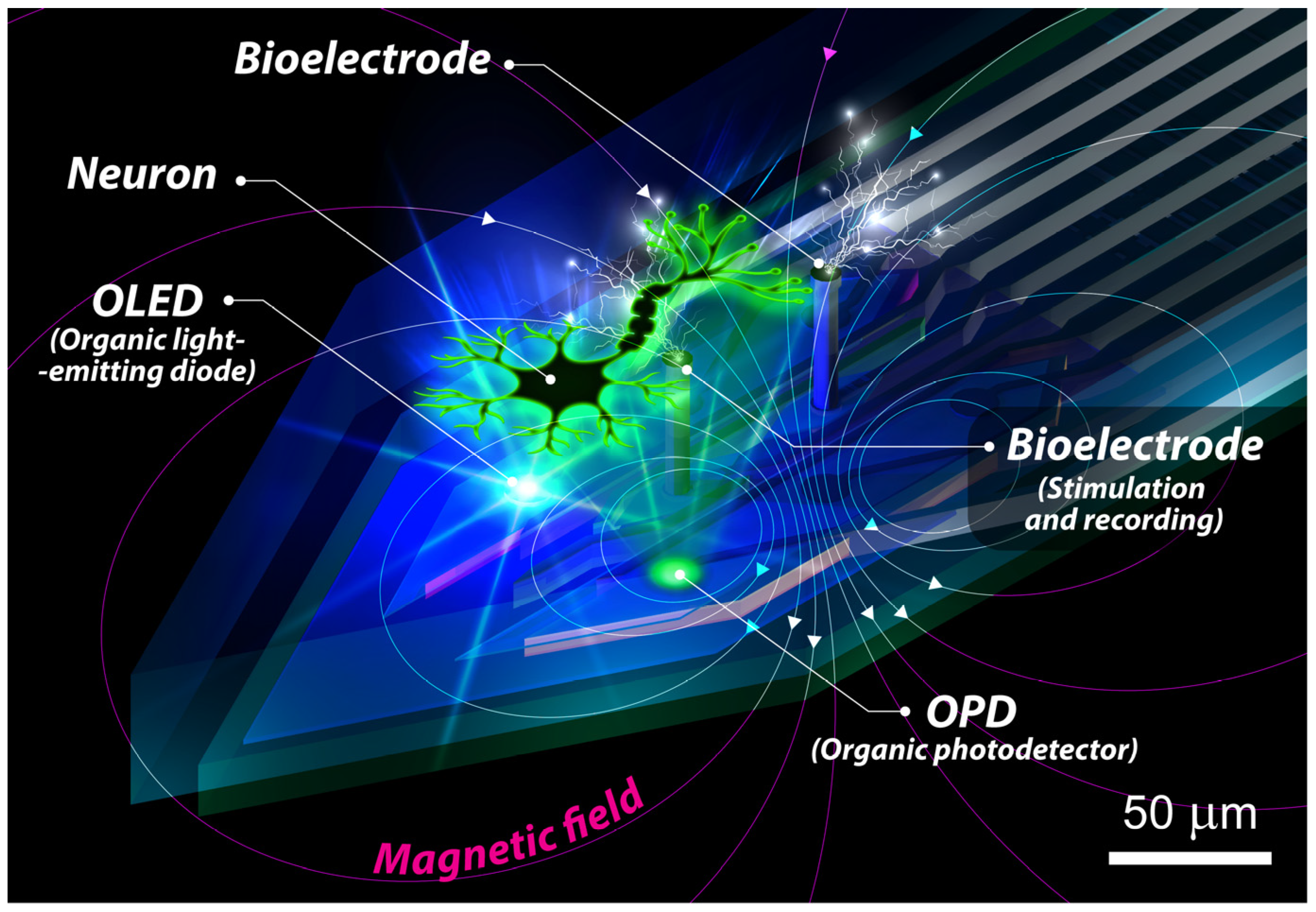
Application of Organic Light-Emitting Diodes and Photodiodes in Optical Control and Detection of Neuronal Activity
Abstract
1. Introduction
2. Tracking Neuronal Activity
2.1. Communication: Action Potentials and Synapses
2.2. Mapping the Brain’s Electrical Activity
2.3. Optical Interrogations of Neural Circuits
2.4. Complementary Role of Magnetic Resonance Imaging
2.5. Advantages of Organic Neural Probes
2.5.1. Optoelectronic Performance
2.5.2. Biocompatibility, Flexibility, and MRI-Compatibility
2.5.3. Multifunctionality
2.6. Applications of OLEDs and OPDs in Mapping Neuronal Activity
2.6.1. Organic Light-Emitting Diodes
2.6.2. Organic Photodiodes
3. Current Challenges and Future Directions
3.1. Flexibility of Implantable Neural Probes
3.2. Transparent Electrodes on Flexible Substrates
3.3. Miniaturization of Devices
3.4. Spectral Overlap of Multifunctional Neural Probes
3.5. Venues for Future Development
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Stringer, C.; Pachitariu, M. Analysis Methods for Large-Scale Neuronal Recordings. Science 2024, 386, eadp7429. [Google Scholar] [CrossRef] [PubMed]
- Breakspear, M. Dynamic Models of Large-Scale Brain Activity. Nat. Neurosci. 2017, 20, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.A.; Steinmetz, N.A. New Perspectives on Dimensionality and Variability from Large-Scale Cortical Dynamics. Curr. Opin. Neurobiol. 2019, 58, 181–190. [Google Scholar] [CrossRef]
- Kerr, C.C.; van Albada, S.J.; Neymotin, S.A.; Chadderdon, G.L.; Robinson, P.A.; Lytton, W.W. Cortical Information Flow in Parkinson’s Disease: A Composite Network/Field Model. Front. Comput. Neurosci. 2013, 7, 45203. [Google Scholar] [CrossRef] [PubMed]
- Adarsh, V.; Gangadharan, G.R.; Fiore, U.; Zanetti, P. Multimodal Classification of Alzheimer’s Disease and Mild Cognitive Impairment Using Custom MKSCDDL Kernel over CNN with Transparent Decision-Making for Explainable Diagnosis. Sci. Rep. 2024, 14, 1774. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, D.; Yan, W. Classification of Alzheimer’s Disease by Combination of Convolutional and Recurrent Neural Networks Using FDG-PET Images. Front. Neuroinform. 2018, 12, 312747. [Google Scholar] [CrossRef]
- Vassanelli, S.; Mahmud, M. Trends and Challenges in Neuroengineering: Toward “Intelligent” Neuroprostheses through Brain-“brain Inspired Systems” Communication. Front. Neurosci. 2016, 10, 438. [Google Scholar] [CrossRef]
- Won, S.M.; Cai, L.; Gutruf, P.; Rogers, J.A. Wireless and Battery-Free Technologies for Neuroengineering. Nat. Biomed. Eng. 2023, 7, 405–423. [Google Scholar] [CrossRef]
- Fan, B.; Li, W. Miniaturized Optogenetic Neural Implants: A Review. Lab Chip 2015, 15, 3838–3855. [Google Scholar] [CrossRef]
- Fekete, Z. Recent Advances in Silicon-Based Neural Microelectrodes and Microsystems: A Review. Sens. Actuators B Chem. 2015, 215, 300–315. [Google Scholar] [CrossRef]
- Streich, L.; Boffi, J.C.; Wang, L.; Alhalaseh, K.; Barbieri, M.; Rehm, R.; Deivasigamani, S.; Gross, C.T.; Agarwal, A.; Prevedel, R. High-Resolution Structural and Functional Deep Brain Imaging Using Adaptive Optics Three-Photon Microscopy. Nat. Methods 2021, 18, 1253–1258. [Google Scholar] [CrossRef]
- Ciccone, G.; Meloni, I.; Fernandez Lahore, R.G.; Vierock, J.; Reineke, S.; Kleemann, H.; Hegemann, P.; Leo, K.; Murawski, C. Tailoring Organic LEDs for Bidirectional Optogenetic Control via Dual-Color Switching. Adv. Funct. Mater. 2022, 32, 2110590. [Google Scholar] [CrossRef]
- Kielar, M.; Gooch, H.; Xu, L.; Pandey, A.K.; Sah, P. Direct Detection of Neuronal Activity Using Organic Photodetectors. ACS Photonics 2021, 8, 228–237. [Google Scholar] [CrossRef]
- Morton, A.; Murawski, C.; Deng, Y.; Keum, C.; Miles, G.B.; Tello, J.A.; Gather, M.C. Photostimulation for In Vitro Optogenetics with High-Power Blue Organic Light-Emitting Diodes. Adv. Biosyst. 2019, 3, 1800290. [Google Scholar] [CrossRef] [PubMed]
- Kielar, M.; Marek, R.; Kenna, M.; Cole, C.M.; Xu, L.; Yambem, S.D.; Sah, P.; Pandey, A.K. Optogenetic Stimulation and Spatial Localization of Neurons Using a Multi-OLED Approach. ACS Photonics 2022, 9, 3279–3290. [Google Scholar] [CrossRef]
- Lai, H.C.; Jan, L.Y. The Distribution and Targeting of Neuronal Voltage-Gated Ion Channels. Nat. Rev. Neurosci. 2006, 7, 548–562. [Google Scholar] [CrossRef]
- Fletcher, A. Action Potential: Generation and Propagation. Anaesth. Intensive Care Med. 2011, 12, 258–262. [Google Scholar] [CrossRef]
- Südhof, T.C. Towards an Understanding of Synapse Formation. Neuron 2018, 100, 276–293. [Google Scholar] [CrossRef]
- Hyman, S.E. Neurotransmitters. Curr. Biol. 2005, 15, R154–R158. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell: Ion Channels and the Electrical Properties of Membranes, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Kenna, M.; Marek, R.; Sah, P. Insights into the Encoding of Memories through the Circuitry of Fear. Curr. Opin. Neurobiol. 2023, 80, 102712. [Google Scholar] [CrossRef]
- Huang, Z. Brief History and Development of Electrophysiological Recording Techniques in Neuroscience. In Signal Processing in Neuroscience; Springer: Singapore, 2016; pp. 1–10. [Google Scholar] [CrossRef]
- Rey, H.G.; Pedreira, C.; Quian Quiroga, R. Past, Present and Future of Spike Sorting Techniques. Brain Res. Bull. 2015, 119, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Robinson, J.T.; Aazhang, B.; Chi, T.; Yang, K.; Li, X.; Rathore, H.; Singer, A.; Yellapantula, S.; Fan, Y.; et al. Recent Advances in Electrical Neural Interface Engineering: Minimal Invasiveness, Longevity, and Scalability. Neuron 2020, 108, 302–321. [Google Scholar] [CrossRef]
- Krauss, J.K.; Lipsman, N.; Aziz, T.; Boutet, A.; Brown, P.; Chang, J.W.; Davidson, B.; Grill, W.M.; Hariz, M.I.; Horn, A.; et al. Technology of Deep Brain Stimulation: Current Status and Future Directions. Nat. Rev. Neurol. 2020, 17, 75–87. [Google Scholar] [CrossRef]
- Frey, J.; Cagle, J.; Johnson, K.A.; Wong, J.K.; Hilliard, J.D.; Butson, C.R.; Okun, M.S.; de Hemptinne, C. Past, Present, and Future of Deep Brain Stimulation: Hardware, Software, Imaging, Physiology and Novel Approaches. Front. Neurol. 2022, 13, 825178. [Google Scholar] [CrossRef] [PubMed]
- Mosley, P.E.; Windels, F.; Morris, J.; Coyne, T.; Marsh, R.; Giorni, A.; Mohan, A.; Sachdev, P.; O’Leary, E.; Boschen, M.; et al. A Randomised, Double-Blind, Sham-Controlled Trial of Deep Brain Stimulation of the Bed Nucleus of the Stria Terminalis for Treatment-Resistant Obsessive-Compulsive Disorder. Transl. Psychiatry 2021, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.R.P.; Olsen, S.; Widge, A.S. Deep Brain Stimulation for Psychiatric Disorders: From Focal Brain Targets to Cognitive Networks. Neuroimage 2021, 225, 117515. [Google Scholar] [CrossRef]
- Holtzheimer, P.E.; Mayberg, H.S. Deep Brain Stimulation for Psychiatric Disorders. Annu. Rev. Neurosci. 2011, 34, 289. [Google Scholar] [CrossRef]
- Chen, T.W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive Fluorescent Proteins for Imaging Neuronal Activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef]
- Zhang, F.; Aravanis, A.M.; Adamantidis, A. Circuit-Breakers: Optical Technologies for Probing Neural Signals and Systems. Nat. Rev. Neurosci. 2007, 8, 577–581. [Google Scholar] [CrossRef]
- Simpson, E.H.; Akam, T.; Patriarchi, T.; Blanco-Pozo, M.; Burgeno, L.M.; Mohebi, A.; Cragg, S.J.; Walton, M.E. Lights, Fiber, Action! A Primer on in Vivo Fiber Photometry. Neuron 2024, 112, 718–739. [Google Scholar] [CrossRef]
- Sridharan, A.; Rajan, S.D.; Muthuswamy, J. Long-Term Changes in the Material Properties of Brain Tissue at the Implant-Tissue Interface. J. Neural Eng. 2013, 10, 066001. [Google Scholar] [CrossRef]
- Yang, H.H.; St-Pierre, F. Genetically Encoded Voltage Indicators: Opportunities and Challenges. J. Neurosci. 2016, 36, 9977–9989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, Y. Organic Semiconductors for Vacuum-Deposited Planar Heterojunction Solar Cells. ACS Omega 2020, 5, 24994–24999. [Google Scholar] [CrossRef]
- Kim, H. Neural Correlates of Explicit and Implicit Memory at Encoding and Retrieval: A Unified Framework and Meta-Analysis of Functional Neuroimaging Studies. Biol. Psychol. 2019, 145, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Boutet, A.; Rashid, T.; Hancu, I.; Elias, G.J.B.; Gramer, R.M.; Germann, J.; Dimarzio, M.; Li, B.; Paramanandam, V.; Prasad, S.; et al. Functional MRI Safety and Artifacts during Deep Brain Stimulation: Experience in 102 Patients. Radiology 2019, 293, 174–183. [Google Scholar] [CrossRef]
- Hargreaves, B.A.; Worters, P.W.; Pauly, K.B.; Pauly, J.M.; Koch, K.M.; Gold, G.E. Metal-Induced Artifacts in MRI. Am. J. Roentgenol. 2011, 197, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.K.; Fincher, C.R.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, A.G. Electrical Conductivity in Doped Polyacetylene. Phys. Rev. Lett. 1977, 39, 1098–1101. [Google Scholar] [CrossRef]
- Chen, L.X. Organic Solar Cells: Recent Progress and Challenges. ACS Energy Lett. 2019, 4, 2537–2539. [Google Scholar] [CrossRef]
- Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J.M.; Bräse, S.; Hong, G.; Gan, X.; Leonhardt, C.; et al. A Brief History of OLEDs—Emitter Development and Industry Milestones. Adv. Mater. 2021, 33, 2005630. [Google Scholar] [CrossRef]
- Ren, H.; Chen, J.D.; Li, Y.Q.; Tang, J.X. Recent Progress in Organic Photodetectors and Their Applications. Adv. Sci. 2021, 8, 2002418. [Google Scholar] [CrossRef]
- Facchetti, A. Semiconductors for Organic Transistors. Mater. Today 2007, 10, 28–37. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Goff, A.L.; Cosnier, S. Nanomaterials for Biosensing Applications: A Review. Front. Chem. 2014, 2, 108707. [Google Scholar] [CrossRef]
- Saggar, S.; Sanderson, S.; Gedefaw, D.; Pan, X.; Philippa, B.; Andersson, M.R.; Lo, S.C.; Namdas, E.B. Toward Faster Organic Photodiodes: Tuning of Blend Composition Ratio. Adv. Funct. Mater. 2021, 31, 2010661. [Google Scholar] [CrossRef]
- Fuentes-Hernandez, C.; Chou, W.F.; Khan, T.M.; Diniz, L.; Lukens, J.; Larrain, F.A.; Rodriguez-Toro, V.A.; Kippelen, B. Large-Area Low-Noise Flexible Organic Photodiodes for Detecting Faint Visible Light. Science 2020, 370, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Kielar, M.; Dhez, O.; Pecastaings, G.; Curutchet, A.; Hirsch, L. Long-Term Stable Organic Photodetectors with Ultra Low Dark Currents for High Detectivity Applications. Sci. Rep. 2016, 6, 39201. [Google Scholar] [CrossRef]
- Fang, Y.; Armin, A.; Meredith, P.; Huang, J. Accurate Characterization of Next-Generation Thin-Film Photodetectors. Nat. Photonics 2019, 13, 1–4. [Google Scholar] [CrossRef]
- Baeg, K.-J.; Binda, M.; Natali, D.; Caironi, M.; Noh, Y. Organic Light Detectors: Photodiodes and Phototransistors. Adv. Mater. 2013, 25, 4267–4295. [Google Scholar] [CrossRef]
- Kim, I.K.; Jo, J.H.; Lee, B.; Choi, Y.J. Detectivity Analysis for Organic Photodetectors. Org. Electron. 2018, 57, 89–92. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, S.H.; Song, I.; Chidchob, P.; Kwon, Y.; Oh, J.H. Chiral Organic Semiconducting Materials for Next-Generation Optoelectronic Sensors. Device 2023, 1, 100176. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, Q.; Miao, Q. Structured and Functionalized Organic Semiconductors for Chemical and Biological Sensors Based on Organic Field Effect Transistors. Mater. Chem. Front. 2020, 4, 3505–3520. [Google Scholar] [CrossRef]
- Borges-González, J.; Kousseff, C.J.; Nielsen, C.B. Organic Semiconductors for Biological Sensing. J. Mater. Chem. C 2019, 7, 1111–1130. [Google Scholar] [CrossRef]
- Kook, G.; Lee, S.W.; Lee, H.C.; Cho, I.J.; Lee, H.J. Neural Probes for Chronic Applications. Micromachines 2016, 7, 179. [Google Scholar] [CrossRef]
- Cellot, G.; Lagonegro, P.; Tarabella, G.; Scaini, D.; Fabbri, F.; Iannotta, S.; Prato, M.; Salviati, G.; Ballerini, L. PEDOT:PSS Interfaces Support the Development of Neuronal Synaptic Networks with Reduced Neuroglia Response in Vitro. Front. Neurosci. 2016, 9, 521. [Google Scholar] [CrossRef]
- Dijk, G.; Rutz, A.L.; Malliaras, G.G. Stability of PEDOT:PSS-Coated Gold Electrodes in Cell Culture Conditions. Adv. Mater. Technol. 2020, 5, 1900662. [Google Scholar] [CrossRef]
- Lan, Z.; Lau, Y.S.; Cai, L.; Han, J.; Suen, C.W.; Zhu, F. Dual-Band Organic Photodetectors for Dual-Channel Optical Communications. Laser Photon. Rev. 2022, 16, 2100602. [Google Scholar] [CrossRef]
- Li, N.; Eedugurala, N.; Azoulay, J.D.; Ng, T.N. A Filterless Organic Photodetector Electrically Switchable between Visible and Infrared Detection. Cell Rep. Phys. Sci. 2022, 3, 100711. [Google Scholar] [CrossRef]
- Cho, H.; Byun, C.-W.; Cho, N.S.; Han, J.-H.; Lee, H.; Choi, S.; Kwon, B.-H.; Lee, J.; Cho, H.; Byun, C.-W.; et al. Color-Tunable Organic Light-Emitting Diodes with Vertically Stacked Blue, Green, and Red Colors for Lighting and Display Applications. Opt. Express 2018, 26, 18351. [Google Scholar] [CrossRef]
- Fröbel, M.; Schwab, T.; Kliem, M.; Hofmann, S.; Leo, K.; Gather, M.C. Get It White: Color-Tunable AC/DC OLEDs. Light Sci. Appl. 2015, 4, e247. [Google Scholar] [CrossRef]
- Deisseroth, K. Optogenetics: 10 Years of Microbial Opsins in Neuroscience. Nat. Neurosci. 2015, 18, 1213–1225. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, Y.; Ameri, S.K.; Jang, H.; Dai, Z.; Huang, Y.; Lu, N. Low-Cost, Micron-Thick, Tape-Free Electronic Tattoo Sensors with Minimized Motion and Sweat Artifacts. npj Flex. Electron. 2018, 2, 6. [Google Scholar] [CrossRef]
- Murawski, C.; Archer, E. A Substrateless, Flexible, and Water-Resistant Organic Light-Emitting Diode. Nat. Commun. 2020, 11, 6250. [Google Scholar] [CrossRef]
- Salatino, J.W.; Ludwig, K.A.; Kozai, T.D.Y.; Purcell, E.K. Glial Responses to Implanted Electrodes in the Brain. Nat. Biomed. Eng. 2017, 1, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yokota, T.; Suzuki, T.; Lee, S.; Woo, T.; Yukita, W.; Koizumi, M.; Tachibana, Y.; Yawo, H.; Onodera, H.; et al. Ultraflexible Organic Light-Emitting Diodes for Optogenetic Nerve Stimulation. Proc. Natl. Acad. Sci. USA 2020, 117, 21138–21146. [Google Scholar] [CrossRef]
- Klapoetke, N.C.; Murata, Y.; Kim, S.S.; Pulver, S.R.; Birdsey-Benson, A.; Cho, Y.K.; Morimoto, T.K.; Chuong, A.S.; Carpenter, E.J.; Tian, Z.; et al. Independent Optical Excitation of Distinct Neural Populations. Nat. Methods 2014, 11, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Steude, A.; Witts, E.C.; Miles, G.B.; Gather, M.C. Arrays of Microscopic Organic LEDs for High-Resolution Optogenetics. Sci. Adv. 2016, 2, e1600061. [Google Scholar] [CrossRef]
- Yuan, W.; Jin, Q.; Du, M.; Duan, L.; Zhang, Y. Tailoring Ultra-Narrowband Tetraborylated Multiple Resonance Emitter for High-Performance Blue OLED. Adv. Mater. 2024, 36, 2410096. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Xin, Y.; Huang, T.; Zhang, Q.; Duan, L.; Zhang, D. Ultra-Low Power-Consumption OLEDs via Phosphor-Assisted Thermally-Activated-Delayed-Fluorescence-Sensitized Narrowband Emission. Nat. Commun. 2025, 16, 30. [Google Scholar] [CrossRef]
- Chen, R.; Liang, N.; Zhai, T. Dual-Color Emissive OLED with Orthogonal Polarization Modes. Nat. Commun. 2024, 15, 1331. [Google Scholar] [CrossRef]
- Ciccone, G.; Weber, J.P.; Meloni, I.; Kleemann, H.; Leo, K.; Murawski, C. Multiplexed Optogenetics with Striped Organic LEDs. Adv. Opt. Mater. 2024, 12, 2301340. [Google Scholar] [CrossRef]
- Lan, Z.; Lei, Y.; Chan, W.K.E.; Chen, S.; Luo, D.; Zhu, F. Near-Infrared and Visible Light Dual-Mode Organic Photodetectors. Sci. Adv. 2020, 6, eaaw8065. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Siegmund, B.; Tang, Z.; Ma, Z.; Kublitski, J.; Xing, S.; Nikolis, V.C.; Ullbrich, S.; Li, Y.; Benduhn, J.; et al. Stacked Dual-Wavelength Near-Infrared Organic Photodetectors. Adv. Opt. Mater. 2021, 9, 2001784. [Google Scholar] [CrossRef]
- Chiba, T.; Kumagai, D.; Udagawa, K.; Watanabe, Y.; Kido, J. Dual Mode OPV-OLED Device with Photovoltaic and Light-Emitting Functionalities. Sci. Rep. 2018, 8, 11472. [Google Scholar] [CrossRef] [PubMed]
- Kielar, M.; Hamid, T.; Wu, L.; Windels, F.; Sah, P.; Pandey, A.K. Organic Optoelectronic Diodes as Tactile Sensors for Soft-Touch Applications. ACS Appl. Mater. Interfaces 2019, 11, 21775–21783. [Google Scholar] [CrossRef]
- Hamid, T.; Kielar, M.; Yambem, S.D.; Pandey, A.K. Multifunctional Diode Operation of Tetracene Sensitized Polymer:Fullerene Heterojunctions with Simultaneous Electroluminescence in Visible and NIR Bands. Adv. Electron. Mater. 2021, 7, 2000824. [Google Scholar] [CrossRef]
- Chen, H.; Huang, Y.; Zhang, R.; Mou, H.; Ding, J.; Zhou, J.; Wang, Z.; Li, H.; Chen, W.; Zhu, J.; et al. Organic Solar Cells with 20.82% Efficiency and High Tolerance of Active Layer Thickness through Crystallization Sequence Manipulation. Nat. Mater. 2025, 24, 444–453. [Google Scholar] [CrossRef]
- Park, J.; Kim, K.; Kim, Y.; Kim, T.S.; Min, I.S.; Li, B.; Cho, Y.U.; Lee, C.; Lee, J.Y.; Gao, Y.; et al. A Wireless, Solar-Powered, Optoelectronic System for Spatial Restriction-Free Long-Term Optogenetic Neuromodulations. Sci. Adv. 2023, 9, eadi8918. [Google Scholar] [CrossRef]
- Pranti, A.S.; Schander, A.; Bödecker, A.; Lang, W. PEDOT: PSS Coating on Gold Microelectrodes with Excellent Stability and High Charge Injection Capacity for Chronic Neural Interfaces. Sens. Actuators B Chem. 2018, 275, 382–393. [Google Scholar] [CrossRef]
- Bianchi, M.; De Salvo, A.; Asplund, M.; Carli, S.; Di Lauro, M.; Schulze-Bonhage, A.; Stieglitz, T.; Fadiga, L.; Biscarini, F. Poly(3,4-Ethylenedioxythiophene)-Based Neural Interfaces for Recording and Stimulation: Fundamental Aspects and In Vivo Applications. Adv. Sci. 2022, 9, 2104701. [Google Scholar] [CrossRef]
- Filho, G.; Júnior, C.; Spinelli, B.; Damasceno, I.; Fiuza, F.; Morya, E. All-Polymeric Electrode Based on PEDOT:PSS for In Vivo Neural Recording. Biosensors 2022, 12, 853. [Google Scholar] [CrossRef]
- Zou, S.J.; Shen, Y.; Xie, F.M.; Chen, J.D.; Li, Y.Q.; Tang, J.X. Recent Advances in Organic Light-Emitting Diodes: Toward Smart Lighting and Displays. Mater. Chem. Front. 2020, 4, 788–820. [Google Scholar] [CrossRef]
- Thejo Kalyani, N.; Dhoble, S.J. Organic Light Emitting Diodes: Energy Saving Lighting Technology—A Review. Renew. Sustain. Energy Rev. 2012, 16, 2696–2723. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Zhang, J.; Tang, W.; Tang, A.; Peng, H.; Xu, Z.; Teng, F.; Wang, Y. Key Issues and Recent Progress of High Efficient Organic Light-Emitting Diodes. J. Photochem. Photobiol. C Photochem. Rev. 2013, 17, 69–104. [Google Scholar] [CrossRef]
- Shuai, Z.; Peng, Q. Organic Light-Emitting Diodes: Theoretical Understanding of Highly Efficient Materials and Development of Computational Methodology. Natl. Sci. Rev. 2017, 4, 224–239. [Google Scholar] [CrossRef]
- Yadav, R.A.K.; Dubey, D.K.; Chen, S.Z.; Liang, T.W.; Jou, J.H. Role of Molecular Orbital Energy Levels in OLED Performance. Sci. Rep. 2020, 10, 9915. [Google Scholar] [CrossRef]
- Riahi, M.; Yoshida, K.; King, L.G.; Samuel, I.D.W. In-Operando Investigation of Purcell Effect on Efficiency Roll-off in Top-Emitting Phosphorescent Organic Light Emitting Diodes. Synth. Met. 2025, 312, 117848. [Google Scholar] [CrossRef]
- Kang, K.; Byeon, I.; Kim, Y.G.; Choi, J.-R.; Kim, D.; Kang, K.; Byeon, I.; Kim, D.; Kim, Y.G.; Choi, J. Nanostructures in Organic Light-Emitting Diodes: Principles and Recent Advances in the Light Extraction Strategy. Laser Photon. Rev. 2024, 18, 2400547. [Google Scholar] [CrossRef]
- Steude, A.; Jahnel, M.; Thomschke, M.; Schober, M.; Gather, M.C. Controlling the Behavior of Single Live Cells with High Density Arrays of Microscopic OLEDs. Adv. Mater. 2015, 27, 7657–7661. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Bawolek, E.; Christen, J.B.; Smith, J.T.; O’Brien, B. Application of Flexible OLED Display Technology for Electro-Optical Stimulation and/or Silencing of Neural Activity. J. Disp. Technol. 2014, 10, 514–520. [Google Scholar] [CrossRef]
- Smith, J.; Shah, A.; Lee, Y.K.; O’Brien, B.; Kullman, D.; Sridharan, A.; Muthuswamy, J.; Blain Christen, J. Optogenetic Neurostimulation of Auricular Vagus Using Flexible OLED Display Technology to Treat Chronic Inflammatory Disease and Mental Health Disorders. Electron. Lett. 2016, 52, 900–902. [Google Scholar] [CrossRef]
- Hillebrandt, S.; Keum, C.; Deng, Y.; Chavas, J.; Galle, C.; Hardin, T.; Galluppi, F.; Gather, M.C. High Brightness, Highly Directional Organic Light-Emitting Diodes as Light Sources for Future Light-Amplifying Prosthetics in the Optogenetic Management of Vision Loss. Adv. Opt. Mater. 2022, 11, 2200877. [Google Scholar] [CrossRef]
- Murawski, C.; Mischok, A.; Booth, J.; Kumar, J.D.; Archer, E.; Tropf, L.; Keum, C.M.; Deng, Y.L.; Yoshida, K.; Samuel, I.D.W.; et al. Narrowband Organic Light-Emitting Diodes for Fluorescence Microscopy and Calcium Imaging. Adv. Mater. 2019, 31, 1903599. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, A.; Shah, A.; Kumar, S.S.; Kyeh, J.; Smith, J.; Blain-Christen, J.; Muthuswamy, J. Optogenetic Modulation of Cortical Neurons Using Organic Light Emitting Diodes (OLEDs). Biomed. Phys. Eng. Express 2020, 6, 025003. [Google Scholar] [CrossRef] [PubMed]
- Kielar, M.; Marek, R.; Gooch, H.; Cole, C.M.; Kenna, M.; Xu, L.; Yambem, S.D.; Pandey, A.K.; Sah, P. Stability, Reliability, and Performance of Organic Light-Emitting Diodes and Photodetectors in Optogenetic Studies. Proc. SPIE 2023, 1266103, 7. [Google Scholar] [CrossRef]
- Matarèse, B.F.E.; Feyen, P.L.C.; de Mello, J.C.; Benfenati, F. Sub-Millisecond Control of Neuronal Firing by Organic Light-Emitting Diodes. Front. Bioeng. Biotechnol. 2019, 7, 483442. [Google Scholar] [CrossRef]
- Ghezzi, D.; Antognazza, M.R.; Dal Maschio, M.; Lanzarini, E.; Benfenati, F.; Lanzani, G. A Hybrid Bioorganic Interface for Neuronal Photoactivation. Nat. Commun. 2011, 2, 166. [Google Scholar] [CrossRef]
- Morton, A.; Murawski, C.; Pulver, S.R.; Gather, M.C. High-Brightness Organic Light-Emitting Diodes for Optogenetic Control of Drosophila Locomotor Behaviour. Sci. Rep. 2016, 6, 31117. [Google Scholar] [CrossRef]
- Murawski, C.; Morton, A.; Samuel, I.D.W.; Pulver, S.R.; Gather, M.C. Organic Light-Emitting Diodes for Optogenetic Stimulation of Drosophila Larvae. In Fourier Transform Spectroscopy; Proceedings, Light, Energy and the Environment; Optica Publishing Group: Washington, DC, USA, 2016; p. JW4A-9. [Google Scholar] [CrossRef]
- Rezaei-Mazinani, S.; Ivanov, A.I.; Proctor, C.M.; Gkoupidenis, P.; Bernard, C.; Malliaras, G.G.; Ismailova, E. Monitoring Intrinsic Optical Signals in Brain Tissue with Organic Photodetectors. Adv. Mater. Technol. 2018, 3, 1700333. [Google Scholar] [CrossRef]
- Murawski, C.; Pulver, S.R.; Gather, M.C. Segment-Specific Optogenetic Stimulation in Drosophila Melanogaster with Linear Arrays of Organic Light-Emitting Diodes. Nat. Commun. 2020, 11, 6248. [Google Scholar] [CrossRef]
- Taal, A.J.; Uguz, I.; Hillebrandt, S.; Moon, C.K.; Andino-Pavlovsky, V.; Choi, J.; Keum, C.; Deisseroth, K.; Gather, M.C.; Shepard, K.L. Optogenetic Stimulation Probes with Single-Neuron Resolution Based on Organic LEDs Monolithically Integrated on CMOS. Nat. Electron. 2023, 6, 669–679. [Google Scholar] [CrossRef]
- Stoltzfus, D.M.; Donaghey, J.E.; Armin, A.; Shaw, P.E.; Burn, P.L.; Meredith, P. Charge Generation Pathways in Organic Solar Cells: Assessing the Contribution from the Electron Acceptor. Chem. Rev. 2016, 116, 12920–12955. [Google Scholar] [CrossRef]
- Simone, G.; Dyson, M.J.; Weijtens, C.H.; Meskers, S.C.; Coehoorn, R.; Janssen, R.A.; Gelinck, G.H. On the Origin of Dark Current in Organic Photodiodes. Adv. Opt. Mater. 2020, 8, 1901568. [Google Scholar] [CrossRef]
- Kielar, M.; Hamid, T.; Wiemer, M.; Windels, F.; Hirsch, L.; Sah, P.; Pandey, A.K. Light Detection in Open-Circuit Voltage Mode of Organic Photodetectors. Adv. Funct. Mater. 2020, 30, 1907964. [Google Scholar] [CrossRef]
- Simone, G.; Di Carlo Rasi, D.; de Vries, X.; Heintges, G.H.; Meskers, S.C.; Janssen, R.A.; Gelinck, G.H. Near-Infrared Tandem Organic Photodiodes for Future Application in Artificial Retinal Implants. Adv. Mater. 2018, 30, 1804678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rózsa, M.; Liang, Y.; Bushey, D.; Wei, Z.; Zheng, J.; Reep, D.; Broussard, G.J.; Tsang, A.; Tsegaye, G.; et al. Fast and Sensitive GCaMP Calcium Indicators for Imaging Neural Populations. Nature 2023, 615, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Mano, O.; Creamer, M.S.; Matulis, C.A.; Salazar-Gatzimas, E.; Chen, J.; Zavatone-Veth, J.A.; Clark, D.A. Using Slow Frame Rate Imaging to Extract Fast Receptive Fields. Nat. Commun. 2019, 10, 4979. [Google Scholar] [CrossRef]
- Baran, D.; Corzo, D.; Blazquez, G.T. Flexible Electronics: Status, Challenges and Opportunities. Front. Electron. 2020, 1, 594003. [Google Scholar] [CrossRef]
- Lewis, J. Material Challenge for Flexible Organic Devices. Mater. Today 2006, 9, 38–45. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef]
- Szafran, K.; Jurak, M.; Mroczka, R.; Wiącek, A.E. Surface Properties of the Polyethylene Terephthalate (PET) Substrate Modified with the Phospholipid-Polypeptide-Antioxidant Films: Design of Functional Biocoatings. Pharmaceutics 2022, 14, 2815. [Google Scholar] [CrossRef]
- Fonrodona, M.; Escarré, J.; Villar, F.; Soler, D.; Asensi, J.M.; Bertomeu, J.; Andreu, J. PEN as Substrate for New Solar Cell Technologies. Sol. Energy Mater. Sol. Cells 2005, 89, 37–47. [Google Scholar] [CrossRef]
- Moss, T.; Greiner, A. Functionalization of Poly(Para-Xylylene)s—Opportunities and Challenges as Coating Material. Adv. Mater. Interfaces 2020, 7, 1901858. [Google Scholar] [CrossRef]
- Vomero, M.; Ciarpella, F.; Zucchini, E.; Kirsch, M.; Fadiga, L.; Stieglitz, T.; Asplund, M. On the Longevity of Flexible Neural Interfaces: Establishing Biostability of Polyimide-Based Intracortical Implants. Biomaterials 2022, 281, 121372. [Google Scholar] [CrossRef] [PubMed]
- Lavazza, J.; Contino, M.; Marano, C. Strain Rate, Temperature and Deformation State Effect on Ecoflex 00-50 Silicone Mechanical Behaviour. Mech. Mater. 2023, 178, 104560. [Google Scholar] [CrossRef]
- Behl, M.; Lendlein, A. Shape-Memory Polymers. Mater. Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Cywar, R.M.; Rorrer, N.A.; Hoyt, C.B.; Beckham, G.T.; Chen, E.Y.X. Bio-Based Polymers with Performance-Advantaged Properties. Nat. Rev. Mater. 2021, 7, 83–103. [Google Scholar] [CrossRef]
- McGlynn, E.; Nabaei, V.; Ren, E.; Galeote-Checa, G.; Das, R.; Curia, G.; Heidari, H. The Future of Neuroscience: Flexible and Wireless Implantable Neural Electronics. Adv. Sci. 2021, 8, 2002693. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, R.; Zhang, H.; Cao, P.; Liu, Z.; Li, Y. Research Progress on the Flexibility of an Implantable Neural Microelectrode. Micromachines 2022, 13, 386. [Google Scholar] [CrossRef]
- Axpe, E.; Orive, G.; Franze, K.; Appel, E.A. Towards Brain-Tissue-like Biomaterials. Nat. Commun. 2020, 11, 3423. [Google Scholar] [CrossRef]
- Weltman, A.; Yoo, J.; Meng, E. Flexible, Penetrating Brain Probes Enabled by Advances in Polymer Microfabrication. Micromachines 2016, 7, 180. [Google Scholar] [CrossRef]
- Zhang, E.N.; Clément, J.P.; Alameri, A.; Ng, A.; Kennedy, T.E.; Juncker, D. Mechanically Matched Silicone Brain Implants Reduce Brain Foreign Body Response. Adv. Mater. Technol. 2021, 6, 2000909. [Google Scholar] [CrossRef]
- Kim, H.; Gilmore, C.M.; Piqué, A.; Horwitz, J.S.; Mattoussi, H.; Murata, H.; Kafafi, Z.H.; Chrisey, D.B. Electrical, Optical, and Structural Properties of Indium–Tin–Oxide Thin Films for Organic Light-Emitting Devices. J. Appl. Phys. 1999, 86, 6451–6461. [Google Scholar] [CrossRef]
- Ellmer, K. Past Achievements and Future Challenges in the Development of Optically Transparent Electrodes. Nat. Photonics 2012, 6, 809–817. [Google Scholar] [CrossRef]
- Chen, Z.; Li, W.; Li, R.; Zhang, Y.; Xu, G.; Cheng, H. Fabrication of Highly Transparent and Conductive Indium-Tin Oxide Thin Films with a High Figure of Merit via Solution Processing. Langmuir 2013, 29, 13836–13842. [Google Scholar] [CrossRef]
- Aleksandrova, M.; Kolev, G.; Cholakova, I.; Dobrikov, G.; Bodurov, G. Photolithography versus Lift off Process for Patterning of Sputtered Indium Tin Oxide for Flexible Displays. Int. J. Thin Film. Sci. Technol. 2013, 2, 67–75. [Google Scholar] [CrossRef]
- Shim, H.; Jang, S.; Yu, C. High-Resolution Patterning of Organic Semiconductors toward Industrialization of Flexible Organic Electronics. Matter 2022, 5, 23–25. [Google Scholar] [CrossRef]
- Chen, Z.; Cotterell, B.; Wang, W.; Guenther, E.; Chua, S.J. A Mechanical Assessment of Flexible Optoelectronic Devices. Thin Solid Films 2001, 394, 201–205. [Google Scholar] [CrossRef]
- Fehse, K.; Walzer, K.; Leo, K.; Lövenich, W.; Elschner, A. Highly Conductive Polymer Anodes as Replacements for Inorganic Materials in High-Efficiency Organic Light-Emitting Diodes. Adv. Mater. 2007, 19, 441–444. [Google Scholar] [CrossRef]
- Krautz, D.; Cheylan, S.; Ghosh, D.S.; Pruneri, V. Nickel as an Alternative Semitransparent Anode to Indium Tin Oxide for Polymer LEDapplications. Nanotechnology 2009, 20, 275204. [Google Scholar] [CrossRef]
- Hu, L.; Kim, H.S.; Lee, J.Y.; Peumans, P.; Cui, Y. Scalable Coating and Properties of Transparent, Flexible, Silver Nanowire Electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef]
- Leem, D.-S.; Edwards, A.; Faist, M.; Nelson, J.; C Bradley, D.D.; de Mello, J.C.; Leem, D.; Edwards, A.; Faist, M.; Nelson, J.; et al. Efficient Organic Solar Cells with Solution-Processed Silver Nanowire Electrodes. Adv. Mater. 2011, 23, 4371–4375. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yim, J.; Wang, X.; Bradley, D.D.C.; Lee, S.; DeMello, J.C. Spin- and Spray-Deposited Single-Walled Carbon-Nanotube Electrodes for Organic Solar Cells. Adv. Funct. Mater. 2010, 20, 2310–2316. [Google Scholar] [CrossRef]
- Chang, H.; Wang, G.; Yang, A.; Tao, X.; Liu, X.; Shen, Y.; Zheng, Z. A Transparent, Flexible, Low-Temperature, and Solution-Processible Graphene Composite Electrode. Adv. Funct. Mater. 2010, 20, 2893–2902. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, L.; Cheng, N.; Andrew, T.L. ITO-Free Transparent Organic Solar Cell with Distributed Bragg Reflector for Solar Harvesting Windows. Energies 2017, 10, 707. [Google Scholar] [CrossRef]
- Galagan, Y.; Rubingh, J.E.J.; Andriessen, R.; Fan, C.C.; Blom, P.W.; Veenstra, S.C.; Kroon, J.M. ITO-Free Flexible Organic Solar Cells with Printed Current Collecting Grids. Sol. Energy Mater. Sol. Cells 2011, 95, 1339–1343. [Google Scholar] [CrossRef]
- Choi, S.; Kippelen, B.; Potscavage, W.J. ITO-Free Large-Area Organic Solar Cells. Opt. Express 2010, 18, A458–A466. [Google Scholar] [CrossRef] [PubMed]
- Hengge, M.; Livanov, K.; Zamoshchik, N.; Hermerschmidt, F.; List-Kratochvil, E.J.W. ITO-Free OLEDs Utilizing Inkjet-Printed and Low Temperature Plasma-Sintered Ag Electrodes. Flex. Print. Electron. 2021, 6, 015009. [Google Scholar] [CrossRef]
- Baierl, D.; Fabel, B.; Lugli, P.; Scarpa, G. Efficient Indium-Tin-Oxide (ITO) Free Top-Absorbing Organic Photodetector with Highly Transparent Polymer Top Electrode. Org. Electron. 2011, 12, 1669–1673. [Google Scholar] [CrossRef]
- Kinner, L.; Dimopoulos, T.; Ligorio, G.; List-Kratochvil, E.J.W.; Hermerschmidt, F. High Performance Organic Light-Emitting Diodes Employing ITO-Free and Flexible TiOx/Ag/Al:ZnO Electrodes. RSC Adv. 2021, 11, 17324–17331. [Google Scholar] [CrossRef]
- Chilvery, A.; Das, S.; Guggilla, P.; Brantley, C.; Sunda-Meya, A. A Perspective on the Recent Progress in Solution-Processed Methods for Highly Efficient Perovskite Solar Cells. Sci. Technol. Adv. Mater. 2016, 17, 650–658. [Google Scholar] [CrossRef]
- Aberra, A.S.; Peterchev, A.V.; Grill, W.M. Biophysically Realistic Neuron Models for Simulation of Cortical Stimulation. J. Neural Eng. 2018, 15, 066023. [Google Scholar] [CrossRef]
- Hillebrandt, S.; Moon, C.-K.; Taal, A.J.; Overhauser, H.; Shepard, K.L.; Gather, M.C.; Hillebrandt, S.; Moon, C.-K.; Gather, M.C.; Taal, A.J.; et al. High-Density Integration of Ultrabright OLEDs on a Miniaturized Needle-Shaped CMOS Backplane. Adv. Mater. 2024, 36, 2300578. [Google Scholar] [CrossRef]
- Li, J.; Ni, Y.; Zhao, X.; Wang, B.; Xue, C.; Bi, Z.; Zhang, C.; Dong, Y.; Tong, Y.; Tang, Q.; et al. Vertically Stacked Skin-like Active-Matrix Display with Ultrahigh Aperture Ratio. Light Sci. Appl. 2024, 13, 177. [Google Scholar] [CrossRef]
- Hou, X.; Chen, S.; Tang, W.; Liang, J.; Ouyang, B.; Li, M.; Song, Y.; Shan, T.; Chen, C.C.; Too, P.; et al. Low-Temperature Solution-Processed All Organic Integration for Large-Area and Flexible High-Resolution Imaging. IEEE J. Electron Devices Soc. 2022, 10, 821–826. [Google Scholar] [CrossRef]
- Dykstra, E.; Fralaide, M.; Zhang, Y.; Biswas, R.; Dennis Slafer, W.; Shinar, J.; Shinar, R. OLEDs on Planarized Light Outcoupling-Enhancing Structures in Plastic. Org. Electron. 2022, 111, 106648. [Google Scholar] [CrossRef]
- Hauss, J.; Bocksrocker, T.; Riedel, B.; Lemmer, U.; Gerken, M. On the Interplay of Waveguide Modes and Leaky Modes in Corrugated OLEDs. Opt. Express 2011, 19, A851–A858. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Choi, B. Enhanced Light Extraction from Bottom Emission OLEDs by High Refractive Index Nanoparticle Scattering Layer. Nanomaterials 2019, 9, 1241. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Dong, G.; Duan, L.; Sun, J.; Zhang, D.; Wang, L. High-Stability Organic Red-Light Photodetector for Narrowband Applications. Laser Photon. Rev. 2016, 10, 473–480. [Google Scholar] [CrossRef]
- Shen, D.; Guan, Z.; Li, M.; Tsang, S.W.; Zhang, W.; Lo, M.F.; Lee, C.S. Trilayer Organic Narrowband Photodetector with Electrically-Switchable Spectral Range and Color Sensing Ability. J. Mater. Chem. C 2021, 9, 3814–3819. [Google Scholar] [CrossRef]
- Xie, B.; Xie, R.; Zhang, K.; Yin, Q.; Hu, Z.; Yu, G.; Huang, F.; Cao, Y. Self-Filtering Narrowband High Performance Organic Photodetectors Enabled by Manipulating Localized Frenkel Exciton Dissociation. Nat. Commun. 2020, 11, 2871. [Google Scholar] [CrossRef]
- Delaporte, P.; Karnakis, D.; Zergioti, I. Laser Processing of Flexible Organic Electronic Materials. In Handbook of Flexible Organic Electronics: Materials. Manufacturing and Applications; Woodhead Publishing: Sawston, UK, 2014; pp. 285–313. [Google Scholar] [CrossRef]
- Teichler, A.; Perelaer, J.; Schubert, U.S. Inkjet Printing of Organic Electronics—Comparison of Deposition Techniques and State-of-the-Art Developments. J. Mater. Chem. C 2013, 1, 1910–1925. [Google Scholar] [CrossRef]
- Park, J.U.; Hardy, M.; Kang, S.J.; Barton, K.; Adair, K.; Mukhopadhyay, D.K.; Lee, C.Y.; Strano, M.S.; Alleyne, A.G.; Georgiadis, J.G.; et al. High-Resolution Electrohydrodynamic Jet Printing. Nat. Mater. 2007, 6, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Sumaiya, S.; Kardel, K.; El-Shahat, A. Organic Solar Cell by Inkjet Printing—An Overview. Technologies 2017, 5, 53. [Google Scholar] [CrossRef]
- Eggenhuisen, T.M.; Galagan, Y.; Biezemans, A.F.K.V.; Slaats, T.M.W.L.; Voorthuijzen, W.P.; Kommeren, S.; Shanmugam, S.; Teunissen, J.P.; Hadipour, A.; Verhees, W.J.H.; et al. High Efficiency, Fully Inkjet Printed Organic Solar Cells with Freedom of Design. J. Mater. Chem. A 2015, 3, 7255–7262. [Google Scholar] [CrossRef]
- Sekitani, T.; Noguchi, Y.; Zschieschang, U.; Klauk, H.; Someya, T. Organic Transistors Manufactured Using Inkjet Technology with Subfemtoliter Accuracy. Proc. Natl. Acad. Sci. USA 2008, 105, 4976–4980. [Google Scholar] [CrossRef]
- Pace, G.; Grimoldi, A.; Rengert, Z.; Bazan, G.C.; Natali, D.; Caironi, M. Inkjet Printed Organic Detectors with Flat Responsivity up to the NIR and Inherent UV Optical Filtering. Synth. Met. 2019, 254, 92–96. [Google Scholar] [CrossRef]
- Azzellino, G.; Grimoldi, A.; Binda, M.; Caironi, M.; Natali, D.; Sampietro, M. Fully Inkjet-Printed Organic Photodetectors with High Quantum Yield. Adv. Mater. 2013, 25, 6829–6833. [Google Scholar] [CrossRef]
- Amruth, C.; Luszczynska, B.; Szymanski, M.Z.; Ulanski, J.; Albrecht, K.; Yamamoto, K. Inkjet Printing of Thermally Activated Delayed Fluorescence (TADF) Dendrimer for OLEDs Applications. Org. Electron. 2019, 74, 218–227. [Google Scholar] [CrossRef]
- Kant, C.; Mahmood, S.; Katiyar, M. Large-Area Inkjet-Printed OLEDs Patterns and Tiles Using Small Molecule Phosphorescent Dopant. Adv. Mater. Technol. 2023, 8, 2201514. [Google Scholar] [CrossRef]
- Noh, Y.Y.; Zhao, N.; Caironi, M.; Sirringhaus, H. Downscaling of Self-Aligned, All-Printed Polymer Thin-Film Transistors. Nat. Nanotechnol. 2007, 2, 784–789. [Google Scholar] [CrossRef]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet Printing-Process and Its Applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Sekitani, T.; Kato, Y.; Kuribara, K.; Zschieschang, U.; Klauk, H.; Yamamoto, T.; Takimiya, K.; Kuwabara, H.; Ikeda, M.; et al. Low-Voltage Organic Transistor with Subfemtoliter Inkjet Source–Drain Contacts. MRS Commun. 2011, 1, 3–6. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D Printing of Conducting Polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Mele, A.; Giardini-Guidoni, A.; Teghil, R. Laser Ablation of Inorganic and Organic Materials. J. Chem. Sci. 1993, 105, 715–733. [Google Scholar] [CrossRef]
- Srinivasan, R.; Braren, B. Ultraviolet Laser Ablation of Organic Polymers. Chem. Rev. 1989, 89, 1303–1316. [Google Scholar] [CrossRef]
- Ravi-Kumar, S.; Lies, B.; Lyu, H.; Qin, H. Laser Ablation of Polymers: A Review. Procedia Manuf. 2019, 34, 316–327. [Google Scholar] [CrossRef]
- Choi, J.; Yoo, Y.; Kim, H.J.; Lee, H.H.; Mottay, E.; Kling, R. Femtosecond Laser Based Manufacturing of Tailored Flexible Electronics for OLED and OPV Applications. 2019 Conf. Lasers Electro-Opt. CLEO 2019-Proc. 2019, 6, 24427. [Google Scholar] [CrossRef]
- Jipa, F.; Zamfirescu, M.; Velea, A.; Popescu, M.; Dabu, R. Femtosecond Laser Lithography in Organic and Non-Organic Materials. In Updates in Advanced Lithography; IntechOpen: London, UK, 2013; pp. 65–94. [Google Scholar] [CrossRef]
- Shim, S.; Park, H.Y.; Choi, G.J.; Shin, H.C.; Kim, S.J. A Simply Fabricated Neural Probe by Laser Machining of a Thermally Laminated Gold Thin Film on Transparent Cyclic Olefin Polymer. ACS Omega 2019, 4, 2590–2595. [Google Scholar] [CrossRef]
- Liu, J.; Gao, M.; Kim, J.; Zhou, Z.; Chung, D.S.; Yin, H.; Ye, L. Challenges and Recent Advances in Photodiodes-Based Organic Photodetectors. Mater. Today 2021, 51, 475–503. [Google Scholar] [CrossRef]
- Kong, L.; Luo, Y.; Wu, Q.; Xiao, X.; Wang, Y.; Chen, G.; Zhang, J.; Wang, K.; Choy, W.C.H.; Zhao, Y.B.; et al. Efficient and Stable Hybrid Perovskite-Organic Light-Emitting Diodes with External Quantum Efficiency Exceeding 40 per Cent. Light Sci. Appl. 2024, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Yang, Y.M.; You, J.; Hong, Z.; Chang, W.H.; Li, G.; Yang, Y. Solution-Processed Hybrid Perovskite Photodetectors with High Detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.P.; et al. Efficient Perovskite Solar Cells via Improved Carrier Management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, C.; Wang, H.; Jiang, Y.; Fang, Y.; Wang, J.; Duan, S.; Fu, X.; Miao, J.; Hu, W. Van Der Waals Integration of Two-Dimensional Materials and Bulk Semiconductors for Infrared Photodetection Technology. MRS Bull. 2023, 48, 914–922. [Google Scholar] [CrossRef]
- Mazaheri, A.; Lee, M.; Van Der Zant, H.S.J.; Frisenda, R.; Castellanos-Gomez, A. MoS2-on-Paper Optoelectronics: Drawing Photodetectors with van Der Waals Semiconductors beyond Graphite. Nanoscale 2020, 12, 19068–19074. [Google Scholar] [CrossRef]
- Li, N.; Jabegu, T.; He, R.; Yun, S.; Ghosh, S.; Maraba, D.; Olunloyo, O.; Ma, H.; Okmi, A.; Xiao, K.; et al. Covalently-Bonded Laminar Assembly of Van Der Waals Semiconductors with Polymers: Toward High-Performance Flexible Devices. Small 2024, 2310175. [Google Scholar] [CrossRef]
- Moon, D.; Lee, W.; Lim, C.; Kim, J.; Kim, J.; Jung, Y.; Choi, H.Y.; Choi, W.S.; Kim, H.; Baek, J.H.; et al. Hypotaxy of Wafer-Scale Single-Crystal Transition Metal Dichalcogenides. Nature 2025, 638, 957–964. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Zhang, S.; Jiang, Q.; Li, P.; Wang, C.; Xiao, R.; Li, X.M.; Song, J. Multifunctional Ultraflexible Neural Probe for Wireless Optogenetics and Electrophysiology. Giant 2024, 18, 100272. [Google Scholar] [CrossRef]
- Yoon, Y.; Shin, H.; Byun, D.; Woo, J.; Cho, Y.; Choi, N.; Cho, I.J. Neural Probe System for Behavioral Neuropharmacology by Bi-Directional Wireless Drug Delivery and Electrophysiology in Socially Interacting Mice. Nat. Commun. 2022, 13, 5521. [Google Scholar] [CrossRef]
- Badrulhisham, F.; Pogatzki-Zahn, E.; Segelcke, D.; Spisak, T.; Vollert, J. Machine Learning and Artificial Intelligence in Neuroscience: A Primer for Researchers. Brain. Behav. Immun. 2024, 115, 470–479. [Google Scholar] [CrossRef]

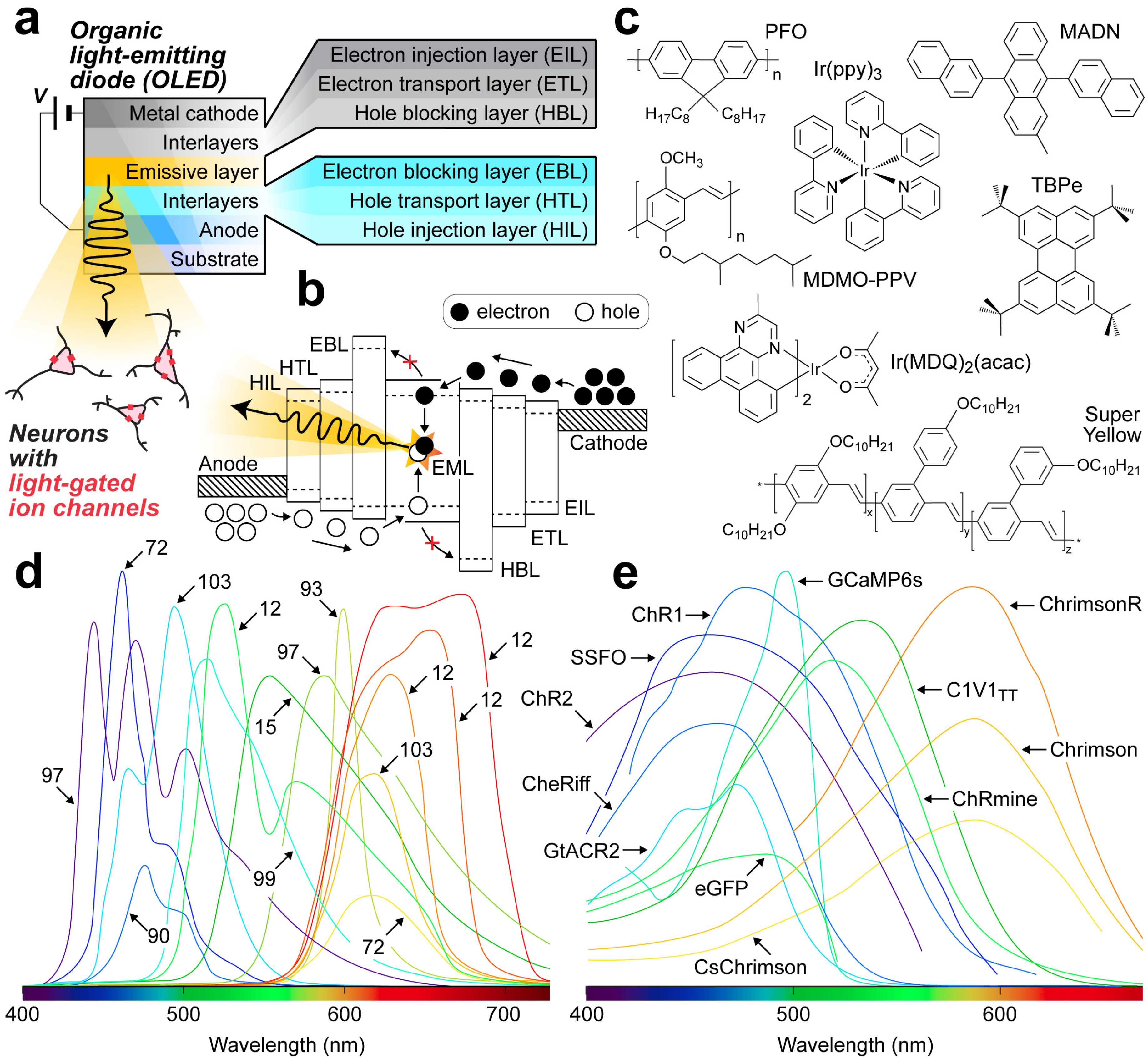

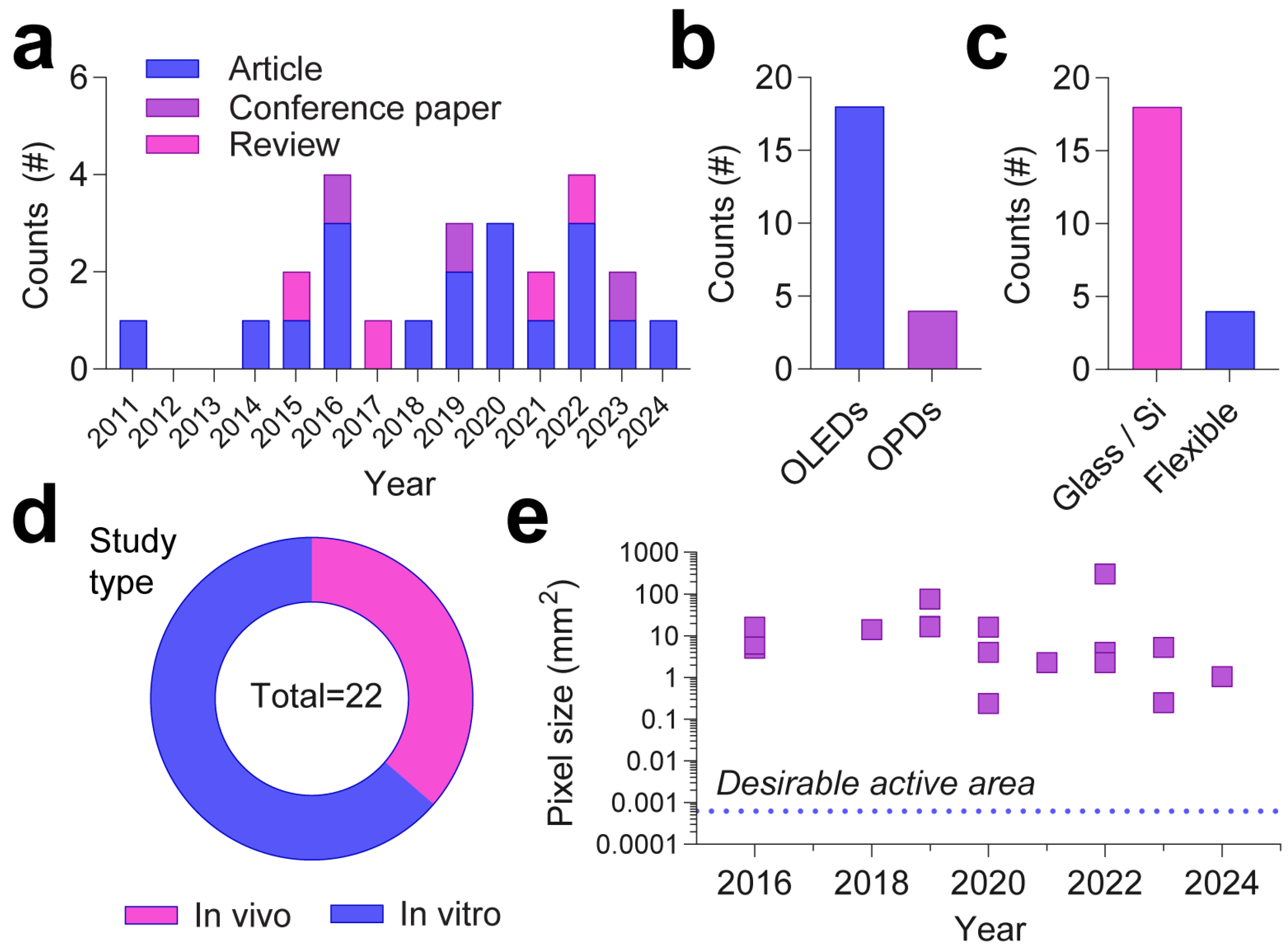
| Year | Device | Active/Emissive Material | Dominant Wavelengths | Study Type/Biological Media | Targeted Indicators | Features | Refs. |
|---|---|---|---|---|---|---|---|
| 2011 | OPD | rr-P3HT:PC61BM | 525–550 nm | Cultured hippocampal neurons (in vitro) | - | Bioorganic interface | [98] |
| 2014 | OLED | n/a | 455 nm | - | ChR2 | Flexible TFT-based OLED display | [91] |
| 2015 | OLED | n/a | 475 nm | Single cells of Chlamydomonas reinhardtii | ChR1, ChR2 | CMOS backplane | [90] |
| 2016 | OLED | n/a | 475 nm | Cultured HEK-293 cells (in vitro) | ChR2, EYFP, mCherry | CMOS backplane | [68] |
| 2016 | OLED | TBPe:MADN Ir(ppy)3 Ir(MDQ)2(acac) | 464 nm 515 nm 606 nm | Locomotor behavior of Drosophila melanogaster (in vivo) | ChR2 | Irradiance: 250–400 μW mm−2 | [99] |
| 2016 | OLED | n/a | 455 nm, (620 nm) | Cultured cortical neurons (in vitro) | ChR2, YFP, (Chrimson) | TFT-based OLED display | [92] |
| 2016 | OLED | TBPe:MADN | 465, 493 nm | Behavioral changes of Drosophila melanogaster | ChR2, (H134R) | Irradiance: 10 μW mm−2 | [100] |
| 2018 | OPD | P3HT:PC61BM | Visible (white) | Changes in transmittance in mice slices (ex vivo) | - | Halogen light source | [101] |
| 2019 | OLED | TBPe:MADN | 460 nm | Fixed tissue slices, live cells and preparations of D. melanogaster | eGFP, GCaMP6s | Narrowband OLEDs | [94] |
| 2019 | OLED | PFO, SO-PPV & others | 469 nm 573 nm | Cultured hippocampal neurons (in vitro) | ChrimsonR SSFO | Irradiance: 100–150 μW mm−2 | [97] |
| 2019 | OLED | MADN:TBPe | 463 nm | Cultured primary neurons (in vitro) | CheRiff | High-power and stable OLEDs. Irradiance: 60–800 μW mm−2 | [14] |
| 2020 | OLED | n/a | 455 nm 520 nm | Cultured cortical neurons (in vitro), and transgenic mouse | ChR2, C1V1tt | Irradiance: 1000 μW mm−2 | [95] |
| 2020 | OLED | n/a (STSB010) | 455 nm | Transgenic rat | ChR2 | Flexible, MRI-compatible OLEDs. Irradiance: 500 μW mm−2 | [66] |
| 2020 | OLED | TBPe:MADN | 460–560 nm | Drosophila melanogaster | CsChrimson, GtACR2 | Locomotor behavior. Irradiance: 15–30 μW mm−2 | [102] |
| 2021 | OPD | Rubrene/C60 | 400–575 nm | Cultured cortical neurons (in vitro) | Cal-520 | Direct detection of neuronal activity Sensitivity: 0.5–20 nW cm−2 | [13] |
| 2022 | OLED | Ir(MDQ)2(acac) | 600 nm | (Retinal cells) | (ChrimsonR) | Tandem-stack architecture, silicon substrate | [93] |
| 2022 | OLED | Super-Yellow | 556 nm | Cultured hippocampal neurons (in vitro) | Chrimson | Spatial localization of neurons. Irradiance: 10–38 μW mm−2 | [15] |
| 2022 | OLED | TBPe:MADN Ir(ppy)2(acac) Ir(MDQ)2(acac) | 462 nm 557 nm 620 nm | ND7/23 cells and neurons in Drosophila melanogaster | ChRmine, GtACR2 & Chrimson (BiPOLEs) | OLED bicolor emission. Irradiance: 1–55 μW mm−2 | [12] |
| 2023 | OLED | TBPe Ir(MDQ)2(acac) | 460, 500 nm 620 nm | Transgenic mice (in vivo) | ChR2 ChRmine | CMOS-based | [103] |
| 2023 | OLED OPD | Rubrene/C60 Super-Yellow | 400–575 nm 556 nm | Cultured cortical and hippocampal neurons (in vitro) | Cal-520, Chrimson | Direct detection and stimulation | [96] |
| 2024 | OLED | MADN:TBPe Ir(MDQ)2(acac) | 460 nm 607 nm | Localized stimulation of Drosophila melanogaster | GtACR2 & Chrimson (BiPOLEs) | Dual-color OLEDs; behavioral change. Irradiance: 134–238 μW mm−2 | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kielar, M.; Kenna, M.; Blanchard, P.; Sah, P. Application of Organic Light-Emitting Diodes and Photodiodes in Optical Control and Detection of Neuronal Activity. Photonics 2025, 12, 281. https://doi.org/10.3390/photonics12030281
Kielar M, Kenna M, Blanchard P, Sah P. Application of Organic Light-Emitting Diodes and Photodiodes in Optical Control and Detection of Neuronal Activity. Photonics. 2025; 12(3):281. https://doi.org/10.3390/photonics12030281
Chicago/Turabian StyleKielar, Marcin, Matthew Kenna, Philippe Blanchard, and Pankaj Sah. 2025. "Application of Organic Light-Emitting Diodes and Photodiodes in Optical Control and Detection of Neuronal Activity" Photonics 12, no. 3: 281. https://doi.org/10.3390/photonics12030281
APA StyleKielar, M., Kenna, M., Blanchard, P., & Sah, P. (2025). Application of Organic Light-Emitting Diodes and Photodiodes in Optical Control and Detection of Neuronal Activity. Photonics, 12(3), 281. https://doi.org/10.3390/photonics12030281





