Phonon Structure Engineering for Intrinsically Spectrally Selective Emitters by Anion Groups
Abstract
1. Introduction
2. Results and Discussions
2.1. Design Concept for Intrinsic SSEs with Anions
2.2. Phonon Structures for Borates Only with [BO3]3− or [BO4]5− Units
2.3. Complementing Phonon Resonances in AW with [BO3]3− and [BO4]5− Units
2.4. Complementing Phonon Resonances in AW with [BO3]3− and [BO3F]4− Units
3. Methods
3.1. Phonon Structure and Electronic Structure Calculations
3.2. Synthesis of K3B6O10Cl and BaAlBO4 Powder and Their IR Emission
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santamouris, M. Regulating the damaged thermostat of the cities-Status, impacts and mitigation challenges. Energy Build. 2015, 91, 43–56. [Google Scholar] [CrossRef]
- Cook, B.I.; Smerdon, J.E.; Seager, R.; Coats, S. Global warming and 21st century drying. Clim. Dyn. 2014, 43, 2607–2627. [Google Scholar] [CrossRef]
- Catalanotti, S.; Cuomo, V.; Piro, G.; Ruggi, D.; Silvestrini, V.; Troise, G. The radiative cooling of selective surfaces. Sol. Energy 1975, 17, 83–89. [Google Scholar] [CrossRef]
- Fan, S.; Li, W. Photonics and thermodynamics concepts in radiative cooling. Nat. Photon. 2022, 16, 182–190. [Google Scholar] [CrossRef]
- Lin, K.-T.; Han, J.; Li, K.; Guo, C.; Lin, H.; Jia, B. Radiative Cooling: Fundamental Physics, Atmospheric Influences, Materials and Structural Engineering, Applications and Beyond. Nano Energy 2021, 80, 105517. [Google Scholar] [CrossRef]
- Smith, G.; Gentle, A. Energy Savings from the Sky. Nat. Energy 2017, 2, 17142. [Google Scholar] [CrossRef]
- Raman, A.P.; Anoma, M.A.; Zhu, L.; Rephaeli, E.; Fan, S. Passive radiative cooling below ambient air temperature under direct sunlight. Nature 2014, 515, 540–544. [Google Scholar] [CrossRef]
- Zhao, D.; Aili, A.; Zhai, Y.; Xu, S.; Tan, G.; Yin, X.; Yang, R. Radiative sky cooling: Fundamental principles, materials, and applications. Appl. Phys. Rev. 2019, 6, 021306. [Google Scholar] [CrossRef]
- Li, W.; Fan, S. Radiative cooling: Harvesting the coldness of the universe. Opt. Photon. News 2019, 30, 32–39. [Google Scholar] [CrossRef]
- Zhai, Y.; Ma, Y.; David, S.N.; Zhao, D.; Lou, R.; Tan, G.; Yang, R.; Yin, X. Scalable-Manufactured Randomized Glass-Polymer Hybrid Metamaterial for Daytime Radiative Cooling. Science 2017, 355, 1062–1066. [Google Scholar] [CrossRef]
- Mandal, J.; Fu, Y.; Overvig, A.C.; Jia, M.; Sun, K.; Shi, N.N.; Zhou, H.; Xiao, X.; Yu, N.; Yang, Y. Hierarchically Porous Polymer Coatings for Highly Efficient Passive Daytime Radiative Cooling. Science 2018, 362, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhu, L.; Raman, A.; Fan, S. Radiative Cooling to Deep Sub-Freezing Temperatures through a 24-h Day-Night Cycle. Nat. Commun. 2016, 7, 13729. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hu, M.K.; Ao, X.Z.; Chen, N.; Pei, G. Radiative cooling: A review of fundamentals, materials, applications, and prospects. Appl. Energy 2019, 236, 489–513. [Google Scholar] [CrossRef]
- Yu, X.X.; Chan, J.Q.; Chen, C. Review of radiative cooling materials: Performance evaluation and design approaches. Nano Energy 2021, 88, 106259. [Google Scholar] [CrossRef]
- Shi, N.N.; Tsai, C.C.; Camino, F.; Bernard, G.D.; Yu, N.; Wehner, R. Keeping Cool: Enhanced Optical Reflection and Radiative Heat Dissipation in Saharan Silver Ants. Science 2015, 349, 298–301. [Google Scholar] [CrossRef]
- Zhou, L.; Song, H.; Liang, J.; Singer, M.; Zhou, M.; Stegenburgs, E.; Zhang, N.; Xu, C.; Ng, T.; Yu, Z.; et al. A polydimethylsiloxane-coated metal structure for all-day radiative cooling. Nat. Sustain. 2019, 2, 718–724. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, J.; Huang, H.; Nan, F.; Zhou, G.; Ou, Q. Flexible Polymer Photonic Films with Embedded Microvoids for High-Performance Passive Daytime Radiative Cooling. ACS Photonics 2021, 8, 3301–3307. [Google Scholar] [CrossRef]
- Zhou, K.; Li, W.; Patel, B.B.; Tao, R.; Chang, Y.; Fan, S.; Diao, Y.; Cai, L. Three-Dimensional Printable Nanoporous Polymer Matrix Composites for Daytime Radiative Cooling. Nano Lett. 2021, 21, 1493–1499. [Google Scholar] [CrossRef]
- Wang, T.; Wu, Y.; Shi, L.; Hu, X.; Chen, M.; Wu, L. A structural polymer for highly efficient all-day passive radiative cooling. Nat. Commun. 2021, 12, 365. [Google Scholar] [CrossRef]
- Li, T.; Zhai, Y.; He, S.; Gan, W.; Wei, Z.; Heidarinejad, M.; Dalgo, D.; Mi, R.; Zhao, X.; Song, J. A Radiative Cooling Structural Material. Science 2019, 364, 760–763. [Google Scholar] [CrossRef]
- Li, D.; Liu, X.; Li, W.; Lin, Z.; Zhu, B.; Li, Z.; Li, J.; Li, B.; Fan, S.; Xie, J.; et al. Scalable and hierarchically designed polymer film as a selective thermal emitter for high-performance all-day radiative cooling. Nat. Nanotechnol. 2021, 16, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, W.; Zhang, Q.; Li, D.; Liu, X.; Wang, Y.; Xu, N.; Wu, Z.; Li, J.; Li, X.; et al. Subambient daytime radiative cooling textile based on nanoprocessed silk. Nat. Nanotechnol. 2021, 16, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Pian, S.; Su, M.; Wang, Z.; Wu, M.; Liu, X.; Chen, M.; Xiang, Y.; Wu, J.; Zhang, M.; et al. Hierarchical-morphology metafabric for scalable passive daytime radiative cooling. Science 2021, 373, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Li, Y.; Chi, C.; Kwon, Y.S.; Huang, J.; Wu, Z.; Zheng, J.; Liu, G.; Tso, C.Y.; Chao, C.Y.; et al. A Solution-Processed Inorganic Emitter with High Spectral Selectivity for Efficient Subambient Radiative Cooling in Hot Humid Climates. Adv. Mater. 2022, 34, 2109350. [Google Scholar] [CrossRef]
- Rephaeli, E.; Raman, A.; Fan, S. Ultrabroadband photonic structures to achieve high-performance daytime radiative cooling. Nano Lett. 2013, 13, 1457–1461. [Google Scholar] [CrossRef]
- Hossain, M.M.; Jia, B.H.; Gu, M. A Metamaterial Emitter for Highly Efficient Radiative Cooling. Adv. Optical Mater. 2015, 3, 1047–1051. [Google Scholar] [CrossRef]
- Chae, D.; Lim, H.; So, S.; Son, S.; Ju, S.; Kim, W.; Rho, J.; Lee, H. Spectrally Selective Nanoparticle Mixture Coating for Passive Daytime Radiative Cooling. ACS Appl. Mater. Interfaces 2021, 13, 21119–21126. [Google Scholar] [CrossRef]
- Chae, D.; Son, S.; Liu, Y.T.; Lim, H.Y.; Lee, H. High-Performance Daytime Radiative Cooler and Near-Ideal Selective Emitter Enabled by Transparent Sapphire Substrate. Adv. Sci. 2020, 7, 2001577. [Google Scholar] [CrossRef]
- Li, Y.; Lin, C.J.; Huang, J.Y.; Chi, C.; Huang, B.L. Spectrally Selective Absorbers/Emitters for Solar Steam Generation and Radiative Cooling-Enabled Atmospheric Water Harvesting. Glob. Chall. 2021, 5, 2000058. [Google Scholar] [CrossRef]
- Robitaille, P.-M. Kirchhoff’s Law of Thermal Emission: 150 Years. Progr. Phys. 2009, 4, 3–13. [Google Scholar]
- Aili, A.; Wei, Z.Y.; Chen, Y.Z.; Zhao, D.L.; Yang, R.G.; Yin, X.B. Selection of polymers with functional groups for daytime radiative cooling. Mater. Today Phys. 2019, 10, 100127. [Google Scholar] [CrossRef]
- Mutailipu, M.; Poeppelmeier, K.R.; Pan, S.L. Borates: A Rich Source for Optical Materials. Chem. Rev. 2021, 121, 1130–1202. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Yu, H.W.; Rondinelli, J.M.; Poeppelmeier, K.R.; Halasyamani, P.S. Deep Ultraviolet Nonlinear Optical Materials. Chem. Mater. 2016, 28, 5238–5258. [Google Scholar] [CrossRef]
- Mutailipu, M.; Pan, S.L. Emergent Deep-Ultraviolet Nonlinear Optical Candidates. Angew. Chem. Int. Ed. 2020, 59, 20302–20317. [Google Scholar] [CrossRef]
- Wu, L.; Sun, J.C.; Zhang, Y.; Jin, S.F.; Kong, Y.F.; Xu, J.J. Structure Determination and Relative Properties of Novel Chiral Orthoborate KMgBO3. Inorg. Chem. 2010, 49, 2715–2720. [Google Scholar] [CrossRef]
- Guo, S.; Liu, L.; Xia, M.; Kang, L.; Huang, Q.; Li, C.; Wang, X.; Lin, Z.; Chen, C. Be2BO3F: A Phase of Beryllium Fluoride Borate Derived from KBe2BO3F2 with Short UV Absorption Edge. Inorg. Chem. 2016, 55, 6586–6591. [Google Scholar] [CrossRef]
- Chen, X.L.; Zhang, F.; Shi, Y.; Sun, Y.; Yang, Z.; Pan, S. MBaYB6O12 (M = Rb, Cs): Two new rare-earth borates with large birefringence and short ultraviolet cutoff edges. Dalton Trans. 2018, 47, 750–757. [Google Scholar] [CrossRef]
- Wu, L.; Chen, X.L.; Xu, Y.P.; Sun, Y.P. Structure Determination and Relative Properties of Novel Noncentrosymmetric Borates MM’4(BO3)3 (M = Na, M’ = Ca and M = K, M’ = Ca, Sr). Inorg. Chem. 2006, 45, 3042–3047. [Google Scholar] [CrossRef]
- Parise, J.B.; Gier, T.E. Hydrothermal Syntheses and Stuctural Refinements of Single Crystal LiBGeO4 and LiBSiO4. Chem. Mater. 1992, 4, 1065–1067. [Google Scholar] [CrossRef]
- Wu, H.P.; Pan, S.; Poeppelmeier, K.R.; Li, H.; Jia, D.; Chen, Z.; Fan, X.; Yang, Y.; Rondinelli, J.M.; Luo, H. K3B6O10Cl: A New Structure Analogous to Perovskite with a Large Second Harmonic Generation Response and Deep UV Absorption Edge. J. Am. Chem. Soc. 2011, 133, 7786–7790. [Google Scholar] [CrossRef]
- Guo, F.J.; Han, J.; Cheng, S.; Yu, S.; Yang, Z.; Pan, S. Transformation of the B-O Units from Corner-Sharing to Edge-Sharing Linkages in BaMBO4 (M = Ga, Al). Inorg. Chem. 2019, 58, 8237–8244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-H.; Kong, F.; Yang, B.-P.; Mao, J.-G. A series of boroselenite-based open frameworks mediated by the cationic sizes of the alkali metals. CrystEngComm. 2012, 14, 8727–8733. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Hu, C.-L.; Xu, X.; Kong, F.; Mao, J.-G. New Second-Order NLO Materials Based on Polymeric Borate Clusters and GeO4 Tetrahedra: A Combined Experimental and Theoretical Study. Inorg. Chem. 2011, 50, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Luo, S.; Lin, Z.; Yao, J.; He, R.; Yue, Y.; Chen, C. ReBe2B5O11 (Re = Y, Gd): Rare-Earth Beryllium Borates as Deep-Ultraviolet Nonlinear-Optical Materials. Inorg. Chem. 2014, 53, 1952–1954. [Google Scholar] [CrossRef]
- Mutailipu, M.; Xie, Z.; Su, X.; Zhang, M.; Wang, Y.; Yang, Z.; Janjua, M.R.; Pan, S. Chemical Cosubstitution-Oriented Design of Rare-Earth Borates as Potential Ultraviolet Nonlinear Optical Materials. J. Am. Chem. Soc. 2017, 139, 18397–18405. [Google Scholar] [CrossRef]
- Jin, S.F.; Cai, G.; Wang, W.; He, M.; Wang, S.; Chen, X. Stable Oxoborate with Edge-Sharing BO4 Tetrahedra Synthesized under Ambient Pressure. Angew. Chem. Int. Ed. 2010, 49, 4967–4970. [Google Scholar] [CrossRef]
- Hu, C.-L.; Chen, J.; Fang, Z.; Tang, R.-L.; Mao, J.-G. LiB2O3F: A Beryllium-Free Deep-Ultraviolet Nonlinear Optical Material Designed Based on a Boron-Rich Strategy. Chem. Mater. 2021, 33, 4783–4791. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Wang, Y.; Zhang, B.B.; Yang, Z.H.; Pan, S.L. CaB5O7F3: A Beryllium-Free Alkaline-Earth Fluorooxoborate Exhibiting Excellent Nonlinear Optical Performances. Inorg. Chem. 2018, 57, 4820–4823. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.B.; Yang, Z.H.; Pan, S.L. Cation-Tuned Synthesis of Fluorooxoborates: Towards Optimal Deep-Ultraviolet Nonlinear Optical Materials. Angew. Chem. Int. Ed. 2018, 57, 2150–2154. [Google Scholar] [CrossRef]
- Li, X.Y.; Peoples, J.; Yao, P.Y.; Ruan, X.L. Ultrawhite BaSO4 Paints and Films for Remarkable Daytime Subambient Radiative Cooling. ACS Appl. Mater. Interfaces 2021, 13, 21733–21739. [Google Scholar] [CrossRef]
- Tong, Z.; Peoples, J.; Li, X.; Yang, X.; Bao, H.; Ruan, X. Electronic and phononic origins of BaSO4 as an ultra-efficient radiative cooling paint pigment. Mater. Today Phys. 2022, 24, 100658. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Togo, A.; Oba, F.; Tanaka, I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B 2008, 78, 134106. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1. [Google Scholar] [CrossRef]
- Skelton, J.M.; Burton, L.A.; Jackson, A.J.; Oba, F.; Parker, S.C.; Walsh, A. Lattice dynamics of the tin sulphides SnS2, SnS and Sn2S3: Vibrational spectra and thermal transport. Phys. Chem. Chem. Phys. 2017, 19, 12452. [Google Scholar] [CrossRef]
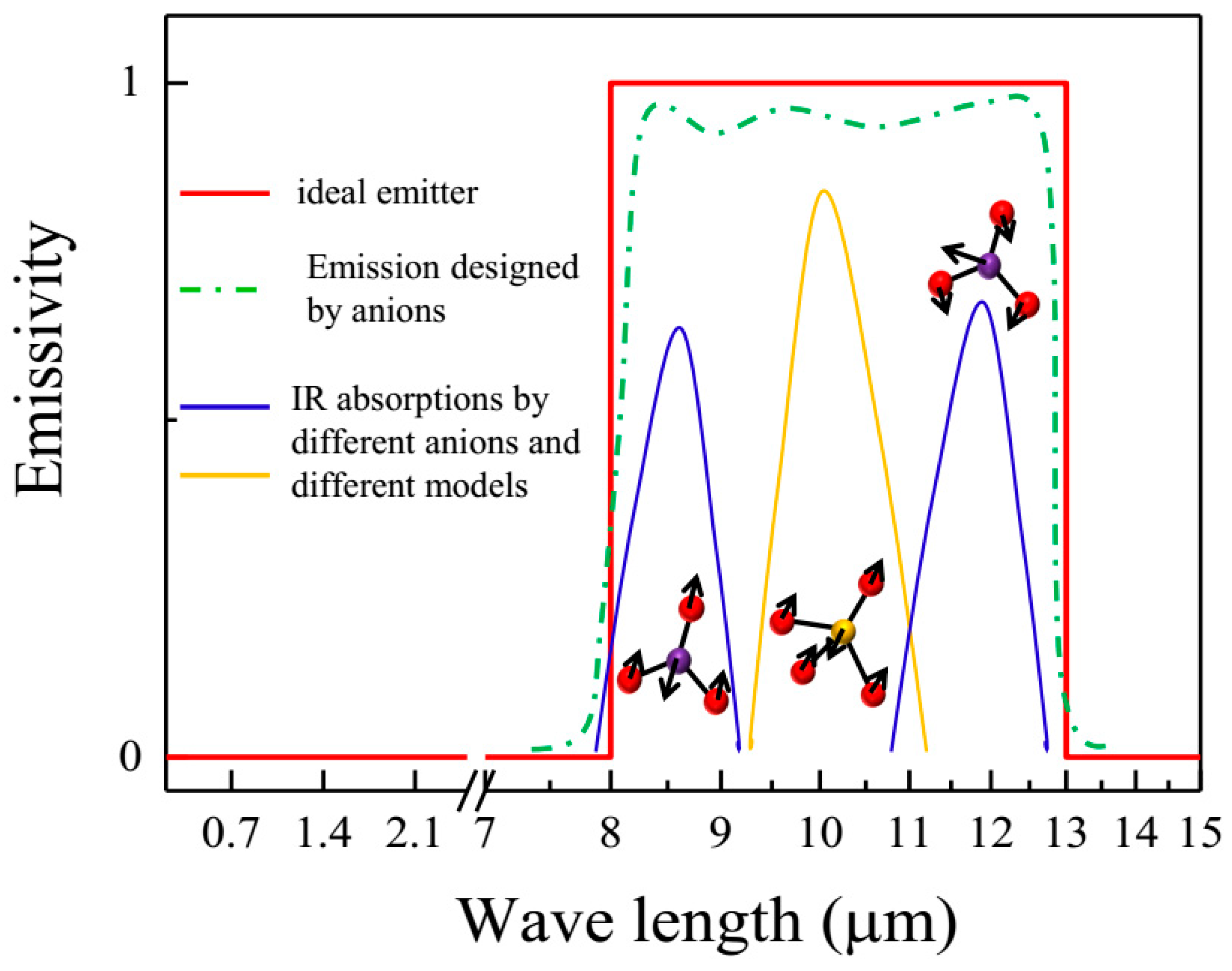

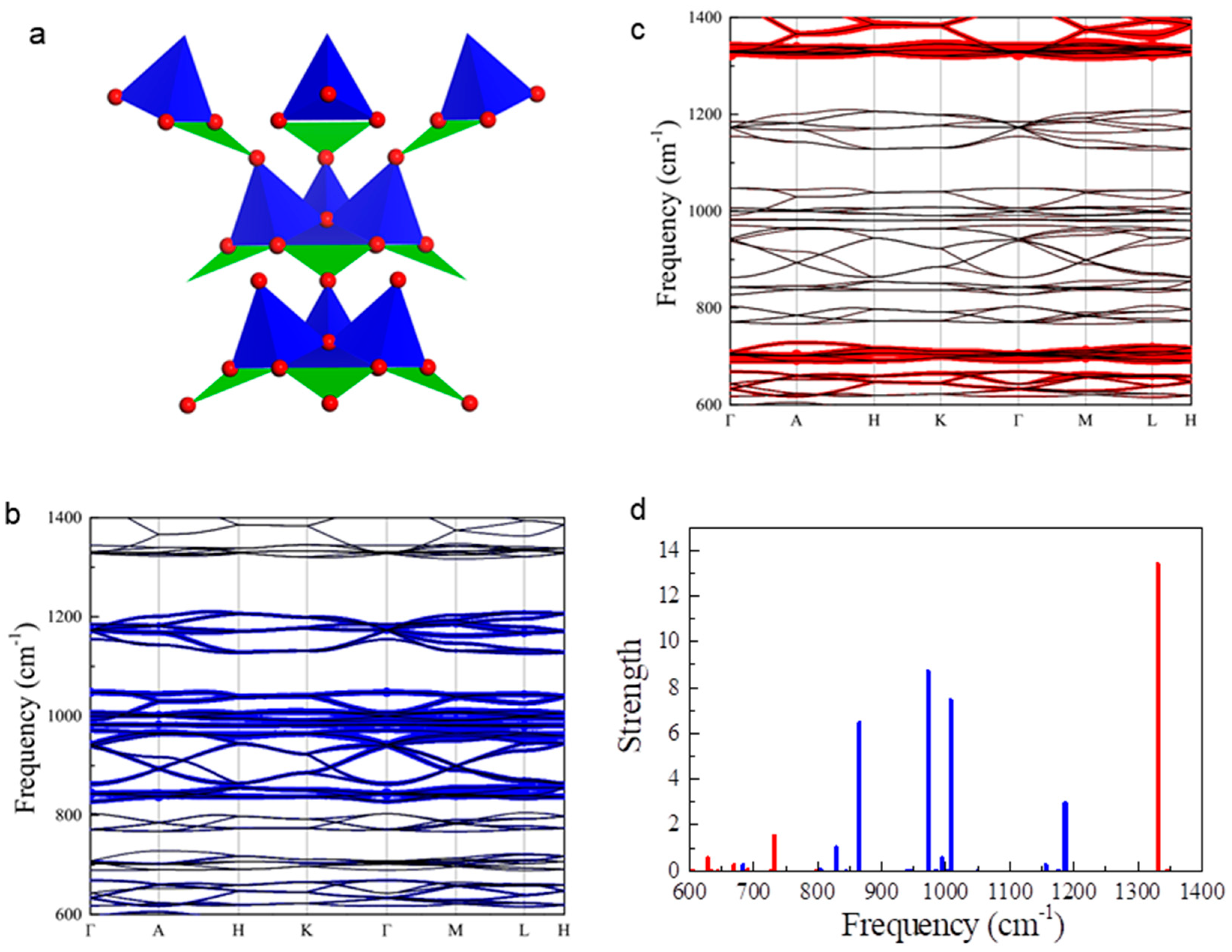
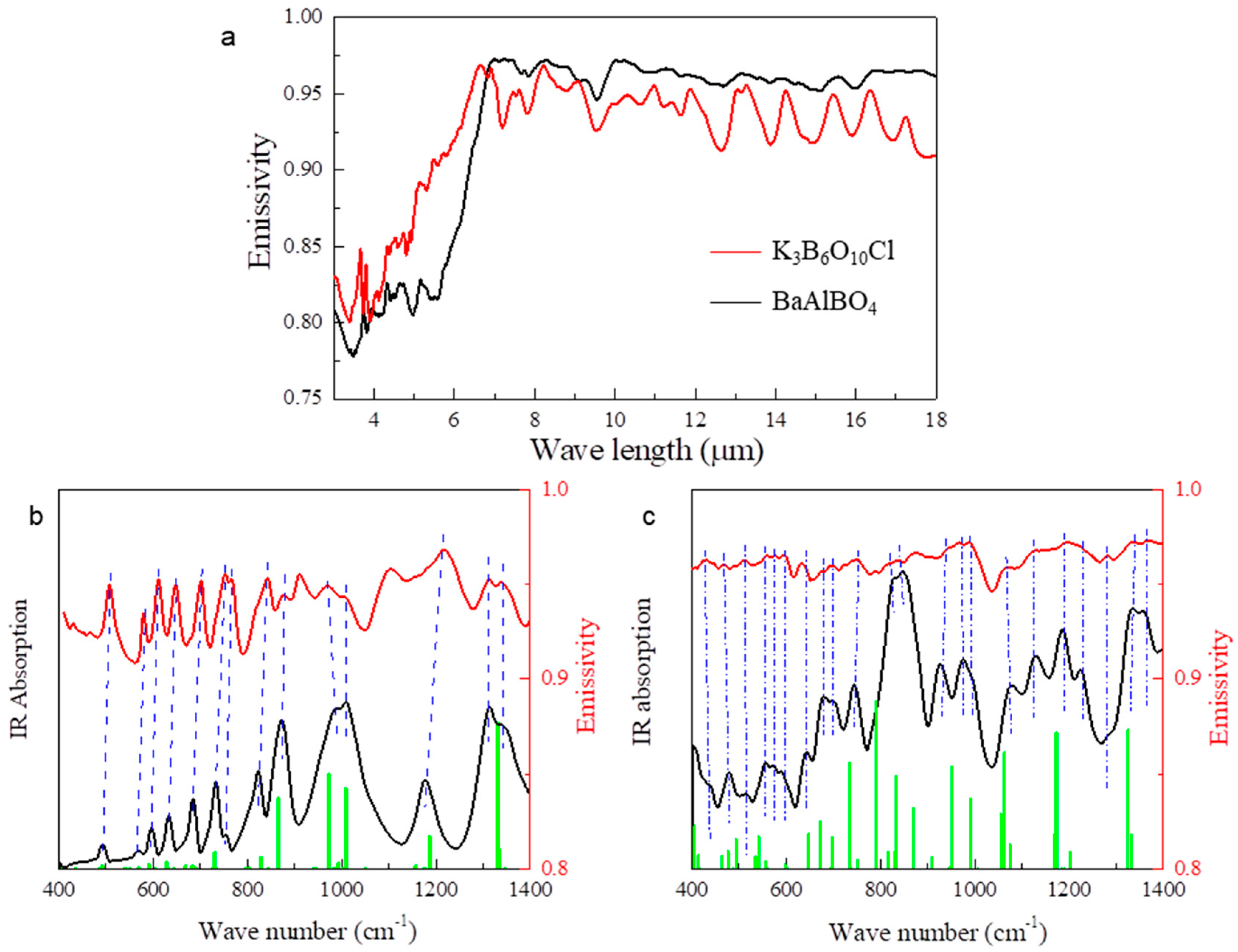

| Anion Groups | Typical IR Absorption Peaks (cm−1) |
|---|---|
| [SO4]2− | 1210~1025, 1000~950 |
| [PO4]3− | 1100~1050, 970~940 |
| [NO3]− | 1510~1210, 1060~1020, 840~800, 760~715 |
| [SiO4]4− | 1100~1000 |
| [BO3]3− | 1375~1150, 750~600 |
| [BO4]5− | ~1100, 1045~1000, 954~800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Huang, E.; Zhong, W.; Xu, B. Phonon Structure Engineering for Intrinsically Spectrally Selective Emitters by Anion Groups. Photonics 2025, 12, 597. https://doi.org/10.3390/photonics12060597
Zhang R, Huang E, Zhong W, Xu B. Phonon Structure Engineering for Intrinsically Spectrally Selective Emitters by Anion Groups. Photonics. 2025; 12(6):597. https://doi.org/10.3390/photonics12060597
Chicago/Turabian StyleZhang, Rui, Enhui Huang, Wenying Zhong, and Bo Xu. 2025. "Phonon Structure Engineering for Intrinsically Spectrally Selective Emitters by Anion Groups" Photonics 12, no. 6: 597. https://doi.org/10.3390/photonics12060597
APA StyleZhang, R., Huang, E., Zhong, W., & Xu, B. (2025). Phonon Structure Engineering for Intrinsically Spectrally Selective Emitters by Anion Groups. Photonics, 12(6), 597. https://doi.org/10.3390/photonics12060597





