Label-Free Detection of Cellular Senescence in Fibroblasts via Third Harmonic Generation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Induction of Senescence
2.2. Immunocytochemical Assay
2.3. SA-β-Galactosidase Staining
2.4. Confocal Image Analysis
2.5. Nonlinear Microscope
2.6. Quantification of the THG Signals Specific to the Lipid Content of the Cells
2.7. Statistical Analysis
3. Results
3.1. Establishing a Senescent Phenotype of Fibroblasts
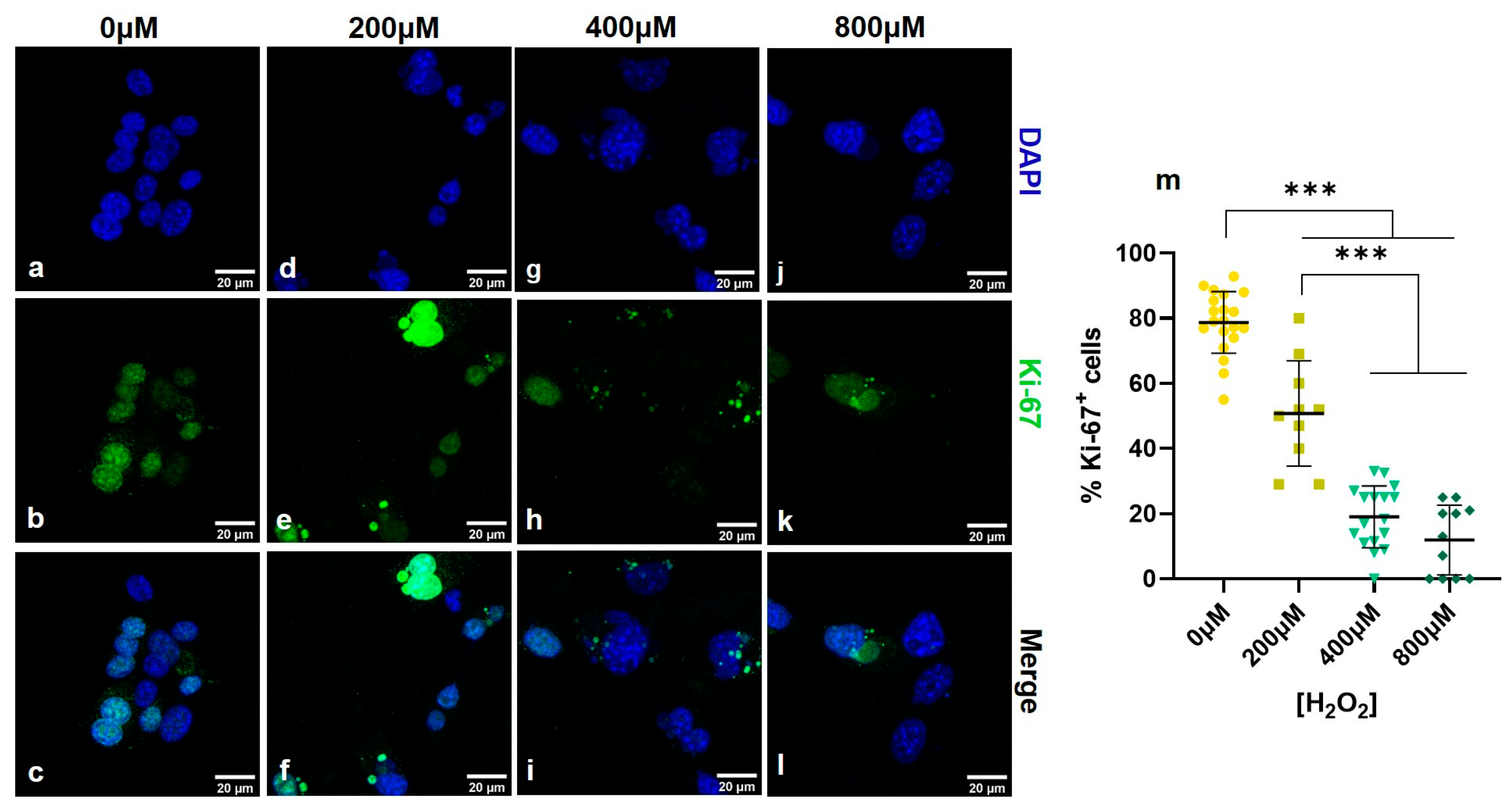

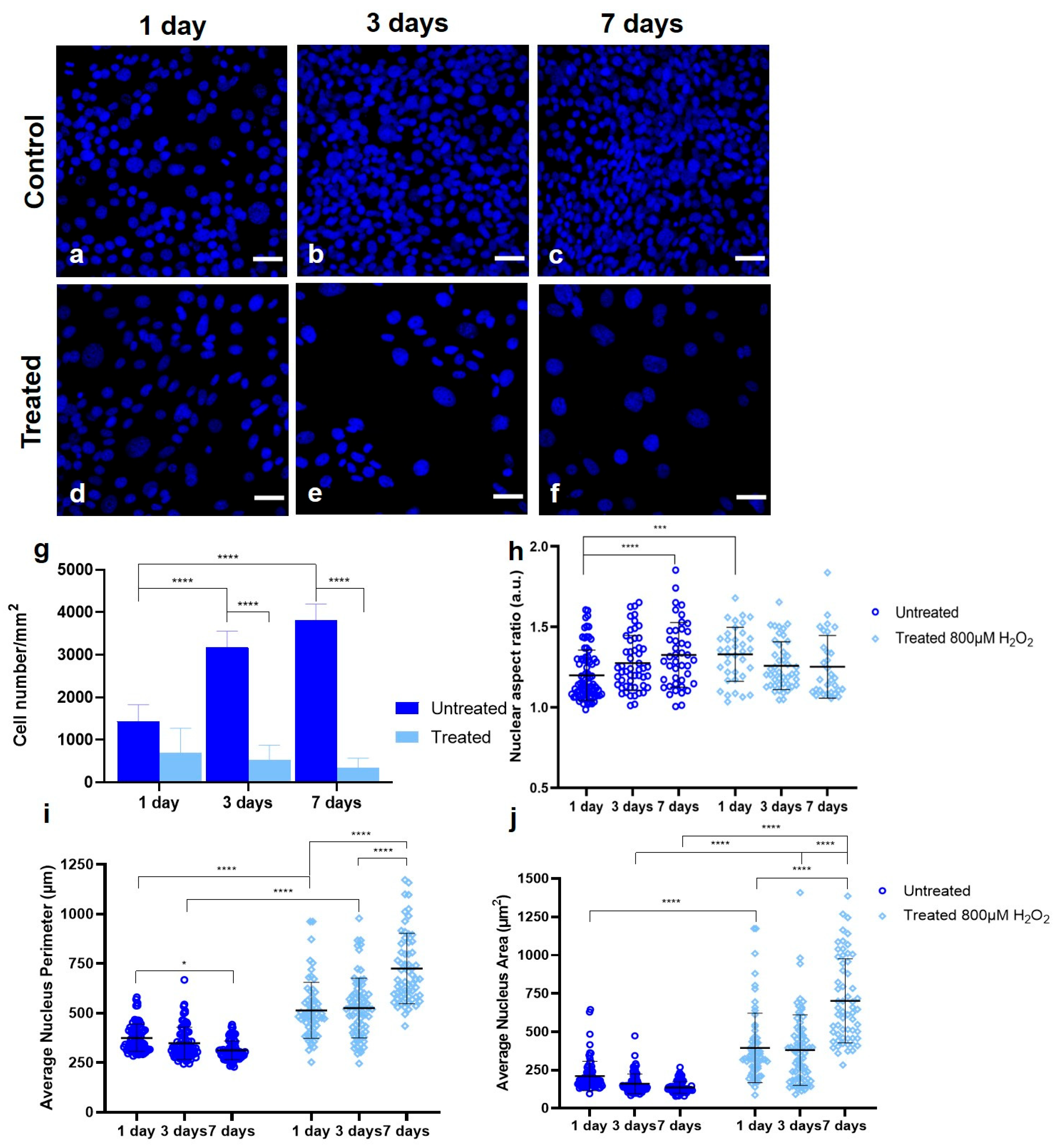
3.2. Simultaneous THG Imaging of Unstained Lipids and TPEF of Ki-67-Positive Nuclei

4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021, 288, 56–80. [Google Scholar] [CrossRef]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Naylor, R.M.; Baker, D.J.; Van Deursen, J.M. Senescent cells: A novel therapeutic target for aging and age-related diseases. Clin. Pharmacol. Ther. 2013, 93, 105–116. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; Van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Rossiello, F.; Clerici, M.; Barozzi, S.; Cittaro, D.; Kaplunov, J.M.; Bucci, G.; Dobreva, M.; Matti, V.; Beausejour, C.M.; et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol. 2012, 14, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, O.; Bourdeau, V.; Roux, A.; Deschênes-Simard, X.; Ferbeyre, G. Mitochondrial Dysfunction Contributes to Oncogene-Induced Senescence. Mol. Cell. Biol. 2009, 29, 4495–4507. [Google Scholar] [CrossRef]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16(INK4a). Cell 1997, 88, 593–602. [Google Scholar] [CrossRef]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chang, S. Role of telomeres and telomerase in genomic instability, senescence and cancer. Lab. Investig. 2007, 87, 1071–1076. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Wang, L.; Lankhorst, L.; Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 2022, 22, 340–355. [Google Scholar] [CrossRef]
- Herranz, N.; Gil, J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Pack, L.R.; Daigh, L.H.; Meyer, T. Putting the brakes on the cell cycle: Mechanisms of cellular growth arrest. Curr. Opin. Cell Biol. 2019, 60, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Zhu, Y.; Langhi, L.G.P.; Tchkonia, T.; Krüger, P.; Fielder, E.; Victorelli, S.; Ruswhandi, R.A.; Giorgadze, N.; Pirtskhalava, T.; et al. Obesity-Induced Cellular Senescence Drives Anxiety and Impairs Neurogenesis. Cell Metab. 2019, 29, 1061–1077.e8. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Bloom, J.; Cross, F.R. Multiple levels of cyclin specificity in cell-cycle control. Nat. Rev. Mol. Cell Biol. 2007, 8, 149–160. [Google Scholar] [CrossRef]
- Wang, N.; He, Y.; Liu, S.; Makarcyzk, M.J.; Lei, G.; Chang, A.; Alexander, P.G.; Hao, T.; Padget, A.M.; de Pedro, N.; et al. Engineering osteoarthritic cartilage model through differentiating senescent human mesenchymal stem cells for testing disease-modifying drugs. Sci. China Life Sci. 2022, 65, 309–327. [Google Scholar] [CrossRef]
- Frippiat, C.; Dewelle, J.; Remacle, J.; Toussaint, O. Signal transduction in H2O2-induced senescence-like phenotype in human diploid fibroblasts. Free Radic. Biol. Med. 2002, 33, 1334–1346. [Google Scholar] [CrossRef]
- Petrova, N.V.; Velichko, A.K.; Razin, S.V.; Kantidze, O.L. Small molecule compounds that induce cellular senescence. Aging Cell 2016, 15, 999–1017. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, D.H.; Chevalier, F.; Groetz, J.E.; Durantel, F.; Thuret, J.Y.; Mann, C.; Saintigny, Y. Comparable Senescence Induction in Three-dimensional Human Cartilage Model by Exposure to Therapeutic Doses of X-rays or C-ions. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 139–146. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Y.; Wang, J.; Zhai, J.; He, F.; Zhu, G. Irradiation-induced senescence of bone marrow mesenchymal stem cells aggravates osteogenic differentiation dysfunction via paracrine signaling. Am. J. Physiol.-Cell Physiol. 2020, 318, C1005–C1017. [Google Scholar] [CrossRef]
- Bielak-Zmijewska, A.; Wnuk, M.; Przybylska, D.; Grabowska, W.; Lewinska, A.; Alster, O.; Korwek, Z.; Cmoch, A.; Myszka, A.; Pikula, S.; et al. A comparison of replicative senescence and doxorubicin-induced premature senescence of vascular smooth muscle cells isolated from human aorta. Biogerontology 2014, 15, 47–64. [Google Scholar] [CrossRef]
- Millner, A.; Ekin Atilla-Gokcumen, G. Lipid players of cellular senescence. Metabolites 2020, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- Lizardo, D.Y.; Lin, Y.L.; Gokcumen, O.; Atilla-Gokcumen, G.E. Regulation of lipids is central to replicative senescence. Mol. Biosyst. 2017, 13, 498–509. [Google Scholar] [CrossRef]
- Saitou, M.; Lizardo, D.Y.; Taskent, R.O.; Millner, A.; Gokcumen, O.; Atilla-Gokcumen, G.E. An evolutionary transcriptomics approach links CD36 to membrane remodeling in replicative senescence. Mol. Omics 2018, 14, 237–246. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Hamsanathan, S.; Gurkar, A.U. Lipids as Regulators of Cellular Senescence. Front. Physiol. 2022, 13, 796850. [Google Scholar] [CrossRef]
- Mutlu, A.S.; Duffy, J.; Wang, M.C. Lipid metabolism and lipid signals in aging and longevity. Dev. Cell 2021, 56, 1394–1407. [Google Scholar] [CrossRef]
- Papsdorf, K.; Brunet, A. Linking Lipid Metabolism to Chromatin Regulation in Aging. Trends Cell Biol. 2019, 29, 97–116. [Google Scholar] [CrossRef]
- Fukumoto, S.; Fujimoto, T. Deformation of lipid droplets in fixed samples. Histochem. Cell Biol. 2002, 118, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Cirulis, J.T.; Strasser, B.C.; Scott, J.A.; Ross, G.M. Optimization of staining conditions for microalgae with three lipophilic dyes to reduce precipitation and fluorescence variability. Cytom. Part A 2012, 81A, 618–626. [Google Scholar] [CrossRef]
- Khoury, S.; Canlet, C.; Lacroix, M.Z.; Berdeaux, O.; Jouhet, J.; Bertrand-Michel, J. Quantification of lipids: Model, reality, and compromise. Biomolecules 2018, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Neuberger, T.; Rolletschek, H.; Schiebold, S.; Nguyen, T.H.; Borisjuk, N.; Börner, A.; Melkus, G.; Jakob, P.; Borisjuk, L. A noninvasive platform for imaging and quantifying oil storage in submillimeter tobacco seed. Plant Physiol. 2013, 161, 583–593. [Google Scholar] [CrossRef]
- Brescia, M.A.; Pugliese, T.; Hardy, E.; Sacco, A. Compositional and structural investigations of ripening of table olives, Bella della Daunia, by means of traditional and magnetic resonance imaging analyses. Food Chem. 2007, 105, 400–404. [Google Scholar] [CrossRef]
- Syed, A.; Smith, E.A. Raman imaging in cell membranes, lipid-rich organelles, and lipid bilayers. Annu. Rev. Anal. Chem. 2017, 10, 271–291. [Google Scholar] [CrossRef]
- Zumbusch, A.; Holtom, G.R.; Xie, X.S. Three-dimensional vibrational imaging by coherent anti-stokes raman scattering. Phys. Rev. Lett. 1999, 82, 4142–4145. [Google Scholar] [CrossRef]
- Cheng, J.X.; Volkmer, A.; Book, L.D.; Xie, X.S. Multiplex coherent anti-stokes Raman scattering microspectroscopy and study of lipid vesicles. J. Phys. Chem. B 2002, 106, 8493–8498. [Google Scholar] [CrossRef]
- Freudiger, C.W.; Min, W.; Saar, B.G.; Lu, S.; Holtom, G.R.; He, C.; Tsai, J.C.; Kang, J.X.; Xie, X.S. Label-free biomedical imaging with high sensitivity by stimulated raman scattering microscopy. Science 2008, 322, 1857–1861. [Google Scholar] [CrossRef]
- Rinia, H.A.; Burger, K.N.J.; Bonn, M.; Müller, M. Quantitative label-free imaging of lipid composition and packing of individual cellular lipid droplets using multiplex CARS microscopy. Biophys. J. 2008, 95, 4908–4914. [Google Scholar] [CrossRef] [PubMed]
- Débarre, D.; Supatto, W.; Pena, A.M.; Fabre, A.; Tordjmann, T.; Combettes, L.; Schanne-Klein, M.C.; Beaurepaire, E. Imaging lipid bodies in cells and tissues using third-harmonic generation microscopy. Nat. Methods 2006, 3, 47–53. [Google Scholar] [CrossRef]
- Palikaras, K.; Mari, M.; Petanidou, B.; Pasparaki, A.; Filippidis, G.; Tavernarakis, A.N. Ectopic fat deposition contributes to age-associated pathology in caenorhabditis elegans. J. Lipid Res. 2017, 58, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Palikaras, K.; Mari, M.; Ploumi, C.; Princz, A.; Filippidis, G.; Tavernarakis, N. Age-dependent nuclear lipid droplet deposition is a cellular hallmark of aging in Caenorhabditis elegans. Aging Cell 2023, 22, e13788. [Google Scholar] [CrossRef]
- Gavgiotaki, E.; Filippidis, G.; Markomanolaki, H.; Kenanakis, G.; Agelaki, S.; Georgoulias, V.; Athanassakis, I. Distinction between breast cancer cell subtypes using third harmonic generation microscopy. J. Biophotonics 2017, 10, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Gavgiotaki, E.; Filippidis, G.; Tsafas, V.; Bovasianos, S.; Kenanakis, G.; Georgoulias, V.; Tzardi, M.; Agelaki, S.; Athanassakis, I. Third Harmonic Generation microscopy distinguishes malignant cell grade in human breast tissue biopsies. Sci. Rep. 2020, 10, 11055. [Google Scholar] [CrossRef]
- Rehberg, M.; Krombach, F.; Pohl, U.; Dietzel, S. Label-free 3D visualization of cellular and tissue structures in intact muscle with second and third harmonic generation microscopy. PLoS ONE 2011, 6, e28237. [Google Scholar] [CrossRef]
- Zipfel, W.R.; Williams, R.M.; Webb, W.W. Nonlinear magic: Multiphoton microscopy in the biosciences. Nat. Biotechnol. 2003, 21, 1369–1377. [Google Scholar] [CrossRef]
- Barad, Y.; Eisenberg, H.; Horowitz, M.; Silberberg, Y. Nonlinear scanning laser microscopy by third harmonic generation. Appl. Phys. Lett. 1997, 70, 922–924. [Google Scholar] [CrossRef]
- Weigelin, B.; Bakker, G.J.; Friedl, P. Third harmonic generation microscopy of cells and tissue organization. J. Cell Sci. 2016, 129, 245–255. [Google Scholar] [CrossRef]
- Gavgiotaki, E.; Filippidis, G.; Kalognomou, M.; Tsouko, A.A.; Skordos, I.; Fotakis, C.; Athanassakis, I. Third Harmonic Generation microscopy as a reliable diagnostic tool for evaluating lipid body modification during cell activation: The example of BV-2 microglia cells. J. Struct. Biol. 2015, 189, 105–113. [Google Scholar] [CrossRef]
- Kyvelidou, C.; Tserevelakis, G.J.; Filippidis, G.; Ranella, A.; Kleovoulou, A.; Fotakis, C.; Athanassakis, I. Following the course of pre-implantation embryo patterning by non-linear microscopy. J. Struct. Biol. 2011, 176, 379–386. [Google Scholar] [CrossRef]
- Mari, M.; Filippidis, G.; Palikaras, K.; Petanidou, B.; Fotakis, C.; Tavernarakis, N. Imaging ectopic fat deposition in caenorhabditis elegans muscles using nonlinear microscopy. Microsc. Res. Tech. 2015, 78, 523–528. [Google Scholar] [CrossRef]
- Gavgiotaki, E.; Filippidis, G.; Zerva, I.; Kenanakis, G.; Archontakis, E.; Agelaki, S.; Georgoulias, V.; Athanassakis, I. Detection of the T cell activation state using nonlinear optical microscopy. J. Biophotonics 2019, 12, e201800277. [Google Scholar] [CrossRef] [PubMed]
- Mari, M.; Voutyraki, C.; Zacharioudaki, E.; Delidakis, C.; Filippidis, G. Lipid content evaluation of Drosophila tumour associated haemocytes through Third Harmonic Generation measurements. J. Biophotonics 2023, 16, e202300171. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef] [PubMed]
- Kamentsky, L.; Jones, T.R.; Fraser, A.; Bray, M.A.; Logan, D.J.; Madden, K.L.; Ljosa, V.; Rueden, C.; Eliceiri, K.W.; Carpenter, A.E. Improved structure, function and compatibility for cellprofiler: Modular high-throughput image analysis software. Bioinformatics 2011, 27, 1179–1180. [Google Scholar] [CrossRef]
- Stirling, D.R.; Swain-Bowden, M.J.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A.; Goodman, A. CellProfiler 4: Improvements in speed, utility and usability. BMC Bioinform. 2021, 22, 433. [Google Scholar] [CrossRef]
- Peán, C.B.; Schiebler, M.; Tan, S.W.S.; Sharrock, J.A.; Kierdorf, K.; Brown, K.P.; Maserumule, M.C.; Menezes, S.; Pilátová, M.; Bronda, K.; et al. Regulation of phagocyte triglyceride by a STAT-ATG2 pathway controls mycobacterial infection. Nat. Commun. 2017, 8, 14642. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, M.; Fairn, G.D. Molecular probes to visualize the location, organization and dynamics of lipids. J. Cell Sci. 2014, 127, 4801–4812. [Google Scholar] [CrossRef] [PubMed]
- Rambold, A.S.; Cohen, S.; Lippincott-Schwartz, J. Fatty acid trafficking in starved cells: Regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell 2015, 32, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Supatto, W.; Truong, T.V.; Débarre, D.; Beaurepaire, E. Advances in multiphoton microscopy for imaging embryos. Curr. Opin. Genet. Dev. 2011, 21, 538–548. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mari, M.; Kanakousaki, E.; Stampouli, K.; Kordas, A.; Manganas, P.; Fotakis, C.; Filippidis, G.; Ranella, A. Label-Free Detection of Cellular Senescence in Fibroblasts via Third Harmonic Generation. Photonics 2025, 12, 919. https://doi.org/10.3390/photonics12090919
Mari M, Kanakousaki E, Stampouli K, Kordas A, Manganas P, Fotakis C, Filippidis G, Ranella A. Label-Free Detection of Cellular Senescence in Fibroblasts via Third Harmonic Generation. Photonics. 2025; 12(9):919. https://doi.org/10.3390/photonics12090919
Chicago/Turabian StyleMari, Meropi, Eleni Kanakousaki, Kyriaki Stampouli, Antonis Kordas, Phanee Manganas, Costas Fotakis, George Filippidis, and Anthi Ranella. 2025. "Label-Free Detection of Cellular Senescence in Fibroblasts via Third Harmonic Generation" Photonics 12, no. 9: 919. https://doi.org/10.3390/photonics12090919
APA StyleMari, M., Kanakousaki, E., Stampouli, K., Kordas, A., Manganas, P., Fotakis, C., Filippidis, G., & Ranella, A. (2025). Label-Free Detection of Cellular Senescence in Fibroblasts via Third Harmonic Generation. Photonics, 12(9), 919. https://doi.org/10.3390/photonics12090919





