Dual-Component Beat-Frequency Quartz-Enhanced Photoacoustic Spectroscopy Gas Detection System
Abstract
1. Introduction
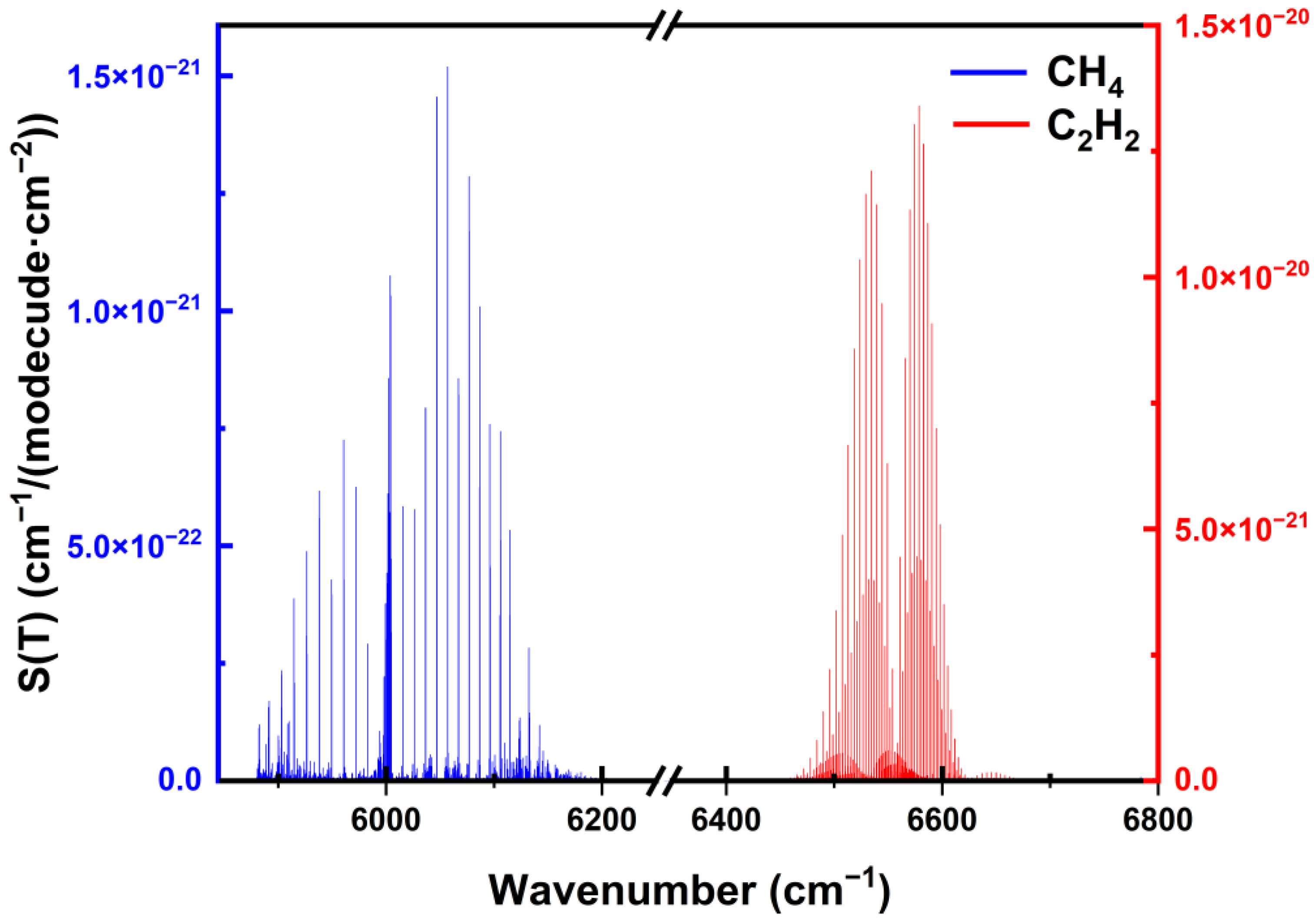
2. Selection of Absorption Lines
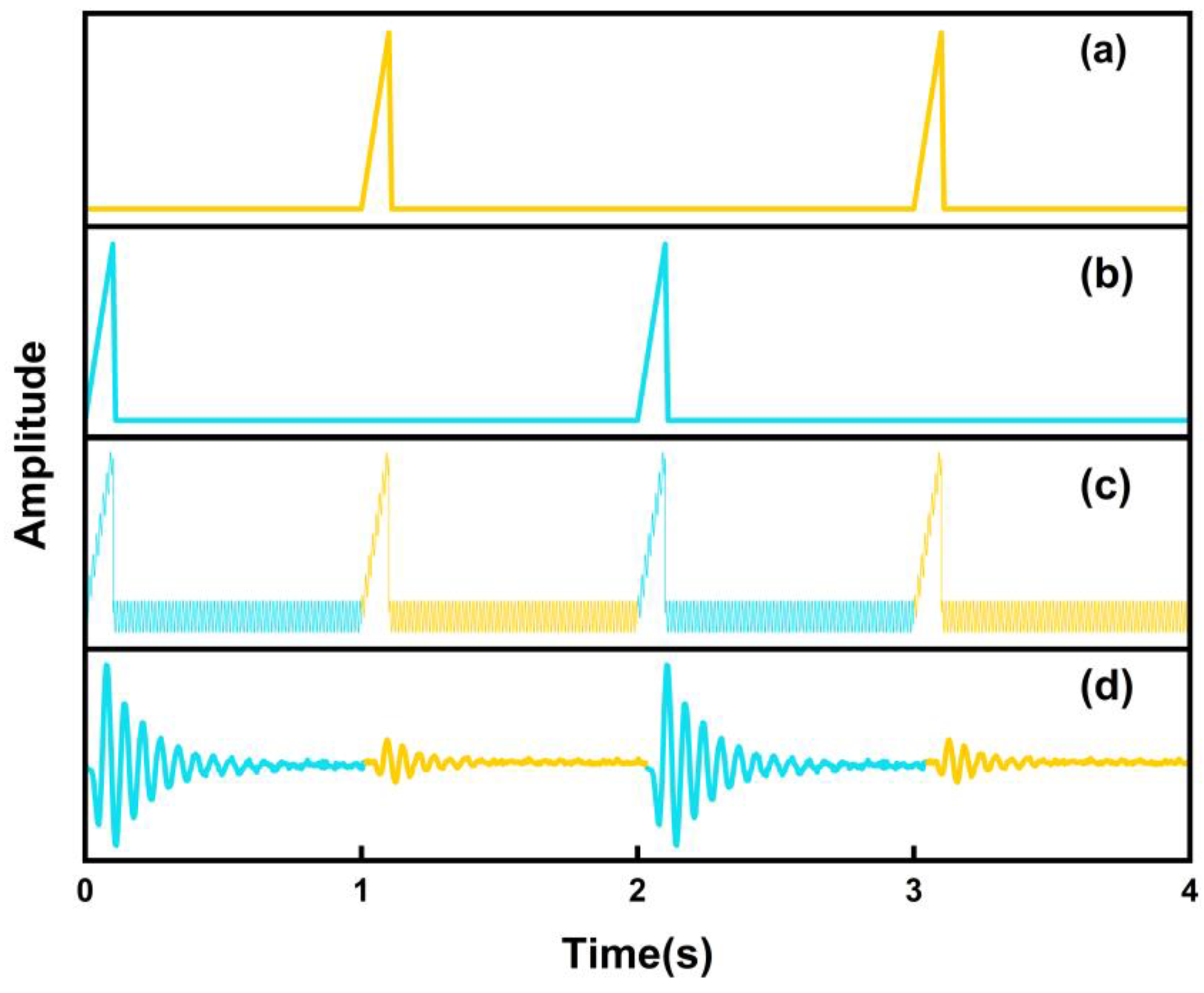
3. Principle of BF-QEPAS
4. Design of Sensor System
5. Experimental Results and Discussion
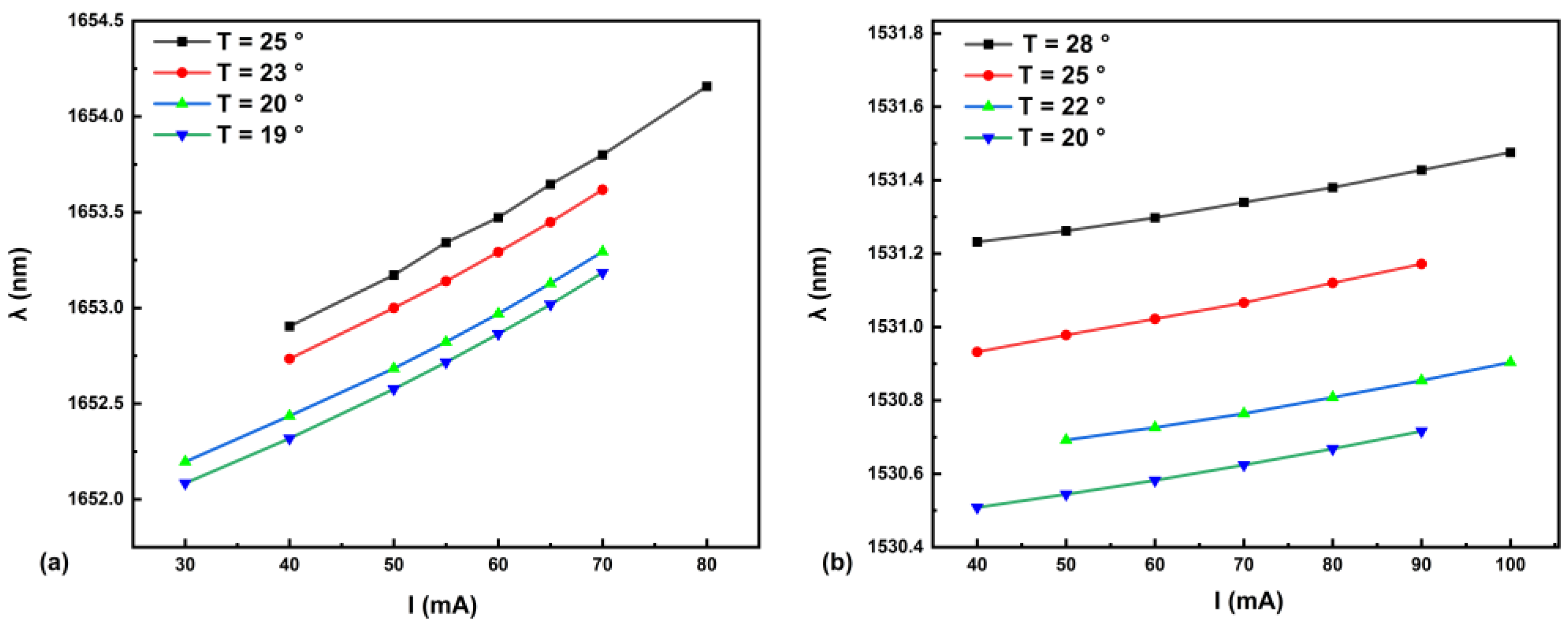
5.1. Determination of QTF Resonant Frequency via Heterodyne Demodulation
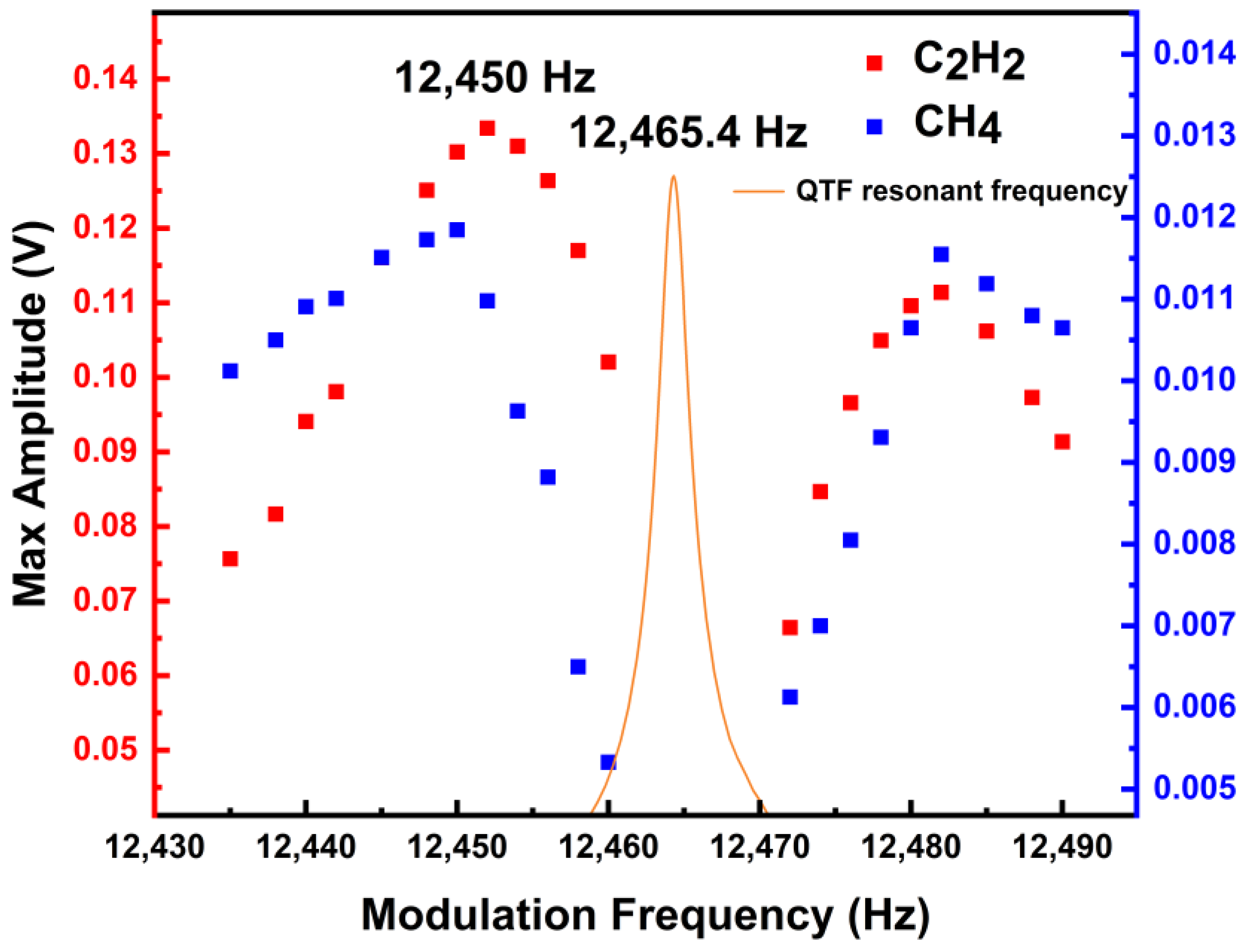
5.2. Modulation Frequency Optimization
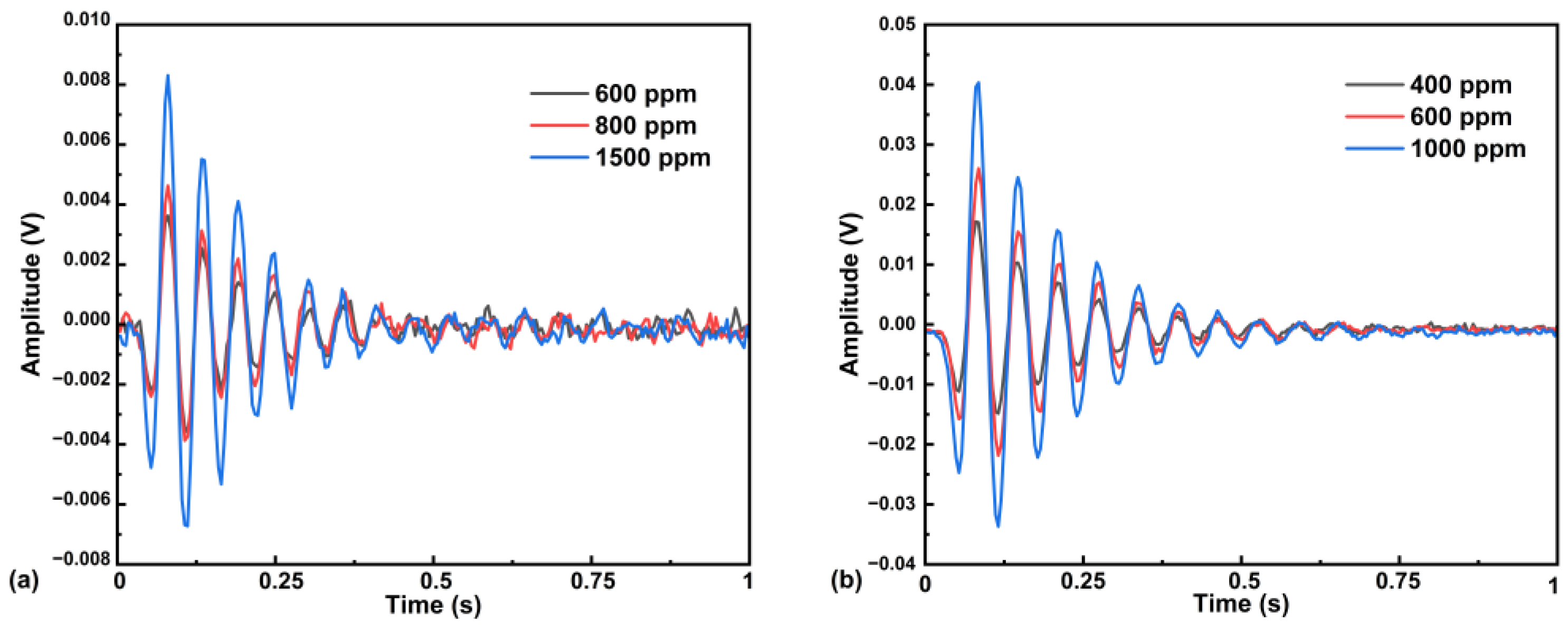
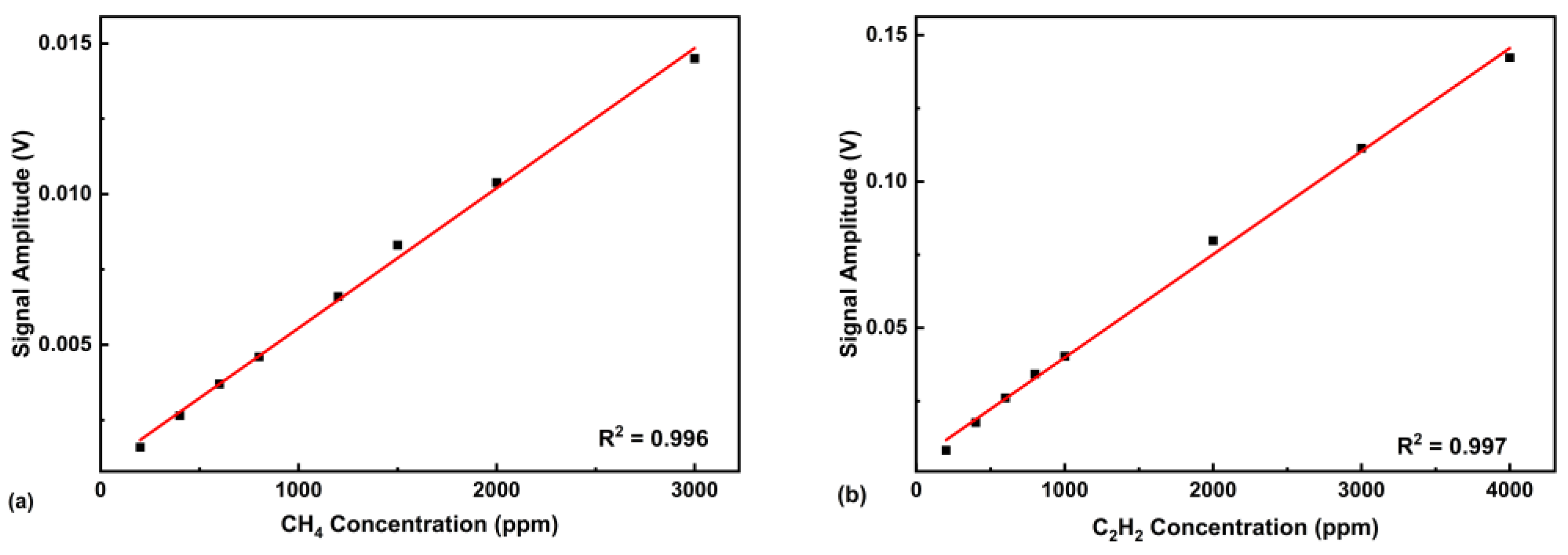
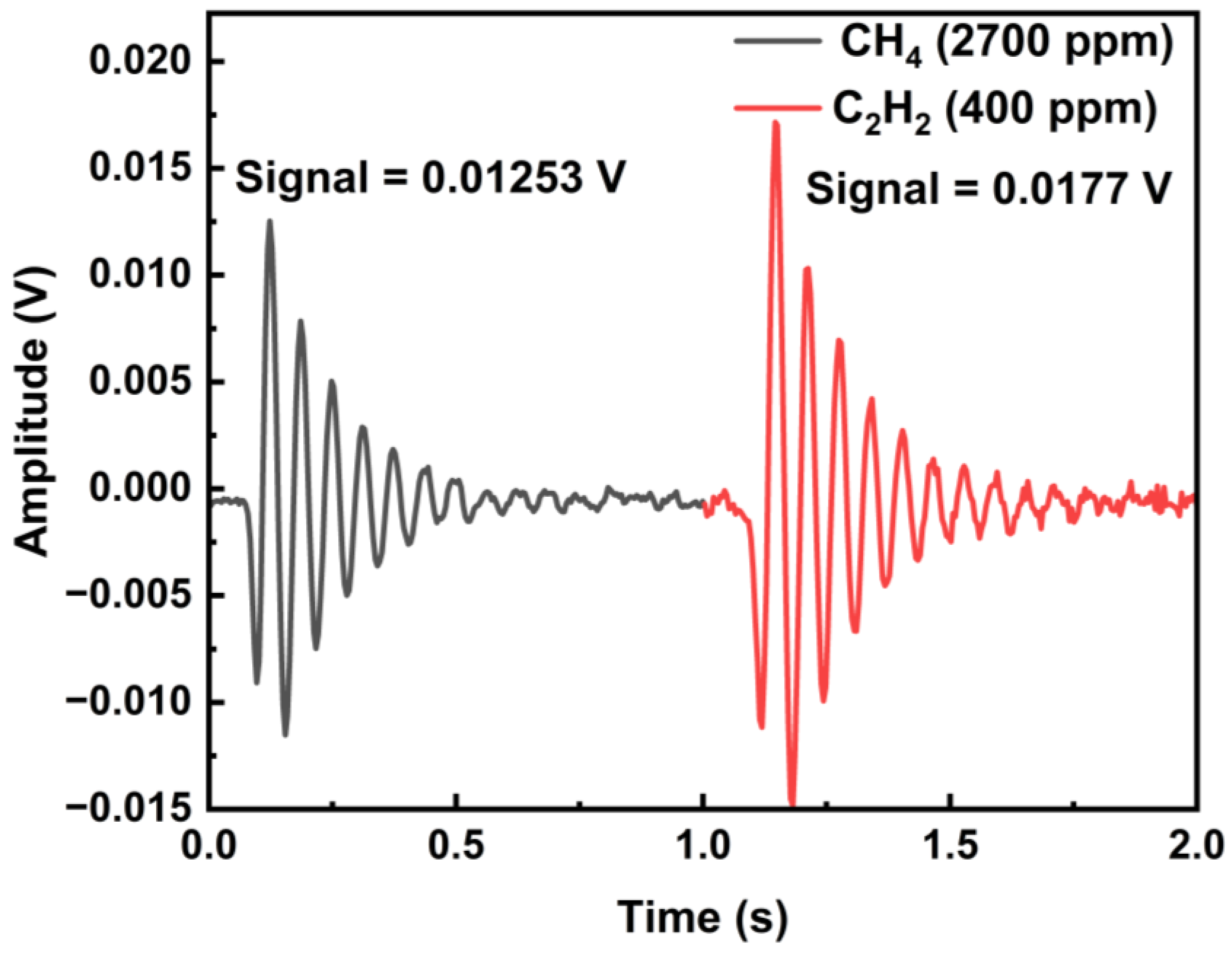
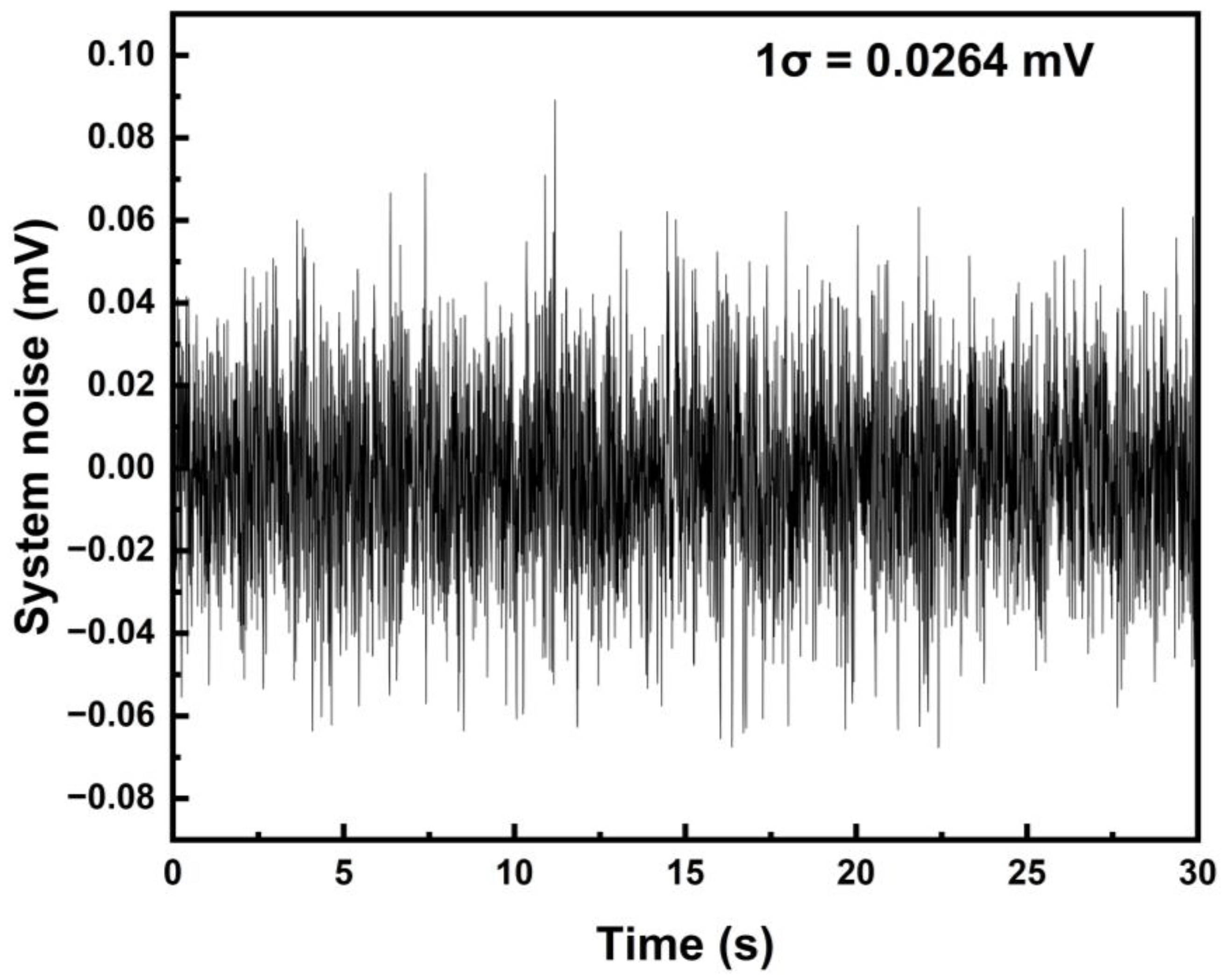
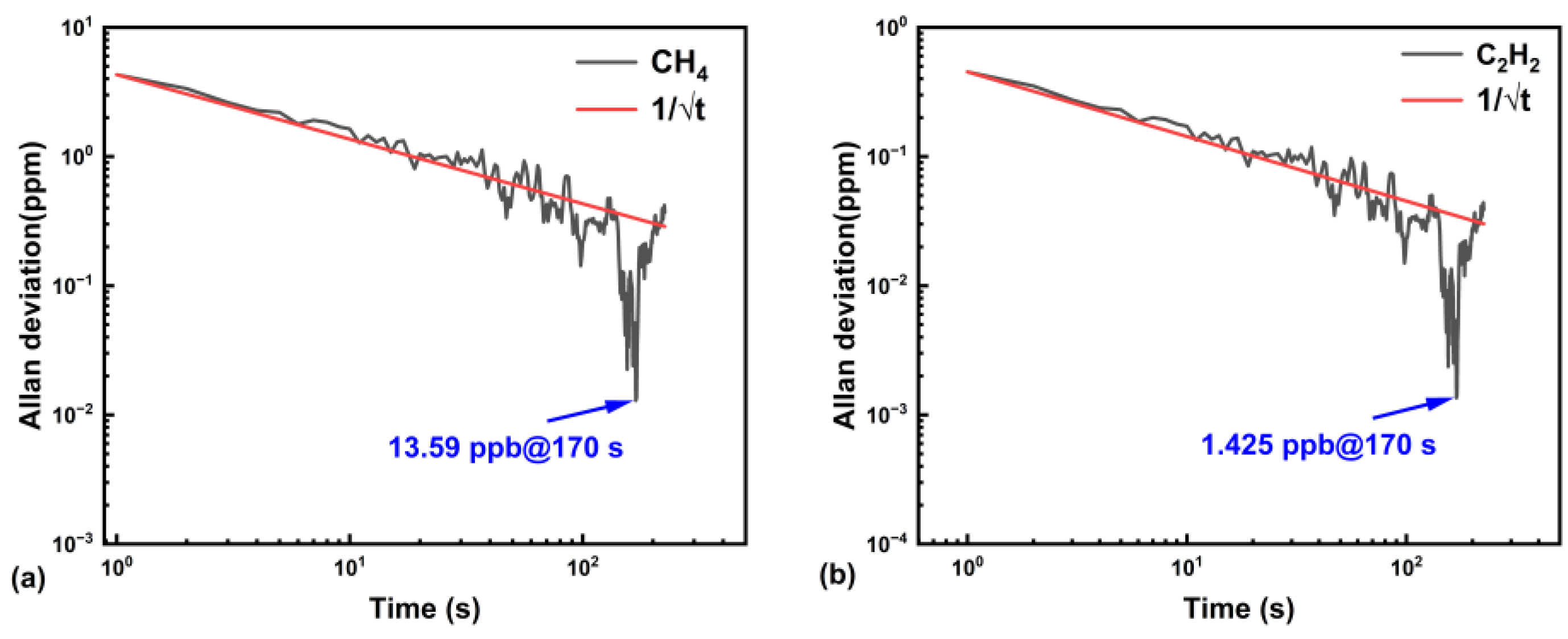
5.3. System Linearity Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BF-QEPAS | beat-frequency quartz-enhanced photoacoustic spectroscopy |
| TDM | time-division multiplexing |
| QTF | quartz tuning fork |
| LIA | lock-in amplifier |
| DFB | distributed feedback |
| GDS | gas dilution system |
| ADM | acoustic detection module |
| R2 | coefficient of determination |
References
- Aldhafeeri, T.; Tran, M.-K.; Vrolyk, R.; Pope, M.; Fowler, M. A Review of Methane Gas Detection Sensors: Recent Developments and Future Perspectives. Inventions 2020, 5, 28. [Google Scholar] [CrossRef]
- Matindoust, S.; Baghaei-Nejad, M.; Abadi, M.H.S.; Zou, Z.; Zheng, L.-R. Food quality and safety monitoring using gas sensor array in intelligent packaging. Sens. Rev. 2016, 36, 169–183. [Google Scholar] [CrossRef]
- Pijolat, C.; Pupier, C.; Sauvan, M.; Tournier, G.; Lalauze, R. Gas detection for automotive pollution control. Sens. Actuators B Chem. 1999, 59, 195–202. [Google Scholar] [CrossRef]
- Hall, J.E.; Hooker, P.; Jeffrey, K.E. Gas detection of hydrogen/natural gas blends in the gas industry. Int. J. Hydrogen Energy 2021, 46, 12555–12565. [Google Scholar] [CrossRef]
- Cristescu, S.M.; Persijn, S.T.; Hekkert, S.T.L.; Harren, F.J.M. Laser-based systems for trace gas detection in life sciences. Appl. Phys. B 2008, 92, 343. [Google Scholar] [CrossRef]
- Schmid, T. Photoacoustic spectroscopy for process analysis. Anal. Bioanal. Chem. 2006, 384, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Patimisco, P.; Scamarcio, G.; Tittel, F.; Spagnolo, V. Quartz-Enhanced Photoacoustic Spectroscopy: A Review. Sensors 2014, 14, 6165–6206. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y. Review of Recent Advances in QEPAS-Based Trace Gas Sensing. Appl. Sci. 2018, 8, 1822. [Google Scholar] [CrossRef]
- Kosterev, A.A.; Dong, L.; Thomazy, D.; Tittel, F.K.; Overby, S. QEPAS for chemical analysis of multi-component gas mixtures. Appl. Phys. B 2010, 101, 649–659. [Google Scholar] [CrossRef]
- Levy, R.; Duquesnoy, M.; Melkonian, J.-M.; Raybaut, M.; Aoust, G. New Signal Processing for Fast and Precise QEPAS Measurements. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2020, 67, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Zhang, X.; Zhang, Z.; Liu, X.; Qin, L.; Shi, J.; Zhao, Y.; Wang, Z. A light-induced thermoelastic spectroscopy using surface mounted device quartz tuning fork. Photoacoustics 2025, 42, 100686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gu, Y.; Zhang, Q.; Wei, Y.; Wang, L.; Li, C.; Wang, W.; Lv, J.; Feng, Y. Performance improvement of quartz-enhanced photoacoustic spectroscopy gas system using ICEEMDAN-PE-WTD. Infrared Phys. Technol. 2025, 145, 105650. [Google Scholar] [CrossRef]
- Wu, H.; Dong, L.; Zheng, H.; Yu, Y.; Ma, W.; Zhang, L.; Yin, W.; Xiao, L.; Jia, S.; Tittel, F.K. Beat frequency quartz-enhanced photoacoustic spectroscopy for fast and calibration-free continuous trace-gas monitoring. Nat. Commun. 2017, 8, 15331. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, R.; Maurin, N.; Trzpil, W.; Bahriz, M.; Vicet, A. Quartz Tuning Fork Resonance Tracking and application in Quartz Enhanced Photoacoustics Spectroscopy. Sensors 2019, 19, 5565. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Feng, C.; Wu, H.; Jia, S.; Dong, L. Calibration-free mid-infrared exhaled breath sensor based on BF-QEPAS for real-time ammonia measurements at ppb level. Sens. Actuators B Chem. 2022, 358, 131510. [Google Scholar] [CrossRef]
- Ye, W.; He, L.; Liu, W.; Yuan, Z.; Zheng, K.; Li, G. Optomechanical energy enhanced BF-QEPAS for fast and sensitive gas sensing. Photoacoustics 2025, 41, 100677. [Google Scholar] [CrossRef] [PubMed]
- Aspromonte, J.; Mascrez, S.; Eggermont, D.; Purcaro, G. Solid-phase microextraction coupled to comprehensive multidimensional gas chromatography for food analysis. Anal. Bioanal. Chem. 2024, 416, 2221–2246. [Google Scholar] [CrossRef] [PubMed]
- Wan, E.; Zhang, Q.; Li, L.; Xie, Q.; Li, X.; Liu, Y. The online in situ detection of indoor air pollution via laser induced breakdown spectroscopy and single particle aerosol mass spectrometer technology. Opt. Lasers Eng. 2024, 174, 107974. [Google Scholar] [CrossRef]
- Singh, R.; Gupta, R.; Bansal, D.; Bhateria, R.; Sharma, M. A Review on Recent Trends and Future Developments in Electrochemical Sensing. ACS Omega 2024, 9, 7336–7356. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Cui, D.; Qi, H.; Yang, L.; Zhao, X. Multi-component Freon gas detection based on infrared tunable Fabry-Perot detector. Sens. Actuators B Chem. 2024, 420, 136488. [Google Scholar] [CrossRef]
- Li, C.; Han, X.; Ma, F.; Zhao, X.; Wang, Z.; Qi, H.; Guo, M.; Chen, K. Multiplexed fiber-optic photoacoustic sensors for simultaneous detection of multi-point gases. Sens. Actuators B Chem. 2024, 399, 134801. [Google Scholar] [CrossRef]
- Guo, S.; Li, J.; Wei, Y.; Yang, Y.; Lu, Z.; Xing, X.; Ren, W.; Yao, S. A multi-laser hybrid absorption sensor for simultaneous measurement of NH3, NO, and temperature in denitrification flue gas. Infrared Phys. Technol. 2024, 136, 105034. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Cong, Z.; Sun, J.; Wang, Z. QEPAS Sensor for Simultaneous Measurements of H2O, CH4, and C2H2 Using Different QTFs. IEEE Photonics J. 2018, 10, 1–8. [Google Scholar] [CrossRef]
- Yu, Y.; Tian, C.; Wang, Z.; Qian, S.; Yan, C.; Zhang, H. Multicomponent gas detection technology of FDM and TDM based on photoacoustic spectroscopy. Appl. Opt. 2021, 60, 838. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Bi, S.; Chen, R.; Zhao, Y.; Shi, J.; Zhang, H.; Wang, Z. Two-Component Gas Sensor of Time-Division Multiplexing Technique Based on QEPAS and LITES. IEEE Photon. Technol. Lett. 2024, 36, 1085–1088. [Google Scholar] [CrossRef]
- Chen, K.; Guo, M.; Yang, B.; Jin, F.; Wang, G.; Ma, F.; Li, C.; Zhang, B.; Deng, H.; Gong, Z. Highly Sensitive Optical Fiber Photoacoustic Sensor for In Situ Detection of Dissolved Gas in Oil. IEEE Trans. Instrum. Meas. 2021, 70, 1–8. [Google Scholar] [CrossRef]
- He, H.; Xu, X. Study on transformer oil dissolved gas online monitoring and fault diagnosis method. In 2012 IEEE International Conference on Condition Monitoring and Diagnosis; IEEE: New York, NY, USA, 2012; pp. 593–596. [Google Scholar]
- Ye, W.; Xia, Z.; Hu, L.; Luo, W.; Liu, W.; Xu, X.; Zheng, C. Infrared dual-gas CH4/C2H2 sensor system based on dual-channel off-beam quartz-enhanced photoacoustic spectroscopy and time-division multiplexing technique. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 285, 121908. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ma, F.; Sun, C.; Qi, H.; Han, X.; Guo, M.; Chen, K. In-situ detection of dissolved C2H2/CH4 with frequency-division-multiplexed fiber-optic photoacoustic sensor. Sens. Actuators B Chem. 2025, 435, 137651. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Feng, Y.; Chen, Z.; Zhuang, Z.; Xia, J.; Zhao, Y.; Zhang, S. Dual-Component Beat-Frequency Quartz-Enhanced Photoacoustic Spectroscopy Gas Detection System. Photonics 2025, 12, 747. https://doi.org/10.3390/photonics12080747
Xu H, Feng Y, Chen Z, Zhuang Z, Xia J, Zhao Y, Zhang S. Dual-Component Beat-Frequency Quartz-Enhanced Photoacoustic Spectroscopy Gas Detection System. Photonics. 2025; 12(8):747. https://doi.org/10.3390/photonics12080747
Chicago/Turabian StyleXu, Hangyu, Yiwen Feng, Zihao Chen, Zhenzhao Zhuang, Jinbao Xia, Yiyang Zhao, and Sasa Zhang. 2025. "Dual-Component Beat-Frequency Quartz-Enhanced Photoacoustic Spectroscopy Gas Detection System" Photonics 12, no. 8: 747. https://doi.org/10.3390/photonics12080747
APA StyleXu, H., Feng, Y., Chen, Z., Zhuang, Z., Xia, J., Zhao, Y., & Zhang, S. (2025). Dual-Component Beat-Frequency Quartz-Enhanced Photoacoustic Spectroscopy Gas Detection System. Photonics, 12(8), 747. https://doi.org/10.3390/photonics12080747



