Laser Power Modulation of Fiber Coated with Multilayer-Graphene Based on Lithium Intercalation Method
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Device Fabrication for Characterization
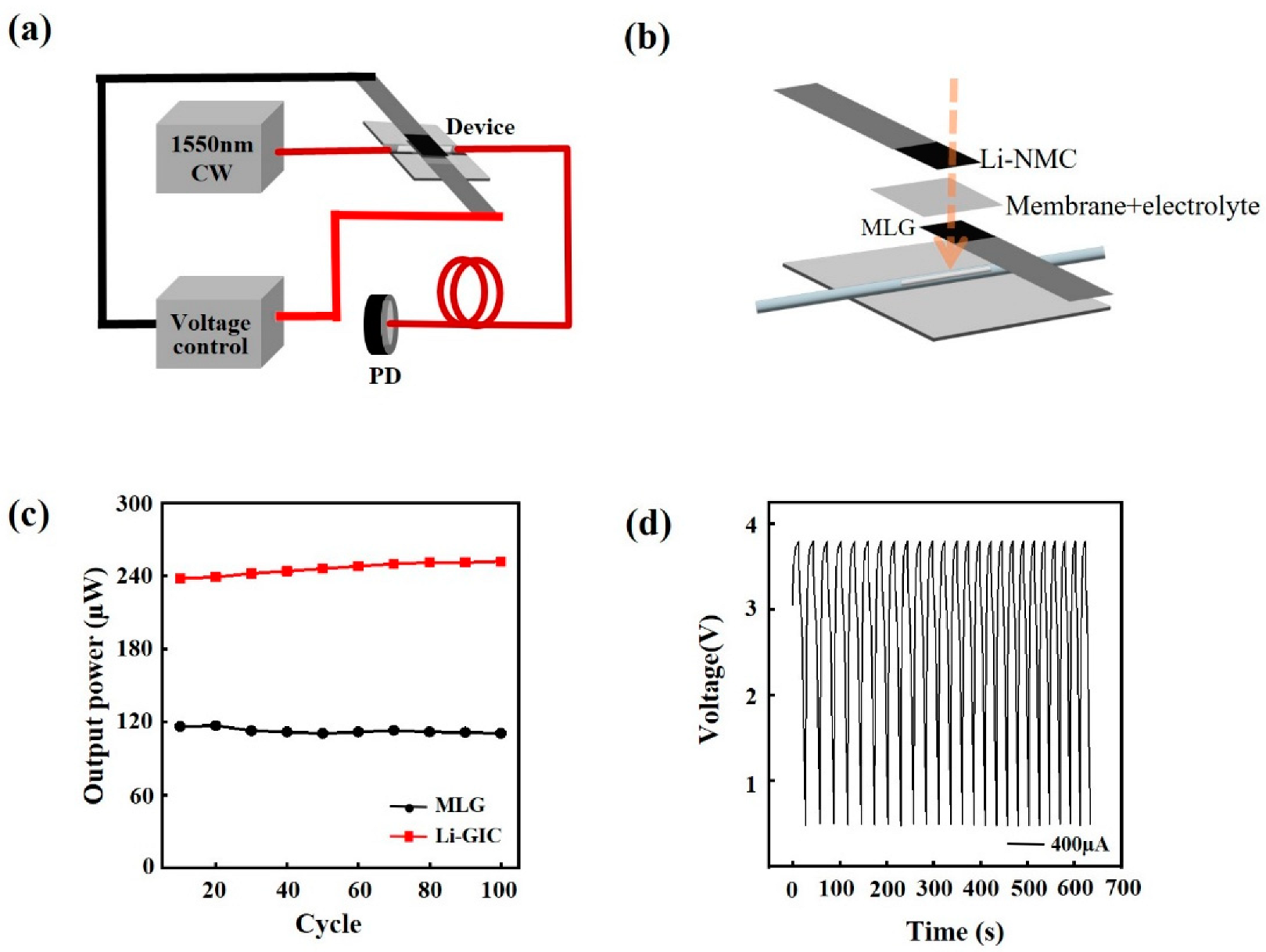
2.3. Device Fabrication for Laser Power Modulation
2.4. Sample Characterization
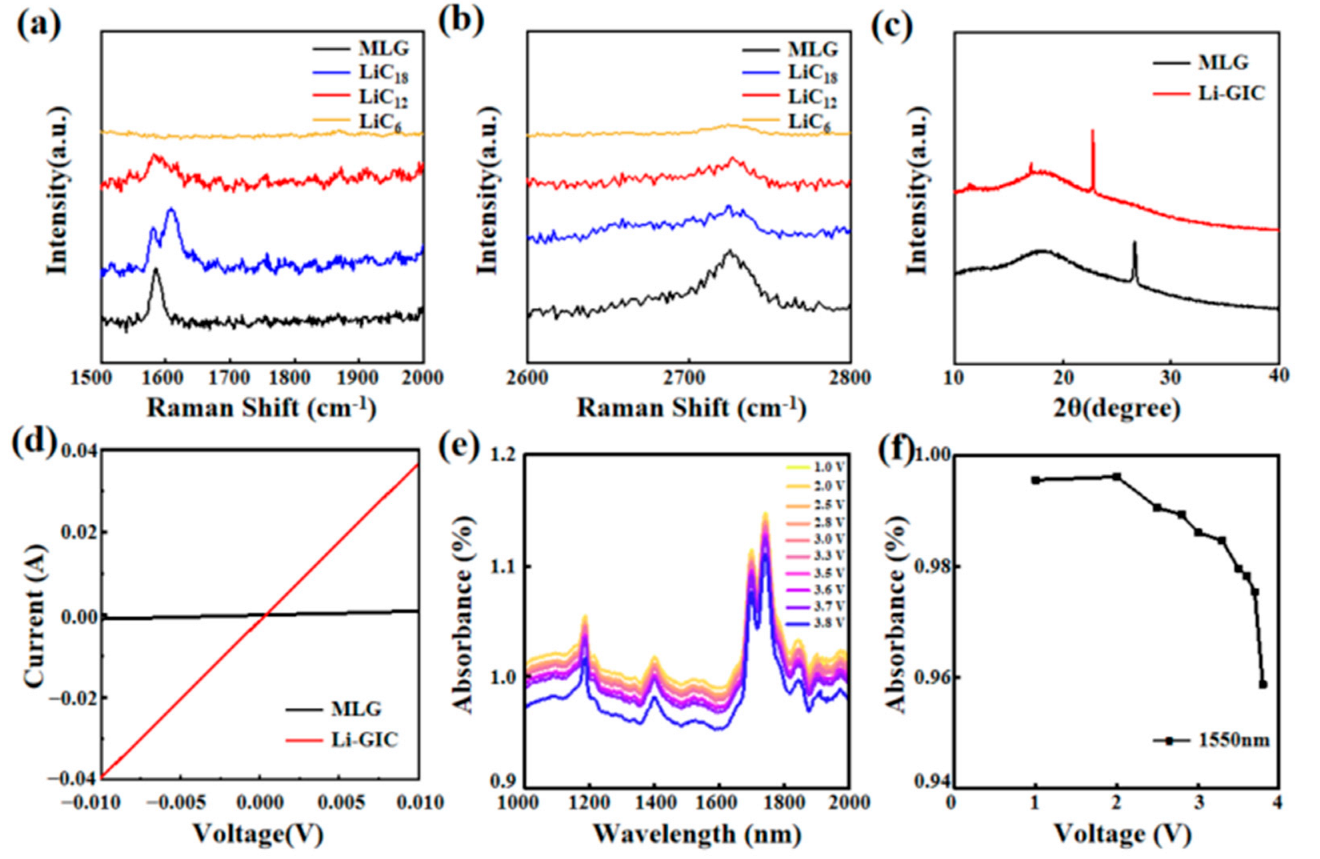
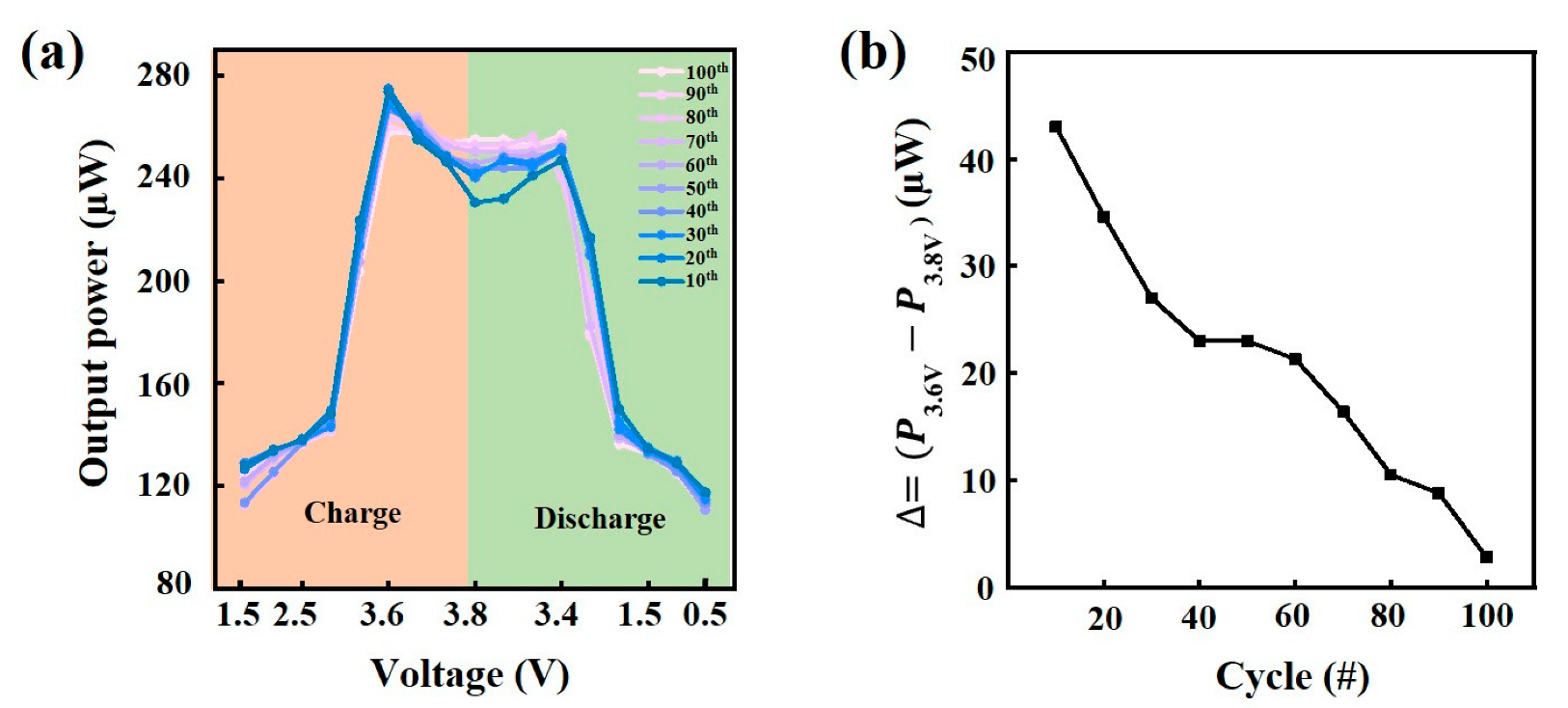
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, Y.; Hansson, J.; Liu, Y.; Chen, S.; Zehri, A.; Samani, M.K.; Wang, N.; Ni, Y.; Zhang, Y.; Zhang, Z.-B.; et al. Graphene related materials for thermal management. 2D Mater. 2019, 7, 012001. [Google Scholar] [CrossRef]
- Li, X.; Li, B.-H.; He, Y.-B.; Kang, F.-Y. A review of graphynes: Properties, applications and synthesis. New Carbon. Mater. 2020, 35, 619–629. [Google Scholar] [CrossRef]
- Lin, L.; Deng, B.; Sun, J.; Peng, H.; Liu, Z. Bridging the Gap between Reality and Ideal in Chemical Vapor Deposition Growth of Graphene. Chem. Rev. 2018, 118, 9281–9343. [Google Scholar] [CrossRef]
- Naumis, G.G.; Barraza-Lopez, S.; Oliva-Leyva, M.; Terrones, H. Electronic and optical properties of strained graphene and other strained 2D materials: A review. Rep. Prog. Phys. 2017, 80, 096501. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.; Wang, D.N.; Chen, Q. Passively mode-locked fiber laser based on graphene covered single-mode fiber with inner short waveguides. Opt. Commun. 2022, 505, 127520. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Zhang, X.; Mateos, X.; Guo, S.; Wang, Z.; Xu, X.; Loiko, P.; Bae, J.E.; Rotermund, F.; Sotor, J.J.O.E. Graphene and SESAM mode-locked Yb:CNGS lasers with self-frequency doubling properties. Opt. Express 2019, 27, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, Y.; Jiang, M.; Xu, X.; Wu, H.; Tian, Y.; Bai, J.; Ren, Z. Passively Q-switched and mode-locked fiber laser research based on graphene saturable absorbers. Opt. Quantum Electron. 2017, 49, 137. [Google Scholar] [CrossRef]
- Liu, S.; Xu, S.; Guan, J.; Qiu, G.; Tong, Z.; Yang, M.; Wei, Z.; Tan, C.; Wang, F.; Meng, H. Interferometric fiber sensor for lead ion detection based on MoS2 hydrogel coating. Opt. Commun. 2024, 559, 130416. [Google Scholar]
- Chen, K.; Zhou, X.; Cheng, X.; Qiao, R.; Cheng, Y.; Liu, C.; Xie, Y.; Yu, W.; Yao, F.; Sun, Z.; et al. Graphene photonic crystal fibre with strong and tunable light–matter interaction. Nat. Photonics 2019, 13, 754–759. [Google Scholar] [CrossRef]
- Chen, R.; Liu, W.; Huang, G.; Wang, D.; Qin, X.; Feng, W. Hydrogen sulfide sensor based on tapered fiber sandwiched between two molybdenum disulfide/citric acid composite membrane coated long-period fiber gratings. Appl. Opt. 2018, 57, 9755–9759. [Google Scholar] [CrossRef] [PubMed]
- Hajiani, T.; Shirkani, H.; Sadeghi, Z. Surface plasmon resonance photonic crystal fiber biosensor: Utilizing silver-phosphorene nanoribbons for Near-IR detection. Opt. Commun. 2024, 569, 130801. [Google Scholar] [CrossRef]
- Qin, Z.; Xie, G.; Ma, J.; Yuan, P.; Qian, L. 28 μm all-fiber Q-switched and mode-locked lasers with black phosphorus. Photonics Res. 2018, 6, 1074–1078. [Google Scholar] [CrossRef]
- Wang, S.; Su, M.; Tang, L.; Li, X.; Li, X.; Bai, H.; Niu, P.; Shi, J.; Yao, J. Graphene-coated D-shaped terahertz fiber modulator. Front. Phys. 2023, 11, 1202839. [Google Scholar] [CrossRef]
- Bakhshi, A.; Jalaly, M.; Vahedi, M. The effect of GO–Fe3O4 hybrid coating on the magnetic field detection by a tapered optical fiber sensor. Opt. Fiber Technol. 2022, 74, 103134. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Ding, E.J.; Xu, S.C.; Li, Z.H.; Wang, X.X.; Song, F. Sensitization of an optical fiber methane sensor with graphene. Opt. Fiber Technol. 2017, 37, 26–29. [Google Scholar] [CrossRef]
- Hou, L.; Bi, K.; Li, Q.; Zhang, S.; Guo, M.; Zhuang, Y.; Huang, D.; Zhang, S.; Han, S.; Mei, L. Terahertz absorption properties of different graphene layers based on the Salisbury effect. Opt. Express 2024, 32, 23907–23915. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Sun, M.; Zhang, W.; Zhang, W. D-Shaped photonic crystal fiber with graphene coating for terahertz polarization filtering and sensing applications. Opt. Fiber Technol. 2023, 79, 103373. [Google Scholar]
- Qi, Z.; Zhu, X.; Jin, H.; Zhang, T.; Kong, X.; Ruoff, R.S.; Qiao, Z.; Ji, H. Rapid Identification of the Layer Number of Large-Area Graphene on Copper. Chem. Mater. 2018, 30, 2067–2073. [Google Scholar] [CrossRef]
- Wu, D.; Sun, M.; Zhang, W.; Zhang, W. Simultaneous Regulation of Surface Properties and Microstructure of Graphene Oxide Membranes for Enhanced Nanofiltration Performance. ACS Appl. Mater. Interfaces, 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Yankowitz, M. Unravelling the magic of twisted trilayer graphene. Nat. Mater. 2023, 22, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Y.Y.; Zhang, S.; Cai, H.; Tong, L.H.; Liao, W.Y.; Zou, R.J.; Xue, S.M.; Tian, Y.; Chen, T.; et al. Layer-dependent evolution of electronic structures and correlations in rhombohedral multilayer graphene. Nat. Nanotechnol. 2025, 20, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Shu, H.; Shen, Z.; Hu, H.; Wang, J.; Chen, X. Electrochemical Lithiation Mechanism of Two-Dimensional Transition-Metal Dichalcogenide Anode Materials: Intercalation versus Conversion Reactions. J. Phys. Chem. C 2019, 123, 2139–2146. [Google Scholar] [CrossRef]
- Mak, K.F.; Ju, L.; Wang, F.; Heinz, T.F. Optical spectroscopy of graphene: From the far infrared to the ultraviolet. Solid State Commun. 2012, 152, 1341–1349. [Google Scholar] [CrossRef]
- Chen, C.; Li, N.W.; Wang, B.; Yuan, S.; Yu, L. Advanced pillared designs for two-dimensional materials in electrochemical energy storage. Nanoscale Adv. 2020, 2, 5496–5503. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Matsumoto, R.; Liu, Q.; Solis-Fernandez, P.; Siao, M.D.; Chiu, P.W.; Ago, H.; Suenaga, K. Alkali metal bilayer intercalation in graphene. Nat. Commun. 2024, 15, 425. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Tan, P.H.; Liu, J.; Ferrari, A.C. Intercalation of few-layer graphite flakes with FeCl3: Raman determination of Fermi level, layer by layer decoupling, and stability. J. Am. Chem. Soc. 2011, 133, 5941–5946. [Google Scholar] [CrossRef] [PubMed]
- Bonavolonta, C.; Vettoliere, A.; Pannico, M.; Crisci, T.; Ruggiero, B.; Silvestrini, P.; Valentino, M. Investigation of Graphene Single Layer on P-Type and N-Type Silicon Heterojunction Photodetectors. Sensors 2024, 24, 6068. [Google Scholar] [CrossRef] [PubMed]
- Di Gaspare, A.; Pogna, E.A.A.; Salemi, L.; Balci, O.; Cadore, A.R.; Shinde, S.M.; Li, L.; di Franco, C.; Davies, A.G.; Linfield, E.H.; et al. Tunable, Grating-Gated, Graphene-On-Polyimide Terahertz Modulators. Adv. Funct. Mater. 2020, 31, 2008039. [Google Scholar] [CrossRef]
- Bao, W.; Wan, J.; Han, X.; Cai, X.; Zhu, H.; Kim, D.; Ma, D.; Xu, Y.; Munday, J.N.; Drew, H.D. Approaching the limits of transparency and conductivity in graphitic materials through lithium intercalation. Nat. Commun. 2014, 5, 4224. [Google Scholar] [CrossRef]
- Li, B.; Zheng, L.; Gong, Y.; Kang, P. Intercalation-driven tunability in two-dimensional layered materials: Synthesis, properties, and applications. Mater. Today 2024, 81, 118–136. [Google Scholar] [CrossRef]
- Yu, X.; Bakan, G.; Guo, H.; Ergoktas, M.S.; Steiner, P.; Kocabas, C. Reversible Ionic Liquid Intercalation for Electrically Controlled Thermal Radiation from Graphene Devices. ACS Nano 2023, 17, 11583–11592. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yin, L.; Ren, D.; Wang, L.; Ren, Y.; Xu, W.; Lapidus, S.; Wang, H.; He, X.; Chen, Z.; et al. In situ observation of thermal-driven degradation and safety concerns of lithiated graphite anode. Nat. Commun. 2021, 12, 4235. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Zhang, R.; Sui, Y.; Li, X.; OuYang, H.; Pu, M.; Chen, H.; Ma, X.; Cheng, X.; Yan, W.; et al. Tunable Optical Display of Multilayer Graphene through Lithium Intercalation. ACS Appl. Mater. Interfaces 2023, 15, 53688–53696. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Zhang, R.; Sui, Y.; Li, X.; OuYang, H.; Pu, M.; Chen, H.; Ma, X.; Cheng, X.; Yan, W.; et al. Inversion Symmetry Breaking in Lithium Intercalated Graphitic Materials. ACS Appl. Mater. Interfaces 2020, 12, 28561–28567. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef]
- Liang, T.; Peng, G.; Zhang, X.; Wei, Y.; Zheng, X.; Luo, W.; Dai, M.; Deng, C.; Zhang, X. Modulating visible-near-infrared reflectivity in ultrathin graphite by reversible Li-ion intercalation. Opt. Mater. 2021, 121, 111517. [Google Scholar] [CrossRef]
- Song, H.; Xie, H.; Xu, C.; Kang, Y.; Li, C.; Zhang, Q. In Situ Measurement of Strain Evolution in the Graphene Electrode during Electrochemical Lithiation and Delithiation. J. Phys. Chem. C 2019, 123, 18861–18869. [Google Scholar] [CrossRef]
- Yadegari, H.; Koronfel, M.A.; Wang, K.; Thornton, D.B.; Stephens, I.E.L.; Molteni, C.; Haynes, P.D.; Ryan, M.P. Operando Measurement of Layer Breathing Modes in Lithiated Graphite. ACS Energy Lett. 2021, 6, 1633–1638. [Google Scholar] [CrossRef]
- Ergoktas, M.S.; Bakan, G.; Steiner, P.; Bartlam, C.; Malevich, Y.; Ozden-Yenigun, E.; He, G.; Karim, N.; Cataldi, P.; Bissett, M.A.; et al. Graphene-Enabled Adaptive Infrared Textiles. Nano Lett. 2020, 20, 5346–5352. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Y.; Kinoshita, K.; Tran, T.D. Microstructural Character-ization of Lithiated Graphite. J. Electrochem. Soc. 1996, 6, 120–123. [Google Scholar] [CrossRef]
- Muhammad, A.R.; Rusdi, M.F.; Jafry, A.A.A.; Markom, A.M.; Jusoh, Z.; Haris, H.; Harun, S.W.; Yupapin, P. Evanescent field interaction of 1550 nm pulsed laser with silver nanomaterial coated D-shape fiber. Infrared Phys. Technol. 2021, 119, 103920. [Google Scholar] [CrossRef]
- Zeng, L.Z.; Ou, Z.T.; Yang, H.Y. Sensitivity Improvement of Plasmonic Optical Fiber Sensors with Graphene-Metal Nanowire Array. Acta Opt. Sin. 2022, 42, 1906002. [Google Scholar]
- Zhang, W.; Wan, J.; Lie, M.; Luo, Y.; Guo, M. Microfluidic refractive index sensor with D-shape fiber and microtube coupling. Acta Phys. Sin. 2022, 71, 210701. [Google Scholar] [CrossRef]
- Wan, K.T. Quantitative Sensitivity Analysis of Surface Attached Optical Fiber Strain Sensor. IEEE Sens. J. 2014, 14, 1805–1812. [Google Scholar] [CrossRef]
- Zheng, X.; Shi, B.; Zhang, C.-C.; Sun, Y.; Zhang, L.; Han, H. Strain transfer mechanism in surface-bonded distributed fiber-optic sensors subjected to linear strain gradients: Theoretical modeling and experimental validation. Measurement 2021, 179, 109510. [Google Scholar] [CrossRef]
- Zhu, G.L.; Zhao, C.Z.; Huang, J.Q.; He, C.; Zhang, J.; Chen, S.; Xu, L.; Yuan, H.; Zhang, Q. Fast Charging Lithium Batteries: Recent Progress and Future Prospects. Small 2019, 15, e1805389. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.; Zeng, G.; Li, Y.; Wang, Z.; Xiao, L.; Jia, S.; Qin, C. Laser Power Modulation of Fiber Coated with Multilayer-Graphene Based on Lithium Intercalation Method. Photonics 2025, 12, 169. https://doi.org/10.3390/photonics12020169
Fang Z, Zeng G, Li Y, Wang Z, Xiao L, Jia S, Qin C. Laser Power Modulation of Fiber Coated with Multilayer-Graphene Based on Lithium Intercalation Method. Photonics. 2025; 12(2):169. https://doi.org/10.3390/photonics12020169
Chicago/Turabian StyleFang, Zhenyu, Ganying Zeng, Yijie Li, Zixuan Wang, Liantuan Xiao, Suotang Jia, and Chengbing Qin. 2025. "Laser Power Modulation of Fiber Coated with Multilayer-Graphene Based on Lithium Intercalation Method" Photonics 12, no. 2: 169. https://doi.org/10.3390/photonics12020169
APA StyleFang, Z., Zeng, G., Li, Y., Wang, Z., Xiao, L., Jia, S., & Qin, C. (2025). Laser Power Modulation of Fiber Coated with Multilayer-Graphene Based on Lithium Intercalation Method. Photonics, 12(2), 169. https://doi.org/10.3390/photonics12020169




