1. Introduction
Molar incisor hypomineralization (MIH) is a developmental enamel defect of systemic origin that typically affects one to four first permanent molars (FPMs), often accompanied by lesions in permanent incisors and occasionally other teeth [
1]. Initially described by Weerheijm et al. in 2001 as a distinct subset of developmental defects of enamel, MIH presents with sharply demarcated opacities of variable color and severity, with or without post-eruptive enamel breakdown (PEB) [
1].
MIH affects an estimated 13.5% of the global population—over 878 million individuals—representing a substantial burden on pediatric oral health systems worldwide. Its prevalence varies significantly by geographic region and diagnostic methodology, with high incidence in densely populated nations such as India, Indonesia, and Pakistan [
2]. Clinically, MIH is challenging due to its variable presentation, rapid progression, and considerable impact on the quality of life and dental function in affected children [
2].
The etiopathogenesis of MIH is complex and multifactorial. Both genetic predispositions—particularly involving enamel-forming genes such as AMELX and ENAM—and environmental insults during critical stages of amelogenesis have been implicated [
3]. Epigenetic factors may further modulate gene–environment interactions. Prenatal, perinatal, and postnatal risk factors include maternal illness, preterm birth, hypoxia, and early childhood diseases. Retrospective evidence links certain antibiotics and systemic conditions to enamel hypomineralization, though prospective validation remains limited. Interestingly, MIH-like defects have been identified in archaeological remains, suggesting that their origins predate industrial-era pollutants [
3].
Histologically, MIH-affected enamel is characterized by reduced calcium and phosphate content, elevated carbonate levels, and a disordered hydroxyapatite crystal structure. Protein content is significantly increased, particularly serum-derived albumin—indicating impaired ameloblast activity or inadequate matrix degradation due to deficient proteases such as MMP-20 and KLK4 [
4]. These changes lead to increased enamel porosity, lower hardness and elastic modulus, hypersensitivity, and mechanical failure. Clinically, lesion color correlates with mineral and protein composition, with brown opacities typically representing the most compromised tissue [
5].
Diagnostic criteria for MIH have been established by the European Academy of Paediatric Dentistry (EAPD), emphasizing the presence of well-demarcated opacities on at least one FPM, often with similar defects in incisors. The presence of PEB and atypical restorations—those that deviate from the pattern of caries—is a key diagnostic indicator of severity. EAPD also stratifies MIH into mild and severe forms based on clinical expression, enamel loss, hypersensitivity, and anesthetic response [
3,
6]. Accurate diagnosis and lesion documentation remain critical for effective management and long-term monitoring.
Traditional diagnostic methods—visual–tactile examination and bitewing radiography—offer limited sensitivity for early-stage or subsurface enamel defects [
7]. To address this, several light-based diagnostic tools have emerged. These include Quantitative Light-Induced Fluorescence (QLF), Digital Imaging Fiber-Optic Transillumination (DIFOTI), laser fluorescence (e.g., DIAGNOdent), and near-infrared imaging (NIR). These techniques exploit light–tissue interactions to identify mineral loss or changes in tissue density, providing non-invasive alternatives to conventional methods. However, their diagnostic performance can be affected by staining, restorations, and operator variability [
8,
9].
Among the NIR modalities, Optical Coherence Tomography (OCT) represents a highly advanced variant, as it leverages low-coherence near-infrared light for cross-sectional imaging with micrometric resolution.
By measuring backscattered near-infrared light, OCT generates high-resolution, cross-sectional images of hard and soft dental tissues in real time [
10,
11]. As a quintessential photonic technology, OCT offers a unique ability to detect internal enamel and dentin abnormalities, including demineralization, structural disorganization, and defects at the dentino–enamel junction (DEJ), all without tissue disruption. Swept-source OCT (SS-OCT), a recent advancement, enhances imaging depth and acquisition speed, reducing motion artifacts and enabling real-time in vivo imaging of dental tissues. While its axial resolution and tissue contrast are generally lower than those of spectral-domain OCT (SD-OCT), SS-OCT remains highly suitable for detecting enamel cracks, age-related structural changes, and the complex patterns typical of MIH [
12,
13].
Compared to radiographic imaging, OCT provides superior contrast in early lesions, higher sensitivity for subtle tissue changes, and repeatability for longitudinal monitoring—features especially valuable in pediatric or pregnant populations. Moreover, as photonics and biomedical imaging converge, OCT offers a platform for integration with artificial intelligence, enabling automated lesion detection and classification [
14].
While several optical technologies have been explored for enamel defect detection, diagnostic challenges remain particularly pronounced in mild MIH lesions, where enamel alterations often present as well-demarcated opacities easily overlooked by conventional visual–tactile examination, leading to underdiagnosis and delayed intervention [
3].
Mild MIH lesions represent an early stage of enamel pathology: although clinically detectable as demarcated opacities, these lesions may already exhibit underlying microstructural disorganization, mineral loss, and increased porosity [
4]. The ability to detect such subsurface changes is clinically relevant, as timely identification can prevent lesion progression towards post-eruptive enamel breakdown, structural failure, and complex restorative needs [
15]. Therefore, advanced non-invasive diagnostic tools capable of capturing these early structural alterations are needed to support preventive and minimally invasive treatment strategies.
In this context, this exploratory observational case–control study aims to identify recurring OCT-based diagnostic patterns specific to mild MIH lesions, through an in vivo descriptive analysis of the structural characteristics of MIH-affected and healthy teeth. Unlike previous in vitro or animal-model investigations, our approach enables real-time, non-invasive clinical visualization of enamel defects in pediatric patients. By leveraging the intrinsic optical properties of dental tissues—where radiation penetrates more deeply in enamel than in dentin due to their different optical responses—this study uses OCT to achieve detailed structural visualization and qualitative characterization of MIH-affected teeth, supporting preventive and clinical management.
2. Materials and Methods
2.1. Study Design and Ethical Considerations
This observational case-control study was conducted from May 2024 to May 2025 at the Pediatric Dentistry and Oral Medicine Unit of the University Hospital “P. Giaccone” of Palermo, in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Institutional Review Board of the University Hospital “Policlinico Paolo Giaccone” in Palermo, Italy (approval number 11/2016). Written informed consent was obtained from the parents or legal caregivers of all participants prior to enrollment.
2.2. Sample Characteristics and Eligibility Criteria
No formal sample size calculation was performed due to the exploratory and qualitative nature of this descriptive imaging study. Participants were children aged between 6 and 11 years, corresponding to the mixed dentition phase. Patients were consecutively recruited during the study period. Clinical eligibility was assessed during the initial dental examination.
Two clearly defined groups were established: cases (MIH-affected teeth) and controls (clinically healthy teeth), matched by tooth type (first permanent molars and permanent incisors) and eruption stage. Controls were carefully selected from pediatric patients attending routine dental check-ups without clinical signs of MIH or other enamel defects. Inclusion criteria for the MIH group were as follows:
- -
The presence of at least one FPM and/or one permanent incisor with clinical signs of mild MIH, based on the EAPD’s criteria;
- -
The presence of well-demarcated opacities without PEB (clinically intact enamel surfaces);
- -
No previous restorative or therapeutic interventions performed for MIH;
- -
Patients in good general health, with no known systemic or genetic conditions affecting enamel formation;
- -
Adequate compliance with clinical procedures.
Exclusion criteria for the MIH group included the following:
- -
Carious lesions or restorations on the index teeth;
- -
Moderate to severe MIH with structural enamel loss, based on the EAPD’s criteria;
- -
The presence of enamel defects of other origin, such as fluorosis or amelogenesis imperfecta;
- -
Previous remineralization treatment involving the affected teeth;
- -
History of systemic fluoride supplementation (tablets or drops) or clinical signs of fluorosis, to avoid confounding effects on enamel mineralization.
The control group comprised permanent incisors and FPMs, carefully selected to match the tooth types examined in the MIH group. These teeth were collected from healthy pediatric subjects with no clinical signs of MIH or other enamel pathologies. Control teeth showed no evidence of enamel hypomineralization, carious lesions, structural anomalies, restorations, or previous dental trauma, and matched for eruption stage with the MIH-affected teeth.
All clinical examinations were conducted under standardized lighting conditions using dental mirrors and air drying to ensure consistency in identifying enamel defects.
2.3. Optical Coherence Tomography (OCT) Imaging Protocol
High-resolution OCT imaging was performed using the VivoSight® swept-source OCT system (Michelson Diagnostics Ltd., Kent, UK), operating at a central wavelength of 1305 nm, which falls within the second near-infrared (NIR-II, 1000–1350 nm) therapeutic window, thereby maximizing tissue transparency and penetration depth. Although originally designed for dermatological imaging, the VivoSight® swept-source OCT system provides sufficient resolution and imaging depth for enamel and dentin visualization in pediatric dental applications. The system provides an axial resolution of approximately 7.5 µm and a lateral resolution of approximately 5 µm in tissue, with an imaging depth of up to 2 mm. The handheld probe was mounted on a positioning support to ensure stable, perpendicular alignment with the buccal surface of each tooth. For each tooth, scans were performed on the mid-buccal surface, focusing specifically on the central vestibular areas most representative of the MIH lesions, as clinically identified during the initial examination. In control teeth, scanning was conducted on the corresponding anatomical area. Imaging was performed using a cross-sectional scanning protocol (B-scan), with a fixed lateral width of 6 mm, composed of 120 slices acquired at 50 µm intervals, resulting in an overall acquisition time of 12 s per tooth. This approach enabled detailed vertical sectioning of enamel and the underlying dentino–enamel junction (DEJ).
Prior to imaging, the tooth surface was gently air-dried for 5–10 s to reduce surface moisture and enhance optical contrast. The procedure was conducted without the use of anesthesia, mechanical preparation, or ionizing radiation, and was well tolerated by all participants. All images were obtained in a single session, immediately following clinical assessment, under standardized lighting conditions.
2.4. Image Analysis
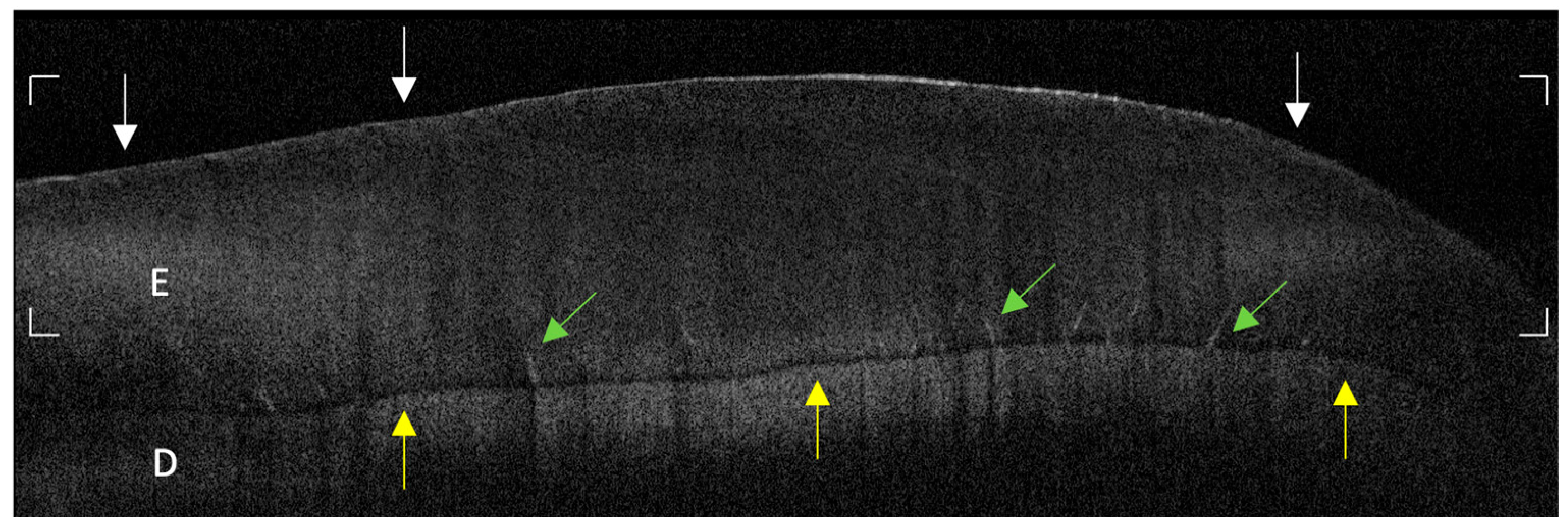
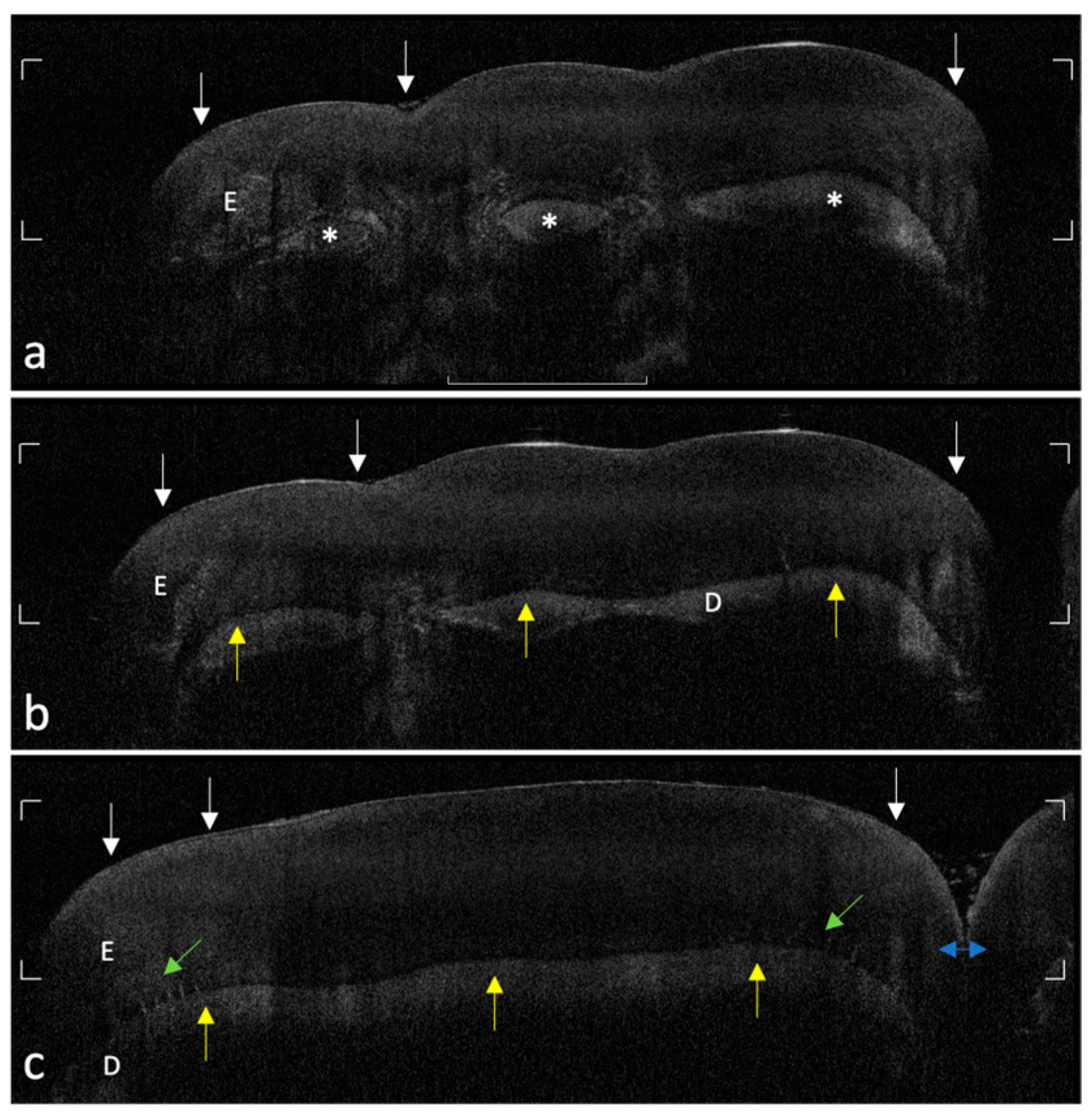
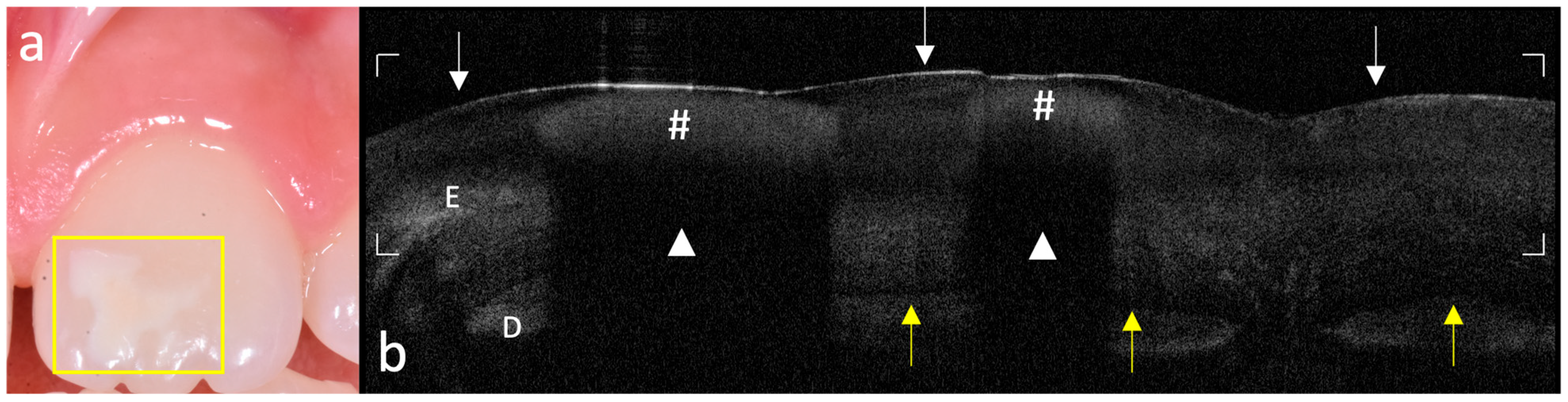
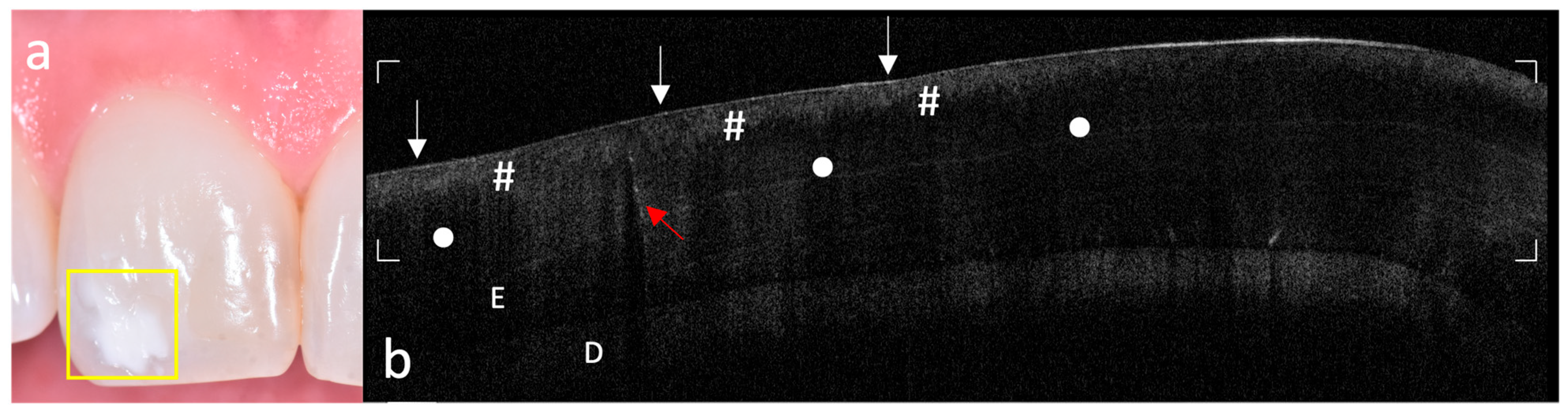
The acquired OCT images were qualitatively evaluated to assess enamel integrity and microstructural patterns in both MIH-affected and control teeth. Analysis focused on identifying optical and subsurface structural alterations relevant to early enamel alterations. Key aspects investigated included the following:
- -
Surface and subsurface reflectivity, particularly the presence of localized hyper-reflective bands indicative of hypomineralized areas;
- -
Hypo-reflective regions indicative of subsurface porosities or voids;
- -
Vertical optical discontinuities, interpreted as potential microcracks within the enamel layer;
- -
Continuity and definition of the DEJ, used as an indicator of enamel–dentin interface integrity;
- -
Visualization of internal enamel structures, such as tufts and spindles, where discernible.
Two examiners with expertise in oro-dental OCT image interpretation [
11,
16,
17,
18] independently analyzed the images. Before the evaluation, a calibration session was conducted using a representative set of 30 images, comprising both MIH-affected and control teeth. This session aimed to standardize assessment criteria and ensure inter-examiner consistency. Any discrepancies in interpretation were resolved through consensus discussions. The overall objective of the analysis was to identify recurring OCT features associated with mild MIH and to compare them with the structural patterns of clinically sound dental hard tissues.
4. Discussion
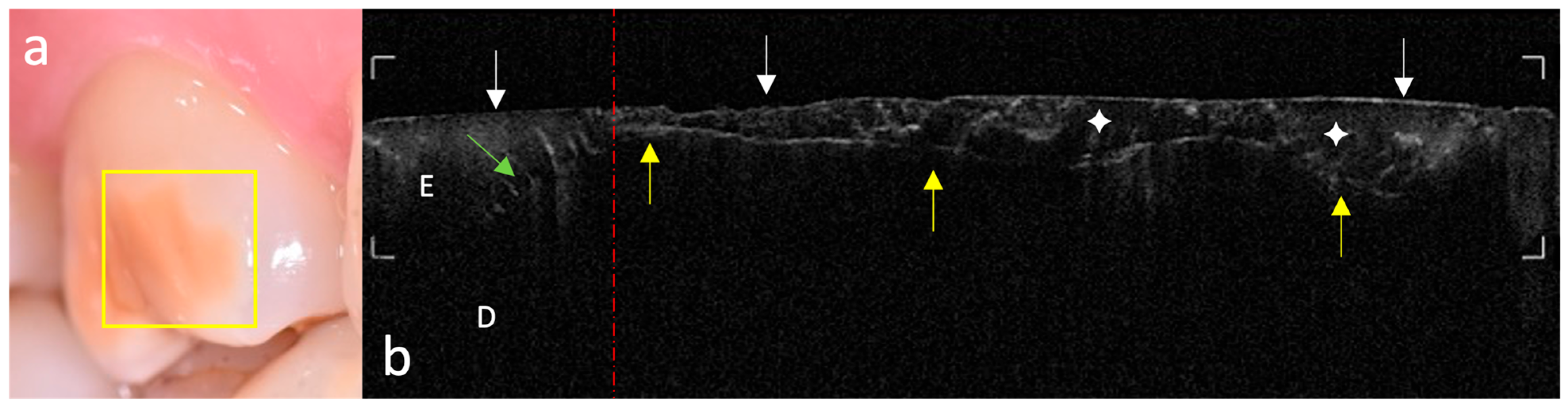
This study highlights the potential of OCT in the non-invasive characterization of MIH, providing in vivo, high-resolution, cross-sectional, real-time imaging of visualization of structural alterations in enamel with micrometric precision.
The systematic acquisition and interpretation of OCT scans demonstrated that MIH lesions, particularly those with clinically mild presentation, exhibit reproducible subsurface hyper-reflective bands confined to the outer third of enamel, which were not detectable by visual inspection alone. These features, absent in healthy controls, may serve as diagnostic optical signatures of MIH-related hypomineralized zones and structural enamel disorganization reported in the literature [
19].
The ability of OCT to differentiate between sound and early MIH-affected enamel is grounded in the physics of light scattering. The increased backscattering signal in affected regions reflects the presence of porosities and discontinuities in enamel prisms, which result from defective mineral deposition during amelogenesis [
10,
20].
Oliveira et al. demonstrated a strong correlation between OCT signal intensity and mineral density profiles obtained by micro-computed tomography (microCT), thereby validating OCT as a reliable surrogate for the non-invasive assessment of enamel mineralization status [
19]. Building upon this foundation, the same group applied grayscale digital analysis and confirmed that hypomineralized enamel exhibits significantly altered optical properties compared to sound tissue, both in signal intensity and homogeneity [
19]. Our findings are in strong agreement with these observations: hyper-reflective regions were consistently observed in MIH-affected enamel, supporting the hypothesis that increased light scattering in OCT images arises from prism disarray, subsurface porosities, and reduced mineral density typical of hypomineralized structures. It is important to emphasize that OCT signal intensity reflects these optical phenomena rather than mechanical hardness per se, although both are linked to the same structural alterations. Furthermore, anisotropy in light scattering, related to the orientation of enamel prisms and microstructural irregularities, may also contribute to variations in OCT signal intensity.
The strength of our OCT findings lies in their ability to detect subtle, subsurface enamel changes even in the absence of visible breakdown. These optical patterns were particularly evident in mild lesions, which often escape visual–tactile detection, and suggest a gradient of mineral integrity progressing from the outer enamel surface toward the DEJ. This observation aligns with previous reports by Espigares et al., who demonstrated that OCT can effectively delineate lesion depth and surface layer thickness in naturally occurring enamel demineralizations, showing high correlation with micro-CT data [
21]. In our study, MIH lesions typically appeared as well-defined superficial hyper-reflective zones with variable penetration depth and lateral extension. Moreover, several scans revealed focal areas of delamination near the DEJ, indicating that structural weakening may extend apically and laterally beyond the clinically visible extent. These findings reinforce the advantage of OCT in capturing subsurface integrity loss that remains undetectable using conventional diagnostic modalities.
Such fine architectural disruptions, including vertical microcracks and subclinical optical voids, were detected in our study and appear as signal interruptions or vertical shadows in the B-scans. These phenomena, also documented by Al-Azri et al., may reflect inter-prismatic discontinuities or stress-related enamel fragmentation [
10]. Their identification is clinically significant: they may represent predisposing zones for PEB, particularly under masticatory load.
From a clinical standpoint, the early detection of structural enamel weaknesses has significant implications for patient care and long-term prognosis. MIH-affected teeth are particularly vulnerable to PEB and accelerated caries progression due to their compromised mechanical properties [
15]. It should be noted, however, that OCT reflectivity is not a direct measure of these mechanical properties. Both reduced mechanical strength and increased backscattering in hypomineralized enamel arise from the same underlying microstructural changes, such as prism disorganization, porosity, and mineral loss. Beyond the biological implications, Gevert et al. also emphasized the psychosocial and functional burden associated with MIH in children and adolescents, including dentinal hypersensitivity, pain during brushing, aesthetic concerns, and increased difficulty in behavioral management [
15]. By revealing structural compromise before cavitation or symptomatic deterioration becomes evident, OCT imaging offers the opportunity to intervene earlier in the disease course. Such early detection may support the adoption of minimally invasive preventive strategies—such as topical remineralization, resin infiltration, or selective adhesive restoration—potentially halting lesion progression and reducing the long-term restorative burden. This proactive approach aligns with the principles of minimally invasive dentistry and personalized pediatric care.
Furthermore, the worldwide prevalence of MIH underscores the need for improved diagnostic protocols. A recent meta-analysis estimated global MIH prevalence at approximately 13.5%, with substantial regional variation [
22]. This epidemiological heterogeneity may be compounded by the limitations of visual-based indices. Incorporating OCT into routine clinical assessments could enable standardized, operator-independent, and reproducible diagnosis across populations.
Compared to conventional radiography, OCT provides superior diagnostic performance in early enamel lesions without exposing children to ionizing radiation. Shimada et al. reported that 3D SS-OCT could clearly depict enamel lesions at smooth tooth surfaces as bright zones, based on increased backscattering signal, and showed higher sensitivity for the diagnosis of remineralized, deep non-cavitated, and cavitated enamel lesions, with significantly greater diagnostic accuracy than visual inspection (
p < 0.05) [
23]. Balhaddad et al. confirmed OCT’s advantage in a systematic review, reporting sensitivity and specificity as high as 74.1% and 95.7%, respectively [
24]. In our study, OCT enabled visualization of MIH-specific features even in lesions classified as mild by visual criteria, suggesting a meaningful potential improvement in early detection compared to traditional indices.
Beyond qualitative structural visualization, SS-OCT enables real-time, radiation-free imaging, which is particularly advantageous in pediatric dentistry. Shimada et al. demonstrated that OCT could detect fine enamel defects—including cracks, early enamel subsurface changes, and age-related structural variations—that may remain undetected by conventional methods [
23]. The integration of handheld OCT probes in clinical workflows may enhance early screening and longitudinal monitoring without compromising safety or comfort.
While the diagnostic performance of OCT is well established, its clinical translation still faces several methodological challenges. Interpretation of OCT signal patterns requires specific training, and while classification systems exist for caries-related findings, no validated schemes are currently available for MIH-specific optical features. Popescu et al. addressed this issue in a preclinical animal model, showing that OCT can effectively differentiate between hypoplasia and hypomineralization based on signal homogeneity and depth, and emphasized the potential of 3D reconstructions and software-assisted visualization to enhance lesion characterization and diagnostic precision [
20]. These technological advances may support future efforts to standardize OCT interpretation and increase diagnostic reproducibility in both clinical and research settings. A promising frontier lies in the automated detection and classification of enamel defects through machine learning. Kim et al. developed an algorithm using the Hough transform for the automatic identification of enamel cracks in OCT images, achieving high sensitivity in detecting defects that often escape conventional observation [
13]. Applying similar AI-driven approaches to MIH detection may increase diagnostic standardization, overcome inter-operator variability, and support large-scale screening initiatives, ultimately laying the foundation for real-time, computer-assisted diagnostics in pediatric dental care and broader epidemiological or preventive programs.
Despite these promising technological advances, several limitations still characterize both the OCT technique and its clinical application, including those directly reflected in the present study. A primary limitation is that the OCT device employed (VivoSight), originally optimized for dermatological imaging, was adapted for dental applications. While its imaging depth and acquisition speed are advantageous for in vivo scanning, its axial resolution and tissue contrast are slightly lower compared to SD-OCT systems. Nevertheless, the performance of this SS-OCT device proved fully adequate for the diagnostic objectives of this study. Furthermore, OCT signal interpretation remains operator-dependent, and the absence of validated classification criteria for MIH-specific optical patterns may limit diagnostic standardization across settings. Future studies should integrate quantitative OCT metrics (e.g., attenuation coefficients or depth-resolved reflectivity analysis) and automated classification approaches to enhance objectivity and reproducibility.
Although highly informative, histological or mineral density validation was not feasible in this clinical context due to ethical and practical constraints. Similarly, radiographic imaging was not included, as intraoral radiographs and bitewings offer only a single two-dimensional projection of enamel defects that are inherently three-dimensional, providing limited diagnostic information for early MIH lesions. Moreover, radiographs cannot accurately depict subsurface microstructural changes and would have required unnecessary ionizing exposure in children, which contrasts with the non-invasive objectives of this study.
The single-center design and the deliberate focus on mild MIH cases—selected to ensure homogeneous samples and avoid imaging artifacts caused by PEB typically observed in more severe forms—were intended to maximize image quality and reproducibility. Focusing on mild lesions is also a strength of this study, as these early-stage defects are the most challenging to detect with conventional diagnostic approaches and highlight the potential of OCT for early, non-invasive enamel evaluation. Nevertheless, the single-center design may introduce selection bias, and future studies should consider multi-center cohorts to validate and expand these findings across broader populations.
In addition, this preliminary exploratory study employed a convenience sample based on clinical feasibility rather than an a priori statistical power calculation, which may limit the generalizability of the findings. This design choice reflects the exploratory nature of this work, which represents the first in vivo OCT characterization of MIH structural patterns. Future studies with larger sample sizes will incorporate formal power analyses and quantitative evaluation to validate and expand these preliminary observations. These studies will also evaluate the diagnostic performance of the qualitative OCT features identified in the present work, integrating accuracy metrics once validated on larger cohorts. Although matching was performed by tooth type and eruption stage, the presence of residual confounding cannot be entirely excluded.
Nevertheless, a major strength of this study lies in its translational nature. To our knowledge, it represents the first in vivo investigation evaluating the application of OCT for the evaluation of MIH. Previous studies have been limited to in vitro or animal models, which do not fully replicate the optical behavior or clinical variability of human enamel. The ability to assess structural changes directly in a clinical setting—without the need for extraction or destructive processing—confirms the feasibility of OCT imaging in real-world pediatric scenarios. Furthermore, the inclusion of clinically mild MIH cases—often underrepresented in OCT research—highlights the sensitivity of this modality in capturing early and subclinical enamel alterations.
Building upon these preliminary findings, future research should aim to develop and validate standardized OCT-based classification systems for MIH, enabling reproducible diagnosis across institutions and operators. The application of quantitative imaging analysis—including reflectivity indices, lesion volume estimation, and structural segmentation—will be essential to improve diagnostic objectivity and support longitudinal monitoring. Expanding the sample to a multi-center cohort that includes severe MIH cases will enhance external validity. Finally, the integration of artificial intelligence for automated lesion recognition and real-time clinical decision-making is currently under investigation, with the goal of improving diagnostic consistency and supporting large-scale screening programs.