New High Light Yield and Fast Ceramic Scintillator Y3Al2.5Ga2.5O12:Ce, Mg
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Autrata, R.; Schauer, P.; Kuapil, J.; Kuapil, J. A Single Crystal of YAG-New Fast Scintillator in SEM. J. Phys. E 1978, 11, 707. [Google Scholar] [CrossRef]
- Greskovich, C.; Duclos, S. Ceramic scintillators. Annu. Rev. Mater. Res. 1997, 27, 69–88. [Google Scholar] [CrossRef]
- Yanagida, T.; Takahashi, H.; Ito, T.; Kasama, D.; Enoto, T.; Sato, M.; Hirakuri, S.; Kokubun, M.; Makishima, K.; Yanagitani, T.; et al. Evaluation of Properties of YAG (Ce) Ceramic Scintillators. IEEE Trans. Nucl. Sci. 2005, 52, 1836–1841. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Y.; Zhang, Z.; Su, L.; Kou, H.; Wang, Y.; Wu, A. Optical and Scintillation Properties of Ce:Y3Al5O12 Single Crystal Fibers Grown by Laser Heated Pedestal Growth Method. J. Rare Earths 2021, 39, 1533–1539. [Google Scholar] [CrossRef]
- Kucera, M.; Nitsch, K.; Kubova, M.; Solovieva, N.; Nikl, M.; Mares, J.A. Ce-doped YAG and LuAG Epitaxial Films for Scintillation Detectors. IEEE Trans. Nucl. Sci. 2008, 55, 1201–1205. [Google Scholar] [CrossRef]
- Zorenko, Y.; Mares, J.A.; Prusa, P.; Nikl, M.; Gorbenko, V.; Savchyn, V.; Kucerkova, R.; Nejezchleb, K. Luminescence and scintillation characteristics of YAG:Ce single crystalline films and single crystals. Radiat. Meas. 2010, 45, 389–391. [Google Scholar] [CrossRef]
- Zorenko, Y.; Gorbenko, V.; Konstankevych, I.; Voloshinovskii, A.; Stryganyuk, G.; Mikhailin, V.; Kolobanov, V.; Spassky, D. Single-crystalline films of Ce-doped YAG and LuAG phosphors: Advantages over bulk crystals analogues. J. Lumin. 2005, 114, 85–94. [Google Scholar] [CrossRef]
- Mares, J.A.; Nikl, M.; Beitlerova, A.; Horodysky, P.; Blazek, K.; Bartos, K.; D’Ambrosio, C. Scintillation Properties of Ce3+- and Pr3+-Doped LuAG, YAG and Mixed LuxY1−xAG Garnet Crystals. IEEE Trans. Nucl. Sci. 2012, 59, 2120–2125. [Google Scholar] [CrossRef]
- Mihóková, E.; Nikl, M.; Mareš, J.A.; Beitlerová, A.; Vedda, A.; Nejezchleb, K.; Blažek, K.; D’Ambrosio, C. Luminescence and scintillation properties of YAG:Ce single crystal and optical ceramics. J. Lumin. 2007, 126, 77–80. [Google Scholar] [CrossRef]
- Lucchini, M.T.; Pauwels, K.; Blazek, K.; Ochesanu, S.; Auffray, E. Radiation tolerance of LuAG:Ce and YAG:Ce crystals under high levels of gamma- and proton-irradiation. IEEE Trans. Nucl. Sci. 2016, 63, 586–590. [Google Scholar] [CrossRef]
- Moszyński, M.; Ludziejewski, T.; Wolski, D.; Klamra, W.; Norlin, L.O. Properties of the YAG:Ce scintillator. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1994, 345, 461–467. [Google Scholar] [CrossRef]
- Ludziejewski, T.; Moszyński, M.; Kapusta, M.; Wolski, D.; Klamra, W.; Moszyńska, K. Investigation of Some Scintillation Properties of YAG:Ce Crystals. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1997, 398, 287–294. [Google Scholar] [CrossRef]
- Nikl, M. Scintillation detectors for x-rays. Meas. Sci. Technol. 2006, 17, R37–R54. [Google Scholar] [CrossRef]
- Zych, E.; Brecher, C.; Glodo, J. Kinetics of cerium emission in a YAG:Ce single crystal: The role of traps. J. Phys. Condens. Matter 2000, 12, 1947–1958. [Google Scholar] [CrossRef]
- Zapadlík, O.; Nikl, M.; Polák, J.; Průša, P.; Linhart, V. Engineering of YAG:Ce to improve its scintillation properties. Opt. Mater. X 2022, 15, 100165. [Google Scholar] [CrossRef]
- Advatech-UK. Available online: https://www.advatech-uk.co.uk/yag_ce.html (accessed on 5 April 2025).
- Crytur. Available online: https://www.crytur.cz/materials/yagce/ (accessed on 5 April 2025).
- Sreebunpeng, K.; Chewpraditkul, W.; Chewpraditkul, W.; Kucerkova, R.; Beitlerova, A.; Nikl, V.; Szczesniak, T.; Grodzicka-Kobylka, M.; Zhu, D.; Hu, C.; et al. Luminescence and scintillation properties of fast Ce,Mg:Lu2YGaxAl5-xO12 ceramic scintillators fabricated from co-precipitated powders. Opt. Mater. 2024, 152, 115418. [Google Scholar] [CrossRef]
- Chewpraditkul, W.; Pattanaboonmee, N.; Chewpraditkul, W.; Kamada, K.; Kim, K.-J.; Yoshikawa, A.; Makowski, M.; Witkowski, V.; Drozdowski, W.; Beitlerova, A.; et al. Optical and scintillation characteristics of Lu2Y(Al5-xGax)O12:Ce,Mg multicomponent garnet crystals. Opt. Mater. 2022, 134, 113186. [Google Scholar] [CrossRef]
- Korzhik, M.; Alenkov, V.; Buzanov, O.; Dosovitskiy, G.; Fedorov, A.; Kozlov, D.; Mechinsky, V.; Nargelas, S.; Tamulaitis, G.; Vaitkevičius, A. Engineering of a new single-crystal multi-ionic fast and high-light-yield scintillation material (Gd0.5–Y0.5)3Al2Ga3O12:Ce,Mg. CrystEngComm 2020, 22, 2502–2506. [Google Scholar] [CrossRef]
- Ueda, J.; Tanabe, S. (INVITED) Review of luminescent properties of Ce3+-doped garnet phosphors: New insight into the effect of crystal and electronic structure. Opt. Mater. X 2019, 1, 100018. [Google Scholar] [CrossRef]
- Chewpraditkul, W.; Pattanaboonmee, N.; Chewpraditkul, W.; Szczesniak, T.; Moszynski, M.; Kamada, K.; Yoshikawa, A.; Kucerkova, R.; Nikl, M. Optical and scintillation properties of LuGd2Al2Ga3O12:Ce, Lu2GdAl2Ga3O12:Ce, and Lu2YAl2Ga3O12:Ce single crystals: A comparative study. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2021, 1004, 165381. [Google Scholar] [CrossRef]
- Chewpraditkul, W.; Horiai, T.; Beitlerova, A.; Kucerkova, R.; Babin, V.; Yoshikawa, A.; Chewpraditkul, W.; Nikl, M. Temperature dependence of photo- and radio-luminescence and scintillation properties of Lu2YAl2.5Ga2.5O12:Ce,Mg multicomponent garnet crystals. Opt. Mater. 2024, 147, 114738. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.; Feng, Y.; Li, X.; Chen, H.; Xie, T.; Kou, H.; Kučerková, R.; Beitlerová, A.; Mihóková, E.; et al. Microstructure evolution in two-step-sintering process toward transparent Ce:(Y,Gd)3(Ga,Al)5O12 scintillation ceramics. J. Alloys Compd. 2020, 846, 156377. [Google Scholar] [CrossRef]
- Smyslova, V.G.; Retivov, V.M.; Dubov, V.V.; Ermakova, L.V.; Ivanov, V.K.; Karpyuk, P.V.; Komendo, I.Y.; Lelekova, D.E.; Mechinsky, V.A.; Vasiliev, A.N.; et al. Effect of nanostructuring of coprecipitated precursors on the morphology and scintillation properties of multication ceramics with a garnet structure. Nanosyst. Phys. Chem. Math. 2024, 15, 2220–8054. [Google Scholar] [CrossRef]
- Korzhik, M.; Retivov, V.; Dubov, V.; Ivanov, V.; Komendo, I.; Lelekova, D.; Karpyuk, P.; Mechinsky, V.; Postupaeva, A.; Smyslova, V.; et al. Compositional disordering: Nanoscale engineering of advanced crystalline scintillation materials. J. Appl. Phys. 2024, 135, 0021–8979. [Google Scholar] [CrossRef]
- Korzhik, M.; Tamulaitis, G.; Vasil’ev, A.N. Physics of Fast Processes in Scintillators; Particle Acceleration and Detection; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Retivov, V.; Dubov, V.; Kuznetsova, D.; Ismagulov, A.; Korzhik, M. Gd3+ content optimization for mastering high light yield and fast GdxAl2Ga3O12:Ce3+ scintillation ceramics. J. Rare Earths 2022, 41, 1911–1918. [Google Scholar] [CrossRef]
- Moses, W.W.; Derenzo, S.E.; Fyodorov, A.; Korzhik, M.; Gektin, A.; Minkov, B.; Aslanov, V. LuAlO3:Ce-a high density, high speed scintillator for gamma detection. IEEE Trans. Nucl. Sci. 1995, 42, 275–279. [Google Scholar] [CrossRef]
- Nargelas, S.; Solovjovas, A.; Talochka, Y.; Podlipskas, Ž.; Kucera, M.; Lucenicova, Z.; Tamulaitis, G. Influence of heavy magnesium codoping on emission decay in Ce-doped multicomponent garnet scintillators. J. Mater. Chem. C 2023, 11, 12007–12015. [Google Scholar] [CrossRef]
- Korzhik, M.V.; Trower, W.P. Origin of scintillation in cerium_doped oxide crystals. Appl. Phys. Lett. 1995, 66, 2327. [Google Scholar] [CrossRef]
- Hayes, W.; Yamaga, M.; Robbins, D.J.; Cocklane, B. Optical detection of excitation EPR in LiYF4. Solid-State Phys. 1980, 13, L1085. [Google Scholar] [CrossRef]
- Kuznetsov, A.I.; Namosov, B.R.; Murk, V.V. Relaxed electronicstates in Al2O3, Y3Al5O12, YAlO3. Solid State Phys (USSR) 1985, 27, 3030–3035. [Google Scholar]
- Kuznetsov, A.I.; Abramov, V.N.; Murk, V.V.; Namosov, B.R. States of the self-trapped excitons in complex oxides. Sov. Phys. Solid State 1991, 33, 2000–2004. [Google Scholar]
- Wood, R.L.; Hayes, W. A study of recombination centres in YAIO3, EuIAIO3, LaAlO3 and Y2O3 using ODMR. Radiat. Eff. 1983, 72, 195. [Google Scholar] [CrossRef]
- Andriessen, J.; Dorenbos, P.; van Eijk, C.W.E. Ce3+ Doped Scintillation Materials. Scintillators and Phosphor Materials; Weber, M.J., Lecoq, P., Ruchti, R.C., Woody, C., Yen, W.M., Zhu, R.-Y., Eds.; Material Research Society: Pittsburgh, PA, USA, 1994; p. 355. [Google Scholar]
- Yamaji, A.; Yanagida, T.; Yokota, Y.; Fujimoto, Y.; Sugiyama, M.; Yoshikawa, A. Comparative study on scintillation properties of LGG, YGG and GGG. In Proceedings of the 2010 IEEE Nuclear Science Symposium, Medical Imaging Conference, Knoxville, TN, USA, 30 October–6 November 2010; pp. 179–181. [Google Scholar] [CrossRef]
- Zorenko, Y.; Zorenko, T.; Vistovskyy, V.; Grinberg, M.; Łukasiewicz, T. Time-resolved spectroscopy of intrinsic luminescence of Y3Ga5O12 and (LaLu)3Lu2Ga3O12 single crystals. Opt. Mater. 2009, 31, 1835–1838. [Google Scholar] [CrossRef][Green Version]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. 2009, 21, 395502. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
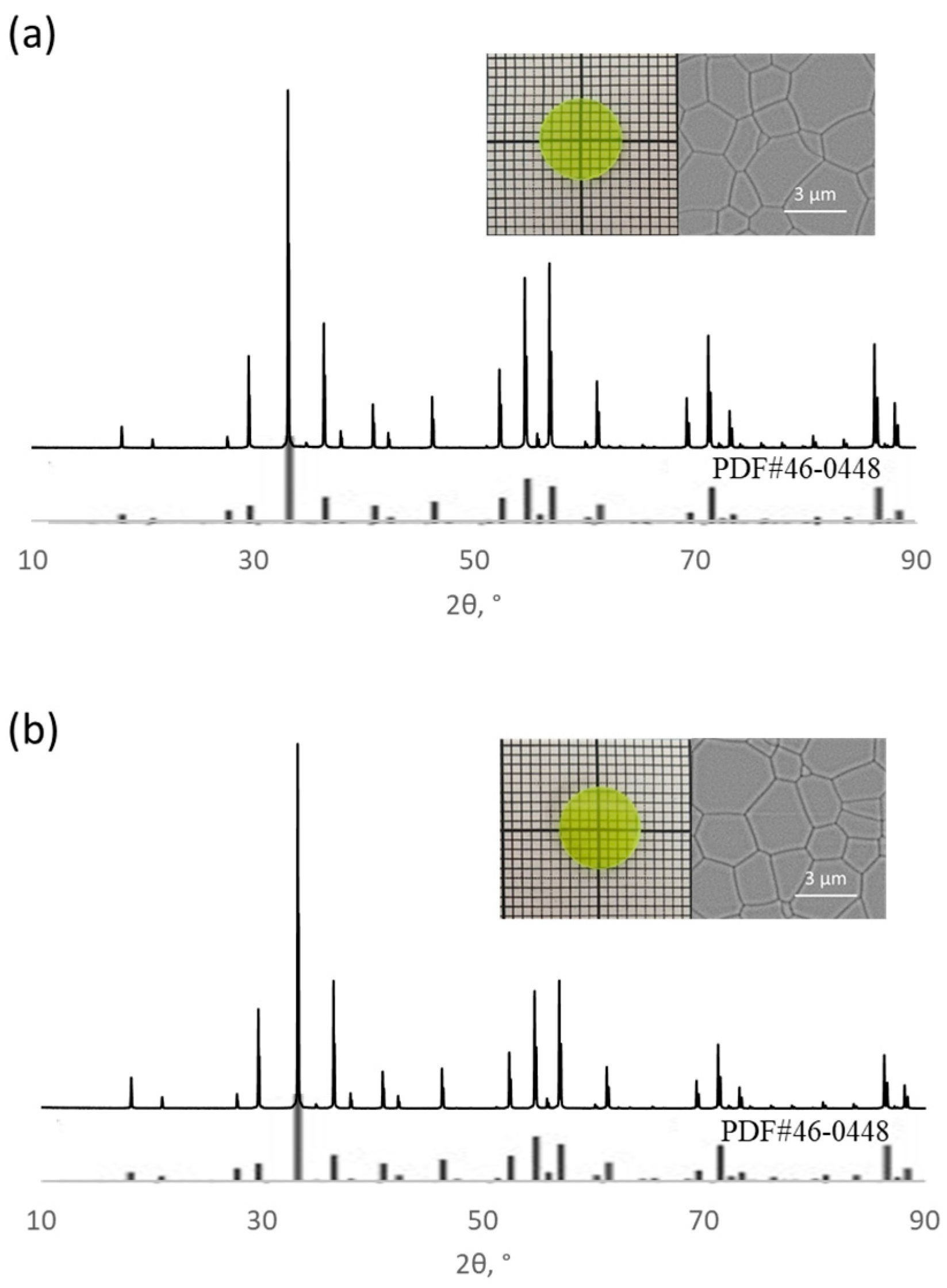

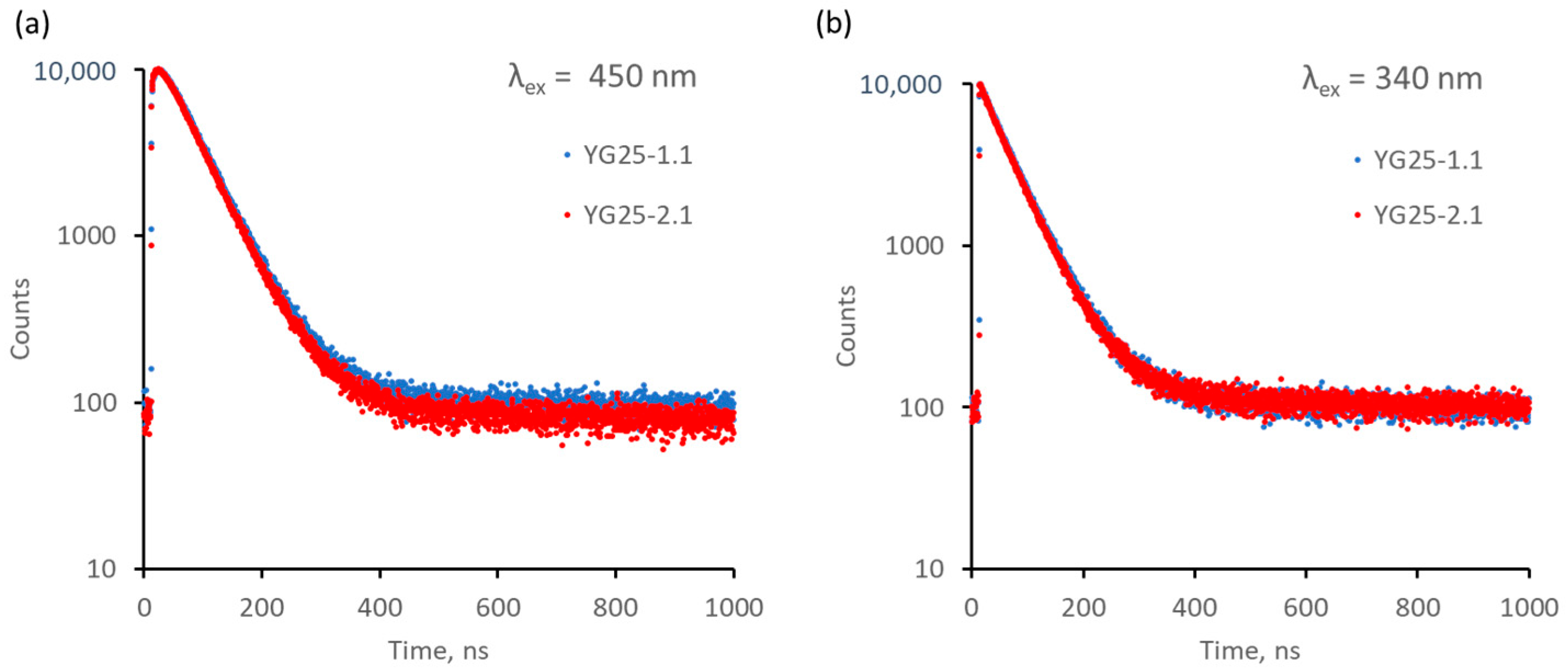
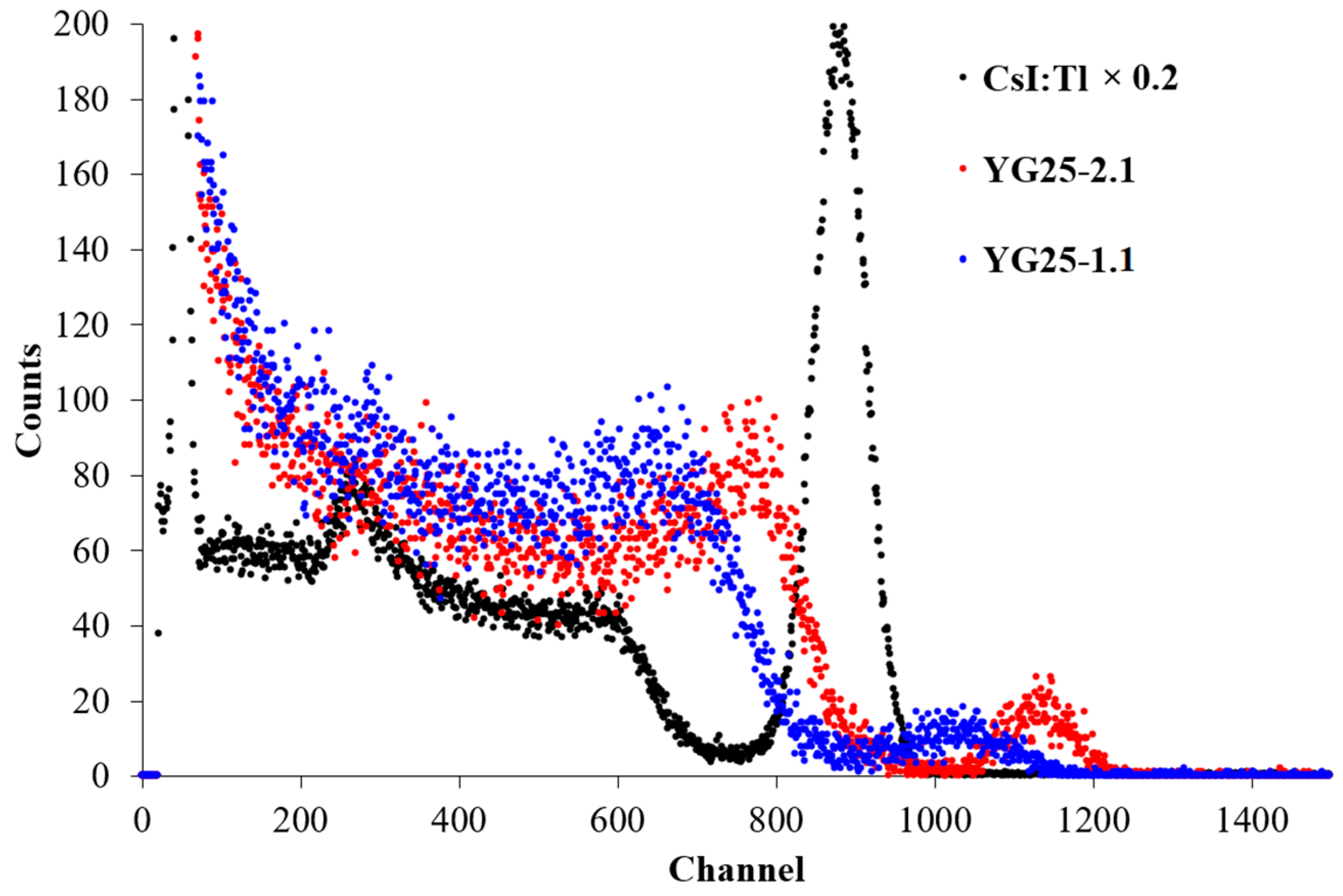
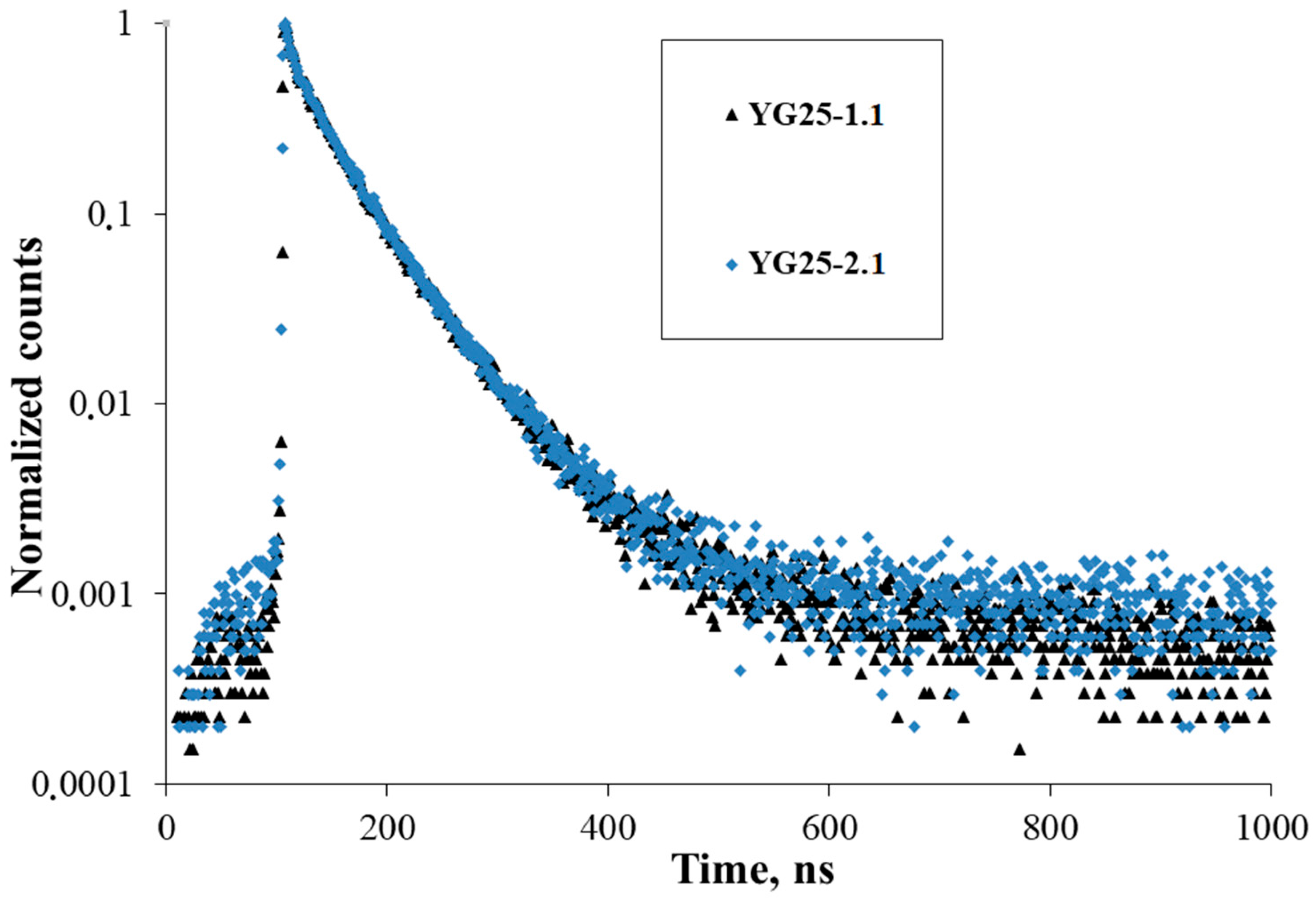

| Sample | Rietveld Factor | a, Å | ρ, g/cm3 |
|---|---|---|---|
| YG25-1.1 | 2.67 | 12.165 | 5.17 |
| YG25-2.1 | 3.74 | 12.162 | 5.17 |
| Sample | τ1 (Fraction), ns (%) | τ2 (Fraction), ns (%) | τ1 (Fraction), ns (%) |
|---|---|---|---|
| λexc 340 nm | λexc 340 nm | λexc 450 nm | |
| YG25-1.1 | 57 (91) | 660 (4) | 57 (100) |
| YG25-2.1 | 54 (96) | 234 (3) | 56 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smyslova, V.; Bondarau, A.; Fedorov, A.; Borisevich, E.; Lagutskiy, I.; Karpuyk, P.; Komendo, I.; Kalinov, V.; Mechinsky, V.; Retivov, V.; et al. New High Light Yield and Fast Ceramic Scintillator Y3Al2.5Ga2.5O12:Ce, Mg. Photonics 2025, 12, 680. https://doi.org/10.3390/photonics12070680
Smyslova V, Bondarau A, Fedorov A, Borisevich E, Lagutskiy I, Karpuyk P, Komendo I, Kalinov V, Mechinsky V, Retivov V, et al. New High Light Yield and Fast Ceramic Scintillator Y3Al2.5Ga2.5O12:Ce, Mg. Photonics. 2025; 12(7):680. https://doi.org/10.3390/photonics12070680
Chicago/Turabian StyleSmyslova, Valentina, Aliaksei Bondarau, Andrei Fedorov, Elizaveta Borisevich, Ilya Lagutskiy, Petr Karpuyk, Ilia Komendo, Vladimir Kalinov, Vitaly Mechinsky, Vasilii Retivov, and et al. 2025. "New High Light Yield and Fast Ceramic Scintillator Y3Al2.5Ga2.5O12:Ce, Mg" Photonics 12, no. 7: 680. https://doi.org/10.3390/photonics12070680
APA StyleSmyslova, V., Bondarau, A., Fedorov, A., Borisevich, E., Lagutskiy, I., Karpuyk, P., Komendo, I., Kalinov, V., Mechinsky, V., Retivov, V., Talochko, Y., Vasil’ev, A., & Korzhik, M. (2025). New High Light Yield and Fast Ceramic Scintillator Y3Al2.5Ga2.5O12:Ce, Mg. Photonics, 12(7), 680. https://doi.org/10.3390/photonics12070680






