Abstract
This study synthesizes and comparatively investigates two squaric acid-based phthalocyanine-like dyes, SNF and its long-chain alkylated derivative LNF, to systematically elucidate the influence of peripheral hydrophobic groups on their third-order nonlinear optical (NLO) properties. The NLO characteristics were comprehensively characterized using femtosecond Z-scan and I-scan techniques at both 800 nm and 900 nm. Both dyes exhibited strong saturable absorption (SA), confirming their potential as saturable absorbers. Critically, the comparative analysis revealed that SNF exhibits a significantly greater nonlinear absorption coefficient (β) compared to LNF under identical conditions. For instance, at 800 nm, the β of SNF was approximately 3–5 times larger than that of LNF. This result conclusively demonstrates that the introduction of long hydrophobic alkyl chains attenuates the NLO response. Furthermore, I-scan measurements revealed excellent SA performance, with high modulation depths (e.g., LNF: 43.0% at 900 nm) and low saturation intensities. This work not only clarifies the structure–property relationship in these D-A-D dyes but also presents a clear strategy for modulating the NLO properties of organic chromophores for applications in near-infrared pulsed lasers.
1. Introduction
Nonlinear optical materials with high coefficients and fast response times are crucial for advancements in various optoelectronic and laser applications, including optical limiting [1,2], optical switching [3], and, particularly, laser mode-locking [4]. The development of efficient saturable absorbers, key components in generating ultrashort laser pulses, relies heavily on materials exhibiting strong nonlinear absorption, specifically saturation absorption [5,6,7]. While a range of materials, including inorganic and organic materials [8,9,10,11,12], insulators [13], and crystals [14], have been investigated for nonlinear optical properties, the ongoing search for novel materials with tailored characteristics remains a central focus. This pursuit is driven by the need to optimize device performance and explore new functionalities in areas like high-power lasers [15,16], ultrafast spectroscopy [17], and bioimaging [18]. Understanding the fundamental mechanisms governing nonlinear light–matter interactions is essential for designing and synthesizing materials with enhanced nonlinear optical responses.
Owing to their distinctive structural and electronic characteristics, squaric acid dyes and their derivatives have attracted attention as promising materials for nonlinear optical applications [19,20]. These molecules, often exhibiting a D-A-D (donor–acceptor–donor) architecture, are characterized by extended π-conjugation, high photothermal stability, and strong absorption in the near-infrared and visible spectral regions [21,22,23]. The extended conjugation facilitates efficient delocalization of electrons, leading to enhanced nonlinear optical effects. Furthermore, the synthetic versatility of squaric acid dyes allows for precise tuning of their optical properties through structural modifications, enabling control over parameters such as the absorption wavelength, nonlinear optical coefficient, and response time [20,24,25,26]. This tunability makes them attractive building blocks for various photonic applications.
While previous studies have extensively investigated two-photon absorption (2PA) in squaric acid derivatives [27], reports on saturation absorption, a phenomenon crucial for saturable absorber applications, are comparatively scarce. For instance, Ballestas-Barrientos et al. [28] synthesized a series of indole-based squaric acid dyes and demonstrated high 2PA cross-sections, highlighting their potential for two-photon imaging. Bondar et al. [29] reported efficient NIR emission and 2PA in a novel squaric acid derivative, indicating its suitability for two-photon fluorescence bioimaging. Zhou et al. [30] explored the ultrafast nonlinear properties of an indole squaric acid, emphasizing its potential for optical limiting based on excited-state absorption. However, the observation and systematic study of saturation absorption in these materials remain relatively unexplored.
While previous studies have primarily reported excited-state absorption and two-photon absorption in squaric acid derivatives, observation of saturation absorption has been less common. In this work, we present two novel D-A-D-structured squaric acid materials and thoroughly investigate their nonlinear optical properties using femtosecond Z/I-scan techniques. These materials exhibit significant saturable absorption signals, demonstrating the potential of squaric acid analogs as saturable absorbers for near-infrared laser applications. Furthermore, we investigate how hydrophobicity affects the nonlinear optical properties of these materials. These findings not only advance the fundamental understanding of nonlinear optical processes in squaric acid dyes but also potentially lead to advancements in areas such as ultrafast laser technology.
2. Experimental Section
2.1. Sample Preparation and Analytical Instruments
The synthesis methods for the samples studied have been extensively detailed in the literature [20,31]. The molecular structures of LNF and SNF are shown in Figure 1. A concise summary is provided here. SNF was synthesized by refluxing a mixture of 1,8-naphtholactimide (donor) and squaric acid (acceptor) in 6 mL of a 1:1 (v/v) toluene/butanol solution for 2 h. After cooling, the resulting product was obtained by filtration and rinsed with ether. Column chromatography, eluting with a 9:1 dichloromethane/methanol mixture, was employed for purification. The purified product was subsequently recrystallized from a methanol/hexane mixture. LNF was synthesized from SNF via alkylation of the lactam nitrogen. This involved reacting SNF with a long-chain alkyl halide in the presence of a base, such as triethylamine, in either DMF or DMA. Column chromatography was employed to purify the resulting product. Both samples were dissolved in dichloromethane and contained in quartz cuvettes of 1 mm thickness. UV–visible absorption spectra were recorded at room temperature using a spectrophotometer (UV-2600i, Shimadzu, Kyoto, Japan) equipped with halogen and deuterium lamps as light sources. Photoluminescence (PL) spectroscopy measurements were conducted using a steady-state/transient fluorescence spectrometer (FLS1000, Edinburgh Instruments, Edinburgh, Scotland, UK).
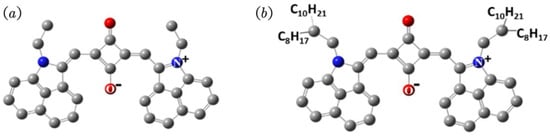
Figure 1.
Schematic representation of the molecular structures of SNF (a) and LNF (b).
2.2. NLO Measurement
The nonlinear optical measurement was conducted using a custom-built femtosecond Z-scan system in our laboratory [32], as depicted in Figure 2. The excitation source was a femtosecond laser setup comprising a Ti/sapphire regenerative amplifier (Solstice Ace, Spectra-Physics, Milpitas, CA, USA), which was seeded by a mode-locked Ti/sapphire oscillator (Mai Tai SP, Spectra-Physics, Milpitas, CA, USA) and driven by a solid-state Q-switched laser (Ascend, Spectra-Physics, Milpitas, CA, USA). The laser fires with the center wavelength of an 800 nm beam (1 kHz repetition rate and 35 fs pulse width), which could be frequency-converted by an optical parametric amplifier (TOPAS Prime, Spectra-Physics, Milpitas, CA, USA) to produce a tunable pump laser beam in the 240–1600 nm wavelength range. In the Z-scan setup, all mirrors used are aluminum-coated with a reflectivity exceeding 90%. The incident laser beam first passes through a beam splitter (BS1,r:t = 50:50). The reflected portion is directed through a neutral-density filter and detected by a reference detector (D1) to monitor power fluctuations, serving as a reference signal to reduce noise caused by laser power instability. The transmitted portion of the beam first passes through a rotatable neutral-density filter (NDF1) for adjustable optical density control, and then through a telescope system composed of a convex lens (L1, f = 350 mm) and a concave lens (L2, f = 75 mm) to reduce the beam size. After being redirected by two aluminum mirrors (M1 and M2), the beam becomes parallel to the translation stage and is then focused onto the sample by a convex lens (L3, f = 200 mm). The transmitted light from the sample is split into two paths by a beam splitter (BS2, r:t = 50:50): one is directed to an open-aperture detection arm consisting of a focusing lens (L4, f = 350 mm) and a silicon photodiode (D2); the other is directed to a closed-aperture detection arm, where the beam passes through an aperture diaphragm before reaching the detector (D3). The laser beam, after being focused by lens L3, produced a focal spot radius (beam waist, ω0) of 31.9 μm for 800 nm light and 39.0 μm for 900 nm light, with corresponding Rayleigh lengths of 4 mm and 5.3 mm, respectively. The excitation power of the laser was controlled by adjusting a rotatable neutral-density filter (NDF1), and the transmitted power was monitored in real time using a power meter (PM100D, Thorlabs, Newton, NJ, USA). The sample was translated along the z-axis to obtain the Z-scan curve, with the scanning range set from –5 cm to 5 cm. In I-scan mode, the sample was fixed at the focal plane (z = 0) to ensure consistent beam irradiation, and a continuously adjustable attenuator was used to change the laser power irradiated on the sample with the same laser beam size. The laser power was adjusted by translating a linear-density neutral-density filter mounted on a motorized stage (console). A reference scan without the sample is first performed to obtain the excitation power as the horizontal axis. Then, a second scan is carried out with the sample in place. The ratio of the two signals gives the transmittance, which is plotted as the vertical axis.
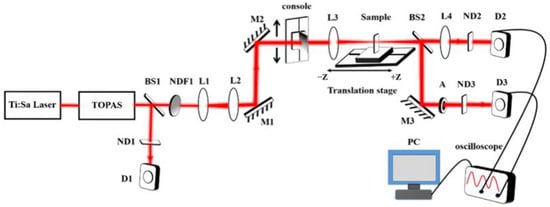
Figure 2.
The optical path diagram of the Z-scan setup. NDF stands for continuously adjustable attenuator, BS stands for beam splitter, M stands for mirror, L stands for lens, A stands for aperture, D stands for detector, and Translation stage stands for sample displacement platform. The focused beam waist (ω0) at z = 0 was 31.9 μm for 800 nm light and 39.0 μm for 900 nm light.
3. Results and Discussion
3.1. Optical Property Characterization of Phthalocyanine-like Squaraine Dyes
The UV–visible absorption spectra of SNF and LNF are presented in Figure 3. And the optical bandgaps of SNF and LNF were determined from the Tauc plots shown in Figure S3 of the Supplementary Materials, indicating values of 1.316 eV and 1.281 eV, respectively. Both compounds exhibit distinct double absorption peaks in the near-infrared (NIR) region. Specifically, SNF displays a maximum absorption peak at approximately 880 nm and a shoulder peak at 790 nm. In contrast, LNF, which incorporates a long-chain alkane moiety, demonstrates a red-shifted absorption spectrum with a maximum peak near 900 nm and a shoulder peak at 808 nm. DFT calculations (Figures S4 and S5) show that the branched alkyl substituents do not contribute to the frontier orbitals but induce a slight twist of the π-conjugated backbone (Δθ ≈ 3.787°). This conformational perturbation narrows the HOMO–LUMO gap by approximately 0.011 eV and increases polarizability, collectively producing the red-shift observed in the absorption spectrum. Both SNF and LNF possess extensive conjugated structures that facilitate efficient delocalization of electrons within the molecules, enabling effective charge transfer, as evidenced by their long-wavelength absorption characteristics. Moreover, both compounds exhibit broad double emission peaks in the NIR region: around 970 nm and 1030 nm for SNF, and approximately 965 nm and 1025 nm for LNF. Importantly, fluorescence emission from both compounds extends into the NIR-II region, indicating successful development of donor–acceptor–donor (D-A-D) systems as emitters within this spectral range. These findings suggest that the incorporation of the hydrophobic alkane chain in LNF not only red-shifts the absorption spectrum but also influences the electronic structure and charge transfer dynamics of the molecule, thereby enhancing its potential as an NIR-II emitter.
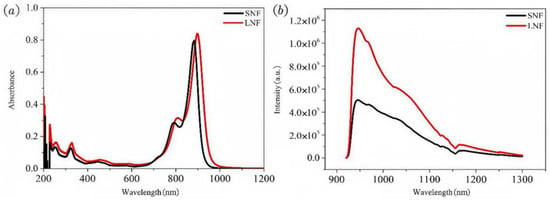
Figure 3.
(a) UV-vis absorption and (b) photoluminescence spectra of SNF and LNF in CH2Cl2 (5 mg/L).
3.2. Nonlinear Optical Properties of Phthalocyanine-like Squaraine Dyes
3.2.1. Analysis of Z-Scan Results of Phthalocyanine-like Squaraine Dyes
To explore the nonlinear optical (NLO) properties of SNF and LNF, open-aperture Z-scan experiments were conducted at wavelengths of 800 nm and 900 nm using a femtosecond laser. Both fall within the strong near-infrared absorption bands of the compounds, enabling resonant nonlinear processes such as saturable absorption. Specifically, 800 nm corresponds to the shorter-wavelength shoulder of the absorption profile, typically associated with H-aggregates, while 900 nm aligns with the monomeric absorption peak. This dual-wavelength approach allows for a comparative analysis of the NLO response across the resonance curve and provides insight into the distinct behaviors of monomer and aggregate species. It also helps clarify how molecular aggregation modulates the overall NLO performance. The reference sample, pure solvent, exhibited no discernible nonlinear optical signals at these wavelengths, confirming that the observed signals originated from the material itself. Figure 4 illustrates the nonlinear absorption signal of the sample at both wavelengths.
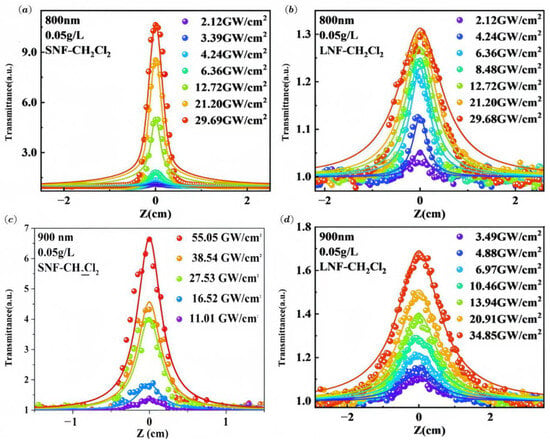
Figure 4.
Open-aperture Z-scan results of SNF (a,c) and LNF (b,d) at 800 and 900 nm wavelengths in CH2Cl2 (c = 5 mg/L).
The normalized optical transmittance of the sample exhibits centrosymmetric behavior about the x-axis and increases as it approaches beam focus. This characteristic is indicative of nonlinear saturable absorption (SA) performance, suggesting that squaraine cyanine dye can effectively absorb low-intensity light while remaining transparent to high-intensity light at 800 nm and 900 nm. To provide a more intuitive understanding of SNF and LNF’s nonlinear characteristics, the nonlinear absorption coefficients (β) were quantitatively determined in this study by fitting the open-aperture Z-scan data obtained at 800 nm and 900 nm using Equation (1) [33]:
where β represents the nonlinear absorption coefficient, T is the nonlinear transmittance, Leff is the effective sample length [, where L is the thickness of the sample and α0 is the linear absorption coefficient of the sample], and z0 is the Rayleigh diffraction length of the laser beam (i.e., , with ω0 being the beam waist radius at the focus).
The experimental data were fitted with the Levenberg–Marquardt nonlinear least-squares routine in MATLAB R2021a. Thermal lensing was evaluated by performing Z-scan at 100 Hz and 1 kHz; no obvious difference in β was observed, confirming negligible thermal contribution. To provide a comprehensive understanding of the experimental results, we estimate the overall uncertainty in the determination of the nonlinear absorption coefficient β to be approximately 15%. The main sources of error include the following: (1) laser power fluctuations (<5%) despite the use of a reference detector; (2) ~5% uncertainty in the beam waist radius (ω0), leading to ~10% uncertainty in peak intensity (I0 ∝ 1/ω02); and (3) uncertainties from sample concentration, path length, and numerical fitting. The nonlinear absorption coefficients of the fitted samples at 800 nm and 900 nm are presented in Table 1. The negative values of these coefficients are indicative of saturation absorption. LNF, compared to SNF, incorporates long-chain alkane groups, imparting greater hydrophobicity and potentially facilitating the encapsulation of hydrophilic liposomes in biological applications. To evaluate the influence of these hydrophobic groups on nonlinear optical properties, the nonlinear absorption of SNF and LNF was compared at identical wavelengths. The results demonstrate a stronger nonlinear absorption coefficient for SNF, suggesting that the introduction of hydrophobic groups diminishes the nonlinear optical response. This attenuation of nonlinear absorption in LNF may be attributed to several factors. Firstly, the introduction of hydrophobic groups could alter the electronic delocalization within the dye molecules. This alteration might lead to a reduction in the effective conjugation length, consequently weakening the nonlinear optical response. Secondly, these hydrophobic groups promote dye molecule aggregation in solution. In our previous work [31], we found that LNF tends to form aggregates easily in organic solvents. Such aggregation could decrease the effective chromophore concentration, thus reducing the observed nonlinear absorption. Finally, the presence of hydrophobic groups could influence the dye solubility in the chosen solvent. This change in solubility might alter the local environment surrounding the chromophores, ultimately affecting their nonlinear optical properties. Our results introduce a novel approach to modulating the nonlinear optical behavior of organic dyes, emphasizing the critical role of hydrophobicity in the design of materials for nonlinear optical applications.

Table 1.
Nonlinear absorption coefficients of SNF and LNF at 800 and 900 nm wavelengths in CH2Cl2 (c = 5 mg/L).
3.2.2. Analysis of I-Scan Results of Phthalocyanine-like Squaraine Dyes
Based on the experimental results of the I-scans conducted at 800 nm and 900 nm, the dyes exhibit high transmittance under intense excitation, indicative of saturable absorption behavior. To quantitatively assess the performance of the saturable absorbing materials, key parameters such as saturation intensity, modulation depth, and nonsaturable loss are essential. Upon increasing the input energy density, the sample transmittance gradually rises, as shown in Figure 5. After reaching a certain intensity level, the transmittance stabilizes, characteristic of saturable absorption. To quantitatively evaluate the sample’s saturable absorption response, the Equation (2) was employed [32]:
where IS, ΔT, and Tns are the saturation intensity of SA, modulation depth, and nonsaturation loss of the sample, respectively. All I-scan results are presented in Table 2.
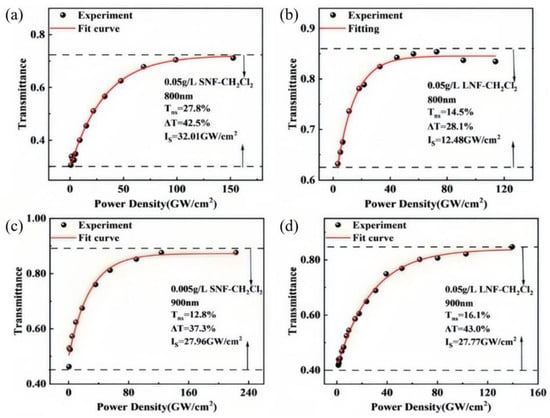
Figure 5.
I-scan results of SNF at 800 nm (a), LNF at 800 nm (b), SNF at 900 nm (c) and LNF at 900 nm (d).

Table 2.
The modulation depth, nonsaturated loss, and saturation light intensity of SNF and LNF.
Squaraine dyes exhibit exceptional nonlinear optical properties, including high modulation depths, low saturation intensities, and minimal nonsaturable losses. These characteristics make them highly suitable as saturable absorbers for passive mode-locking and Q-switching in ultrafast lasers, enabling the generation of laser pulses with short durations and high repetition rates. In ultrafast laser systems, the modulation depth represents the maximum change in optical loss achievable by the material, directly influencing the efficiency of pulse generation. A higher modulation depth allows for a broader range of transmittance variation, enhancing the optical modulation performance of the laser. Additionally, a lower saturation intensity enables the laser to produce ultrashort pulses even at lower pump intensities, thereby improving overall laser performance. The excellent saturable absorption properties of squaraine dyes position them as promising candidates for near-infrared laser applications in the field of ultrafast lasers. Their high modulation depths and low saturation intensities facilitate the generation of laser pulses with desirable characteristics, such as short durations and high repetition rates, which are essential for various applications in photonics and optoelectronics. In conclusion, squaraine dyes hold significant potential as near-infrared laser dyes in the field of ultrafast lasers, offering advantages that can lead to advancements in laser technology and its diverse applications.
4. Conclusions
The third-order nonlinear absorption properties of SNF and LNF at 800 nm and 900 nm were investigated using the open-aperture Z-scanning technique. This study also explored the effects of hydrophobic groups on the nonlinear optical properties of the samples. The results indicate that both SNF and LNF exhibit excellent third-order nonlinear saturable absorption properties, with the effect gradually increasing with excitation intensity within a certain optical intensity range, highlighting their potential for use as saturable absorbers in pulsed lasers. Additionally, it was found that SNF has stronger nonlinear absorption coefficients compared to LNF, suggesting that hydrophobic groups weaken the nonlinear optical properties of the materials. These findings propose new ideas for the nonlinear optical modulation of organic dyes. Furthermore, the I-scan results demonstrated that these materials have great potential for application in near-infrared ultrafast lasers due to their ultra-high modulation depth and small saturation intensity at 800 nm and 900 nm wavelengths.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/photonics12080779/s1, Figure S1: The specific synthetic route of SNF; Figure S2: The specific synthetic route of SNF; Figure S3: Tauc plots of (a) SNF and (b) LNF. The estimated optical bandgaps are 1.316 eV and 1.281 eV, respectively; Figure S4: Optimised geometries and frontier-orbital isosurfaces of SNF and LNF(b3lyp/6-311g(d,p), PCM = CH2Cl2). Isosurface = 0.002; blue = positive, green = negative. Average dihedral angle (phenyl–squaraine plane) increases from −3.196° (SNF) to 0.591° (LNF). Calculated HOMO-LUMO gaps: 1.605 eV (SNF) and 1.594 eV (LNF); Figure S5: Electron centroid map: the blue circle indicates HOMO, and the green circle indicates LUMO.After alkyl chain substitution, the centroid shift decreases from 0.441 Å to 0.432 Å.
Author Contributions
Conceptualization, Z.L., H.W. and B.J.; Methodology, Z.L., H.W. and B.J.; Software, M.Z. and A.L.; Validation, F.Z., Y.W., L.S., W.G., M.Q., M.Z., H.W. and B.J.; Formal analysis, F.Z., W.S., X.L., W.G., M.Q., M.Z., J.M., A.L., Z.L. and B.J.; Investigation, F.Z., W.S., X.L., W.G. and M.Q.; Data curation, A.L.; Writing—review & editing, F.Z., W.S., X.L., Y.W., H.W. and B.J.; Supervision, H.W. and B.J. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was funded by the National Natural Science Foundation of China (Grant No: 21873114, 22063005), the Natural Science Foundation of Jiangxi Province (No. 20212ACBA203012, No. 20224BAB214003, No. 20232BAB203031), the Interdisciplinary Innovation Fund of Natural Science, Nanchang University (No. 9167-27060003-ZD2101, No. 9167-28220007-YB2113), the Foundation of Jiangxi Provincial Key Laboratory of Functional Crystalline Materials Chemistry (No. 2024SSY05162), Opening Foundation of Key Laboratory of Laser & Infrared System (Shangdong University), and the Fundamental Research Funds of Shandong University.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Husain, A.; Ganesan, A.; Sebastian, M.; Makhseed, S. Large ultrafast nonlinear optical response and excellent optical limiting behaviour in pyrene-conjugated zinc(II) phthalocyanines at a near-infrared wavelength. Dye. Pigment. 2021, 184, 108787. [Google Scholar] [CrossRef]
- Samad, F.A.; Mahmoud, A.; El-Khouly, M.E.; Apsari, R.; Mohamed, T. Nonlinear optical studies of perylenediimide nanowires using femtosecond laser pulses for optical limiter application. J. Mol. Liq. 2025, 418, 126679. [Google Scholar] [CrossRef]
- Zidan, M.D.; EL-Daher, M.S.; Al-Ktaifani, M.M.; Allahham, A.; Ghanem, A. Spatial phase modulation and all-optical switching of tris(2′,2-bipyridyl)iron(II) tetrafluoroborate. Optik 2020, 219, 165275. [Google Scholar] [CrossRef]
- Verrone, R.N.; Moisset, C.; Lemarchand, F.; Campos, A.; Cabié, M.; Perrin-Pellegrino, C.; Lumeau, J.; Natoli, J.Y.; Iliopoulos, K. Thickness-dependent optical nonlinearities of nanometer-thick Sb2Te3 thin films: Implications for mode-locking and super-resolved direct laser writing. ACS Appl. Nano Mater. 2020, 3, 7963–7972. [Google Scholar] [CrossRef]
- Khazaeinezhad, R.; Hosseinzadeh Kassani, S.; Paulson, B.; Jeong, H.; Gwak, J.; Rotermund, F.; Yeom, D.I.; Oh, K. Ultrafast nonlinear optical properties of thin-solid DNA film and their application as a saturable absorber in femtosecond mode-locked fiber laser. Sci. Rep. 2017, 7, 41480. [Google Scholar] [CrossRef]
- Huang, B.; Yi, J.; Jiang, G.; Miao, L.; Hu, W.; Zhao, C.; Wen, S. Passively Q-switched vectorial fiber laser modulated by hybrid organic-inorganic perovskites. Opt. Mater. Express 2017, 7, 1220. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, H.; Wang, Y.; Ni, Z.; Yan, Y.; Shen, Z.X.; Loh, K.P.; Tang, D.Y. Atomic-layer graphene as a saturable absorber for ultrafast pulsed lasers. Adv. Funct. Mater. 2009, 19, 3077–3083. [Google Scholar] [CrossRef]
- Maji, T.K.; Aswin, J.R.; Mukherjee, S.; Alexander, R.; Mondal, A.; Das, S.; Sharma, R.K.; Chakraborty, N.K.; Dasgupta, K.; Sharma, A.M.R.; et al. Combinatorial large-area MoS2/Anatas–TiO2 interface: A pathway to emergent optical and optoelectronic functionalities. ACS Appl. Mater. Interfaces 2020, 12, 44345–44359. [Google Scholar] [CrossRef]
- Qiao, J.; Chuang, M.Y.; Lan, J.C.; Lin, Y.Y.; Sung, W.H.; Fan, R.; Wu, M.Y.; Lee, C.Y.; Chen, C.H.; Liu, H.; et al. Two-photon absorption within layered Bi2Te3 topological insulators and the role of nonlinear transmittance therein. J. Mater. Chem. C 2019, 7, 7027–7034. [Google Scholar] [CrossRef]
- John, J.S.; Arumanayagam, T.; Murugakoothan, P.; Sajan, D.; Joy, N.; Philip, R. Synthesis, growth and characterization of guanidinium hippurate monohydrate single crystals: A new third order nonlinear optical material. Opt. Mater. 2020, 110, 110493. [Google Scholar] [CrossRef]
- Shanu, M.; Acharyya, J.N.; Kuriakose, A.; Banerjee, D.; Soma, V.R.; Vijaya Prakash, G. Ultrafast dynamics, optical nonlinearities, and chemical sensing application of free-standing porous silicon-based optical microcavities. ACS Appl. Mater. Interfaces 2024, 16, 16996–17006. [Google Scholar] [CrossRef]
- Yahya, M.; Nural, Y.; Seferoğlu, Z. Recent advances in the nonlinear optical (NLO) properties of phthalocyanines: A review. Dye. Pigment. 2022, 198, 109960. [Google Scholar] [CrossRef]
- Jia, L.; Cui, D.; Wu, J.; Feng, H.; Yang, Y.; Yang, T.; Qu, Y.; Du, Y.; Hao, W.; Jia, B.; et al. High third-order Kerr optical nonlinearity in BiOBr 2D films measured by the Z-scan method. APL Photonics 2019, 4, 092101. [Google Scholar]
- Zhou, Y.; Huang, Y.; Xu, X.; Fan, Z.; Khurgin, J.B.; Xiong, Q. Nonlinear optical properties of halide perovskites and their applications. Appl. Phys. Rev. 2020, 7, 041313. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, X.; Hu, W.; Jiang, Y.; Chen, C.; Dong, N.; Wang, J.; Liang, Y.; Zhu, B.; Zhang, H.; et al. Ultra-high nonlinear saturable absorption responses and ultra-fast carrier dynamics of organic DAST. Adv. Opt. Mater. 2023, 11, 2202241. [Google Scholar] [CrossRef]
- Vahala, K.J. Optical microcavities. Nature 2003, 424, 839–846. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Q.; Qiu, J. Emerging low-dimensional materials for nonlinear optics and ultrafast photonics. Adv. Mater. 2017, 29, 1605886. [Google Scholar] [CrossRef]
- Tian, T.; Fang, Y.; Wang, W.; Yang, M.; Tan, Y.; Xu, C.; Zhang, S.; Chen, Y.; Xu, M.; Cai, B.; et al. Durable organic nonlinear optical membranes for thermotolerant lightings and in vivo bioimaging. Nat. Commun. 2023, 14, 4429. [Google Scholar] [CrossRef]
- Hutchings, M.G.; Ferguson, I.; Allen, S.; Zyss, J.; Ledoux, I. Non-linear optical properties of squarate esters and amides. J. Chem. Res. 1998, 5, 244–245. [Google Scholar] [CrossRef]
- Ilina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine Dyes: Molecular Design for Different Applications and Remaining Challenges. Bioconjug. Chem. 2020, 31, 194–213. [Google Scholar] [CrossRef]
- Inoue, T.; Pandey, S.S.; Fujikawa, N.; Yamaguchi, Y.; Hayase, S. Synthesis and characterization of squaric acid based NIR dyes for their application towards dye-sensitized solar cells. J. Photochem. Photobiol. Chem. 2010, 213, 23–29. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, H.; Cai, S.; Yang, X.; Gao, X. Single-bond-linked and vinylene-bridged azulenyl bis(squaraine) dyes: Design, synthesis and molecular self-assembly behaviors. Org. Chem. Front. 2024, 11, 7059–7068. [Google Scholar] [CrossRef]
- Krishna, M.B.M.; Rao, D.N. Influence of solvent contribution on nonlinearities of near infra-red absorbing croconate and squaraine dyes with ultrafast laser excitation. J. Appl. Phys. 2013, 114, 133103. [Google Scholar] [CrossRef]
- Sarasiya, S.; Sarasiya, S.; Henary, M. Exploration of NIR squaraine contrast agents containing various heterocycles: Synthesis, optical properties and applications. Pharmaceuticals 2023, 16, 1299. [Google Scholar] [CrossRef]
- MacCuaig, W.M.; Wickizer, C.; Van, R.S.; Buabeng, E.R.; Lerner, M.R.; Grizzle, W.E.; Shao, Y.; Henary, M.; McNally, L.R. Influence of structural moieties in squaraine dyes on optoacoustic signal shape and intensity. Chemistry 2024, 10, 713–729. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, X.; Xu, J.; Li, J.; Yang, J.; Wang, Y.; Zhang, X.; Xiao, J.; Song, Y. Modulation of the nonlinear optical response in squaraine derivatives via alkyl cyclization: Near-infrared ultrafast nonlinear refraction and absorption. Dye. Pigment. 2024, 225, 112058. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Y.; Chen, Z.; Li, H.; Hu, R.; Qu, J.; Lu, Y.; Liu, L. NIR-II light-activated two-photon squaric acid dye with type I photodynamics for antitumor therapy. Nanophotonics 2022, 11, 5089–5100. [Google Scholar] [CrossRef]
- Ballestas-Barrientos, A.R.; Woodward, A.W.; Moreshead, W.V.; Bondar, M.V.; Belfield, K.D. Synthesis and linear and nonlinear photophysical characterization of two symmetrical pyrene-terminated squaraine derivatives. J Phys. Chem. C 2016, 120, 7829–7838. [Google Scholar] [CrossRef]
- Bondar, M.V.; Faryadras, S.; Munera, N.; Chang, H.J.; Uddin, M.; Belfield, K.D.; Kachkovsky, O.D.; Van Stryland, E.W.; Hagan, D.J. New two-photon absorbing squaraine derivative with efficient near-infrared fluorescence, superluminescence, and high photostability. J. Phys. Chem. B 2022, 126, 3897–3907. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, X.; Ma, P.; Zhou, F.; Li, Z.; Niu, R.; Yang, J.; Wang, Y.; Zhang, X.; Song, Y.; et al. Enhanced ultrafast nonlinear absorption and optical limiting of indolium squaraine for laser protection. Opt. Mater. 2022, 126, 112178. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, G.; Wang, J.; Wang, M.; Guo, W.; Tan, M.; Si, L.; Yang, Y.; Wang, H.; Wang, H. A synergetic strategy of NIR-II squaraine dyes with ultrahigh photothermal conversion efficiency for photothermal therapy. Sci. China Chem. 2024, 67, 612–621. [Google Scholar] [CrossRef]
- Li, X.; Zhou, M.; Li, S.; Zhang, F.; Li, Z.; Li, Z.; Jin, B. Solvent effects and self-assembled aggregation modulate nonlinear optical effects in indocyanine green-like dyes. Opt. Mater. 2024, 150, 115132. [Google Scholar] [CrossRef]
- Sheik-Bahae, M.; Said, A.A.; Wei, T.H.; Hagan, D.J.; Van Stryland, E.W. Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 1990, 26, 760–769. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).