The Potential of Tunable Femtosecond Laser Light to Prevent Melanoma A375 Cell Growth: An In Vitro Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
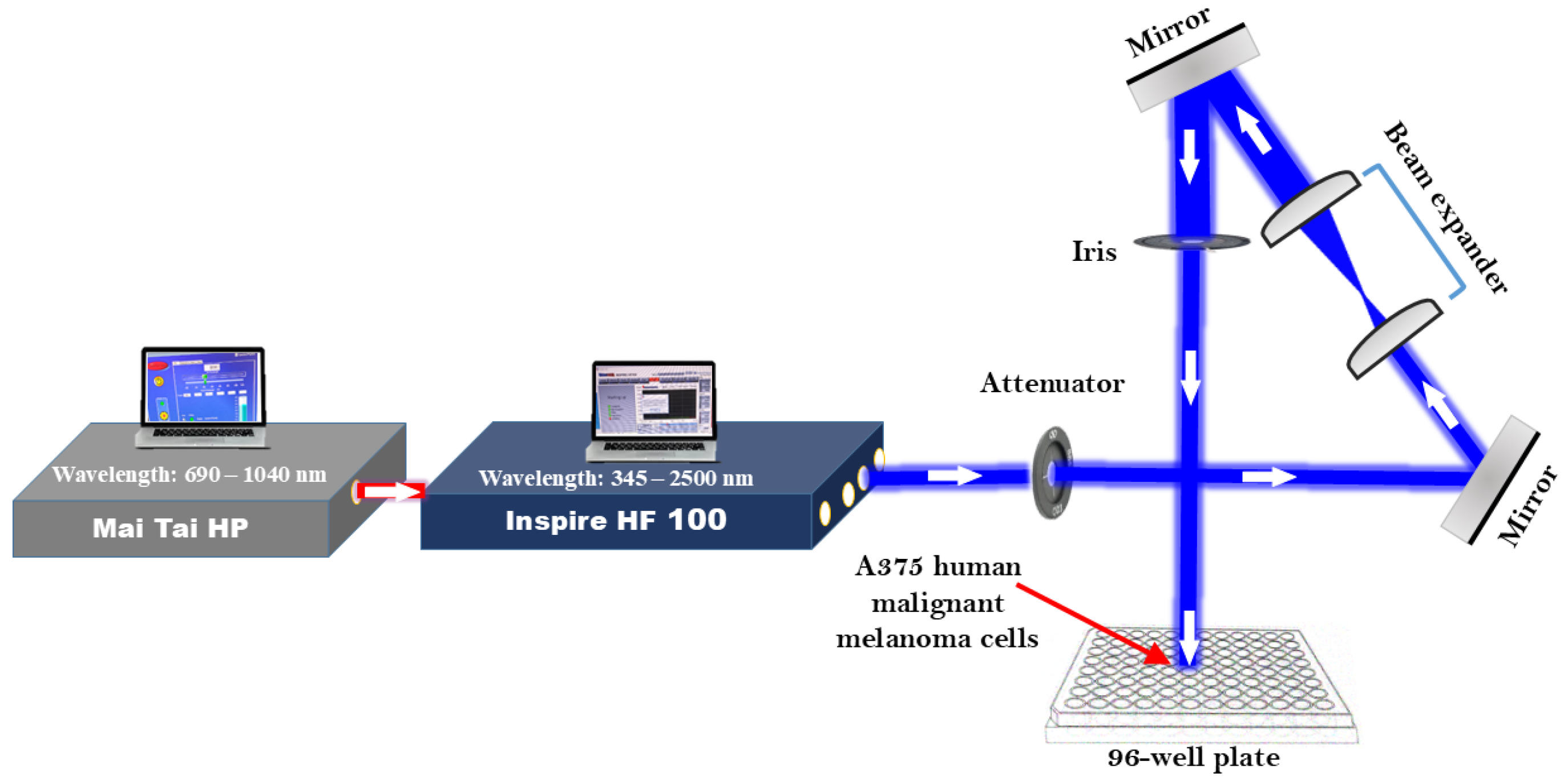
2.2. Setting up and Configuring Laser Systems
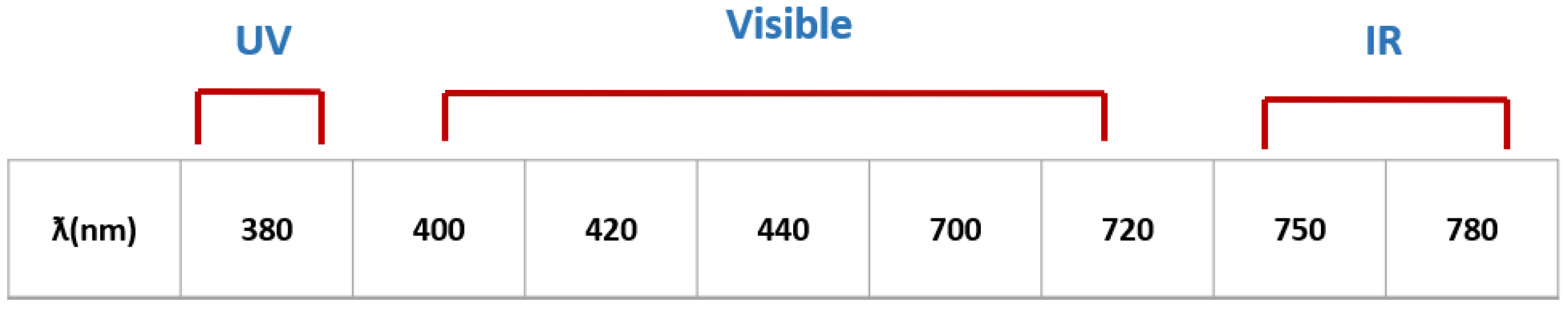
2.3. Femtosecond Laser Irradiation of A375 Cells
2.4. Cell Viability Evaluation via MTT Assay
2.5. Statistical Analysis
3. Results
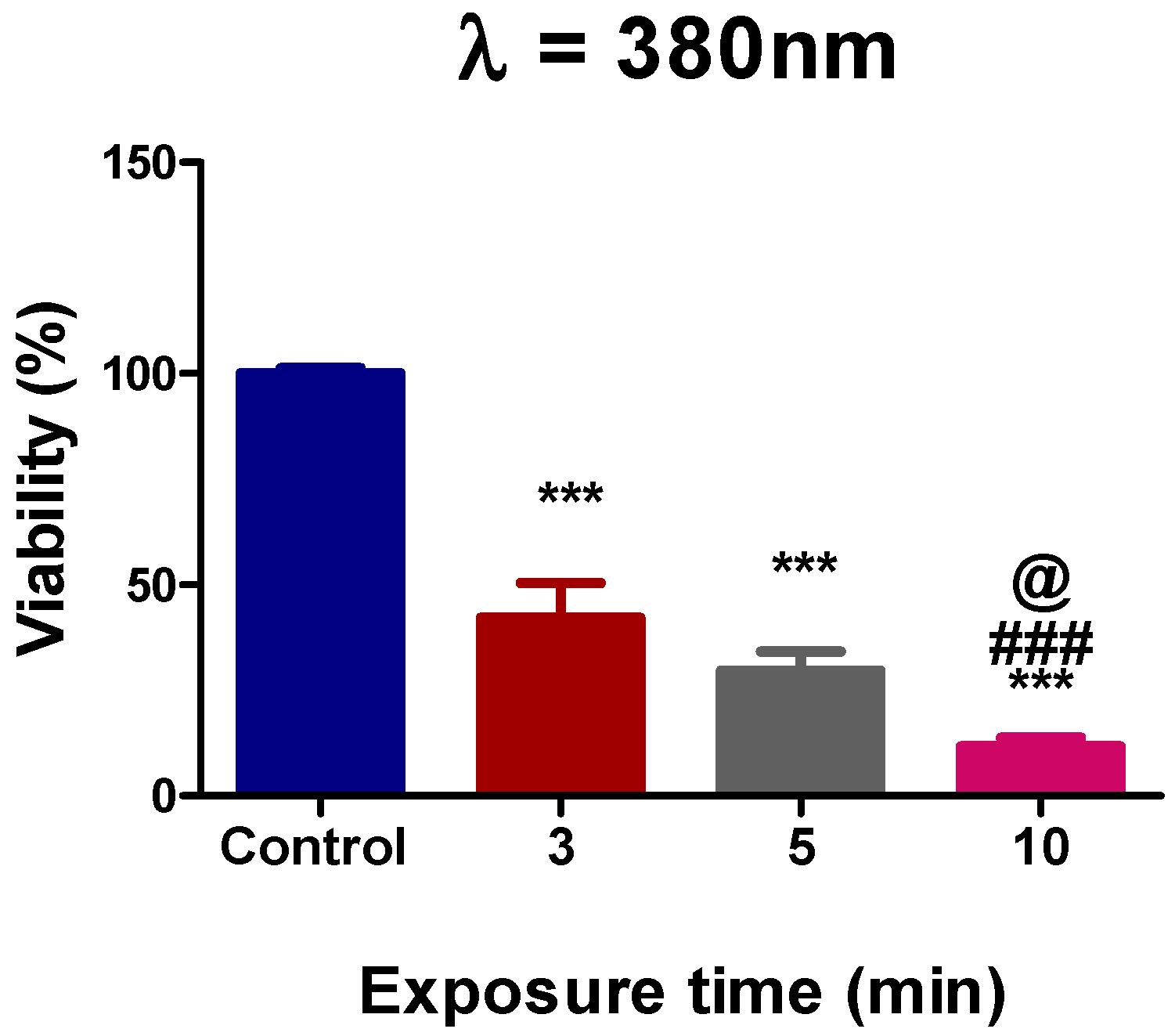
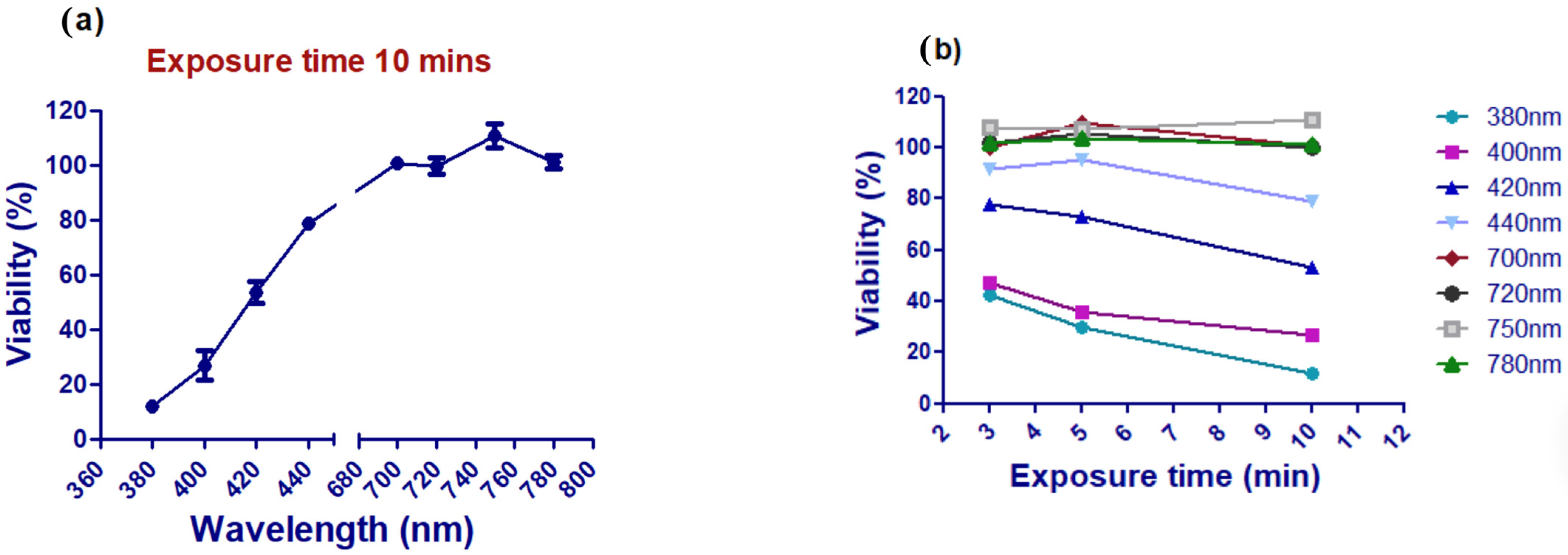
3.1. Assessment of Ultraviolet Femtosecond Laser Light Effects on A375 Cell Viability
3.2. Assessment of A375 Cell Viability Following Visible Femtosecond Laser Light Exposure
3.3. Assessment of A375 Cell Viability Following Near-Infrared Femtosecond Laser Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jassim, A.; Rahrmann, E.P.; Simons, B.D.; Gilbertson, R.J. Cancers make their own luck: Theories of cancer origins. Nat. Rev. Cancer 2023, 23, 710–724. [Google Scholar] [CrossRef]
- Shakhova, O. Neural crest stem cells in melanoma development. Curr. Opin. Oncol. 2014, 26, 215–221. [Google Scholar] [CrossRef]
- Couto, G.K.; Junior, J.C.R.; Pacheco, B.S.; Simoes, L.D.; Paschoal, J.D.; Seixas, F.K.; Acunha, T.V.; Iglesias, B.A.; Collares, T. Zinc (II), copper (II) and nickel (II) ions improve the selectivity of tetra-cationic platinum (II) porphyrins in photodynamic therapy and stimulate antioxidant defenses in the metastatic melanoma lineage (A375). Photodiagnosis Photodyn. Ther. 2020, 31, 101942. [Google Scholar] [CrossRef]
- Schadendorf, D.; Fisher, D.E.; Garbe, C.; Gershenwald, J.E.; Grob, J.-J.; Halpern, A.; Herlyn, M.; Marchetti, M.A.; McArthur, G.; Ribas, A.; et al. Melanoma. Nat. Rev. Dis. Primers 2015, 1, 1–20. [Google Scholar] [CrossRef]
- Mundra, V.; Peng, Y.; Rana, S.; Natarajan, A.; Mahato, R.I. Micellar formulation of indocyanine green for phototherapy of melanoma. J. Control. Release 2015, 220, 130–140. [Google Scholar] [CrossRef]
- Najafabad, B.K.; Attaran, N.; Mahmoudi, M.; Sazgarnia, A. Effect of photothermal and photodynamic therapy with cobalt ferrite superparamagnetic nanoparticles loaded with ICG and PpIX on cancer stem cells in MDA-MB-231 and A375 cell lines. Photodiagnosis Photodyn. Ther. 2023, 43, 103648. [Google Scholar] [CrossRef]
- Szlasa, W.; Supplitt, S.; Drąg-Zalesińska, M.; Przystupski, D.; Kotowski, K.; Szewczyk, A.; Kasperkiewicz, P.; Saczko, J.; Kulbacka, J. Effects of curcumin based PDT on the viability and the organization of actin in melanotic (A375) and amelanotic melanoma (C32)–in vitro studies. Biomed. Pharmacother. 2020, 132, 110883. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Gore, M. Randomized adjuvant therapy trials in melanoma: Surgical and systemic. Semin. Oncol. 2007, 34, 509–515. [Google Scholar] [CrossRef]
- Khelladi, M. Femtosecond Laser Pulses: Generation, Measurement and Propagation. In Recent Advances in Numerical Simulations; IntechOpen: London, UK, 2021. [Google Scholar]
- Niemz, M.H. Laser-Tissue Interactions; Springer: Cham, Switzerland, 2007; Volume 322. [Google Scholar]
- Krüger, J.; Kautek, W.; Newesely, H. Femtosecond-pulse laser ablation of dental hydroxyapatite and single-crystalline fluoroapatite. Appl. Phys. A 1999, 69, S403–S407. [Google Scholar] [CrossRef]
- Ryu, S.; Jun, I.; Kim, T.-I.; Seo, K.Y.; Kim, E.K. Prediction accuracy of conventional and total keratometry for intraocular lens power calculation in femtosecond laser-assisted cataract surgery. Sci. Rep. 2021, 11, 12869. [Google Scholar] [CrossRef]
- Yoon, J.; Ryu, S.-w.; Lee, S.; Choi, C. Cytosolic irradiation of femtosecond laser induces mitochondria-dependent apoptosis-like cell death via intrinsic reactive oxygen cascades. Sci. Rep. 2015, 5, 8231. [Google Scholar] [CrossRef] [PubMed]
- Thøgersen, J.; Knudsen, C.S.; Maetzke, A.; Knak Jensen, S.J.; Keiding, S.R.; Alsner, J.; Overgaard, J. Reproductive death of cancer cells induced by femtosecond laser pulses. Int. J. Radiat. Biol. 2007, 83, 289–299. [Google Scholar] [CrossRef]
- Tirlapur, U.K.; König, K. Femtosecond near-infrared laser pulse induced strand breaks in mammalian cells. Cell. Mol. Biol. 2001, 47, OL131–OL134. [Google Scholar]
- Gabel, C.V. Femtosecond lasers in biology: Nanoscale surgery with ultrafast optics. Contemp. Phys. 2008, 49, 391–411. [Google Scholar] [CrossRef]
- Ahmed, E.; El-Gendy, A.O.; Moniem Radi, N.A.; Mohamed, T. The bactericidal efficacy of femtosecond laser-based therapy on the most common infectious bacterial pathogens in chronic wounds: An in vitro study. Lasers Med Sci. 2021, 36, 641–647. [Google Scholar] [CrossRef]
- El-Gendy, A.O.; Samir, A.; Ahmed, E.; Enwemeka, C.S.; Mohamed, T. The antimicrobial effect of 400 nm femtosecond laser and silver nanoparticles on gram-positive and gram-negative bacteria. J. Photochem. Photobiol. B Biol. 2021, 223, 112300. [Google Scholar] [CrossRef]
- Zecha, J.A.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Sonis, S.T.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.J.; et al. Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: Part 1: Mechanisms of action, dosimetric, and safety considerations. Support. Care Cancer 2016, 24, 2781–2792. [Google Scholar] [CrossRef] [PubMed]
- Kars, M.D.; Yildirim, G.; Gundogdu, Y.; Gonce, F.; Ayan, E.; Kilic, H.S. Revealing the therapeutic effects of aminolevulinate mediated femtosecond laser induced photo-chemotherapy in different cancer cells. EuroBiotech J. 2020, 4, 207–215. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Sharma, S.K.; Carroll, J.; Hamblin, M.R. Biphasic dose response in low level light therapy—An update. Dose-Response 2011, 9, 602–618. [Google Scholar] [CrossRef]
- Taha, S.; Mohamed, W.R.; Elhemely, M.A.; El-Gendy, A.O.; Mohamed, T. Breast cancer and laser therapy. Laser Innov. Res. Appl. 2024, 1, 43–66. [Google Scholar] [CrossRef]
- Ilina, I.V.; Sitnikov, D.S. Application of Ultrashort Lasers in Developmental Biology: A Review. Photonics 2022, 9, 914. [Google Scholar] [CrossRef]
- Golovynska, I.; Golovynskyi, S.; Qu, J. Comparing the impact of NIR, visible and UV light on ROS upregulation via photoacceptors of mitochondrial complexes in normal, immune and cancer cells. Photochem. Photobiol. 2023, 99, 106–119. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldan-Varona, P.; Rodriguez-Cobo, L.; López-Higuera, J.M. Light technology for efficient and effective photodynamic therapy: A critical review. Cancers 2021, 13, 3484. [Google Scholar] [CrossRef] [PubMed]
- Nevola, L.; Martín-Quirós, A.; Eckelt, K.; Camarero, N.; Tosi, S.; Llobet, A.; Giralt, E.; Gorostiza, P. Light-regulated stapled peptides to inhibit protein–protein interactions involved in clathrin-mediated endocytosis. Angew. Chem. Int. Ed. Engl. 2013, 52, 7704–7708. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.-Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci. Rep. 2017, 7, 7781. [Google Scholar] [CrossRef]
- Kushibiki, T.; Hirasawa, T.; Okawa, S.; Ishihara, M.J. Blue laser irradiation generates intracellular reactive oxygen species in various types of cells. Photomed. Laser Surg. 2013, 31, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, F.; Antonini, G.; Sarti, P.; Brunori, M. Structure and function of a molecular machine: Cytochrome c oxidase. Biophys. Chem. 1995, 54, 1–33. [Google Scholar] [CrossRef]
- Ushenko, V.A.; Hogan, B.T.; Dubolazov, A.; Piavchenko, G.; Kuznetsov, S.L.; Ushenko, A.G.; Ushenko, Y.O.; Gorsky, M.; Bykov, A.; Meglinski, I. 3D Mueller matrix mapping of layered distributions of depolarisation degree for analysis of prostate adenoma and carcinoma diffuse tissues. Sci. Rep. 2021, 11, 5162. [Google Scholar] [CrossRef]
- Mason, M.G.; Nicholls, P.; Cooper, C.E. Re-evaluation of the near infrared spectra of mitochondrial cytochrome c oxidase: Implications for non invasive in vivo monitoring of tissues. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 1882–1891. [Google Scholar] [CrossRef]
- Oh, P.-S.; Hwang, H.; Jeong, H.-S.; Kwon, J.; Kim, H.-S.; Kim, M.; Lim, S.; Sohn, M.-H.; Jeong, H.-J. Blue light emitting diode induces apoptosis in lymphoid cells by stimulating autophagy. Int. J. Biochem. Cell Biol. 2016, 70, 13–22. [Google Scholar] [CrossRef]
- Zhuang, J.; Liu, Y.; Yuan, Q.; Liu, J.; Liu, Y.; Li, H.; Wang, D. Blue light-induced apoptosis of human promyelocytic leukemia cells via the mitochondrial-mediated signaling pathway. Oncol. Lett. 2018, 15, 6291–6296. [Google Scholar] [CrossRef]
- Oh, P.-S.; Na, K.S.; Hwang, H.; Jeong, H.-S.; Lim, S.; Sohn, M.-H.; Jeong, H.-J. Effect of blue light emitting diodes on melanoma cells: Involvement of apoptotic signaling. J. Photochem. Photobiol. B Biol. 2015, 142, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Gupta, A.; Huang, Y.-Y.; Yin, R.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Tegos, G.P.; Hamblin, M.R. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: Efficacy, safety, and mechanism of action. Antimicrob. Agents Chemother. 2013, 57, 1238–1245. [Google Scholar] [CrossRef]
- Dai, T.; Gupta, A.; Murray, C.K.; Vrahas, M.S.; Tegos, G.P.; Hamblin, M.R. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist. Updat. 2012, 15, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Habit, H.A.H.; Suardi, N.; Mahmud, S.; Mydin, R.B.S.; Bakhori, S.K.M. In vitro toxicity of low-level green laser irradiation effects on human breast cancer cell lines. Indian J. Biochem. Biophys. 2020, 57, 627–633. [Google Scholar]
- Taha, S.; Mohamed, W.R.; Elhemely, M.A.; El-Gendy, A.O.; Mohamed, T. Tunable femtosecond laser suppresses the proliferation of breast cancer in vitro. J. Photochem. Photobiol. B Biol. 2023, 240, 112665. [Google Scholar] [CrossRef] [PubMed]
- Frigo, L.; Luppi, J.S.; Favero, G.M.; Maria, D.A.; Penna, S.C.; Bjordal, J.M.; Bensadoun, R.J.; Lopes-Martins, R.A. The effect of low-level laser irradiation (In-Ga-Al-AsP-660 nm) on melanoma in vitro and in vivo. BMC Cancer 2009, 9, 1–8. [Google Scholar] [CrossRef]
- Bamps, M.; Dok, R.; Nuyts, S. Low-level laser therapy stimulates proliferation in head and neck squamous cell carcinoma cells. Front. Oncol. 2018, 8, 343. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Nelson, S.T.; Strahan, J.R. Photobiomodulation and cancer: What is the truth? Photomed. Laser Surg. 2018, 36, 241–245. [Google Scholar] [CrossRef]
- Sommer, A.P.; Pinheiro, A.L.; Mester, A.R.; Franke, R.-P.; Whelan, H.T. Biostimulatory windows in low-intensity laser activation: Lasers, scanners, and NASA’s light-emitting diode array system. J. Clin. Laser Med. Surg. 2001, 19, 29–33. [Google Scholar] [CrossRef]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.-Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.B.; Mishra, A.; Hu, K.; An, J.; Kim, Y.-J.; Yoon, Y.-J. Femtosecond-laser-based 3D printing for tissue engineering and cell biology applications. ACS Biomater. Sci. Eng. 2017, 3, 2198–2214. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, R.M.; Spooner, G.J.; Sletten, K.R.; Yen, K.G.; Sayegh, S.I.; Loesel, F.H.; Horvath, C.; Liu, H.; Elner, V.; Cabrera, D. Ophthalmic applications of femtosecond lasers. In Commercial and Biomedical Applications of Ultrafast Lasers; SPIE: Bellingham, WA, USA, 1999. [Google Scholar]
- Malviya, R.; Meenakshi, D.U.; Goyal, P. Laser Therapy in Healthcare: Advances in Diagnosis and Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2024. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, S.; T. Nawaf, K.; Rifaat, H.M.; El-Gendy, A.O.; Mohamed, T. The Potential of Tunable Femtosecond Laser Light to Prevent Melanoma A375 Cell Growth: An In Vitro Investigation. Photonics 2025, 12, 694. https://doi.org/10.3390/photonics12070694
Taha S, T. Nawaf K, Rifaat HM, El-Gendy AO, Mohamed T. The Potential of Tunable Femtosecond Laser Light to Prevent Melanoma A375 Cell Growth: An In Vitro Investigation. Photonics. 2025; 12(7):694. https://doi.org/10.3390/photonics12070694
Chicago/Turabian StyleTaha, Safaa, Khalid T. Nawaf, Hala M. Rifaat, Ahmed O. El-Gendy, and Tarek Mohamed. 2025. "The Potential of Tunable Femtosecond Laser Light to Prevent Melanoma A375 Cell Growth: An In Vitro Investigation" Photonics 12, no. 7: 694. https://doi.org/10.3390/photonics12070694
APA StyleTaha, S., T. Nawaf, K., Rifaat, H. M., El-Gendy, A. O., & Mohamed, T. (2025). The Potential of Tunable Femtosecond Laser Light to Prevent Melanoma A375 Cell Growth: An In Vitro Investigation. Photonics, 12(7), 694. https://doi.org/10.3390/photonics12070694






