Collaboration of Two UV-Absorbing Dyes in Cholesteric Liquid Crystals Films for Infrared Broadband Reflection and Ultraviolet Shielding
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Preparation of Samples and Cells
3. Results
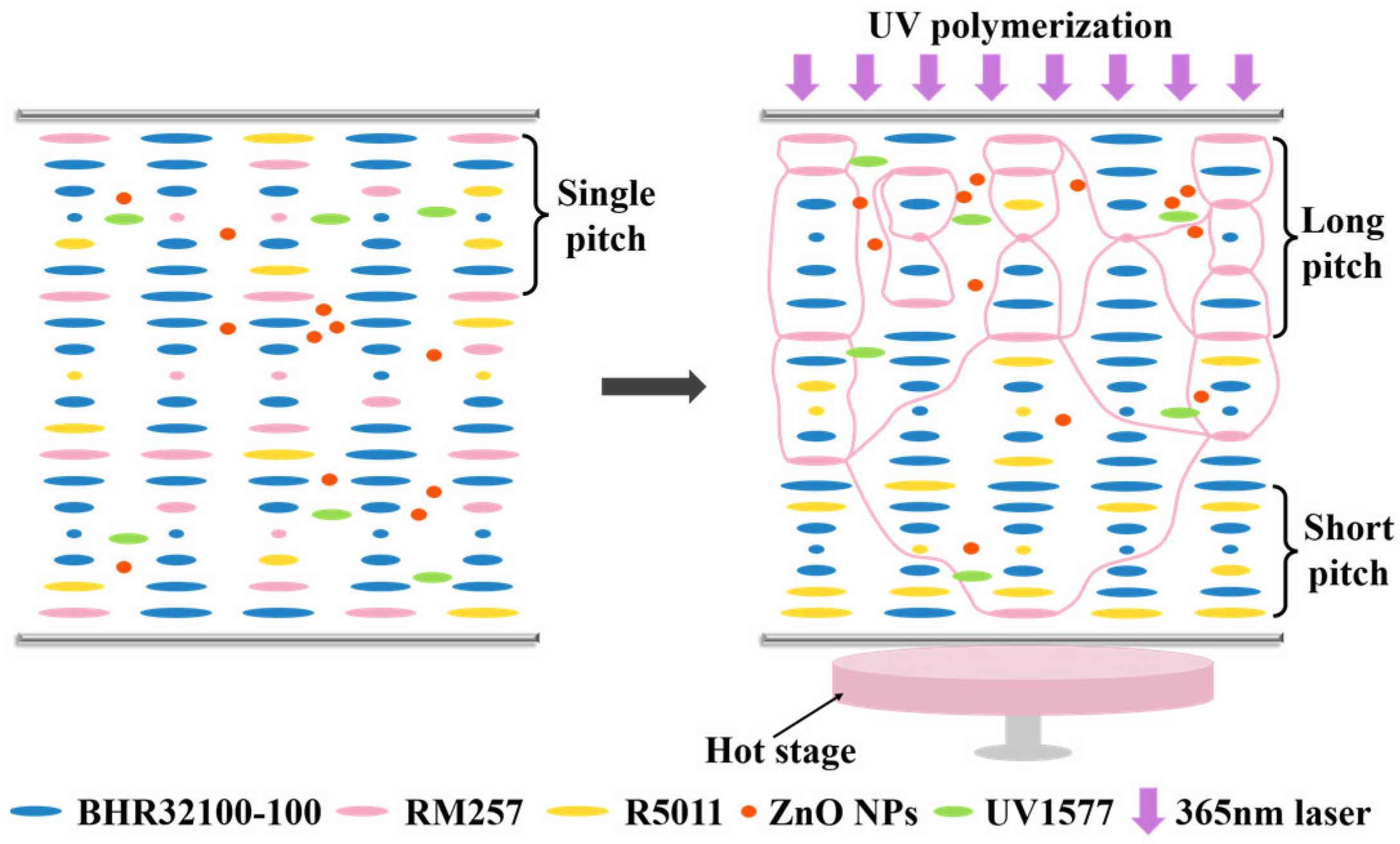
3.1. Pitch Gradient-Driven Broadband Reflection Mechanism in CLC Films
3.2. The Influence of UV-1577/ZnO NPs Mixtures on the Reflection Broadband of Samples
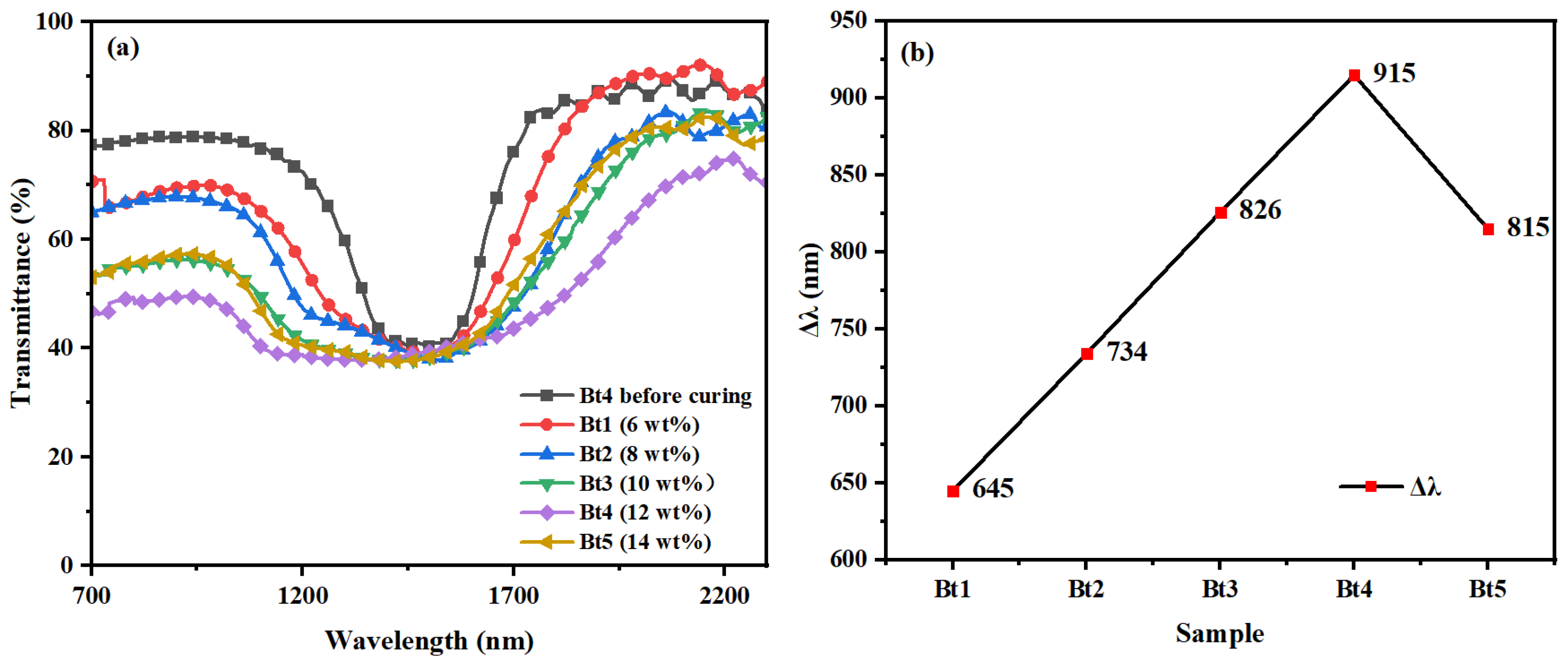
3.3. The Influence of RM257 Concentration on the Reflection Bandwidth of Samples
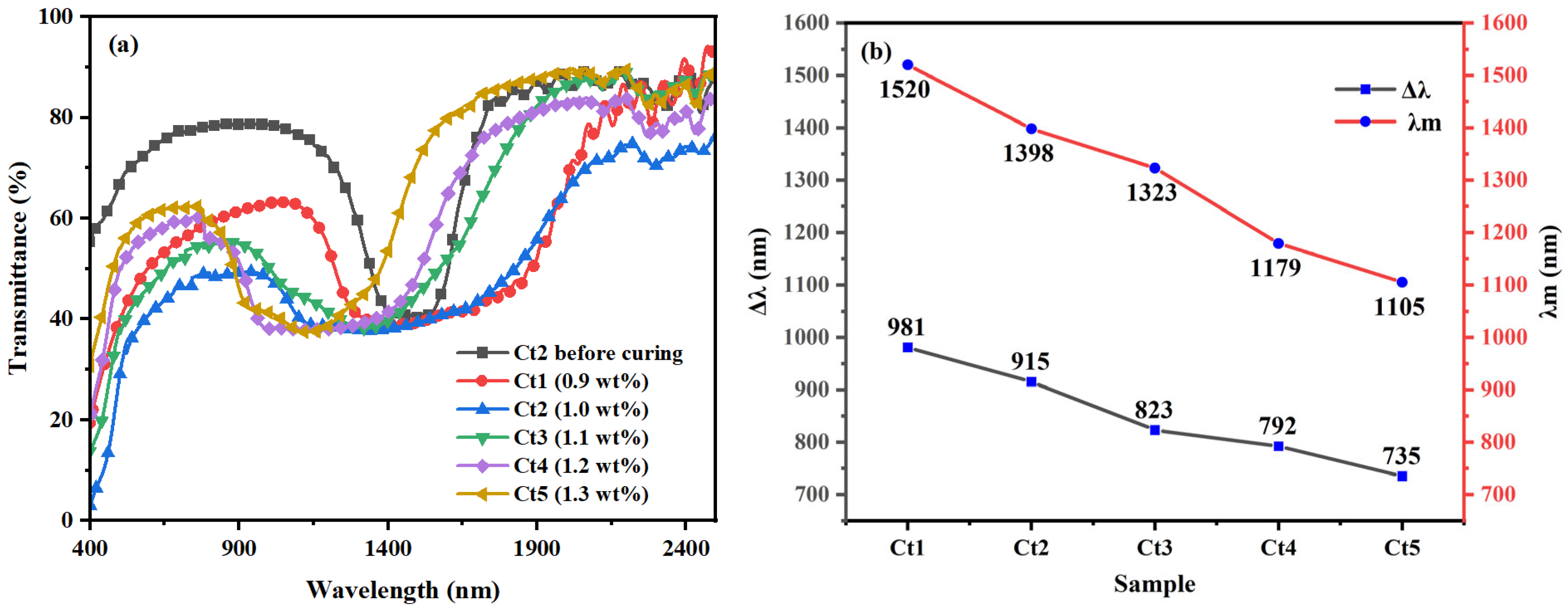
3.4. The Influence of R5011 Concentration on the Reflection Bandwidth of Samples
3.5. The Influence of Polymerization Temperature on the Reflection Bandwidth of Samples
3.6. The Influence of UV Intensity on the Reflection Bandwidth of Samples
3.7. The Influence of Polymerization Time on the Reflection Bandwidth of Samples
3.8. Comparison of the Optimal Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ZnO NPs | zinc oxide nanoparticles |
| CLC | cholesteric liquid crystal |
| BP | benzophenone |
| POM | polarized light microscopy |
| SEM | scanning electron microscopy |
| PET | polyethylene terephthalate |
References
- Berreman, D.W.; Scheffer, T.J. Bragg reflection of light from single-domain cholesteric liquid-crystal films. Phys. Rev. Lett. 1970, 25, 577–580. [Google Scholar] [CrossRef]
- De Vries, H. Rotatory power and other optical properties of certain liquid crystals. Acta Crystallogr. 1951, 4, 219–226. [Google Scholar] [CrossRef]
- Cheng, S.; Li, W.; Zhang, H.; Akhtar, M.N.; Yi, Z.; Zeng, Q.; Ma, C.; Sun, T.; Wu, P.; Ahmad, S. High sensitivity five band tunable metamaterial absorption device based on block like Dirac semimetals. Opt. Commun. 2024, 569, 130816. [Google Scholar] [CrossRef]
- Broer, D.J.; Lub, J.; Mol, G.N. Wide-band reflective polarizers from cholesteric polymer networks with a pitch gradient. Nature 1995, 378, 467–469. [Google Scholar] [CrossRef]
- Zheng, Z.-g.; Wang, C.; Shen, D. Dichroic-dye-doped polymer stabilized optically isotropic chiral liquid crystals. J. Mater. Chem. C 2013, 1, 6471–6478. [Google Scholar] [CrossRef]
- Mitov, M. Cholesteric Liquid Crystals with a Broad Light Reflection Band. Adv. Mater. 2012, 24, 6260–6276. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-g.; Liu, B.-w.; Zhou, L.; Wang, W.; Hu, W.; Shen, D. Wide tunable lasing in photoresponsive chiral liquid crystal emulsion. J. Mater. Chem. C 2015, 3, 2462–2470. [Google Scholar] [CrossRef]
- Zheng, Z.-G.; Lu, Y.-Q.; Li, Q. Photoprogrammable mesogenic soft helical architectures: A promising avenue toward future chiro-optics. Adv. Mater. 2020, 32, 1905318. [Google Scholar] [CrossRef]
- Dai, L.; Li, W.; Cao, J.; Li, J.; Liu, S. Formation, tuning and application of chiral nematic liquid crystal phase based on nanocrystalline cellulose. Prog. Chem. 2015, 27, 861–869. [Google Scholar] [CrossRef]
- He, Y.; Lin, S.; Guo, J.; Li, Q. Circularly polarized luminescent self-organized helical superstructures: From materials and stimulus-responsiveness to applications. Aggregate 2021, 2, e141. [Google Scholar] [CrossRef]
- Ma, L.-L.; Wu, S.-B.; Hu, W.; Liu, C.; Chen, P.; Qian, H.; Wang, Y.; Chi, L.; Lu, Y.-Q. Self-assembled asymmetric microlenses for four-dimensional visual imaging. ACS Nano 2019, 13, 13709–13715. [Google Scholar] [CrossRef]
- Schmidtke, J.; Juennemann, G.; Keuker-Baumann, S.; Kitzerow, H.-S. Electrical fine tuning of liquid crystal lasers. Appl. Phys. Lett. 2012, 101, 051117. [Google Scholar] [CrossRef]
- Palto, S.P.; Shtykov, N.M.; Umanskii, B.A.; Barnik, M.I. Multiwave out-of-normal band-edge lasing in cholesteric liquid crystals. J. Appl. Phys. 2012, 112, 013105. [Google Scholar] [CrossRef]
- Davies, D.J.D.; Vaccaro, A.R.; Morris, S.M.; Herzer, N.; Schenning, A.P.H.J.; Bastiaansen, C.W.M. A Printable optical time-temperature integrator based on shape memory in a chiral nematic polymer network. Adv. Funct. Mater. 2013, 23, 2723–2727. [Google Scholar] [CrossRef]
- Lin, S.; Tang, Y.; Kang, W.; Bisoyi, H.K.; Guo, J.; Li, Q. Photo-triggered full-color circularly polarized luminescence based on photonic capsules for multilevel information encryption. Nat. Commun. 2023, 14, 3005. [Google Scholar] [CrossRef]
- Mitov, M.; Dessaud, N. Going beyond the reflectance limit of cholesteric liquid crystals. Nat. Mater. 2006, 5, 361–364. [Google Scholar] [CrossRef]
- Guillard, H.; Sixou, P. Active broadband polymer stabilized liquid crystals. Liq. Cryst. 2001, 28, 933–944. [Google Scholar] [CrossRef]
- Ai, Z.; Liu, H.; Cheng, S.; Zhang, H.; Yi, Z.; Zeng, Q.; Wu, P.; Zhang, J.; Tang, C.; Hao, Z. Four peak and high angle tilted insensitive surface plasmon resonance graphene absorber based on circular etching square window. J. Phys. D Appl. Phys. 2025, 58, 185305. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.; Yi, Z.; Wang, J.; Cheng, S.; Li, B.; Wu, P. High absorption broadband ultra-long infrared absorption device based on Nanoring-Nanowire metasurface structure. Photonics 2025, 12, 451. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Luo, D. Optical thermal sensor based on cholesteric film refilled with mixture of toluene and ethanol. Opt. Eng. 2017, 25, 26349–26355. [Google Scholar] [CrossRef]
- Wu, P.-C.; Wu, G.-W.; Timofeev, I.V.; Zyryanov, V.Y.; Lee, W. Electro-thermally tunable reflective colors in a self-organized cholesteric helical superstructure. Photonics Res. 2018, 6, 1094–1100. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, Y.; Wu, S.-T. Broadband circular polarizer using stacked chiral polymer films. Opt. Eng. 2007, 15, 6414–6419. [Google Scholar] [CrossRef]
- Kralik, J.C.; Fan, B.; Vithana, H.; Li, L.; Faris, S.M. Backlight output enhancement using cholesteric liquid crystal films. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A-Mol. Cryst. Liq. Cryst. 1997, 301, 249–254. [Google Scholar] [CrossRef]
- Li, Y.-C.; Lin, B.-H.; Neyts, K.; Beeckman, J.; Wang, C.-T. Multiband reflectors based on stacked single-pitch cholesteric liquid crystal films. Opt. Laser Technol. 2025, 190, 113290. [Google Scholar] [CrossRef]
- Relaix, S.; Bourgerette, C.; Mitov, M. Broadband reflective liquid crystalline gels due to the ultraviolet light screening made by the liquid crystal. Appl. Phys. Lett. 2006, 89, 251907. [Google Scholar] [CrossRef]
- Sixou, P.; Gautier, C. Passive broadband reflector using photocrosslinkable liquid crystal molecules. Polym. Adv. Technol. 2002, 13, 329–338. [Google Scholar] [CrossRef]
- Xiao, J.; Cao, H.; He, W.; Ma, Z.; Geng, J.; Wang, L.; Wang, G.; Yang, H. Wide-band reflective polarizers from cholesteric liquid crystals with stable optical properties. J. Appl. Polym. Sci. 2007, 105, 2973–2977. [Google Scholar] [CrossRef]
- Guo, J.; Sun, J.; Zhang, L.; Li, K.; Cao, H.; Yang, H.; Zhu, S. Broadband reflection in polymer stabilized cholesteric liquid crystal cells with chiral monomers derived from cholesterol. Polym. Adv. Technol. 2008, 19, 1504–1512. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q. Light-driven chiral molecular switches or motors in liquid crystals. Adv. Mater. 2012, 24, 1926–1945. [Google Scholar] [CrossRef]
- Xiang, J.; Li, Y.; Li, Q.; Paterson, D.A.; Storey, J.M.D.; Imrie, C.T.; Lavrentovich, O.D. Electrically tunable selective reflection of light from ultraviolet to visible and infrared by heliconical cholesterics. Adv. Mater. 2015, 27, 3014–3018. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Li, Q. Light-driven liquid crystalline materials: From photo -induced phase transitions and property modulations to applications. Chem. Rev. 2016, 116, 15089–15166. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Bunning, T.J.; Li, Q. Stimuli-driven control of the helical axis of self-organized soft helical superstructures. Adv. Mater. 2018, 30, 1706512. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhao, H.; Song, L.; Yang, Z.; Cao, H.; Cheng, Z.; Liu, Q.; Yang, H. Electrically controllable selective reflection of chiral nematic liquid crystal/chiral ionic liquid composites. Adv. Mater. 2010, 22, 468–472. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Yang, H.; Wang, J.; Li, B.; Zhang, H.; Yi, Y. TiN-Only metasurface absorber for solar energy harvesting. Photonics 2025, 12, 443. [Google Scholar] [CrossRef]
- Rosenhauer, R.; Fischer, T.; Stumpe, J.; Giminez, R.; Pinol, M.; Serrano, J.-L.; Broer, D.J. Light-induced orientation of dyes in azobenzene containing LC polymers. In Liquid Crystals VI; SPIE: Pune, India, 2002; pp. 121–135. [Google Scholar] [CrossRef]
- Broer, D.J. On the history of reactive mesogens: Interview with Dirk J. Broer. Adv. Mater. 2020, 32, 1905144. [Google Scholar] [CrossRef]
- Chisvert, A.; Benede, J.L.; Salvador, A. Current trends on the determination of organic UV filters in environmental water samples based on microextraction techniques—A review. Anal. Chim. Acta 2018, 1034, 22–38. [Google Scholar] [CrossRef]
- van Heeswijk, E.P.A.; Kloos, J.J.H.; de Heer, J.; Hoeks, T.; Grossiord, N.; Schenning, A.P.H.J. Well-adhering, easily producible photonic reflective coatings for plastic substrates. ACS Appl. Mater. Interfaces 2018, 10, 30008–30013. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Gan, P.; Zhang, X.; Shi, W.; Li, H.; Cao, H.; Wang, H.; Wang, D.; Yang, Z.; He, W. Optical properties of broadband reflection of cholesteric liquid crystal by thermal diffusion of benzotriazoles. Appl. Opt. 2022, 61, 4486–4493. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Cao, Y.; Cao, H.; Wang, H.; Yang, Z.; Wang, D.; He, W. Near-infrared reflective polymer films based on UV-327-doped zinc oxide nanoparticles. Materials 2023, 16, 7660. [Google Scholar] [CrossRef]
- Uthirakumar, P.; Devendiran, M.; Yun, J.-H.; Kim, G.C.; Kalaiarasan, S.; Lee, I.-H. Role of carbon quantum dots and film thickness on enhanced UV shielding capability of flexible polymer film containing carbon quantum dots/N-doped ZnO nanoparticles. Opt. Mater. 2018, 84, 771–777. [Google Scholar] [CrossRef]
- Ke, Y.; Chen, J.; Lin, G.; Wang, S.; Zhou, Y.; Yin, J.; Lee, P.S.; Long, Y. Smart Windows: Electro-, thermo-, mechano-, photochromics, and beyond. Adv. Energy Mater. 2019, 9, 1902066. [Google Scholar] [CrossRef]
- Wang, S.; Gao, W.; Hu, X.; Shen, Y.; Wang, L. Supramolecular strategy for smart windows. Chem. Commun. 2019, 55, 4137–4149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Runnerstrom, E.L.; Milliron, D.J. Switchable materials for smart windows. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 283–304. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, H.; Loonen, R.; Hensen, J.L.M.; Schenning, A.; Debije, M.G. Application of broadband infrared reflector based on cholesteric liquid crystal polymer bilayer film to windows and its impact on reducing the energy consumption in buildings. J. Mater. Chem. A 2014, 2, 14622–14627. [Google Scholar] [CrossRef]
- DeForest, N.; Shehabi, A.; O’Donnell, J.; Garcia, G.; Greenblatt, J.; Lee, E.S.; Selkowitz, S.; Milliron, D.J. United States energy and CO2 savings potential from deployment of near-infrared electrochromic window glazings. Build. Environ. 2015, 89, 107–117. [Google Scholar] [CrossRef]
- Nundy, S.; Mesloub, A.; Alsolami, B.M.; Ghosh, A. Electrically actuated visible and near-infrared regulating switchable smart window for energy positive building: A review. J. Clean. Prod. 2021, 301, 126854. [Google Scholar] [CrossRef]










| Sample Number | BHR32100-100/RM257/R5011/ IRG651/UV-1577/ZnO NPs (wt%) | Polymerization Temperature (°C) | UV Intensity (mW/cm2) | Polymerization Time (min) |
|---|---|---|---|---|
| At1 | 86.40/12.00/1.00/0.30/0.30/0.00 a | 50 | 2.0 | 20 |
| At2 | 86.40/12.00/1.00/0.30/0.24/0.06 | 50 | 2.0 | 20 |
| At3 | 86.40/12.00/1.00/0.30/0.18/0.12 | 50 | 2.0 | 20 |
| At4 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 20 |
| At5 | 86.40/12.00/1.00/0.30/0.06/0.24 | 50 | 2.0 | 20 |
| At6 | 86.40/12.00/1.00/0.30/0.00/0.30 | 50 | 2.0 | 20 |
| Bt1 | 92.40/6.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 20 |
| Bt2 | 90.40/8.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 20 |
| Bt3 | 88.40/10.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 20 |
| Bt4 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 20 |
| Bt5 | 84.40/14.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 20 |
| Ct1 | 86.50/12.00/0.90/0.30/0.12/0.18 | 50 | 2.0 | 20 |
| Ct2 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 20 |
| Ct3 | 86.30/12.00/1.10/0.30/0.12/0.18 | 50 | 2.0 | 20 |
| Ct4 | 86.20/12.00/1.20/0.30/0.12/0.18 | 50 | 2.0 | 20 |
| Ct5 | 86.10/12.00/1.30/0.30/0.12/0.18 | 50 | 2.0 | 20 |
| Dt1 | 86.40/12.00/1.00/0.30/0.12/0.18 | 30 | 2.0 | 20 |
| Dt2 | 86.40/12.00/1.00/0.30/0.12/0.18 | 40 | 2.0 | 20 |
| Dt3 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 20 |
| Dt4 | 86.40/12.00/1.00/0.30/0.12/0.18 | 60 | 2.0 | 20 |
| Dt5 | 86.40/12.00/1.00/0.30/0.12/0.18 | 70 | 2.0 | 20 |
| Et1 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 0.5 | 20 |
| Et2 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 1.0 | 20 |
| Et3 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 1.5 | 20 |
| Et4 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 20 |
| Et5 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 2.5 | 20 |
| Ft1 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 3 |
| Ft2 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 6 |
| Ft3 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 10 |
| Ft4 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 15 |
| Ft5 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 20 |
| Ft6 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 25 |
| Ft7 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 30 |
| Gt1 | 86.40/12.00/1.00/0.30/0.12/0.18 | 50 | 2.0 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, M.; Liu, Y.; Zhao, X.; Liu, Z.; Zhang, J.; Zuo, D.; Cui, G.; Cao, H.; Li, M. Collaboration of Two UV-Absorbing Dyes in Cholesteric Liquid Crystals Films for Infrared Broadband Reflection and Ultraviolet Shielding. Photonics 2025, 12, 656. https://doi.org/10.3390/photonics12070656
Xie M, Liu Y, Zhao X, Liu Z, Zhang J, Zuo D, Cui G, Cao H, Li M. Collaboration of Two UV-Absorbing Dyes in Cholesteric Liquid Crystals Films for Infrared Broadband Reflection and Ultraviolet Shielding. Photonics. 2025; 12(7):656. https://doi.org/10.3390/photonics12070656
Chicago/Turabian StyleXie, Mengqi, Yutong Liu, Xiaohui Zhao, Zhidong Liu, Jinghao Zhang, Dengyue Zuo, Guang Cui, Hui Cao, and Maoyuan Li. 2025. "Collaboration of Two UV-Absorbing Dyes in Cholesteric Liquid Crystals Films for Infrared Broadband Reflection and Ultraviolet Shielding" Photonics 12, no. 7: 656. https://doi.org/10.3390/photonics12070656
APA StyleXie, M., Liu, Y., Zhao, X., Liu, Z., Zhang, J., Zuo, D., Cui, G., Cao, H., & Li, M. (2025). Collaboration of Two UV-Absorbing Dyes in Cholesteric Liquid Crystals Films for Infrared Broadband Reflection and Ultraviolet Shielding. Photonics, 12(7), 656. https://doi.org/10.3390/photonics12070656





