Abstract
Structural color is a kind of natural color that widely exists in nature. The ordered microstructure of nano materials can absorb or reflect light of specific wavelength, thus showing colorful colors. Structural color is an ideal choice for color contact lens pattern pigment due to its good tinting degree, stability, and nontoxicity. This paper explores a method for synthesis of zinc oxide (ZnO) nanoparticles with a high refractive index and enhancement of the brightness of the structured colors by introducing carbon black nanoparticles. This method is convenient and successful to prepare ZnO ink, which can produce bright structural colors, and to produce color patterns through rubber pad printing. It is worth mentioning that ZnO nanoparticles can be self-assembled and arranged in contact lens ink without subsequent complicated processing. At the same time, the color only comes from ZnO and carbon black. While there is no other organic matter, the presence of nanoparticles plays a certain role in sterilization. Blue contact lenses prepared by this method have bright structural color, high oxygen permeability, and high hydrophilicity. At the same time, a cell viability test showed that the contact lenses prepared by this method had low adsorption capacity for lipids and proteins, reflecting the photonic crystal’s high biocompatibility. In summary, a trend for future research is to use high-refractive-index zinc oxide nanoparticles to produce structural colors rather than employing conventional contact lens pigments.
1. Introduction
Structural color [1,2,3] and chemical pigment are two sources of color in nature. Compared with traditional chemical pigments, structural color is green, stable, and environmentally friendly, and will not undergo photochemical decomposition. At the same time, the hue and light intensity of structural colors change in response to angular patterns, different light intensities, and structural distances [4]. At present, the application of structural color in textiles [2], cosmetics [5], paints [6], and anti-counterfeiting patterns [7] has been studied, but there is still a challenge regarding multiple application scenarios for structural colors. The unique coloring mechanism of structural color provides conditions for high corrosion resistance, high bleaching, and high biocompatibility of structural color. Therefore, it has great potential to replace traditional chemical pigments in the fields of medical treatment, textiles [2,8,9,10,11], and high-efficiency filters.
Most of the applications of photonic crystal structural color are mainly focused on polystyrene (PS), polymethylmethacrylate [12,13,14] (PMMA), and silicon dioxide [15,16,17] (SiO2). However, low-refractive-index (<1.6) nanoparticles such as photonic crystal silicon dioxide [18,19,20] and polystyrene [11,21,22], which are commonly prepared at present, are similar to the refractive index of contact lens ink, resulting in the poor color effect of photonic crystal (PC) structures formed by diffraction in the main body of contact lens [11,23]. In order to solve the problem of the low refractive index of organic nanoparticles, this study proposed to prepare high-refractive-index (>2.0) zinc oxide (ZnO) nanoparticles [24,25,26]. By doping contact lens adhesive ink in the self-assembly system [27,28,29], patterned photonic crystals with both structural color effect and stability were prepared on contact lenses. These research results will provide a practical basis for the application of photonic crystal chromogenic structure in contact lenses.
This study demonstrates the controlled fabrication of ZnO nanoscale architectures through optimized sol-gel chemistry protocols, establishing a tunable platform for photonic material development [30,31,32,33,34]. The size of ZnO nanoparticles was adjusted by adjusting the concentration of diethylene glycol, zinc acetate, and supernatant, so as to control structural color. ZnO PC-based films with periodic structure were obtained by interfacial gravity self-assembly. The observed chromatic dilution in photonic crystalline systems primarily stems from pronounced optoelectronic disparity between ZnO nanostructures and ophthalmic polymeric matrices, inducing non-resonant scattering phenomena that yield leucocratic optical manifestations [35]. Researchers found that the incoherent scattering can be absorbed by doping black materials, so as to improve the color saturation of PC structures [36,37,38,39].
Colored contact lenses are popular because of their beauty, convenience, and vision correction function. It is worth noting that the chemical pigment molecules of traditional contact lenses are at risk of leakage [40], and long-term wearing may cause eye problems such as corneal ulcer and keratitis [41,42,43]. The choice of using photonic crystals to make colored contact lenses can not only reduce the risk of pigment leakage and eye disease caused by biocompatibility, but also produce a colorful structural color better than a single traditional color in the sunlight. A study reported a method of fabricating SiO2 nanoparticles and adding an appropriate amount of carbon black for scattering in other wavelength regions [28,29]. At the same time, the stability of the nanoparticles was enhanced by high temperature, which provided a good idea for the application of photonic crystal structural color in contact lenses. Current constraints including prohibitive fabrication expenses, multistep processing requirements, and the fact that non-coherent light dispersion effects frequently induce opalescent discoloration, severely impeding practical implementation. This technological impasse motivates our proposition of implementing autonomously organized ZnO nanocrystals—characterized by eco-benign synthesis and facile fabrication protocols—within ophthalmic substrates. Strategic nanocarbon integration enables plasmonic resonance amplification, intensifying structural chromatic fidelity in corneal devices. Such engineered photonic architectures demonstrate potential as sustainable alternatives to conventional pigment-based coloration systems in next-generation ocular prosthetics.
2. Experimental Section
2.1. Materials
Zinc acetate dihydrate ((CH3COO)2Zn·2H2O) and diethylene glycol (DEG) were commercially sourced from Macklin Biochemical (Shanghai, China). Anhydrous ethanol was supplied by Sinopharm Chemical Reagent (Shanghai, China), while hexadecane and hexamethylene diisocyanate (HDI) were procured from Macklin (Shanghai, China). The ophthalmic polymer precursor (38% 2-hydroxyethyl methacrylate), artificial tear fluid, and proprietary viscous ink formulations (labeled mix/mon/mgl, see Figure S1) constituted confidential industrial-grade materials provided by HYDRON Corporation for flexible lens fabrication. Ultrapure aqueous solvent was produced in-house through double distillation protocols. All reagents met analytical-grade specifications and were employed as received without additional refinement.
2.2. Synthesis of ZnO Nanoparticles
Monodispersed ZnO colloid nanoparticles were synthesized by a modified sol-gel method. The preparation process is shown in Scheme 1. First, 57.5 g DEG and 1.1 g (CH3COO)2Zn·2H2O were added into a 100 mL container. The flask containing the mixture was heated in a constant-temperature oil bath with a condensation reflux device at 160 °C and stirred magnetically for 1 h. The flask was then moved to a water bath to cool to 25 °C while stirring continuously. The emulsion obtained was centrifuged at 8000 r/min for 15 min. The collected supernatant is used as the seed crystal for subsequent ZnO growth. Next, 57.5 g DEG and 1.1 g (CH3COO)2Zn·2H2O were added into a 100 mL three-necked flask. The flask containing the mixture was heated in a constant temperature oil bath at 140 °C using a condensation reflux device and stirred magnetically (300 r/min). ZnO seed crystals were added into a mixed solution system of three-necked flasks at a rate of 15 mL/h using an injection pump. As the volume of zinc oxide seed crystals added changes, the color of the photonic crystal film formed by drop-coating on the countertop changes. Following precursor introduction, thermal processing at 160 °C (1 h duration) facilitated nanocrystal growth, with subsequent ambient cooling and high-speed centrifugal isolation (8000 rpm, 30 min) yielding monodisperse ZnO nanoparticles.
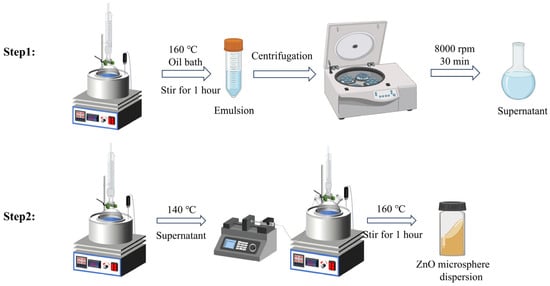
Scheme 1.
Schematic diagram of the synthesis of ZnO nanoparticles.
2.3. Preparation of ZnO Nanoparticle Paint
The pre-mixture was prepared by dissolving 0.3 g “mix”, 1.2 g “mon”, and 0.1 g “mgl” in a 5 mL single-neck culture flask and stirring at room temperature (150 r/min). Taking the preparation of 20 wt% ZnO nanoparticle ink as an example, 0.4 g of pre-mixture was added to 0.1 g of ZnO nanoparticles and stirred for 3 h (150 r/min). Then, 30 μL HDI was added and stirred for 30 min to obtain viscous ZnO nanoparticle ink. A reference formulation was engineered, maintaining equivalent monomeric proportions while deliberately excluding photonic nanostructures, to establish baseline optical performance parameters.
2.4. Preparation of Structural Color Contact Lenses
The fabrication protocol (Scheme 2) initiates with precision deposition of the nanocomposite suspension onto patterned stainless steel substrates, achieving instantaneous film formation via a blade-coating technique to ensure complete pattern replication. This chromatic layer is then transferred to ophthalmic device molds using elastomeric microstamps. Following thermal conditioning at 80 °C (10 h duration), 70 μL of hydrogel precursor is dispensed into the mold cavity under controlled laminar flow to eliminate gaseous inclusions. Immediate photopolymerization occurs through thermal curing at 121 °C (20 min) in precision ovens, culminating in demolding of the final corneal prosthesis. Replicating this methodology enables production of control specimens. All tooling components—engineered steel templates, silicone transfer interfaces, and polymer molds—were sourced from HYDRON Biomedical’s ISO-certified manufacturing platform (Figure S2).
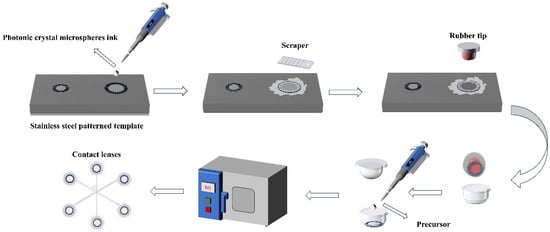
Scheme 2.
Performance testing of structural color contact lenses.
2.5. Performance Testing of Structural Color Contact Lenses
Contact angle, oxygen permeability, cytotoxicity, light transmission, and protein adsorption rate performance tests were performed sequentially. First, contact lenses need to be soaked in a standard saline solution and fully hydrated before testing. The standard aqueous solution was configured from NaCl, Na2HPO4, and NaH2PO4 in a certain ratio. Hydrogel-based ocular prostheses underwent surface hydration modulation via non-linting textile desiccation, followed by precise administration of 2 microliter isotonic electrolyte standard onto the biomimetic interface. Wettability characteristics were quantified through sessile drop goniometry following 180-s equilibration under ambient thermodynamic conditions. The flat oxygen permeability was determined by polarography [44]. Cytotoxicity was assessed by the MTT colorimetric method [45]. Specific procedures for protein adsorption [46], lipid adsorption, and other tests are described in detail in the Supporting Instructions.
2.6. Characterization
Scanning electron microscope (SEM) images were observed on a Tescan Mira4 (Brno, Czech Republic). Optical photographs were taken using an iPhone 13. Reflectance spectra were measured using an Ideaoptics spectrograph (Shanghai, China). Infrared spectral characterization was performed using Fourier-transform methodology across the mid-infrared range (450–4000 cm−1). Initial analyses employed a Shimmering Shale GC-FTIR hybrid system, with subsequent validation conducted on a Shimadzu IRTracer-100 Fourier-transform infrared spectrophotometer to ensure spectroscopic reproducibility. The performance tests of contact lenses were provided by Ocean Optics (Shanghai, China). Material wettability characteristics were quantified via contact angle analysis using OCA 15 Pro, Data Physics (Shanghai, China), while oxygen transmissibility was assessed using a 201 T gas permeameter (CREATECH REHDER, West Lafayette, IN, USA). Optical clarity parameters were determined through UV-Vis spectroscopy, with biomolecular adsorption dynamics on ophthalmic substrates characterized by spectrophotometric quantification (Evolution 220, Thermo Fisher Scientific, Waltham, MA, USA).
3. Results and Discussion
3.1. Microstructure of ZnO Nanoparticles
This study employed a simple sol-gel method to prepare monodispersed ZnO nanoparticles. Compared to traditional synthesis methods, the particle size distribution range is relatively narrow, which not only enhances uniformity, but also improves the structural color generated by the self-assembly of photonic crystals. By simply controlling the volume of the supernatant injected, monodispersed nanoparticles with relatively uniform particle sizes can be obtained. In the reaction system, ZnO monodispersed particles with photonic crystal colors of blue, green, and red were synthesized when 6 mL, 10 mL, and 12 mL of the supernatant were injected, respectively. SEM images revealed the morphology of blue, green, and red ZnO monodispersed particles assembled into micrometer-sized photonic crystal nanoparticles (Figure 1). Lei et al. [28] found that, by stirring with a turbine oscillator, SiO2 was dispersed in the continuous phase of hexadecane. The heating process induced water concentration and evaporation, and sintering enhanced the stability between nanoparticles, ultimately resulting in micrometer-sized photonic crystal nanoparticles assembled via van der Waals forces. This material addressed the issue of traditional photonic crystals being unable to self-assemble in relatively viscous liquids. However, the improved method allows for direct self-assembly of monodispersed ZnO nanoparticles in relatively viscous inks, thereby generating structural colors. Using monodispersed ZnO particles and carbon black as raw materials, photonic crystal nanoparticles were synthesized. Compared to PS and SiO2 nanoparticles, ZnO photonic crystal nanoparticles offer superior safety and unique antibacterial properties. The decorative motifs were fabricated on stainless steel substrates via laser engraving technology. Engraved channels measuring a precisely controlled 16 µm depth maintained optimal material flow characteristics during pattern replication while preserving the structural fidelity of the original artwork (Figure S2). The developed photonic crystal inks possessed appropriate rheological properties for pad printing applications. Incorporation of limited CB quantities effectively minimized light reflectance and internal particle scattering phenomena. The experimental data revealed that an optimized concentration of 0.12 wt% CB substantially enhanced opacity performance in the photonic crystalline nanostructures (Figure S4).
Figure 1.
Digital photographs of ZnO planar crystals under natural light.
Based on the reflection spectra and calculations derived from the Bragg diffraction expression [36,47,48], the size of ZnO particles that can produce different structural colors was determined, consistent with the constituent unit sizes of the photonic crystal nanoparticles (Figure 2a–c). The redshift in the reflectance peak positions observed in Figure 2 is attributed to the size-dependent quantum confinement effects of the ZnO nanoparticles, where smaller particle sizes correlate with a widening bandgap. This phenomenon aligns with calculations from the Bragg Equation (1),
where is the wavelength of the incident light, and is the effective refractive index. For a photonic nanoparticle composed of two different media, ZnO and air, the effective refractive index is given by , where [49] is the volume fraction in the face-centered cubic structure () and represents the interplanar spacing. For the (111) plane, is the diameter of the particles.
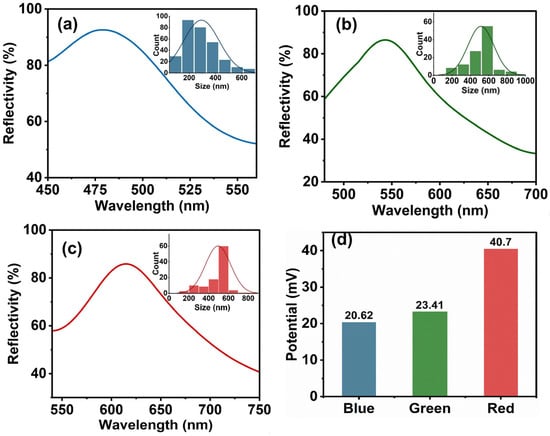
Figure 2.
Reflection spectra of photonic crystal nanoparticles composed of formation of the blue structural color of ZnO nanoparticles (a). Reflection spectra of photonic crystal nanoparticles consisting of formation of the green structural color of ZnO nanoparticles (b). Reflection spectra of photonic crystal nanoparticles composed of formation of the red structural color of ZnO nanoparticles (c). The Zeta potential of zinc oxide of different sizes corresponding to photonic crystals of different colors (d).
Zeta potential serves as a critical indicator for evaluating colloidal stability in dispersed systems, and its value reflects the distribution of surface charges on the dispersed particles. Analyzed from the principle of colloid chemistry, colloidal dispersion systems exhibit enhanced cohesive persistence when their zeta potential magnitude increases, which originates from the formation of the double electric layer structure between the charged particles—upon reaching threshold zeta potential magnitudes, colloidal suspensions achieve stabilized dispersion states (usually ±20 mV). Sufficient electrostatic repulsion can effectively overcome the van der Waals’ attraction, thus maintaining the kinetic stability of the dispersed system. In this study, the potential measurements of the dispersed system of zinc oxide nanoparticles showed (Figure 2d) that all samples exhibited significant positive electrical characteristics, with their values exceeding the stability threshold of 20 mV. This phenomenon not only reveals the charge characteristics of the nanoparticle surface in the dispersion medium, but also verifies the stability of the experimentally synthesized ZnO nanoparticles from an experimental point of view.
Figure 3 presents the Fourier-transform infrared spectroscopic characterization results obtained from the analyzed specimen set. In the ZnO nanoparticle spectra, the strong characteristic absorption peak around 453 cm−1 is due to the Zn-O stretching vibration, which confirms the presence of ZnO. Meanwhile, the bands around 3432 cm−1 and 1626 cm−1 are associated with O-H stretching and bending vibrations caused by adsorbed water [50], which may be due to the small amount of water remaining on the surface of ZnO nanoparticles or the presence of hydroxyl groups. The peaks around 1591 cm−1 and 1413 cm−1 are associated with the residual acetate groups in ZnO. The peaks around 1591 cm−1 and 1413 cm−1 match the C=O and C-O produced by the residual acetate groups in ZnO dihydrate. The above results further confirm the presence of ZnO nanoparticles, while exhibiting any reagents or functional groups associated with the synthesis process.
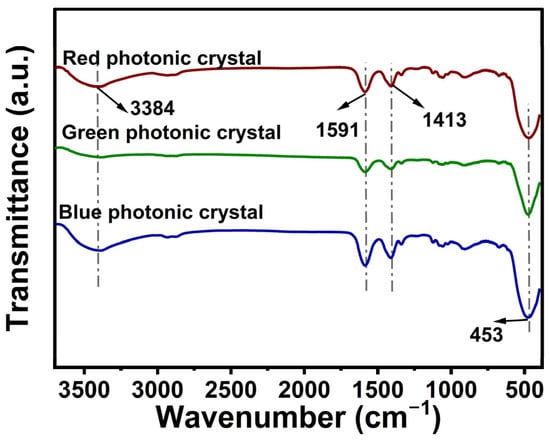
Figure 3.
FTIR of different ZnO nanoparticle sizes.
The crystal structure characteristics of the ZnO nanoparticle material can be obtained after resolution of the X-ray diffraction pattern. As shown in Figure 4a, the diffraction spectrum of the sample presents a series of characteristic peaks at 2θ angles of 31.78°, 34.58°, 36.38°, 47.72°, 56.70°, 63.06°, 66.38°, 68.22°, and 69.10°, and the diffraction peak positions of these peaks are similar to those of the hexagonal fibrillar zincite structure ZnO in the standard card (Figure 4b) (100), (002), (101), (102), (110), (103), (200), (112), and (201) crystallographic family diffractions, in perfect agreement. The experimental data indicate that the prepared ZnO nanoparticles have a typical fibrillar zincite crystalline structure, which has been widely confirmed as the most stable form of ZnO in existence. Notably, the narrow half-height width of the diffraction peaks and the good symmetry of the peak shapes reflect that the material is characterized by a highly ordered crystal arrangement. Meanwhile, no characteristic peaks of other heterogeneous phases were found in the detection range, confirming that the product has high phase purity.
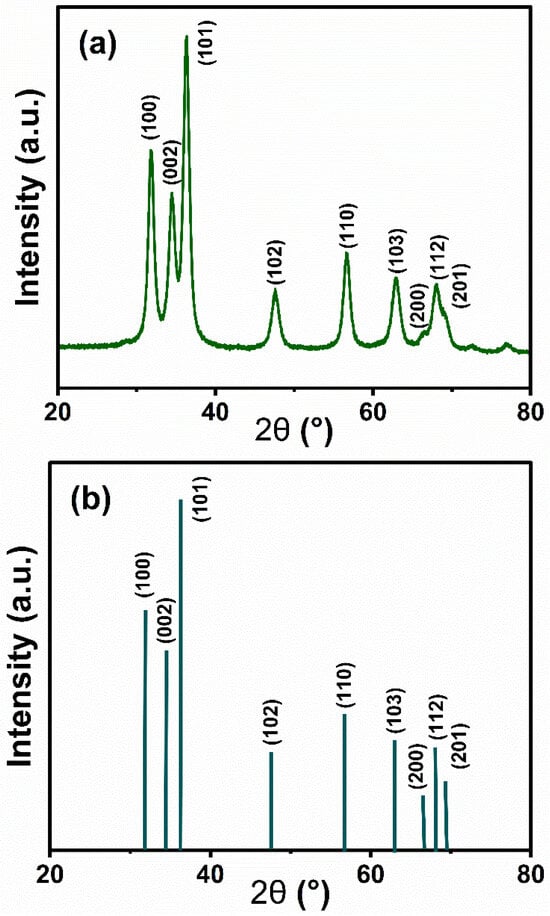
Figure 4.
X-ray diffraction pattern of (a) ZnO nanoparticles with a diameter of 270 nm and (b) ZnO.
3.2. Preparation of Photonic Crystal Contact Lenses
Structural coloration elements are dispersed within polymeric ink matrices through controlled agitation processes, necessitating inherent durability and spontaneous ordering characteristics in the nanostructured components. The morphological analyses in Figure 5a–c reveal pre-sintered colloidal architectures exhibiting periodic boundary configurations with defined geometrical features. However, mechanical stirring during mixing with the ink can easily damage the nanoparticle structure (Figure S3). Thermomechanical consolidation processes induce densification of microstructural components, with thermal processing at 400 °C demonstrating progressive geometric regularization of surface asperities, as evidenced in Figure 5d–f, thereby activating structural reinforcement mechanisms. The sintered nanoparticles are then incorporated into the ink. Without sintering, stirring causes some nanoparticles to lose their spherical structure, resulting in ZnO nanoparticles failing to achieve regular self-assembly. This leads to the ink being unable to display vivid structural colors. Compared with non-sintered precursor formulations, zinc oxide colloidal architectures in processed inks demonstrate periodic lattice organization, enabling wavelength-selective light modulation that manifests as stable photonic coloration on optical substrates. When sintered at 400 °C, the nanoparticle structure becomes visible within the field of view, providing a color source for the lenses (Figure 5).
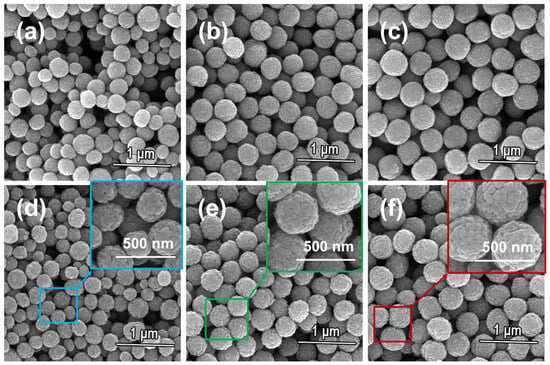
Figure 5.
SEM images of zinc oxide photonic crystal nanoparticles: blue photonic crystal nanoparticles (a); green photonic crystal nanoparticles (b); red photonic crystal nanoparticles (c); blue photonic crystal nanoparticles after sintering (d); green photonic crystal nanoparticles after sintering (e); red photonic crystal nanoparticles after sintering (f).
Advanced colloidal assembly techniques enabled development of wavelength-selective ophthalmic devices exhibiting stable azure photonic responses. Figure 6a,b document angle-resolved optical characteristics under solar irradiation, with Figure 6c,d confirming hue consistency across artificial illumination spectra, demonstrating invariant chromatic performance in multi-photon environments. The comparative analyses in Figure 6e reveal hydration-dependent luminance modulation, where biomimetic tear film simulations induce refractive contrast between aqueous and gaseous phases. The engineered interfaces maintain spectral fidelity under diverse electromagnetic excitation sources. Mechanical robustness was evidenced through standardized abrasion tests using hydrated substrates, with no detectable chromophore displacement observed post-surface contact.
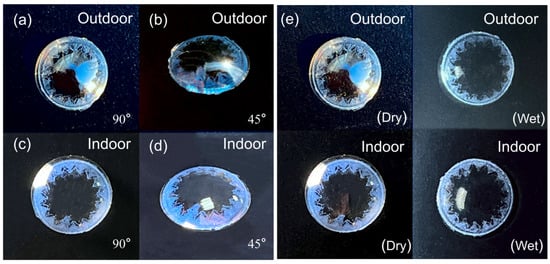
Figure 6.
Effects of lenses under sunlight conditions at 90° (a); effects of lenses under sunlight conditions at 45° (b); effects of lenses under artificial light at 90° (c); effects of lenses under artificial light at 45° (d); effects of dry and wet lenses under different light sources at 90° (e).
3.3. Photonic Crystal Contact Lens Performance
To comprehensively evaluate the performance of ZnO photonic crystal nanoparticle contact lenses, a series of critical tests was conducted, including assessments of light transmittance, oxygen permeability, cytotoxicity, contact angle, and protein adsorption. These tests aimed to ensure the safety, biocompatibility, and wearing comfort of the contact lenses.
3.3.1. Light Transmittance Test
Light transmittance is a crucial parameter for assessing the optical performance of contact lenses, directly affecting the visual clarity and comfort of wearers. Regulatory compliance verification protocols specify a minimum 89% optical transparency threshold for ophthalmic prosthetics [28]. Dual-axis photometric evaluation revealed that ZnO photonic nanostructured ocular devices achieved 94.61% mean luminous transmission values, compared with 98.78% for conventional reference samples (Figure 7a,b). Both values significantly exceeded the standard requirement, indicating that ZnO photonic crystal nanoparticle contact lenses exhibit excellent optical transparency, providing wearers with clear vision and a comfortable wearing experience. High transmittance not only enhances visual correction, but also reduces eye fatigue.
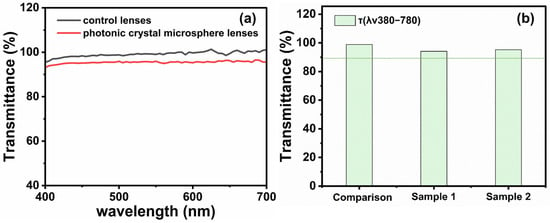
Figure 7.
Photonic crystal nanoparticle contact and control lens transmittance (a); histograms of transmittance for photonic crystal nanoparticle contact lenses, control lenses, and transmittance standards (b).
3.3.2. Cytotoxicity Test
Biocompatibility is a key indicator of the compatibility between contact lens materials and ocular tissues. Cytotoxicity evaluation via mitochondrial metabolic activity assessment revealed that ZnO-based photonic nanostructured ocular interfaces demonstrated statistically significant enhancement in spectrophotometric absorbance metrics (143% relative to reference toxicant benchmarks). Comparative analysis with baseline culture conditions showed that extract-exposed cellular populations maintained ≥93% cytocompatibility indices (Figure 8), confirming the absence of a cytotoxic response and validated biosafety profiles meeting standards [28]. This result is consistent with Lei’s findings [28], suggesting that ZnO nanoparticles are safe for use in contact lenses.
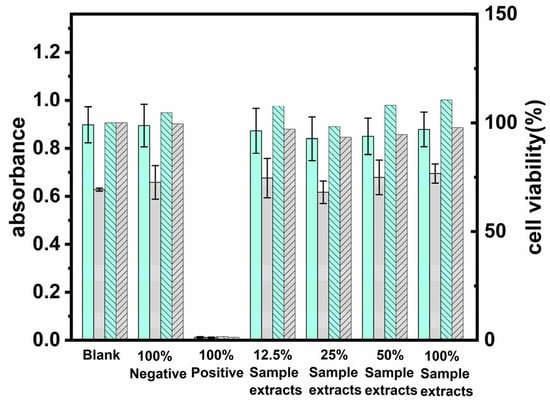
Figure 8.
Cytotoxicity, as assessed by MTT assay.
3.3.3. Protein Adsorption Test
Biomolecular adhesion represents a critical determinant in ophthalmic device biocompatibility and user tolerance. Accumulated protein layers may induce microbial colonization, compromise both gaseous exchange capacity and optical transmission characteristics, and provoke ocular surface pathophysiology. Triplicate biomimetic assays simulating lacrimal fluid dynamics quantified interfacial adsorption efficiencies, revealing 6.60% mean biomolecule retention for ZnO photonic nanostructured ocular interfaces versus 6.36% for conventional counterparts (Figure 9). Statistically equivalent adhesion profiles confirm negligible nanoparticle-induced interfacial alteration, correlating with Lei’s [28] interfacial stabilization mechanism and corroborating the structural inertness of engineered photonic architectures in biological matrices.
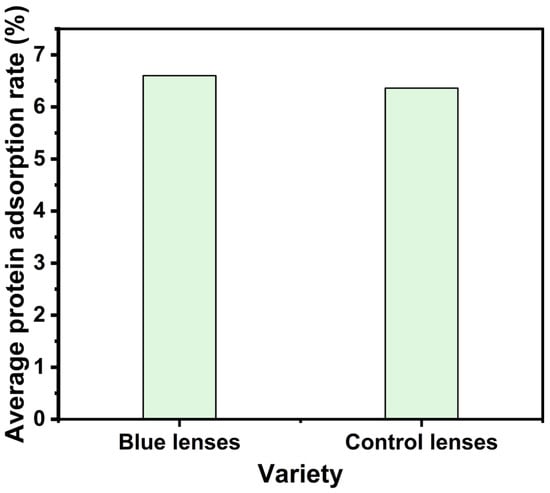
Figure 9.
Histograms of protein adsorption for photonic crystal nanoparticle contact and control lenses.
3.3.4. Oxygen Permeability Test
Ocular metabolic homeostasis fundamentally depends on atmospheric oxygen diffusion, establishing gas-phase transport capacity as an essential physiological determinant for biomaterial evaluation. The oxygen permeability coefficient (Dk/t) of ZnO photonic crystal nanoparticle contact lenses was 13.03 × 10−11 (cm2/s) [mLO2/(mL·mmHg)], while that of the control lenses was 17.09 × 10−11 (cm2/s) [mLO2/(mL·mmHg)] (Table S1). The results indicate that the addition of ZnO photonic crystal nanoparticles does not significantly affect the oxygen permeability of the lenses. This result aligns with Lei’s findings [28], suggesting that ZnO photonic crystal nanoparticles have high oxygen permeability in contact lenses.
3.3.5. Contact Angle Test
The surface hydrophilicity of contact lenses directly affects wearing comfort. The contact angles of the zinc oxide photonic crystal nanoparticles contact lenses were 37.8 and 37.1, while the contact angles of the control contact lenses were 53.7 and 52.8 (Figure 10), respectively. Zinc oxide photonic crystal nanoparticle contact lenses performed very well in hydrophilicity tests, which indicated that the addition of zinc oxide photonic crystal nanoparticles greatly improved the surface hydrophilicity of the contact lenses and made the wearing experience more comfortable. It was shown that zinc oxide photonic crystal nanoparticles are highly hydrophilic in contact lenses.
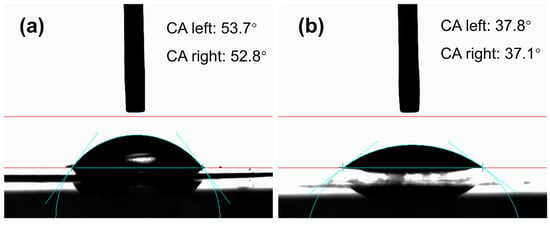
Figure 10.
Contact angle measurements for control lenses (a) and photonic crystal nanoparticle contact lenses (b).
4. Conclusions
In summary, we have developed a blue-colored contact lens utilizing zinc oxide nanoparticles with a high refractive index as the source of the structural color in the ink. By selecting a spherical structural material, we overcame the challenges associated with particle self-assembly and enhanced coloration. The proposed method successfully addresses the limitations of traditional photonic crystals in achieving self-assembly within high-viscosity inks. The mechanical strength of the nanoparticles was improved through a calcination process. Additionally, by optimizing the content of carbon black to absorb incoherent scattering, we achieved high-saturation blue lenses. The inherently antibacterial photonic crystals effectively replaced traditional organic pigments. Furthermore, by employing customized lens pattern transfer printing technology, the lenses demonstrated excellent safety and stability, meeting the standardized requirements for contact lenses. This approach opens new avenues for the application of photonic crystals in biomedical devices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/photonics12060598/s1. References [51,52,53] are cited in Supplementary Materials.
Author Contributions
S.H.: investigation, methodology, formal analysis, writing—original draft, and writing—review and editing. Z.P.: investigation, methodology, formal analysis, and writing—review. L.Z.: investigation and formal analysis. X.-L.H.: resources, supervision, project administration, and funding acquisition. Q.-X.Z.: resources, supervision, project administration, and funding acquisition. S.-N.D.: conceptualization, resources, supervision, writing—review and editing, project administration, and funding acquisition. S.H. and Z.P. contributed equally to this work, and should be regarded as joint first authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (22174015).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Authors Xue-Lian Han, Quan-Xi Zhang were employed by the company Haichang Contact Lens Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kinoshita, S.; Yoshioka, S. Structural Colors in Nature: The Role of Regularity and Irregularity in the Structure. ChemPhysChem 2005, 6, 1442–1459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Fang, Y. Structural colored fabric with superhydrophobicity by assembly SiO2@PPy composite microspheres and spraying PDMS on white fabric. Opt. Mater. 2025, 161, 116826. [Google Scholar] [CrossRef]
- Vigneron, J.P.; Simonis, P. Natural photonic crystals. Phys. B Condens. Matter 2012, 407, 4032–4036. [Google Scholar] [CrossRef]
- Han, P.; Li, Y.; Zhao, B.; Li, H.; Wang, Z.; Liu, X.; Meng, W. Visual and label-free detection of urea based on amorphous photonic films with non-iridescent structural colors. Anal. Chim. Acta 2025, 1345, 343731. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Zhu, L.; Wu, Y.; Si, P.; Zhang, D. Harmless photonic crystal tattoo with angle-independent structural color based on SiO nanoparticles and silk fibroin. J. Appl. Polym. Sci. 2024, 141, e55482. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Lee, K.M.; White, T.J.; Jeong, K.-U. Cholesteric liquid crystal paints: In situ photopolymerization of helicoidally stacked multilayer nanostructures for flexible broadband mirrors. NPG Asia Mater. 2018, 10, 1061–1068. [Google Scholar] [CrossRef]
- Bai, L.; Xie, Z.; Wang, W.; Yuan, C.; Zhao, Y.; Mu, Z.; Zhong, Q.; Gu, Z. Bio-Inspired Vapor-Responsive Colloidal Photonic Crystal Patterns by Inkjet Printing. ACS Nano 2014, 8, 11094–11100. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Q.; Wang, X.; Liu, G.; Chai, L.; Zhou, L.; Shao, J.; Yin, Y. Shear-Induced Assembly of Liquid Colloidal Crystals for Large-Scale Structural Coloration of Textiles. Adv. Funct. Mater. 2021, 31, 2010746. [Google Scholar] [CrossRef]
- He, Y.; Liu, L.; Fu, Q.; Ge, J. Precise Assembly of Highly Crystalline Colloidal Photonic Crystals inside the Polyester Yarns: A Spray Coating Synthesis for Breathable and Durable Fabrics with Saturated Structural Colors. Adv. Funct. Mater. 2022, 32, 2200330. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Hu, M.; Zhou, L.; Chai, L.; Fan, Q.; Shao, J. Patterned SiO2/Polyurethane Acrylate Inverse Opal Photonic Crystals with High Color Saturation and Tough Mechanical Strength. Langmuir 2019, 35, 14282–14290. [Google Scholar] [CrossRef]
- Han, Y.; Meng, Z.; Wu, Y.; Zhang, S.; Wu, S. Structural Colored Fabrics with Brilliant Colors, Low Angle Dependence, and High Color Fastness Based on the Mie Scattering of Cu2O Spheres. ACS Appl. Mater. Interfaces 2021, 13, 57796–57802. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gao, H.; Wang, Y.; Chen, T. Creation of polystyrene nanoparticle patterns for structural color application. Colloids Surf. A Physicochem. Eng. Asp. 2024, 686, 133318. [Google Scholar] [CrossRef]
- Yang, X.; Ge, D.; Wu, G.; Liao, Z.; Yang, S. Production of Structural Colors with High Contrast and Wide Viewing Angles from Assemblies of Polypyrrole Black Coated Polystyrene Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 16289–16295. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zhang, Y.; Fu, G.; Zhou, L.; Fan, Q. Preparation of monodispersed polystyrene microspheres and self-assembly of photonic crystals for structural colors on polyester fabrics. J. Text. Inst. 2014, 105, 938–943. [Google Scholar] [CrossRef]
- Sun, H.; Cui, J.; Wei, L.; Zhang, W.; Jiang, S.; Zhou, H.; Xie, Z. Preparation of structural colors assembled by SiO2@PDA microspheres to achieve inhibition of coffee-ring effect by multilayer coating on paper substrate. Dyes Pigments 2025, 238, 112716. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Yu, W.; Jia, P.; Zhang, X.; Wu, N.; Yu, H.; Song, Y.; Zhou, J. Bio-inspired stretchable and self-healable nanocomposite gelatin hydrogel with low silica nanoparticle content and brilliant angle/strain-independent structural colors. Chem. Eng. J. 2024, 496, 154190. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Vir, A.B.; Lin, M.; Garnier, G. From transparent to structural white: Modulating nanoscale self-assembly in silica and nanocellulose composites. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 131999. [Google Scholar] [CrossRef]
- Okawa, M.; Ikami, T.; Yamagishi, Y.; Watanabe, K.; Nagai, H. High-luminescence polymer/ceramic pressure-sensitive paint employing low-refractive-index silica nanoparticles. Meas. Sci. Technol. 2025, 36, 035104. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Yang, Q.; Liu, S.; Xiao, T.; Jiang, L.; Tan, X.; Lei, Y. Synthesis of hydrophobic silica without traditional aging process to construct the anti-reflective film with ultralow refractive index for improving power conversion efficiency. Colloids Surf. A Physicochem. Eng. Asp. 2024, 703, 135218. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Zhang, H.; Shao, Y.; Zhu, J. Mussel-inspired antimicrobial and antireflective Ag-NPs/PDA/SiO2 coatings via interfacial functionalization. Chem. Eng. J. 2024, 490, 151434. [Google Scholar] [CrossRef]
- Liu, X.; Hu, S.; Tang, Y. Coated High-Refractive-Index Barium Titanate Glass Microspheres for Optically Trapped Microsphere Super-Resolution Microscopy: A Simulation Study. Photonics 2020, 7, 84. [Google Scholar] [CrossRef]
- Vasudevan, K.; Divyasree, M.C.; Chandrasekharan, K. Enhanced nonlinear optical properties of ZnS nanoparticles in 1D polymer photonic crystal cavity. Opt. Laser Technol. 2019, 114, 35–39. [Google Scholar] [CrossRef]
- Chacko, V.; Bansal, S.; Hafiz, A.K. Effect of dispersion on omnidirectional reflection band in zinc oxide-based one-dimensional photonic crystal heterostructures. J. Nanophotonics 2018, 12, 026012. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kim, I.-P. Quasi-bound states in the continuum with high Q-factors in metasurfaces of lower-index dielectrics supported by metallic substrates. RSC Adv. 2022, 12, 1961–1967. [Google Scholar] [CrossRef]
- Sun, X.W.; Kwok, H.S. Optical properties of epitaxially grown zinc oxide films on sapphire by pulsed laser deposition. J. Appl. Phys. 1999, 86, 408–411. [Google Scholar] [CrossRef]
- Kim, S.H.; Oh, G.-Y.; Kim, D.G.; Ki, H.C.; Kim, T.U.; Kim, H.J. Analysis of triangular resonator integrated with zinc oxide thin film. Opt. Quantum Electron. 2013, 45, 755–760. [Google Scholar] [CrossRef]
- Ren, Y.; Guo, Y.; Cheng, Y.; Chu, Y.; Fang, Y.; Liu, Y.; Hou, J.; Liu, Z. Towards visual color display with fast electric field response: Highly chromatic and stable colloidal photonic crystal inks. Opt. Mater. 2022, 129, 112508. [Google Scholar] [CrossRef]
- Lei, X.-Q.; Yang, F.; Han, X.-L.; Chen, P.; Ding, S.-N. Photonic crystal microspheres: Synthesis, characterization, and applications in colored contact lenses. Opt. Mater. 2024, 150, 115229. [Google Scholar] [CrossRef]
- Yang, F.; Wu, S.-T.; Lei, X.-Q.; Han, X.-L.; Chen, P.; Ding, S.-N. Novel SiO2 photonic crystal microspheres as inorganic pigments for structural color contact lenses. Opt. Mater. 2023, 138, 113705. [Google Scholar] [CrossRef]
- Huang, N.; Zhu, M.W.; Gao, L.J.; Gong, J.; Sun, C.; Jiang, X. A template-free sol–gel technique for controlled growth of ZnO nanorod arrays. Appl. Surf. Sci. 2011, 257, 6026–6033. [Google Scholar] [CrossRef]
- Huang, K.-M.; Ho, C.-L.; Chang, H.-J.; Wu, M.-C. Fabrication of inverted zinc oxide photonic crystal using sol–gel solution by spin coating method. Nanoscale Res. Lett. 2013, 8, 306. [Google Scholar] [CrossRef]
- Hammami, Z.; Selmi, A.; Maayoufi, A.E.; Altalhi, T.; Touil, S.; Mezni, A. Structural, optical, and dielectric properties of copper-doped ZnO nanorods prepared by modified sol-gel process. J. Sol-Gel Sci. Technol. 2025, 114, 669–678. [Google Scholar] [CrossRef]
- Rafee, V.; Razaghizadeh, A.; Nakhaei, R.; Hosini, R. Eco-friendly dye-sensitized solar cells: Green synthesis of ZnO nanoparticles using Sargassum algae and performance enhancement through optimized dye combinations. Mater. Sci. Eng. B 2025, 317, 118164. [Google Scholar] [CrossRef]
- Dhahri, R.; Benamara, M.; Nassar, K.I.; Elkenany, E.B.; Al-Syadi, A.M. Zinc oxide-based sensor prepared by modified sol–gel route for detection of low concentrations of ethanol, methanol, acetone, and formaldehyde. Semicond. Sci. Technol. 2024, 39, 115021. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.B.; Choi, Y.H.; Park, S.; Kim, S.-H. Photonic Microbeads Templated by Oil-in-Oil Emulsion Droplets for High Saturation of Structural Colors. Small 2022, 18, 2105225. [Google Scholar] [CrossRef]
- Han, P.; Li, Y.; Liu, J.; Meng, W.; Zhao, B. High-Performance and Broad-Viewing-Angle Structural Colored Films with Carbon Black and Carbon Quantum Dot Doping. Coatings 2024, 14, 1177. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, B.; Chen, A.; Liu, X.; Shi, L.; Zi, J. Using Cuttlefish Ink as an Additive to Produce Non-iridescent Structural Colors of High Color Visibility. Adv. Mater. 2015, 27, 4719–4724. [Google Scholar] [CrossRef]
- Shi, X.; He, J.; Xie, X.; Dou, R.; Lu, X. Photonic crystals with vivid structure color and robust mechanical strength. Dyes Pigments 2019, 165, 137–143. [Google Scholar] [CrossRef]
- Liu, B.; Fan, J.; Liu, W.; Zhang, G.; Wu, Z. Controllable in situ generated carbon black hollow silica (C@h-SiO2) photonic crystal inks with highly saturated structural colors. Dyes Pigments 2023, 208, 110810. [Google Scholar] [CrossRef]
- Stapleton, F.; Bakkar, M.; Carnt, N.; Chalmers, R.; Vijay, A.K.; Marasini, S.; Ng, A.; Tan, J.; Wagner, H.; Woods, C.; et al. CLEAR—Contact lens complications. Contact Lens Anterior Eye 2021, 44, 330–367. [Google Scholar] [CrossRef]
- Poggio, E.C.; Glynn, R.J.; Schein, O.D.; Seddon, J.M.; Shannon, M.J.; Scardino, V.A.; Kenyon, K.R. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N. Engl. J. Med. 1989, 321, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Pan, M.; Yang, X.; Shang, L.; Zhao, Y. Structural Color Contact Lenses from Cholesteric Cellulose Liquid Crystals. Small Methods 2024, 9, 2401582. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Li, L.; Liu, P.; Zheng, F.; Guo, L.; Zhao, Y.; Jin, L.; Li, T.; Gu, Z. Self-Assembled Coffee-Ring Colloidal Crystals for Structurally Colored Contact Lenses. Small 2015, 11, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, M.; Prausnitz, J.M.; Radke, C.J. A single-lens polarographic measurement of oxygen permeability (Dk) for hypertransmissible soft contact lenses. Biomaterials 2007, 28, 4331–4342. [Google Scholar] [CrossRef]
- Bala, R.; Pareek, B.; Umar, A.; Arora, S.; Singh, D.; Chaudhary, A.; Alkhanjaf, A.A.M.; Almadiy, A.A.; Algadi, H.; Kumar, R.; et al. In-vitro cytotoxicity of nickel oxide nanoparticles against L-6 cell-lines: MMP, MTT and ROS studies. Environ. Res. 2022, 215, 114257. [Google Scholar] [CrossRef]
- Ye, A.; Mei, H.; Zhang, Z.; Song, F.; Jiang, L.; Huang, T.; Li, P.; Du, S.; Feng, Y.; Jiang, T.; et al. Corneal first aid lens: Collagen-based hydrogels loading aFGF as contact lens for treating corneal injuries. J. Control. Release 2025, 379, 251–265. [Google Scholar] [CrossRef]
- Han, P.; Li, X.; Zhao, B.; Li, Y.; Li, H.; Wang, Z.; Meng, W. Brilliant non-iridescent structural colors of hierarchical photonic films by hydrophobic substrates assembly design. Dyes Pigments 2023, 219, 111587. [Google Scholar] [CrossRef]
- Matsubara, K.; Watanabe, M.; Takeoka, Y. A thermally adjustable multicolor photochromic hydrogel. Angew. Chem. Int. Ed. 2007, 46, 1688–1692. [Google Scholar] [CrossRef]
- Vigneron, J.P.; Lousse, V. Variation of a photonic crystal color with the Miller indices of the exposed surface. In Proceedings of the Integrated Optoelectronic Devices 2006, San Jose, CA, USA, 1 March 2006; SPIE: Bellingham, WA, USA, 2006. [Google Scholar]
- Kunimatsu, K.; Uchida, H.; Watanabe, M. Interactions between adsorbed hydrogen and water molecules on a polycrystalline Pt electrode studied by in-situ ATR-FTIR spectroscopy. J. Electroanal. Chem. 2024, 954, 118037. [Google Scholar] [CrossRef]
- Young, M.D.; Benjamin, W.J. Calibrated oxygen permeability of 35 conventional hydrogel materials and correlation with water content. Eye Contact Lens 2003, 29, 126–133. [Google Scholar] [CrossRef]
- Menzies, K.L.; Rogers, R.; Jones, L. In vitro contact angle analysis and physical properties of blister pack solutions of daily disposable contact lenses. Eye Contact Lens 2010, 36, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Luensmann, D.; Jones, L. Protein deposition on contact lenses: The past, the present, and the future. Contact Lens Anterior Eye 2012, 35, 53–64. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).