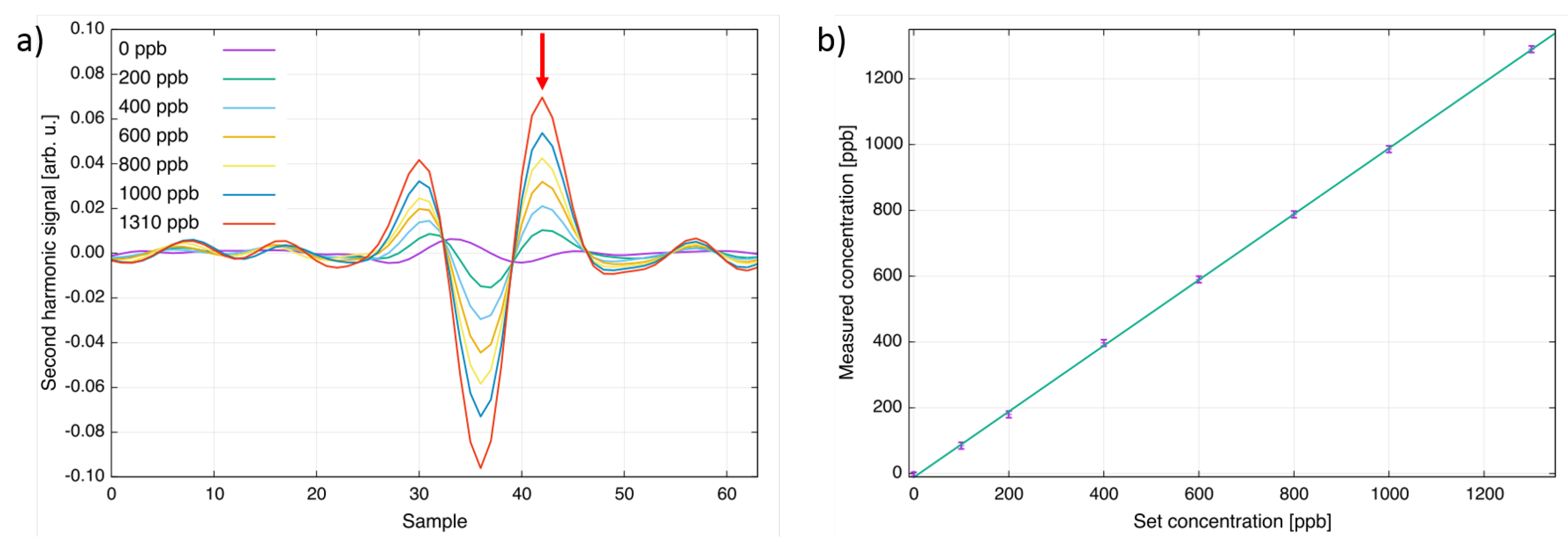
In order to calibrate and characterize the sensor’s analytical performance, it was mounted onto a heated single-pass cell with an OPL of 700 mm and capable of heating gas mixtures up to 400 C. A gas cylinder with 4.65 ppm CF in nitrogen (certified accuracy ±5%) and a gas mixer (HovaGas Digital G6) were used to perform a calibration of the sensor. Different concentrations ranging from 0 ppb to 1310 ppb CF in nitrogen were generated and filled into the absorption cell. Figure 2a shows second harmonic spectra collected at a cell temperature of 125 C. In Figure 2b, the set concentrations of the gas mixer are plotted versus the measured concentration of the developed sensor. As can be seen, the response is linear within the accuracy of the gas mixer (±1%) and the noise level of the CF sensor.
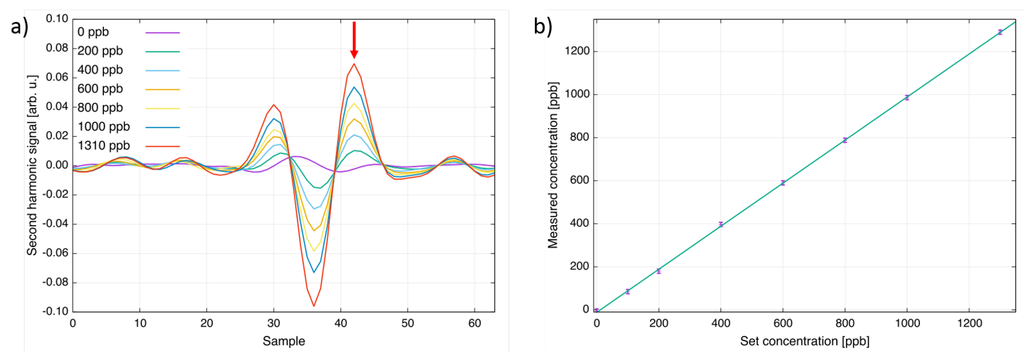
Figure 2.
(a) Second harmonic spectra of CF for different concentration levels at T = 125 C. The peak indicated by an arrow was used for the concentration calculation; (b) Comparison of the concentration set at the gas mixer and the value measured with the QCL CF sensor.
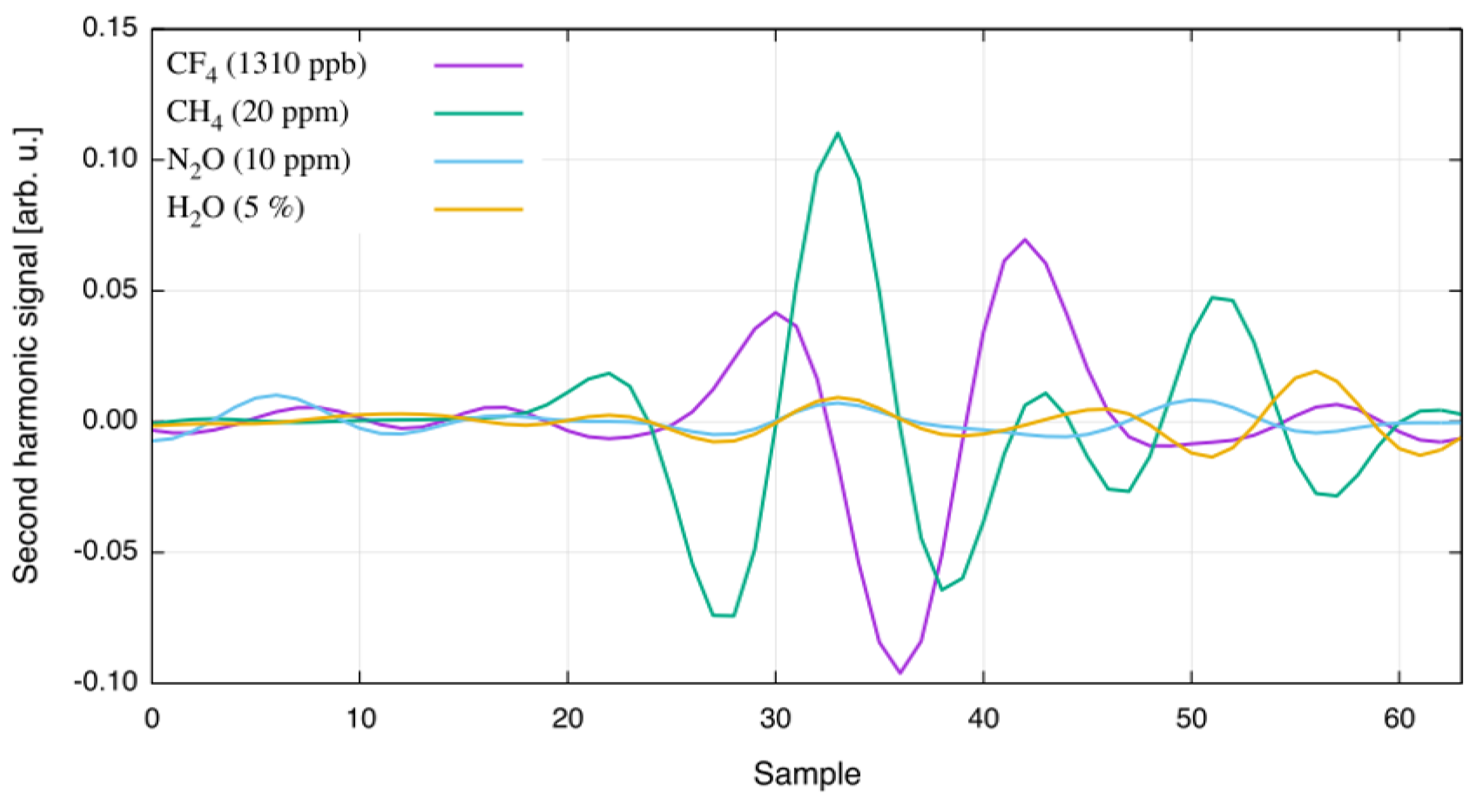
Following analysis with spectra provided by the HITRAN and HITEMP databases, the response of the CF sensor was evaluated with respect to potentially interfering gases by sequentially filling the heated single-pass cell with 20 ppm CH, 10 ppm NO, and 5% HO (generated with a HovaCAL digital 112-MF vapor generator). The recorded second harmonic spectra are plotted in Figure 3. A comparison of the measured spectra and the HITRAN simulation confirms good agreement between line position and absorption strength of the interferents evaluated.
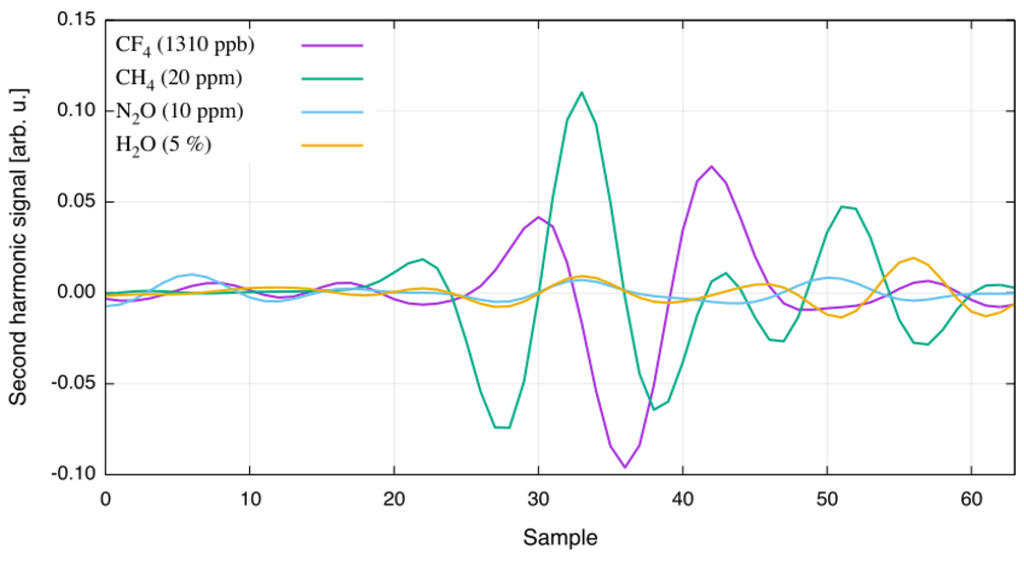
Figure 3.
Second harmonic spectra of interfering gases at T = 125 C. The gases and their concentration are given in the legend.
Table 2 quantifies contributions from the different gases to the CF measurement. Despite the close proximity of interfering absorption lines, the influence on the CF measurement is limited for typical emission gas mixtures in the aluminum industry.

Table 2.
Overview of interference at a gas temperature of 125 C.
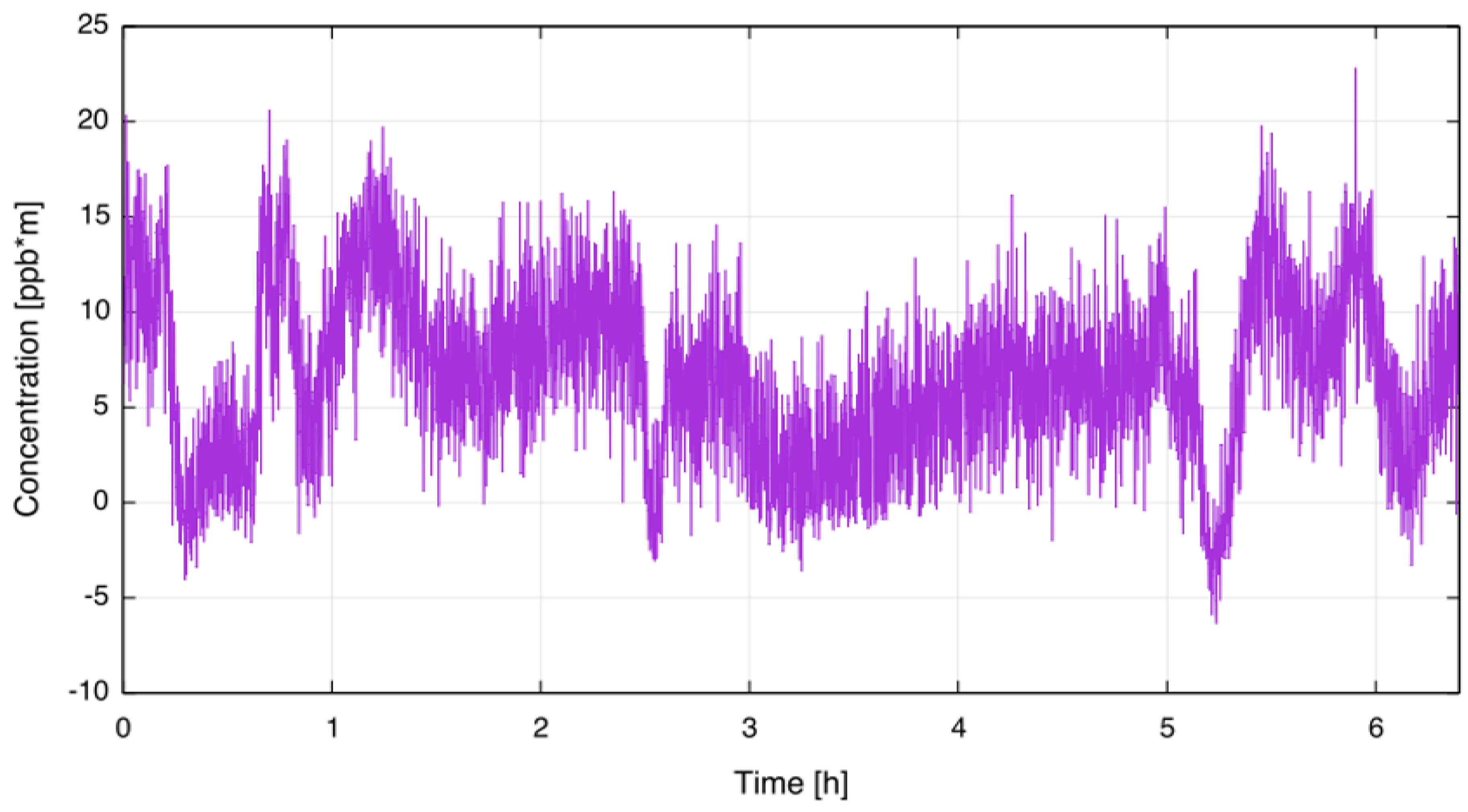
The lower detection limit (LDL) of the sensor was determined by mounting the sensor on an absorption cell (OPL = 735 mm) filled with ambient air, putting it into a climate chamber and measuring the CF concentration for several hours. To simulate real-world ambient conditions, the set temperature of the climate chamber was changed in several steps from ambient to 55 C and back to ambient again. For this test the averaging time of the sensor was set to 5 s. From Figure 4, a short term noise level of less than 10 ppb·m (peak-to-peak value), corresponding to a sensitivity of 3.5 × 10 [rel. abs.], was deducted. The achieved sensitivity is in good agreement with other WMS QCL sensors, as recently reported by, for example, Ren et al. [24] and Cao et al. [25]. In previous work using a similar setup applying traditional WMS to measure sulphur dioxide at 7.5 µm a short term sensitivity of 2 × 10 [rel. abs.] was demonstrated [21]. As expected, the new approach is less sensitive than traditional WMS while providing a higher selectivity for molecules with broad absorption features. Concentration values reported by the QCL sensor remained below 20 ppb·m across the testing period, so it can be assumed that this is the long-term LDL of the QCL CF sensor. The Allan-Werle deviation [26] and thus the theoretical LDL was not investigated since in this context only the LDL for the targeted application was of interest.
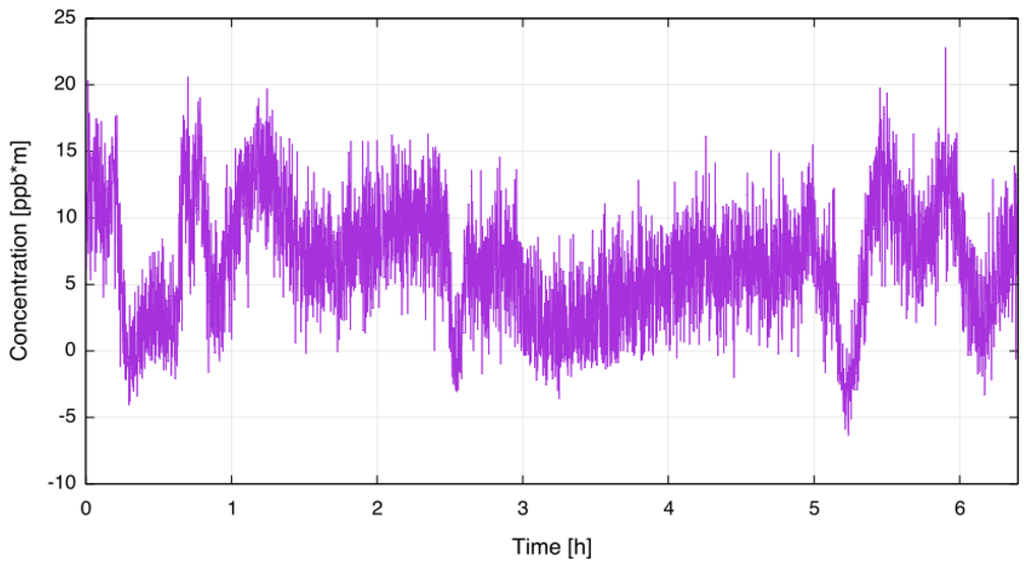
Figure 4.
Determination of lower detection limit. The sensor was mounted inside a climate chamber with varying temperature.
The sensitivity is limited by optical etalons which are seen as long-term variations in Figure 4. Etalons arise from undesired multiple reflections of the laser beam within the transmitter and receiver unit.
4. Field-Test at an Alcoa Aluminum Smelter
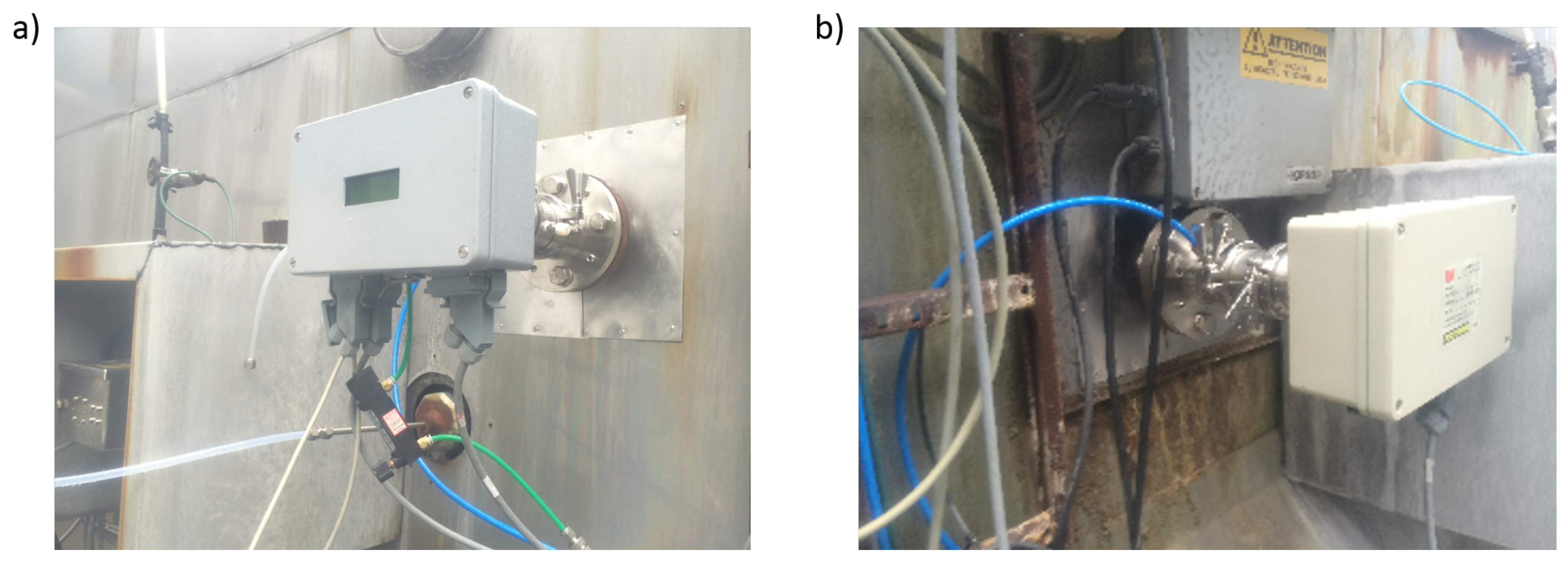
Feasibility of the QCL CF sensor under real-world conditions was tested by installing the unit at an Alcoa aluminum smelter. Transmitter and receiver unit were mounted on opposite sites of an exhaust duct servicing exhaust gas from 72 smelting pots (Figure 5). The duct diameter is about 2.4 m, the pressure is around ambient and the gas temperature varies between 80 C and 100 C. Measurements were performed continuously between 25 September and 19 October 2015.
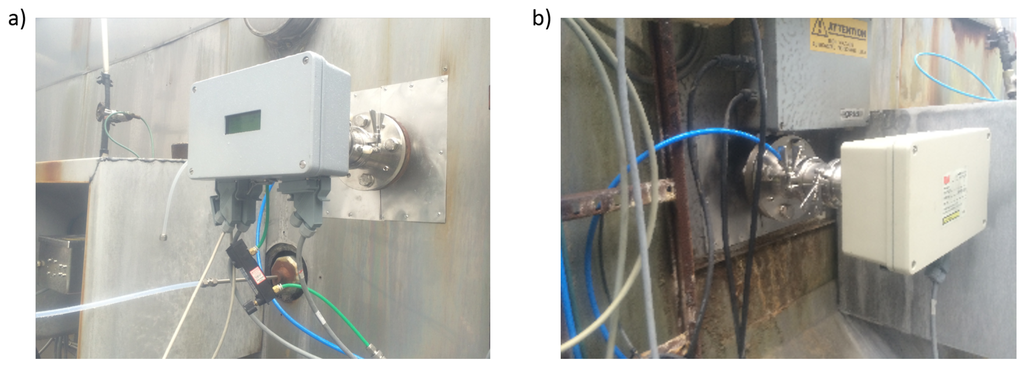
Figure 5.
Photograph of (a) transmitter unit and (b) receiver unit installed at an exhaust duct of an Alcoa Aluminum smelter.
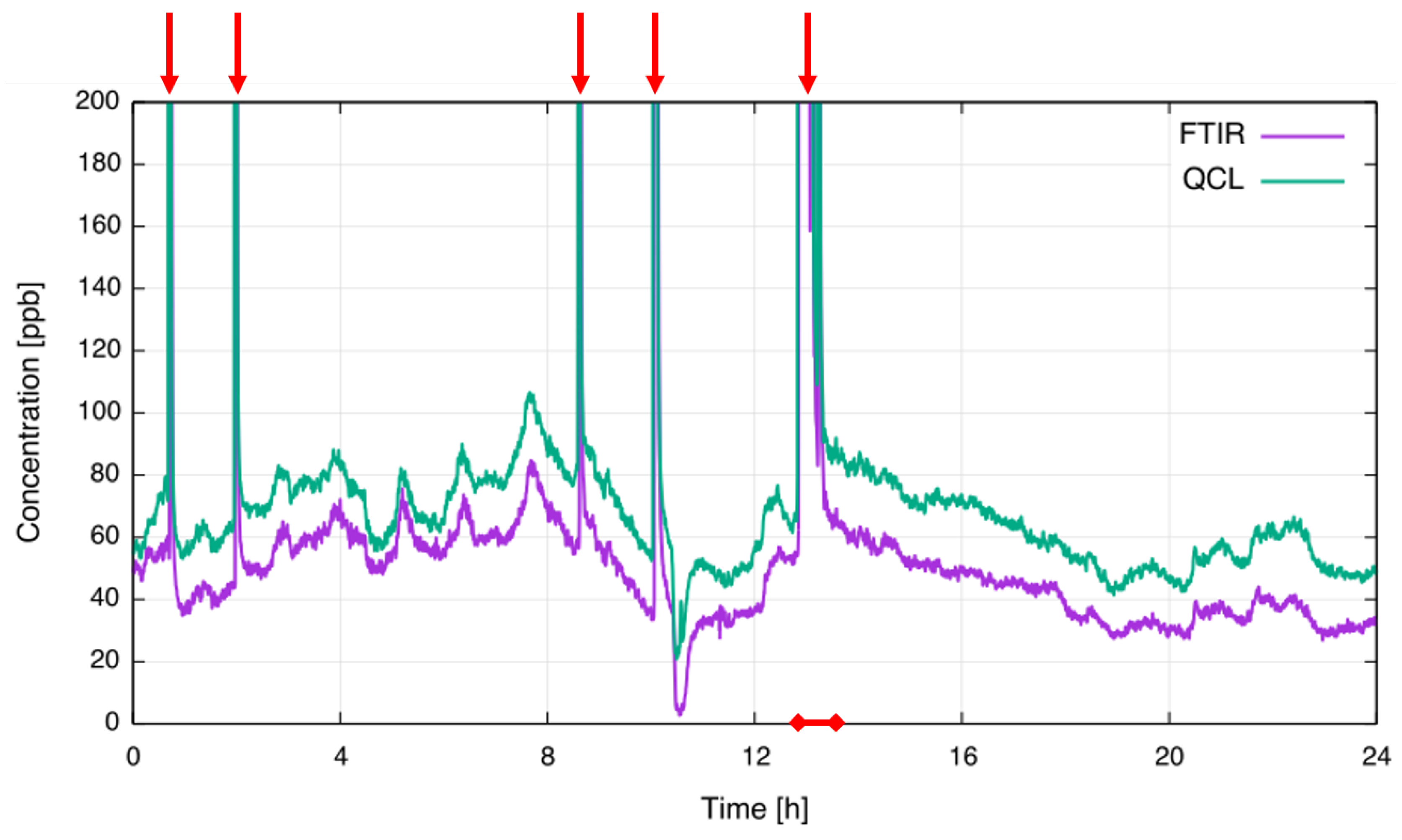
An extractive FTIR spectrometer was installed at the same location in order to perform comparative measurements. Spectral averaging time of the FTIR spectrometer was set to 20 s. To synchronize both sensors, the averaging time of the QCL sensor was configured to be the same. Figure 6 is showing a 24 h plot of the concentration readings from both instruments; the purple trace depicts the FTIR readings and the green trace the concentration obtained with the QCL CF sensor. To make the baseline more visible, the y-axis is limited to a concentration of 200 ppb.
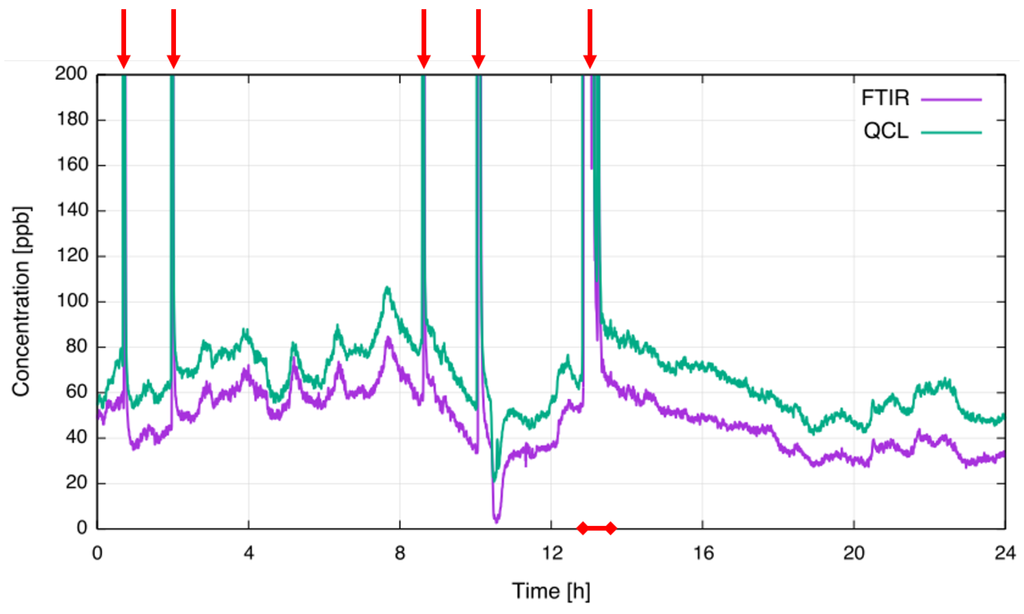
Figure 6.
Comparison of extractive FTIR and in-situ QCL concentration measurements. Anode effects are indicated by red arrows. The red-marked section is plotted in Figure 7 with full scale.
Baseline, LV-PFC emissions ranging 20 ppb to 100 ppb CF and several AEs with concentrations of several parts-per-million (ppm) were recorded during the presented measurement interval. Good agreement between both measurement platforms was observed, with even small fluctuations in emitted values distinctly visible in both profiles. The offset between FTIR and QCL measurement can be attributed to interference from CH (Table 2) and appears to be nearly constant across the measurement time. Within the concentrations of interest to this study, CF levels measured by the FTIR spectrometer are unaffected by CH. In contrast to the QCL sensor, the prediction algorithm in the FTIR spectrometer utilizes a broader spectral range covering the entire spectral feature. As such, gas concentrations measured are less susceptible to the presence of known interferents. At the time no efforts were attempted to compensate for the CH contribution in the QCL sensor. Variations in the offset might arise either from optical etalons slowly moving across the CF absorption (e.g., the offset at around 5:00 is much smaller than during the rest of the measurement time) or from variations of the CH concentration in the exhaust gas. The short term noise level of the QCL sensor is about 5 ppb and is comparable to the lab results taking the optical path length of 2.4 m into account. The value is of the same order as the FTIR noise level.
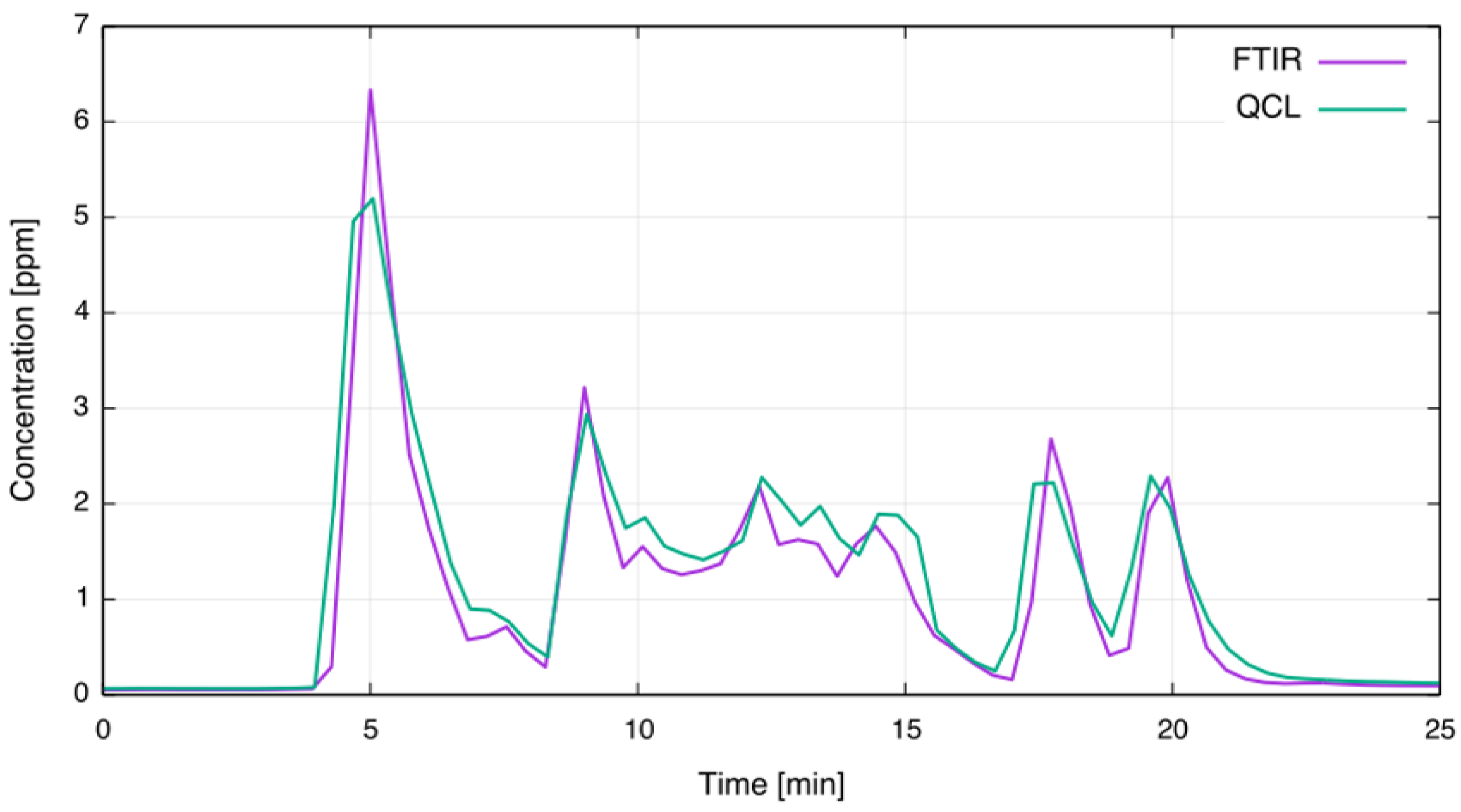
Figure 7 shows the concentration readings in full scale for the time period marked by the red bar in Figure 6; multiple anode effects are occurring. As can be seen, the overall agreement is very good. However, the QCL CF sensor displays a nonlinear behavior at concentrations above 4 ppm. The nonlinearity arises due to strong absorption of the analyte: According to simulations using HITRAN, 4 ppm CF at ambient pressure, gas temperature of 80 C and OPL of 2.4 m results in 45% absorption. For such strong absorptions the linear approximation of WMS signals is no longer valid [10,22]. To overcome this drawback, incorporating quasi-simultaneous direct absorption measurements alongside the WMS can be considered. This approach was recently demonstrated by Klein et al. [27].
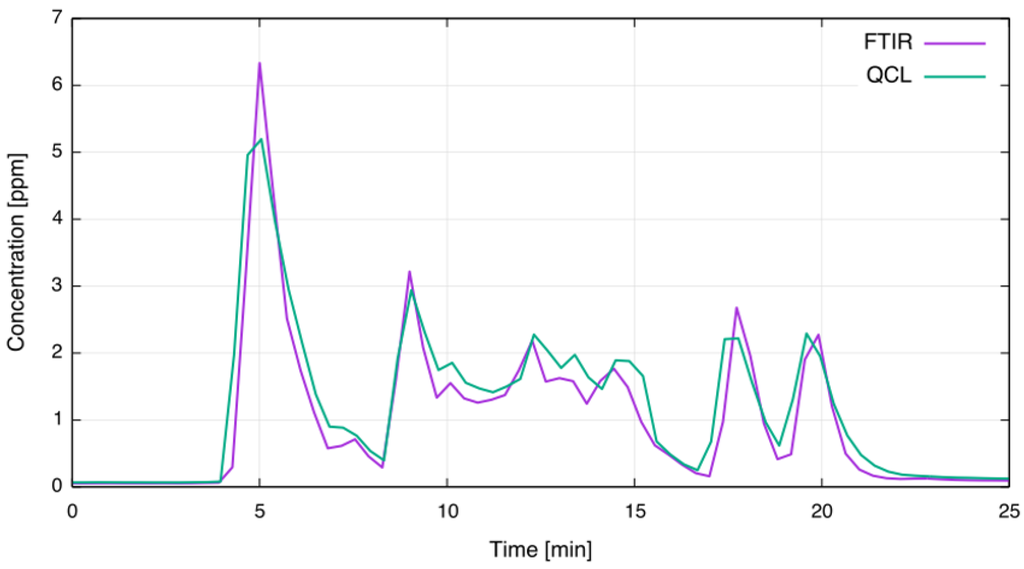
Figure 7.
Comparison of extractive FTIR and in-situ QCL concentration measurements during multiple anode effects.
Additional background information and a detailed evaluation of the obtained measurement data can be found in Espinoza-Nava et al. [28].
5. Conclusions
In summary, the presented QCL sensor demonstrates a unique WMS approach to measure tetrafluoromethane. By scanning only the part with the strongest curvature of the CF absorption feature at 1283 cm interference from water vapor, methane and nitrous oxide was limited to a few ppb. A long-term detection limit of about 20 ppb·m was determined in a laboratory environment; the short-term value is well below 10 ppb·m.
The field-test demonstrated that the sensor has the potential to continuously measure CF emissions at an aluminum smelter. The measurement results were compared to a co-located FTIR spectrometer and a general agreement between the two methods was observed. Deviations of the concentration readings of the QCL system can be attributed to minor interference from methane absorption. During the field-test, no efforts were made to subtract the contribution from methane. Future developments of the technology will include evaluation and implementation of advanced signal processing to minimize the observed interference. Initial tests of the advanced signal processing are very promising and the technique will be transferred to the QCL CF sensor. Results of upcoming field-tests will be reported elsewhere at a later stage.
Acknowledgments
The authors would like to thank Morten Sannerud and Håvar Bakken (NEO Monitors AS, Skedsmokorset, Norway) for their contribution to the field-test. L. Espinoza-Nava and N. Menegazzo would like to acknowledge Alcoa’s Primary Metals and Environmental Management Teams for encouraging publication of environmental research.
Author Contributions
P. Geiser and P. Kaspersen conceived and designed the experiment; P. Geiser and V. Avetisov performed the laboratory experiments and analyzed the data; L. Espinoza-Nava and N. Menegazzo performed the field-test; P. Geiser wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.; Tignor, M.M.; Miller, H.L. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Kim, J.; Fraser, P.J.; Li, S.; Mühle, J.; Ganesan, A.L.; Krummel, P.B.; Steele, L.P.; Park, S.; Kim, S.K.; Park, M.K.; et al. Quantifying aluminum and semiconductor industry perfluorocarbon emissions from atmospheric measurements. Geophys. Res. Lett. 2014, 41, 4787–4794. [Google Scholar] [CrossRef]
- Grjotheim, K.; Kvande, H. Introduction to Aluminum Electrolysis; Aluminum-Verlag: Düsseldorf, Germany, 1993. [Google Scholar]
- Dando, N.R.; Xu, W.; Marks, J. Comparison of PFC Emission for Operating and Newly Started Pots in the Alcoa Fjardaal Point Fed Prebake Smelter. In Light Metals 2009; Bearne, G., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA; pp. 269–273.
- Zarouni, A.; Reverdy, M.; Zarouni, A.A.; Venkatasubramaniam, K. A Study of Low Voltage PFC Emissions at DUBAL. In Light Metals 2013; Sadler, B.A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA; pp. 859–863.
- Dando, N.R.; Menegazzo, N.; Espinoza-Nava, L.; Westendorf, N.; Batista, E. Non Anode Effect PFCs: Measurement Considerations and Potential Impacts. In Light Metals 2015; Hyland, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA; pp. 551–554.
- Dando, N.R.; Xu, W.; Espinoza-Nava, L. In-Plant Performance Comparison of Fourier Transform and Photoacoustic Infra-Red PFC Monitors. In Light Metals 2004; Tabereaux, A.T., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA; pp. 381–385.
- U.S. Environmental Protection Agency; International Aluminium Institute. Available online: http://www3.epa.gov/highgwp/aluminum-pfc/documents/measureprotocol.pdf (accessed on 31 March 2016).
- Gouvernement du Québec. Available online: http://www2.publicationsduquebec.gouv.qc.ca/dynamicSearch/telecharge.php?type=3&file=/Q_2/Q2R15_A.htm (accessed on 31 March 2016).
- Linnerud, I.; Kaspersen, P.; Jaeger, T. Gas monitoring in the process industry using diode laser spectroscopy. Appl. Phys. B 1998, 67, 297–305. [Google Scholar] [CrossRef]
- Rothman, L.; Gordon, I.; Babikov, Y.; Barbe, A.; Benner, D.C.; Bernath, P.; Birk, M.; Bizzocchi, L.; Boudon, V.; Brown, L.; et al. The HITRAN2012 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2013, 130, 4–50. [Google Scholar] [CrossRef]
- Pacific Northwest National Laboratory. Available online: https://secure2.pnl.gov/nsd/nsd.nsf/ (accessed on 31 March 2016).
- Rothman, L.; Gordon, I.; Barber, R.; Dothe, H.; Gamache, R.; Goldman, A.; Perevalov, V.; Tashkun, S.; Tennyson, J. HITEMP, the high-temperature molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 2139–2150. [Google Scholar] [CrossRef]
- Bouchard, G.; Kallmeyer, J.; Marks, J. PFC Emissions Measurements from Canadian Primary Aluminum Production. In Light Metals 2001; Anjier, J.L., Ed.; The Minerals, Metals & Materials Society: Warrendale, PA, USA; pp. 283–288.
- Gamble, H.A.; Mackay, G.I.; Karecki, D.R.; Pisano, J.T.; Schiff, H.I. Development of a TDLAS based Methodology for Monitoring Perfluorocarbon Production during the Aluminium Smelting Process. In Light Metals 2001; Anjier, J.L., Ed.; The Minerals, Metals & Materials Society: Warrendale, PA, USA; pp. 275–281.
- Kimmerle, F.M.; Potvin, G.; Pisano, J.T. Measured Versus Calculated Reduction of the PFC Emissions from Prebaked Hall Héroult Cells. In Light Metals 1997; Huglen, R., Ed.; The Minerals, Metals & Materials Society: Warrendale, PA, USA; pp. 165–171.
- Faist, J. Quantum Cascade Lasers; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Geiser, P. New Opportunities in Mid-Infrared Emission Control. Sensors 2015, 15, 22724–22736. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Sun, K.; Khan, M.A.; Miller, D.J.; Zondlo, M.A. Compact and portable open-path sensor for simultaneous measurements of atmospheric N2O and CO using a quantum cascade laser. Opt. Express 2012, 20, 28106–28118. [Google Scholar] [CrossRef] [PubMed]
- NEO Monitors AS. Available online: http://neomonitors.com/products/lasergas-q-no/ (accessed on 31 March 2016).
- Geiser, P.; Dang, D.; Bohman, A.; Kaspersen, P. Mid-infrared Sulfur Dioxide Measurements at Elevated Temperatures for Emission Control. In Lasers, Sources, and Related Photonic Devices; Optical Society of America: Washington, DC, USA, 2012. [Google Scholar]
- Kluczynski, P.; Gustafsson, J.; Lindberg, Å.M.; Axner, O. Wavelength modulation absorption spectrometry—An extensive scrutiny of the generation of signals. Spectrochim. Acta Part B At. Spectrosc. 2001, 56, 1277–1354. [Google Scholar] [CrossRef]
- Geiser, P.; Kaspersen, P. Quantum cascade laser based tetrafluoromethane and nitrogen oxide measurements for emission monitoring applications. In Imaging and Applied Optics 2014; Optical Society of America: Washington, DC, USA, 2014. [Google Scholar]
- Ren, W.; Jiang, W.; Tittel, F.K. Single-QCL-based absorption sensor for simultaneous trace-gas detection of CH4 and N2O. Appl. Phys. B 2014, 117, 245–251. [Google Scholar] [CrossRef]
- Cao, Y.; Sanchez, N.P.; Griffin, R.; Tittel, F.K.; Dong, L. Mid-infrared detection of atmospheric CH4, N2O and H2O based on a single continuous wave quantum cascade laser. In CLEO: 2015; Optical Society of America: Washington, DC, USA, 2015. [Google Scholar]
- Werle, P. Accuracy and precision of laser spectrometers for trace gas sensing in the presence of optical fringes and atmospheric turbulence. Appl. Phys. B 2010, 102, 313–329. [Google Scholar] [CrossRef]
- Klein, A.; Witzel, O.; Ebert, V. Rapid, Time-Division Multiplexed, Direct Absorption- and Wavelength Modulation-Spectroscopy. Sensors 2014, 14, 21497–21513. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Nava, L.; Menegazzo, N.; Dando, N.R.; Geiser, P. QCL-Based Perfluorocarbon Emission Monitoring. In Light Metals 2016; Williams, E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA; pp. 541–544.
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).