Abstract
Background: Clinical diagnostics, food industries, and biotechnological processes typically use an enzyme called alpha-amylase to metabolize carbohydrates. Objective: The aim of this study is to investigate how low-level laser irradiation (LLLI) affects alpha-amylase activity towards determining the usability of LLLI in non-invasive enzymatic modulation. Methods: Enzyme solutions were irradiated at 10, 20, 30, and 40 J/cm2 utilizing 589 nm and 532 nm diode-pumped solid-state lasers. The iodine–starch colorimetric method was used to quantify post-irradiation enzymatic activity, with inverse correlations found between absorbance and activity levels. Modulation was determined by the wavelength and dosage. Results: Enzymatic activity significantly improved when utilizing 589 nm irradiation at lower doses, maximizing at 120% at 20 J/cm2 (p < 0.01). Neutral or inhibitory effects were revealed when higher doses were applied. Enzymatic activity showed progressive inhibition when 532 nm irradiation was applied, declining to 75% at 40 J/cm2 (p < 0.01). Conclusions: These outcomes indicate that conformational flexibility and catalytic efficiency occur when applying lower-energy photons at 589 nm, whilst oxidative stress and impaired enzymatic function are induced by higher-energy photons at 532 nm. This is consistent with the biphasic dose–response characteristic of photobiomodulation.
1. Introduction
As the key enzyme in the metabolization of carbohydrates, alpha-amylase facilitates the hydrolyzation of starch into glucose and maltose, which are the main sources of energy for biological processes. Mostly found in human saliva, pancreatic secretions, plants, and microorganisms, this enzyme is broadly used in food processing, fermentation, textile industries, and clinical diagnostics [1]. Due to its extensive usage, the modulation of alpha-amylase activity has been broadly considered to optimize industrial processes and therapeutic strategy developments [2].
The modulation of biological processes using low-intensity lasers or light emitting diodes (LEDs) at wavelengths of between 400 and 1000 nm is known as low-level laser irradiation (LLLI) or photobiomodulation therapy (PBMT). LLLI uses non-thermal mechanisms, as opposed to high-intensity lasers, to induce cellular and molecular level photochemical and photophysical changes. LLLI has been proven to promote wound healing, reduce inflammation, enhance tissue regeneration, and modulate cellular metabolism [3,4]. At the molecular level, LLLI mainly targets mitochondrial chromophores like cytochrome c oxidase, thus increasing the production of adenosine triphosphate (ATP), modulating reactive oxygen species (ROS), and activating multiple transcription drivers [3,5]. Collectively, these factors help regulate cellular functions. Nonetheless, there is very little research on LLLI’s effect on single enzymes, most notably alpha-amylase. New findings indicate that LLLI modulates alpha-amylase activity by altering the enzyme’s conformational structures or its substrates’ physicochemical properties [6,7]. The photomodulation of enzymes has been shown to be beneficial for improving biocatalytic stability and efficiency. The modulation of the enzymatic activities of catalase, ATPases, and peroxidases has been induced by LLLI based on wavelength and dosage [8,9]. Specific wavelengths were selected based on their known interactions with biological chromophores and previous reports on wavelength-dependent photobiological effects. The 532 nm (green light) wavelength corresponds to higher photon energy and is strongly absorbed by intracellular pigments like flavins and porphyrins, leading to the generation of reactive oxygen species (ROS) and oxidative stress at higher doses, which may inhibit enzymatic function or alter protein structures [10,11]. In contrast, 589 nm (yellow light) lies at a lower photon energy and is absorbed less aggressively, but it is known to stimulate metabolic activity and protein conformational flexibility at lower dosages without inducing damaging ROS levels [12,13]. These contrasting photon energies and absorption profiles are hypothesized to result in differential effects on enzyme kinetics. Prior research has shown that enzymatic activities, such as those of ATPase, catalase, and lipase, can be selectively modulated by LLLI depending on the wavelength and energy density applied [8,14]. However, little is known about how specific wavelengths influence alpha-amylase, a critical enzyme in carbohydrate metabolism and industrial processes. Exploring these effects may offer insights into the precision regulation of enzymatic reactions for therapeutic and biotechnological purposes. Very few studies have explored the impact of wavelength and energy density on alpha-amylase activity. Research on this matter may elucidate how enzymatic processes can be controlled, therefore improving the accuracy of industrial applications such as baking and brewing or establishing therapeutic strategies for managing metabolic disorders. This current study aims to examine the in vitro impacts of LLLI on alpha-amylase activity at 589 nm and 532 nm wavelengths and multiple energy densities. We hypothesize that LLLI will exert wavelength- and dose-dependent modulatory effects on enzyme activity, offering insights into novel strategies for enzyme regulation and expanding the applications of photobiomodulation in the medical and industrial fields.
2. Materials and Methods
2.1. Materials
- Enzyme Source: Alpha-amylase (from Bacillus species; Sigma-Aldrich, St. Louis, MO, USA);
- Substrate: Soluble starch (analytical grade; Sigma-Aldrich);
- Reagents:
- Iodine-potassium iodide (I2-KI) solution (prepared fresh: 0.2% I2 and 2% KI in distilled water);
- Phosphate-buffer solution (50 mM, pH 6.9; prepared with analytical grade reagents);
- Laser Systems:
- Diode-pumped solid-state laser (DPSSL), 589 nm (Product model F Series, Changchun Dragon Lasers Co., Changchun, China);
- DPSSL, 532 nm (Shannxi Richeng Technology Development Co., Xi’an, China);
- Instrumentation:
- Digital spectrophotometer (UV-Vis model, Shimadzu Corporation, Kyoto, Japan), configured to measure absorbance at 620 nm;
- Calibrated optical power meter (Thorlabs, Newton, NJ, USA) for laser energy density validation;
- Standard laboratory centrifuge, micropipettes, and aseptic working bench.
2.2. Ethical Considerations and Donor Selection
The study protocol was approved by the institutional ethics review board. All procedures followed the ethical standards of the Declaration of Helsinki. Blood samples were collected from 10 healthy, non-smoking adult volunteers (5 males and 5 females aged 20–40 years) after obtaining written informed consent. Exclusion criteria included chronic illness, recent medication use, or infection within the past two weeks.
2.3. Blood Sample Collection and Handling
- Collection: Venous blood samples (10 mL per donor) were collected from 10 healthy adult volunteers under aseptic conditions using sterile disposable syringes. The samples were immediately transferred into heparinized collection tubes to prevent clotting, with a final heparin concentration of 15 U/mL.
- Processing and Preparation: Each donor’s blood sample was handled individually to preserve biological variability. The samples were gently inverted to mix with the anticoagulant and processed within one hour of collection. For each individual sample, aliquots were prepared and separately subjected to the laser irradiation protocol. Following treatment, enzymatic activity assays were independently conducted on each sample.
- Data Representation: All results presented in this study represent the mean ± standard deviation (SD) of the individual responses from the 10 separate donor samples (n = 10), ensuring statistical robustness and accounting for inter-individual variation.
All samples were maintained at ambient room temperature (22–25 °C) and processed within one hour of collection to preserve enzyme integrity and minimize degradation.
2.4. Laser Irradiation Protocol
Aliquots (1.0 mL) of the enzyme solution were irradiated using diode-pumping solid-state lasers at 589 nm and 532 nm. The samples were exposed to energy densities of 10, 20, 30, and 40 J/cm2 at room temperature. The power output was adjusted using a calibrated power meter, and exposure times were calculated based on laser power and beam area. After irradiation, the samples were immediately transferred back into sterile tubes, securely capped, and prepared for enzymatic assay.
2.5. Enzymatic Activity Assay
The enzymatic activity of alpha-amylase was assessed using the iodine–starch colorimetric method as follows [15]:
- Reaction Setup:
- ○
- An amount of 100 µL of the blood sample supernatant (post-centrifugation, if necessary) was mixed with 900 µL of 1% soluble starch solution, prepared in phosphate buffer (pH 6.9).
- ○
- The reaction mixture was incubated at 37 °C for 10 min to facilitate the enzymatic hydrolysis of starch.
- Reaction Termination:
- ○
- The reaction was halted by the addition of 1 mL of iodine–potassium iodide solution, which forms a blue-black complex with any residual starch.
- Measurement:
- ○
- Absorbance was measured using a spectrophotometer capable of measuring visible light from 400 to 620 nm.
- ○
- The degree of color development is inversely proportional to alpha-amylase activity; lower absorbance indicates greater enzymatic hydrolysis of starch, reflecting higher enzymatic activity.
- Controls and Blanks:
- ○
- Each set included a non-irradiated control sample and reagent blanks to account for background absorbance.
All samples were analyzed in triplicate, and average values were used for subsequent statistical analysis. Figure 1 shows a schematic diagram of this study.
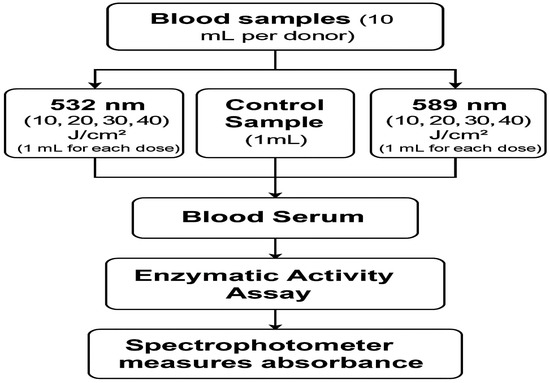
Figure 1.
A block diagram of the study.
2.6. Statistical Analysis
The data are presented as the mean ± standard deviation (SD). Statistical significance between the irradiated and control groups was assessed using a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons. A p-value < 0.05 was considered statistically significant. All analyses were performed using SPSS software (version 2O2O, IBM Corp., Armonk, NY, USA).
3. Results
3.1. Effect of 589 nm LLLI on Alpha-Amylase Activity
Alpha-amylase enzyme activity exhibited a statistically significant enhancement upon irradiation with 589 nm wavelength light at lower energy densities, as summarized in Table 1. At 10 J/cm2, the relative enzymatic activity increased to 115 ± 4% compared to the non-irradiated control, with a p-value < 0.01, indicating a highly significant stimulatory effect. A further increase was observed at 20 J/cm2, where activity reached 120 ± 5%, also with a p-value < 0.01, signifying robust enhancement. However, at 30 J/cm2, the relative activity slightly decreased to 117 ± 3% with a p-value > 0.05, suggesting no statistically significant difference from the control group and indicating a plateau or neutral effect. A decline in enzymatic activity became evident at 40 J/cm2, where it dropped to 95 ± 2% with a p-value < 0.05, reflecting a statistically significant inhibitory effect.

Table 1.
Alpha-amylase activity at 589 nm with different energy densities.
3.2. Effect of 532 nm LLLI on Alpha-Amylase Activity
In contrast to 589 nm irradiation, exposure to 532 nm wavelength light showed a different, primarily inhibitory pattern on enzyme activity, as detailed in Table 2. At 10 J/cm2, activity marginally increased to 102 ± 3% relative to the control; however, this change was not statistically significant (p-value > 0.05), indicating a neutral effect. Increasing the energy density to 20 J/cm2 resulted in a slight inhibition, with activity decreasing to 95 ± 4%, and with a p-value < 0.05. Further escalation to 30 J/cm2 led to more pronounced inhibition (88 ± 3%, p-value < 0.01). At the highest energy density of 40 J/cm2, enzyme activity significantly declined to 75 ± 2% with a p-value < 0.01, showing a strong inhibitory effect.

Table 2.
Alpha-amylase activity at 532 nm with different energy densities.
3.3. Combined Overview of Wavelength Effects
Figure 2 Provides a comprehensive visualization of the effects of LLLI on alpha-amylase activity across different wavelengths (589 nm and 532 nm) and energy densities (10, 20, 30, and 40 J/cm2). The figure clearly illustrates the stimulatory trend at 589 nm, particularly at lower energy densities, where enzymatic activity peaks at 20 J/cm2. In contrast, the 532 nm wavelength shows a consistent inhibitory effect, with a notable decline in activity as energy density increases, reaching its lowest point at 40 J/cm2. Error bars representing standard deviation (SD) highlight the consistency and reproducibility of the experimental data. The observed wavelength-dependent modulation emphasizes the specificity of laser–enzyme interaction, suggesting that lower-energy photons (589 nm) enhance catalytic efficiency, whereas higher-energy photons (532 nm) may induce conformational changes detrimental to enzyme function. These patterns support the hypothesis that LLLI has a wavelength- and dose-dependent photobiomodulatory effect on enzyme activity.
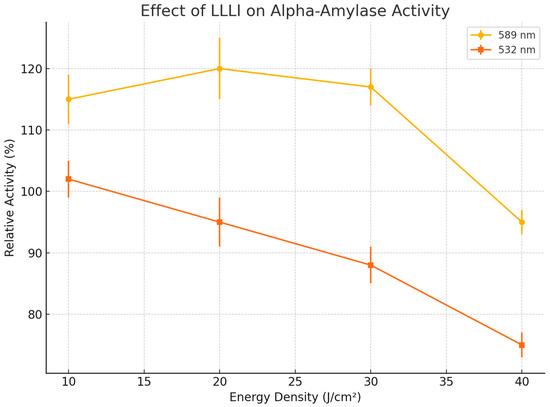
Figure 2.
Effect of LLLI on alpha-amylase activity at different wavelengths and energy densities. Error bars represent standard deviations (SD).
4. Discussion
The present study demonstrates that low-level laser irradiation (LLLI) can modulate alpha-amylase activity in vitro in a manner dependent on both wavelength and energy density. In particular, alpha-amylase activity was significantly enhanced when exposed to LLLI at 589 nm with low energy densities of 10 and 20 J/cm2, but it was neutral or inhibited with high energy densities of 30 and 40 J/cm2. Exposure to LLLI at 532 nm across all the aforementioned energy densities resulted in inhibited enzymatic activity as per the reported differential bioeffects of LLLI [16,17]. The 589 nm biphasic dose–response is consistent with the Arndt-Schulz law or dose–response curve of photobiomodulation, which demonstrates the biologically stimulating effect of low energy doses and the inhibitory effect of high energy doses [18]. Recent research confirms that enzymatic activity is enhanced by low-dosage photobiomodulation but is inhibited by high dosages due to extreme oxidative stress [19]. Exposure to LLLI at 589 nm with low dosages boosts enzymatic activity due to induced changes in protein conformational dynamics, increased efficiency of the substrate binding, and the promotion of catalytic turnover [20]. Research employing advanced spectroscopy and computational models demonstrated the ability of LLLI to increase protein molecular flexibility and alter enzyme hydration shells, thus improving enzymatic reactions [6]. Contrarily, the inhibitory effect of 532 nm LLLI at high dosages of 30 and 40 J/cm2 could be due to the green light’s higher photon energy, which led to a higher production of reactive oxygen species (ROS). Too much ROS can cause the key amino acid residues to be oxidatively modified, resulting in destabilized protein conformation and impaired enzymatic function [21,22]. Green laser irradiation has recently been indicated to promote oxidative stress pathways mediated by ROS, thus inactivating enzymatic systems [23]. Further research on other enzymes like lipase, catalase, and proteases showed a similar pattern of wavelength and dosage significance for modulation, highlighting the specific nature of the interaction between laser and enzyme [24,25]. The findings indicate the need to optimize LLLI parameters to attain specific outcomes for certain enzyme systems. The ability to either improve or inhibit alpha-amylase activity through LLLI is important for the food processing, brewing, pharmaceutical, and biofuel industries, among others, which critically rely on controlled enzymatic reactions [21].
A key issue is the apparent contradiction with the Grotthuss–Draper law, which states that only absorbed light can induce photochemical change. Since alpha-amylase lacks strong chromophores in the visible spectrum, one might question how the modulation of enzymatic activity could occur. This apparent contradiction can be reconciled by considering that the photochemical effects may occur indirectly rather than through direct absorption by the enzyme. It is now well-established that LLLI can influence enzymatic function via indirect photophysical mechanisms. For instance, light absorbed by water molecules, buffer components, or trace ions may alter the enzyme’s microenvironment, including hydrogen bonding networks, electrostatic interactions, and protein hydration shells, thereby leading to structural rearrangements [6,20]. In the current study, all blood components were irradiated using a low-level laser, and the enzyme was not irradiated as a separate boundary from the blood components. This suggests that any observed modulation of alpha-amylase activity may have resulted from laser-induced changes in the surrounding biological matrix rather than direct interaction with the enzyme molecule itself.
These changes may influence substrate binding, transition state stabilization, and catalytic turnover, even if the enzyme does not directly absorb the light. In such cases, the photochemical event occurs in the surrounding matrix, and the enzyme’s structure and function are secondarily altered through molecular signaling or local physicochemical shifts. This aligns with previous studies in which other enzymes, including catalase, peroxidases, and lipase, were similarly modulated by visible light via environmental alterations rather than direct absorption [8,24].
Further examination is underway on the therapeutic modulation of enzymatic pathways, pioneering approaches for the management of metabolic, inflammatory, and degenerative disorders [26]. In summary, this study underpins the utility of photobiomodulation for enzymatic regulation in a non-invasive and controlled manner for biomedical and industrial purposes. Future research should focus on elucidating the precise molecular mechanisms involved and optimizing laser parameters to maximize therapeutic and biotechnological benefits.
5. Conclusions
Low-level laser irradiation influences the activity of alpha-amylase in vitro in a wavelength- and dose-dependent manner. The 589 nm wavelength at 20 J/cm2 enhanced activity, while 532 nm at higher doses showed inhibitory effects. These findings support further exploration of LLLI as a tool for precise enzymatic modulation in biomedical and industrial fields.
Funding
This research received no external funding.
Institutional Review Board Statement
The Research Ethics Committee Members confirm that the research ethics guidelines issued by the Faculty Council (representing the higher scientific committee) have been precisely followed by the above researcher (s) in executing the current study. The study has been assigned the study protocol code (HREC-JMU-FMS)/00003, which should be used for all communication to the HREC-JMU-FMS related to this study. This ethical clearance is valid from 1 November 2024 until 1 June 2025.
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Data Availability Statement
Relevant data are available from the authors upon reasonable request.
Conflicts of Interest
The author declares that there are no conflicts of interest.
References
- Arantes, G. A computational perspective on enzymatic catalysis. Quim. Nova 2008, 31, 377–383. [Google Scholar] [CrossRef]
- Nater, U.M.; La Marca, R.; Erni, K.; Ehlert, U. Alpha-amylase activity in blood increases after pharmacological, but not psychological, activation of the adrenergic system. PLoS ONE 2015, 10, e0130449. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karu, T. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem. Photobiol. 2010, 86, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.S.; Potrich, J.W. Effect of GaAlAs laser irradiation on enzyme activity. Photomed. Laser Surg. 2010, 28, 431–434. [Google Scholar] [CrossRef]
- Gao, X.; Xing, D. Molecular mechanisms of cell proliferation induced by low power laser irradiation. J. Biomed. Sci. 2009, 16, 4. [Google Scholar] [CrossRef]
- Chen, Y.P.; Liu, Y.J.; Wang, X.L.; Ren, Z.Y.; Yue, M. Effect of microwave and He-Ne laser on enzyme activity and biophoton emission of isatis indigotica fort. J. Integr. Plant Biol. 2005, 47, 849–855. [Google Scholar] [CrossRef]
- da Silva, T.G.; Ribeiro, R.S.; Mencalha, A.L.; de Souza Fonseca, A. Photobiomodulation at molecular, cellular, and systemic levels. Lasers Med. Sci. 2023, 38, 136. [Google Scholar] [CrossRef]
- Ang, B.J.; Suardi, N.; Abduraman, M.A. Exploring differentiation-dependent responses to 532 nm green laser photobiomodulation in SHSY5Y neuroblastoma cells. Lasers Med. Sci. 2024, 39, 147. [Google Scholar] [CrossRef]
- Awad, F.S.; Al Musawi, M.S.; Salih, S.F. A comparative study of 589 and 532 nm low level laser irradiation effects on normal human peripheral blood mononuclear cells viability. JPMA J. Pak. Med. Assoc. 2024, 74 (Suppl. 8), S266–S269. [Google Scholar] [CrossRef] [PubMed]
- Al Musawi, M.S.; Al-Gailani, B.T. In vitro biostimulation of low-power diode pumping solid state laser irradiation on human serum proteins. Photobiomodulation Photomed. Laser Surg. 2020, 38, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.H.; Al Musawi, M.S.; Al-Gailani, B.T. A comparison study of 589 and 650 nm on improving stored whole blood stability by irradiation with a low-power diode pumping solid-state laser. JPMA J. Pak. Med. Assoc. 2024, 74 (Suppl. 8), S172–S175. [Google Scholar] [CrossRef]
- Al Musawi, M.S.; Jafaar, M.S.; Ahmed, N.M.; Al-Gailani, B.T.; Suhaimi, F.M. Effects of low power violet laser irradiation on red blood cells volume and erythrocyte sedimentation rate in human blood. In International Conference on Applied Physics and Engineering (Icape2016): Proceedings of the 2nd International Conference on Applied Physics and Engineering, Penang, Malaysia, 2–3 November 2016; AIP Publishing LLC: Melville, NY, USA, 2017; Volume 1875, p. 020005. [Google Scholar]
- Kandra, L. α-Amylases of medical and industrial importance. J. Mol. Struct. THEOCHEM 2003, 666, 487–498. [Google Scholar] [CrossRef]
- AlGhamdi, K.M.; Kumar, A.; Moussa, N.A. Low-level laser therapy: A useful technique for enhancing the proliferation of various cultured cells. Lasers Med. Sci. 2012, 27, 237–249. [Google Scholar] [CrossRef]
- Mussttaf, R.A.; Jenkins, D.F.; Jha, A.N. Assessing the impact of low level laser therapy (LLLT) on biological systems: A review. Int. J. Radiat. Biol. 2019, 95, 120–143. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Chen, A.C.; Carroll, J.D.; Hamblin, M.R. Biphasic dose response in low level light therapy. Dose-Response 2009, 7, 358–383. [Google Scholar] [CrossRef]
- Dellagrana, R.A.; Rossato, M.; Sakugawa, R.L.; Baroni, B.M.; Diefenthaeler, F. Photobiomodulation therapy on physiological and performance parameters during running tests: Dose–response effects. J. Strength Cond. Res. 2018, 32, 2807–2815. [Google Scholar] [CrossRef]
- Kilik, R.; Bober, P.; Ropovik, I.; Beňačka, R.; Genči, J.; Nečas, A.; Sabo, J. Proteomic analysis of plasma proteins after low-level laser therapy in rats. Physiol. Res. 2019, 68, S399–S404. [Google Scholar] [CrossRef]
- Yang, X.; Huang, J.; Guo, J.; Fang, S.; Wang, Z.; Wu, G.; Wu, Y.; Zhong, F. Bridging chemistry and biology for light-driven new-to-nature enantioselective photoenzymatic catalysis. Chem. Soc. Rev. 2025, 54, 5157–5188. [Google Scholar] [CrossRef]
- Ogilby, P.R. Singlet oxygen: There is indeed something new under the sun. Chem. Soc. Rev. 2010, 39, 3181–3209. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology 2002, 181–182, 223–227. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Malavazzi, T.C.; Fernandes, K.P.; Lopez, T.C.; Rodrigues, M.F.; Horliana, A.C.; Bussadori, S.K.; Mesquita-Ferrari, R.A. Effects of the invasive and non-invasive systemic photobiomodulation using low-level laser in experimental models: A systematic review. Lasers Med. Sci. 2023, 38, 137. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.T.; Kang, M.J. Application of capillary electrophoresis with laser-induced fluorescence to immunoassays and enzyme assays. Molecules 2019, 24, 1977. [Google Scholar] [CrossRef] [PubMed]
- Felician, M.C.; Belotto, R.; Tardivo, J.P.; Baptista, M.S.; Martins, W.K. Photobiomodulation: Cellular, molecular, and clinical aspects. J. Photochem. Photobiol. 2023, 17, 100197. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).