1. Introduction
Optical films serve as critical components in laser systems, with their laser-induced damage threshold (LIDT) significantly constraining the advancement of these systems toward higher-power and -energy applications [
1,
2,
3]. Based on fundamental optical principles, a variety of functional films, such as anti-reflection films, high-reflection films, polarizing films, and optical filters, can be fabricated through the rational design of multilayer systems utilizing materials with varying refractive indices. TiO
2 possesses a high refractive index and low absorption and dispersion in the near-infrared and visible light regions, as well as excellent chemical and thermal stability, making it a commonly used material for optical film preparation. In the field of high-LIDT films, since the band gap of TiO
2 (3.2 eV) is not particularly large compared to other high-refractive index materials, it does not provide an advantage to laser damage resistance at short wavelengths. Therefore, research on TiO
2 focuses mainly on the fundamental frequency of the 1064 nm wavelength. The primary methods for preparing TiO
2 films include physical vapor deposition (PVD) and the sol-gel process. Hassanpour et al. deposited TiO
2 films by electron beam evaporation (EBE), and the highest LIDT at 1064 nm and 12 ns was 5.87 J/cm
2 [
4]. Yao et al. deposited TiO
2 films by EBE, and the highest LIDT at 1064 nm and 12 ns was 9.4 J/cm
2 [
5]. The low LIDT of films prepared by PVD is mainly attributed to the substantial introduction of sub-stoichiometric defects during the physical deposition process [
6,
7]. In contrast, films produced via the sol-gel process not only possess significantly enhanced LIDT, but also offer several other advantages, including lower costs, reduced defect density, and superior surface morphology [
8,
9,
10].
Previous studies have shown that the sol-gel method allows for the effective regulation of the film’s structure and properties by adjusting the sol composition [
11]. During the sol preparation process, an inhibitor must be added to coordinate with metal ions, forming a complex to suppress the rate of hydrolysis and polycondensation of the sol [
12,
13]. In addition, it has been found that incorporating organic polymer compounds can alter the structure and properties of films, resulting in higher LIDT and superior performance [
14,
15]. These inhibitors and organic polymer compounds are collectively referred to as additives, and they include acetylacetone (ACAC) [
16], diethanolamine (DEA) [
17], polyethylene glycol 200 (PEG 200) [
18], and polyvinylpyrrolidone (PVP) [
19]. Wang et al. prepared sol-gel ZrO
2 films with ACAC, and the optical properties of the films and their relation to the crystal structure were studied [
16]. Liang et al. introduced DEA and PVP to successfully fabricate dual-additive ZrO
2 films and investigated the effects of PVP concentrations on the properties of the films [
20]. However, few studies have analyzed and compared the effects of different types of additives on the properties of sol-gel-derived films. Particularly scarce are studies that compare the performance of single-additive films with that of dual-additive films and elucidate the specific effects and mechanisms.
In this study, titanium tetraisopropoxide (TTIP) was used as a precursor to prepare sols with four groups of additives: ACAC, a combination of ACAC and PEG 200, DEA, and a combination of DEA and PEG 200. TiO2 films were subsequently fabricated using the dip-coating method. The sol viscosity, optical properties, microstructure, surface topography, LIDT, and damage morphologies of the films were measured and discussed. Furthermore, the effects of various additives on the sol-gel films, as well as their mechanisms, were investigated.
2. Experimental Details
TTIP (99.9%) of reagent grade was purchased from Aladdin. ACAC (99%), DEA (99.5%), and PEG 200 (average molecular weight: 180~220) were purchased from Sinopharm Chemical Reagent. All reagents were used as received without further purification. TiO
2 sols were prepared with the molar ratios of materials listed in
Table 1. All four groups of sols utilized titanium TTIP as the precursor and were distinguished by their specific additives: ACAC, ACAC combined with PEG 200, DEA, and DEA combined with PEG 200. Due to the rapid hydrolysis of TTIP upon contact with atmospheric moisture, we first dissolved the additives in ethanol before adding the mixture to the TTIP precursor. Deionized water was then added dropwise; vigorous stirring was maintained throughout the process. Finally, the above solution was stirred for 1 h and then aged under sealed conditions at 276 K. The four groups containing ACAC, ACAC combined with PEG 200, DEA, or DEA combined with PEG 200 were denoted as A, AP, D, and DP, respectively. Before the films were prepared, BK7 substrates were cleaned carefully with ethanol under ultrasonic conditions. The sols, which had been aged for 7 days, were used to fabricate TiO
2 films by dip-coating at a withdrawal speed of 60 mm/min. The prepared films were annealed at 353 K for 1 h to ensure complete drying.
Sol viscosity was measured by a glass capillary viscometer at a relative humidity of 40% and a temperature of 293 K. The transmittance spectra of the films were measured using a Lambda 900 spectrophotometer (Perkin Elmer, Shelton, CT, USA), and the wavelength accuracy of the device during spectra recording was within 0.08%. The refractive index, extinction coefficient, and film thickness were measured by a UVISEL-ER ellipsometer (Horiba, Oberursel, Germany). The microstructure was characterized by a D8 Advance X-ray diffractometer (XRD) (Bruker, Billerica, MA, USA). The surface topography was measured using a Dimension V atomic force microscope (AFM) (Bruker, Billerica, MA, USA), followed by the calculation of the root mean square (RMS) roughness. Laser damage testing was performed using the “1-on-1” regime according to ISO standard 11254-1, using a 1064 nm Q-switch pulsed laser at a pulse length of 12 ns [
21,
22]. The laser was focused to deliver a far-field circular Gaussian beam with a diameter of 0.306 mm at 1/e
2 of the maximum intensity. Samples were irradiated from the film-coated side. The online damage detection system consisted of a microscope focused on the test area and a CCD camera to determine whether the radiation sites had been damaged. Ten sites on each sample were exposed to the same fluence, and the proportion of damaged sites was recorded. This procedure was repeated at various fluence levels until the range was sufficiently broad to include both zero damage probability and 100% damage probability points, enabling the development of a damage probability versus fluence plot. The testing protocol typically employed a minimum of ten different fluence levels, examining a total of 100 sites. The LIDT was defined as the incident pulse energy density (J/cm
2) when the film was at 0% damage probability. The damage morphologies after laser irradiation were evaluated by a Sirion 200 field emission scanning electron microscope (FESEM) (JEOL, Tokyo, Japan). The laser damage spot depth information was tested by a Dektak XT step profiler (Bruker, Billerica, MA, USA).
3. Results
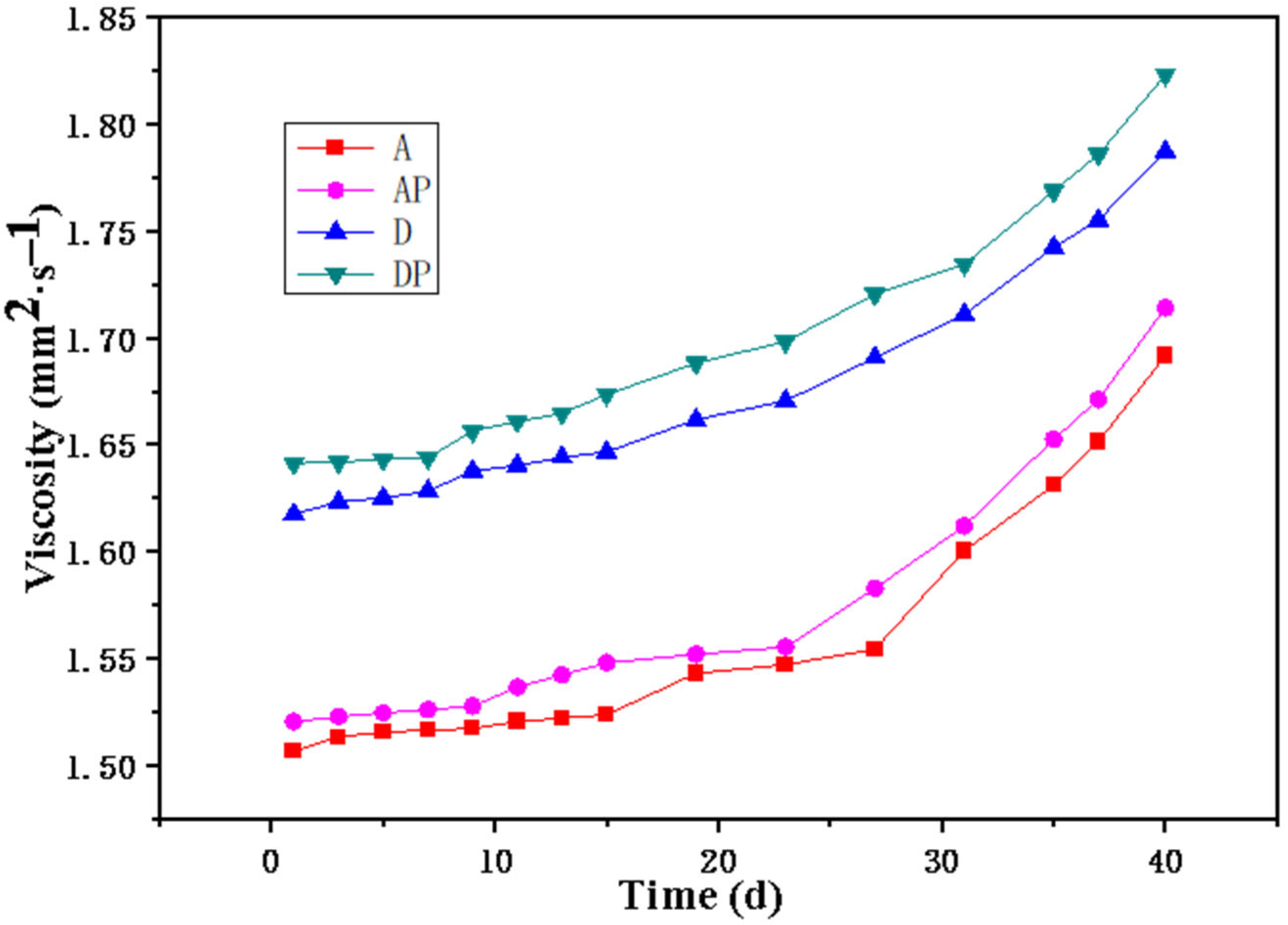
The relationship between sol viscosity and time is shown in
Figure 1. The viscosity of all four sols increases as the aging time extends. Specifically, the viscosity of sols A, AP, D, and DP increases by only 0.186 mm
2·s
−1, 0.194 mm
2·s
−1, 0.170 mm
2·s
−1, and 0.182 mm
2·s
−1, respectively, over a 40-day period, indicating that the sols remains stable with minimal change. However, as illustrated in
Figure 1, it is observed that the viscosity of sols A and AP, which contain ACAC, is lower than those of sols D and DP, which contain DEA. Moreover, the addition of PEG 200 results in a slight increase in viscosity. Additionally, the viscosity curves of sols A and AP exhibit abrupt transition points on the 15th and 9th days, respectively, indicating a rapid increase in viscosity after those days. In contrast, the viscosity curves of sols D and DP exhibit no significant abrupt transition points, demonstrating smooth profiles throughout the measurement range. These findings suggest that DEA exhibits a superior chelating ability for TTIP compared to ACAC, leading to more stable sols.
The transmittance spectra of the TiO
2 films are presented in
Figure 2. Films D and DP both maintain a transmittance of around 89.5% at wavelengths above 350 nm, demonstrating excellent high transmittance properties. Films A and AP show similarly high transmittance within the wavelength range of 570 nm to 900 nm. However, for wavelengths shorter than 570 nm, the transmittance rapidly begins to decrease with the reduction in wavelength. This indicates that, compared to ACAC, the films prepared with DEA exhibit superior optical properties.
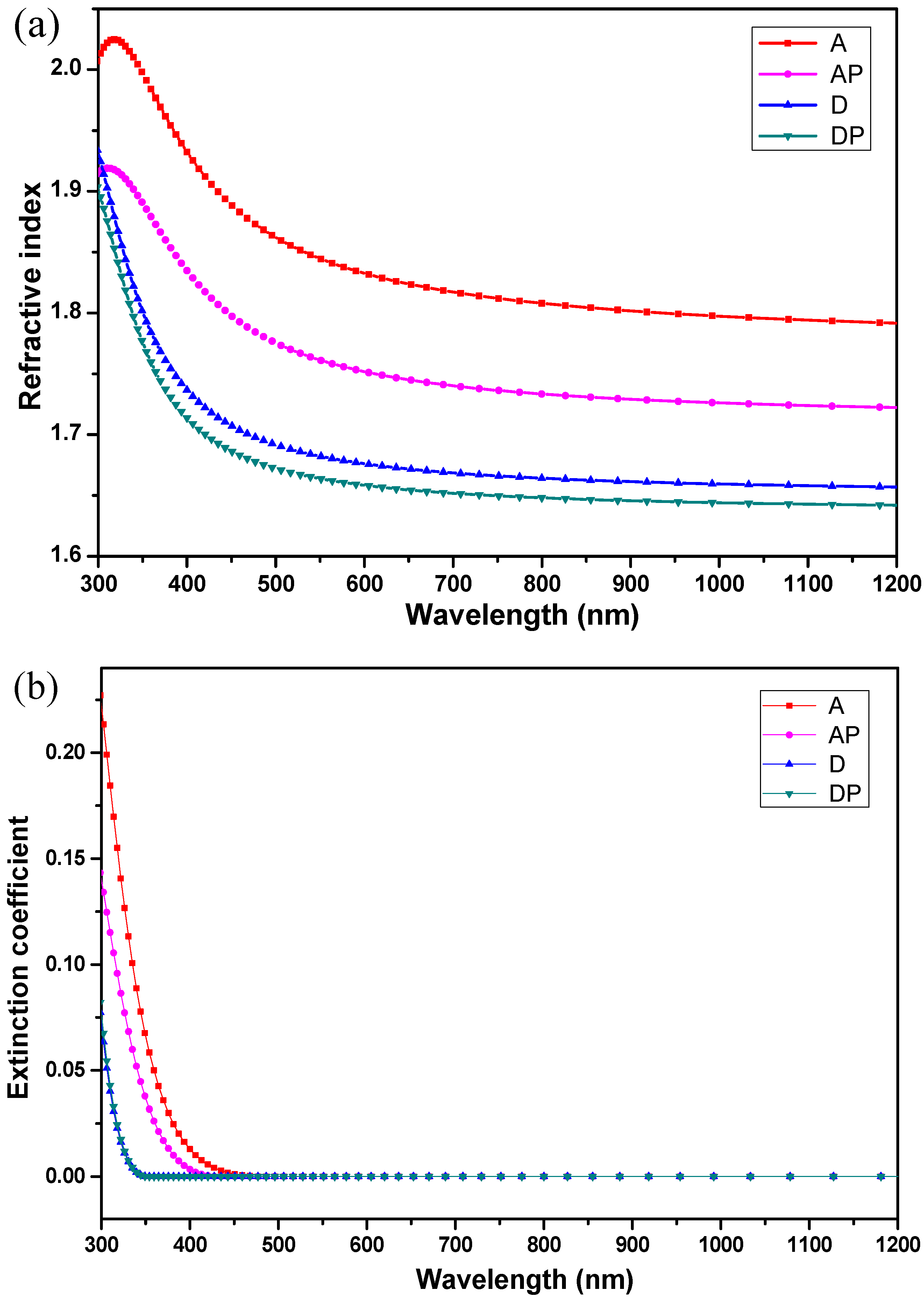
Figure 3 shows the refractive index and extinction coefficient of each film investigated by ellipsometer. As is shown in
Figure 3a, the films with ACAC, A and AP, exhibit higher refractive indices than the films with DEA, D and DP. Additionally, it is observed that the refractive indices of films AP and DP decrease slightly after the addition of PEG 200. A higher refractive index indicates a denser film, while a lower refractive index corresponds to a looser film. Thus, it can be concluded that the addition of PEG 200, with its macromolecular chains, reduces the film’s density, making it more porous. As illustrated in
Figure 3b, the extinction coefficients of films approach zero at a wavelength of 550 nm, indicating negligible optical loss in the visible range. For wavelengths shorter than 450 nm, it can be observed that the extinction coefficient of film A is the highest, followed by film AP, while the extinction coefficients of films D and DP are the lowest, with their curves nearly overlapping.
Table 2 reveals that the thickness increases in the order of film A, film AP, film D, and film DP, which is consistent with the viscosity results. This is mainly due to the fact that the sol viscosity is closely related to the film thickness, and the higher the viscosity, the poorer the sol fluidity, resulting in a thicker film when it is prepared by dip-coating.
Figure 4 shows the XRD patterns of the films. It reveals that none of the films display distinct diffraction peaks, indicating an amorphous structure. This observation suggests that none of the incorporated additives induce crystallization within the film matrices. Generally, the first phase transition of TiO
2 occurs at a temperature of around 573 K. Since the TiO
2 films used in this experiment were prepared by dip-coating at 293 K and the annealing temperature was only 353 K, they all remained in an amorphous state.
The AFM topography of the films is shown in
Figure 5. From
Figure 5a,b, it can be seen that the surface of film A is smoother than that of film AP, with these films’ RMS roughness values being 0.32 nm and 0.33 nm, respectively. From
Figure 5c,d, it can be observed that the surface of film D is noticeably smoother than that of film DP, while the surface of film DP exhibits numerous small protrusions. The RMS values of the two films are 0.42 nm and 0.48 nm, respectively. It is revealed that the surface roughness increases in the order of film A, film AP, film D, and film DP, and the addition of PEG 200 enhances the surface roughness of films. The reason for this is that a lower sol viscosity facilitates easier deposition of the film, resulting in a thinner film with smaller surface undulations and, thus, lower roughness. Conversely, using a sol with higher viscosity results in a thicker film, which in turn increases the surface roughness.
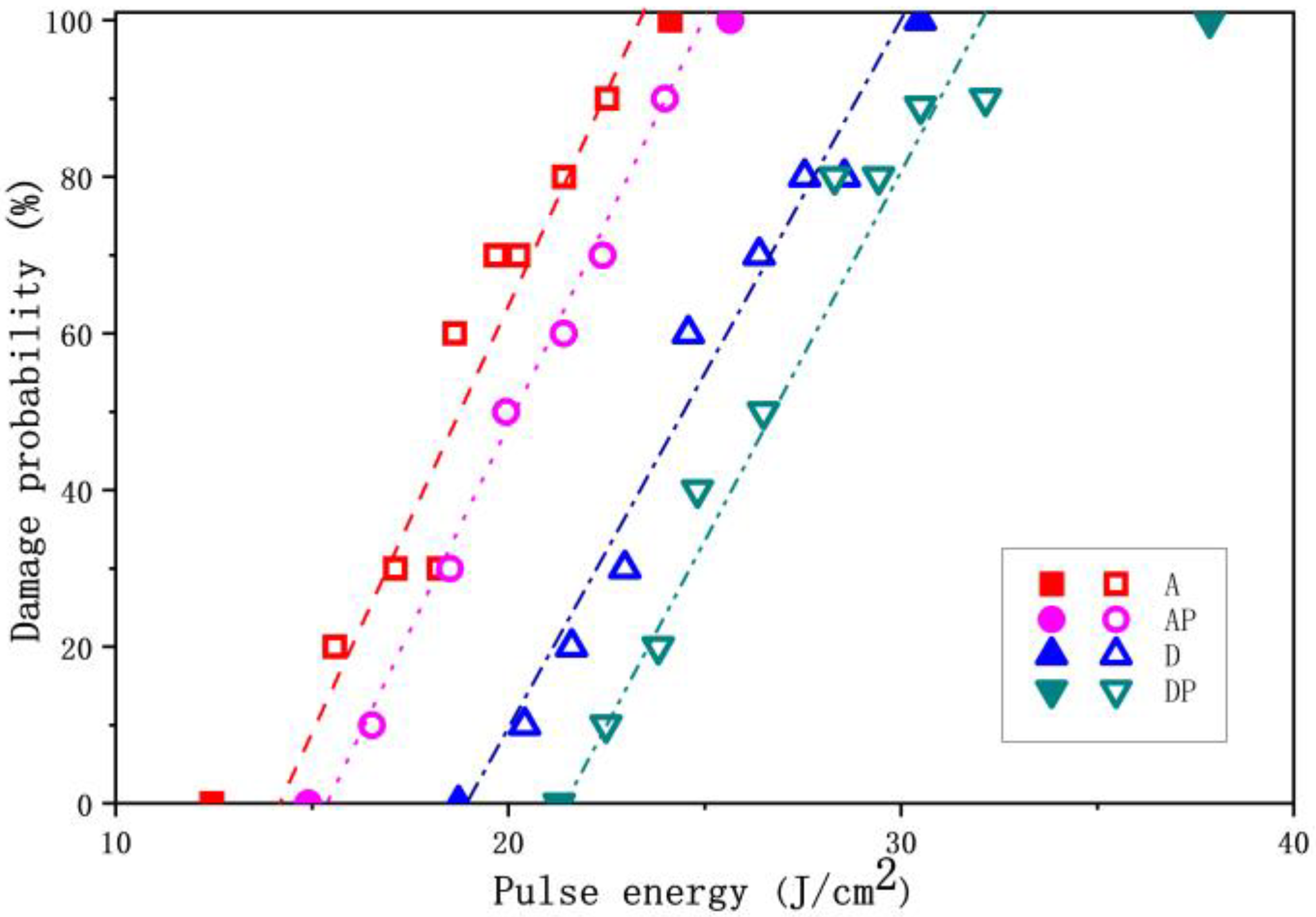
As shown in
Figure 6, the LIDT values of films A, AP, D, and DP are 14.1 J/cm
2, 15.4 J/cm
2, 18.9 J/cm
2, and 21.5 J/cm
2, respectively. It can be observed that the LIDTs of films D and DP, which contain DEA, are significantly higher than those of films A and AP, which contain ACAC. Furthermore, for the dual-additive films AP and DP, prepared with the addition of PEG 200, the LIDT values increase by 9.2% and 13.8%, respectively, compared to their corresponding single-additive films. Additionally, the laser energies corresponding to 0% damage probability for the four groups of films are 12.4 J/cm
2, 14.9 J/cm
2, 18.8 J/cm
2, and 21.3 J/cm
2, respectively. It can be observed that the film containing only DEA exhibits a higher zero-damage-probability energy threshold compared to the film containing only ACAC. After the addition of PEG 200, the dual-additive films demonstrate higher laser energy at 0% damage probability. Furthermore, the laser energy at 100% damage probability for the four groups of films is 20.1 J/cm
2, 25.6 J/cm
2, 30.5 J/cm
2, and 37.9 J/cm
2, respectively, following the same trend observed for the 0% damage probability threshold. These findings demonstrate that DEA exhibits superior laser damage resistance compared to ACAC. Additionally, the incorporation of PEG 200 to create dual-additive film systems further enhances the overall laser damage resistance capabilities.
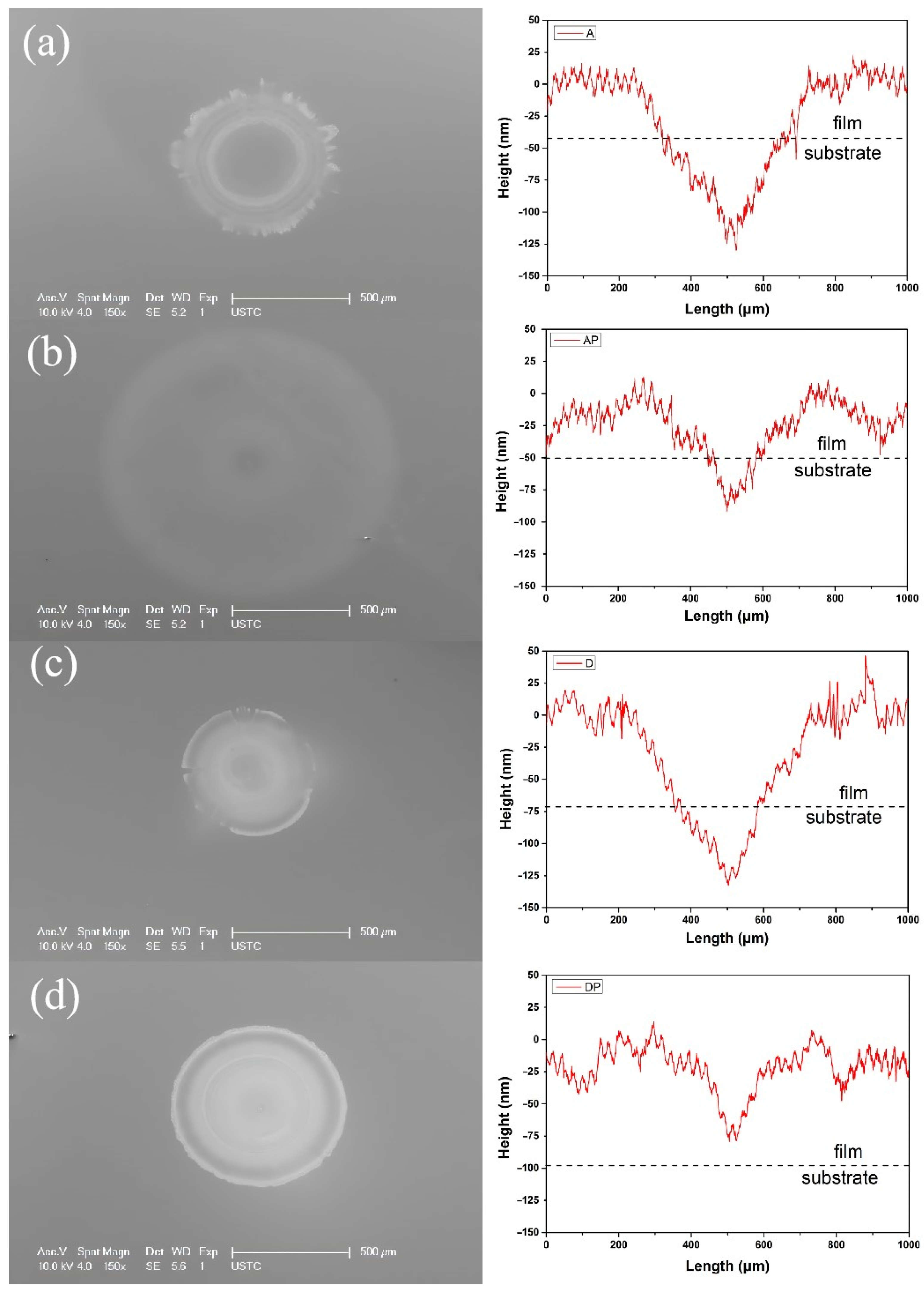
Figure 7 illustrates the SEM damage morphologies of the films under laser fluences of 22.5 J/cm
2, 24.0 J/cm
2, 27.5 J/cm
2, and 30.5 J/cm
2, respectively. All four groups of films exhibit defect-induced damage, where the defects within the films have a higher absorption coefficient. As a result, when the laser irradiates, the temperature rapidly increases, and heat is transferred to the surrounding film material, raising the film’s temperature. When the melting or boiling point of the film material is reached, thermal degradation occurs, resulting in catastrophic structural failure of the film [
23,
24,
25]. It was revealed that the damage spots on the two single-additive films, A and D, are relatively small, with rough edges, indicating stress-type damage patterns, as shown in
Figure 7a,c [
18]. In contrast, the damage spots on the two dual-additive films, AP and DP, are larger, with smooth edges, exhibiting typical melting-type damage characteristics, as illustrated in
Figure 7b,d [
26].
In parallel, a step profiler was used to detect the damage morphologies to give more precise results. As is shown in
Figure 7, the ordinate at zero represents the outer surface of each film, while values below zero correspond to pits caused by the laser, and values above zero indicate protrusions formed at the edges of the damage spots due to localized expansion caused by high temperatures. Furthermore, the interface between the film and the substrate is marked with dashed lines. From
Figure 7, it can be seen that the damage spots on films A and D are more profound, while the damage spots on films AP and DP are shallower. Interestingly, the observed damage depth demonstrates no direct correlation with the overall thickness of the film. Film A displays the most pronounced substrate deterioration, whereas the incorporation of PEG 200 significantly attenuates the degree of substrate damage observed in film AP. In the case of film D, the damage extends beyond the film and penetrates into the substrate, whereas for film DP, the damage remains confined within the film matrix without compromising the underlying substrate. It indicates that the addition of PEG 200 has a certain suppressive effect on damage caused by defects under laser radiation. Additionally, SEM observations show that the damage spots on the dual-additive films are larger than those on the single-additive films. However, step profiler measurements reveal that the crater area caused by laser irradiation is actually smaller in the dual-additive films. This further confirms that the damage in films AP and DP is of the melting type, meaning that despite the larger damage area, the depth variation at the periphery of the damaged region is not significant due to melting effects. In contrast, the damage in films A and D is predominantly of the stress type, resulting in more pronounced overall depth variations across the damaged regions.
To elucidate the underlying damage mechanisms, a theoretical calculation of the temperature evolution of defects under laser radiation in films with various additives was performed based on the defect-induced damage model. We assumed a uniform spherical defect with radius
a embedded within the film, where heat is generated within the sphere for the time
(where
represents the laser pulse duration) at a constant rate
A. The temperatures of both the defect and the surrounding film are governed by the below heat diffusion equation.
Here,
,
and when
t >
tp,
. The suffixes
P and
refer to the defect and the surrounding film, respectively.
T,
K,
κ, and I denote the temperature, thermal conductivity, diffusivity, and laser power intensity, respectively. Q is the absorption efficiency factor, and it can be calculated from the Mie scattering theory [
27]. The boundary conditions are (a) at
,
; (b) when
r =
a,
, and
; and (c)
finite as
and
finite as
r → ∞.
The final solution for the temperature distribution within the defect and the surrounding film can be expressed as follows [
28]:
where
,
,
, and
. To better elucidate the differential effects of the three additives on laser-induced damage, an extreme assumption was made for the films, wherein the defect is considered to be completely encapsulated by the additives ACAC, DEA, and PEG 200, designated as S-A, S-D, and S-P, respectively. The thermophysical parameters of the materials used in the simulation calculations are enumerated in
Table 3.
The laser-induced damage model is depicted in
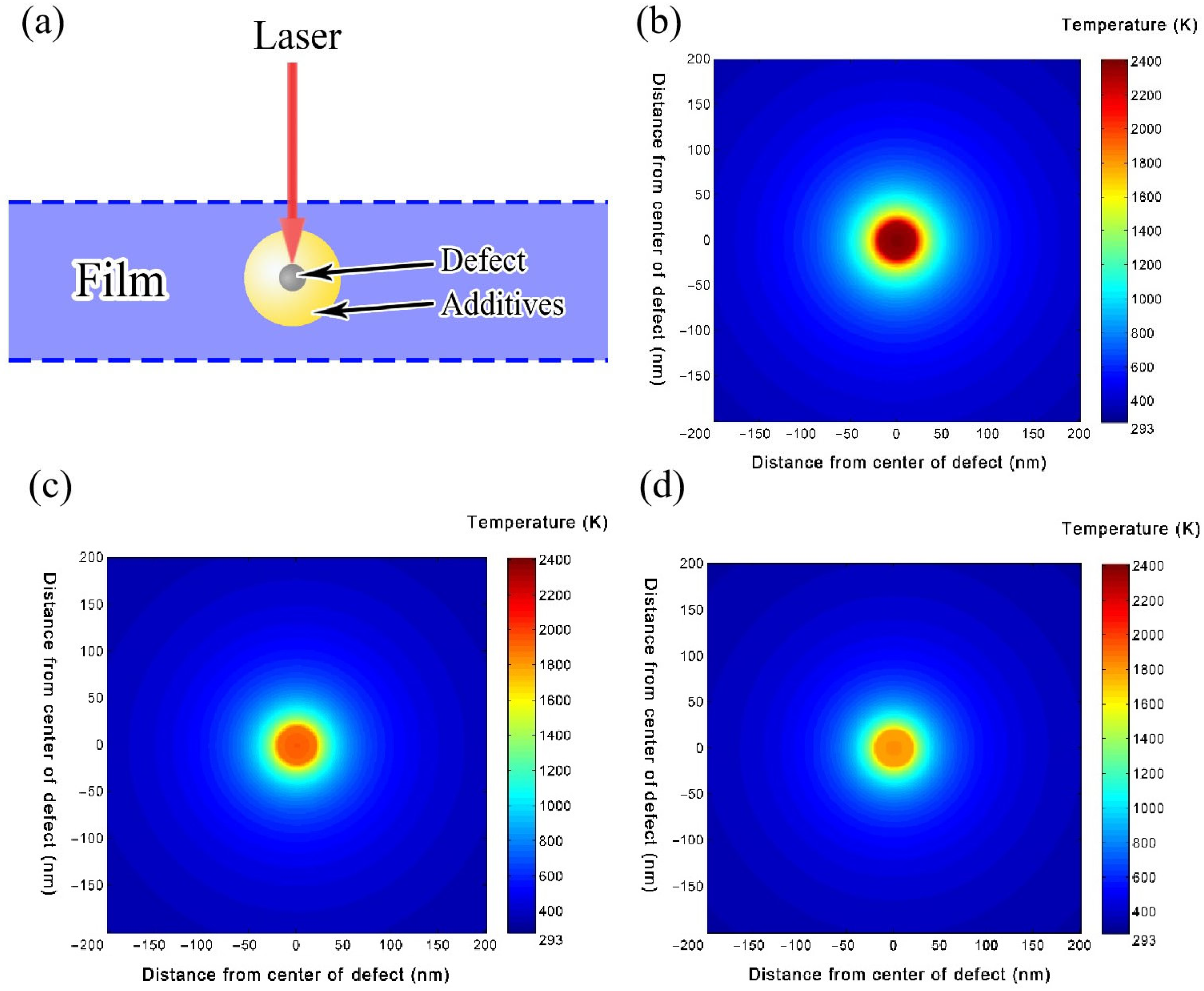
Figure 8a. Here, a defect radius of 20 nm and a film thickness of 100 nm are defined. A laser with a pulse duration of 12 ns, a wavelength of 1064 nm, and an energy density of 20 J/cm
2 is applied to the defect-containing films. The temperature distribution results for films S-A, S-D, and S-P are calculated. As illustrated in
Figure 8b, the maximum temperature of film S-A reaches 2133 K. In contrast, as shown in
Figure 8c,d, the maximum temperatures for films S-D and S-P are 1562 K and 1678 K, respectively, with only a slight difference between them. It is evident that, under the same laser energy, the temperatures of the defects in films S-D and S-P are significantly lower than in film S-A. This is primarily due to the differing thermal conductivities of the additives. ACAC has the lowest thermal conductivity of 0.0015 W/cm/K, while DEA and PEG 200 have higher thermal conductivities of 0.0021 W/cm/K and 0.0019 W/cm/K, respectively. Under laser irradiation, the temperature of the defect increases rapidly and transfers heat to the surrounding film. The heat then spreads outward within the film through thermal conduction. Due to the low thermal conductivity of ACAC, heat diffusion is more difficult, leading to a higher temperature at the defect center within film S-A. In contrast, the higher thermal conductivities of DEA and PEG 200 allow heat to diffuse more efficiently, resulting in lower temperatures at the defect centers of films S-D and S-P. Among them, film S-D has the highest thermal conductivity, leading to the lowest temperature at the defect center. This suggest that films A and AP, containing ACAC, have lower thermal conductivities, resulting in higher temperatures under laser irradiation. Once the temperature reaches the film’s melting point, the film is damaged, which leads to the lower LIDT of films A and AP. On the other hand, films D and DP, with DEA, have much lower temperatures under laser irradiation, leading to a higher LIDT. This is one of the most important reasons why the LIDT of films D and DP is higher than that of films A and AP.
4. Discussion
Analysis of the preceding results unequivocally demonstrates that ACAC and DEA exercise profoundly divergent effects on the intrinsic characteristics of both the precursor sols and their resulting films. First, the sol containing ACAC exhibits lower viscosity compared to that containing DEA, so film A, prepared under the same conditions, is thinner and has a smoother surface than film D. This indicates that using ACAC as an additive is more likely to produce films with better surface quality. However, the transmittance of film A rapidly decreases at shorter wavelengths, resulting in a significant reduction in its overall optical performance. Secondly, when DEA is employed as an additive, the sol demonstrates enhanced stability. The fabricated film exhibits substantially higher transmittance and a lower extinction coefficient compared to its counterpart incorporating ACAC. Consequently, the film containing DEA displays superior optical properties. Finally, SEM images show that all four groups of films experience defect-induced damage under laser irradiation. According to the calculations in
Figure 8, the film with ACAC shows higher temperatures at the defect center due to the lower thermal conductivity of ACAC, making it difficult for the temperature to dissipate within the film, which reduces the laser damage resistance.
In addition, the dual-additive films prepared by further introducing PEG 200 exhibit more interesting properties. The addition of PEG 200 increases the sol viscosity, resulting in thicker films with higher surface roughness and diminished surface quality. This represents the detrimental effect of PEG 200 incorporation on the films’ properties. However, the dual-additive films exhibit better laser damage resistance than the single-additive films. The LIDTs of films A and D are 14.1 J/cm
2 and 15.4 J/cm
2, respectively. Upon PEG 200 incorporation, films AP and DP exhibit enhanced LIDT values of 18.9 J/cm
2 and 21.5 J/cm
2, demonstrating respective increases of 9.2% and 13.8% compared to their unmodified counterparts. Moreover, as demonstrated in
Figure 6, the dual-additive films not only elevate the laser energy threshold required for 0% damage probability, but also significantly increase the energy level necessary to reach 100% damage probability. Here, it can be reasonably approximated that 100% damage probability corresponds to the intrinsic damage of film, meaning that destruction will occur regardless of the presence of internal defects within the film [
29].
Furthermore, it is known that the loose three-dimensional ordered network structure of sol-gel films is the most important reason for their high LIDT [
30]. According to ellipsometer characterization, it can be seen that the density of dual-additive films decreases, and this relatively loose structure can better withstand the local expansion stress that occurs during laser irradiation. As is shown in
Figure 7, the damage spots in single-additive films A and D display stress-type damage patterns, whereas those in dual-additive films AP and DP manifest distinct melting-type damage characteristics. This indicates that the addition of PEG 200 effectively relieves the internal stress generated in the film during laser irradiation, which is another key reason for the higher LIDT of dual-additive films, in addition to the high thermal conductivity of DEA. It should be noted that the dual additives used in this study primarily enhance the damage threshold for fundamental frequency nanosecond laser irradiation, where the damage mechanisms are mainly defect-induced and thermal damage. For other types of lasers, particularly short-wavelength and ultrashort-pulse lasers, these additives may not be effective, and further research is needed.