Highly Sensitive Dual-Polished Dual-Core PCF-Based SPR Sensor for Hemoglobin Detection Using FEM and Machine Learning
Abstract
1. Introduction
2. Sensor Design and Operation
2.1. Device Modeling and Structure
2.2. Experimental Setup and Working Mechanism of the Sensor
3. Results and Discussions
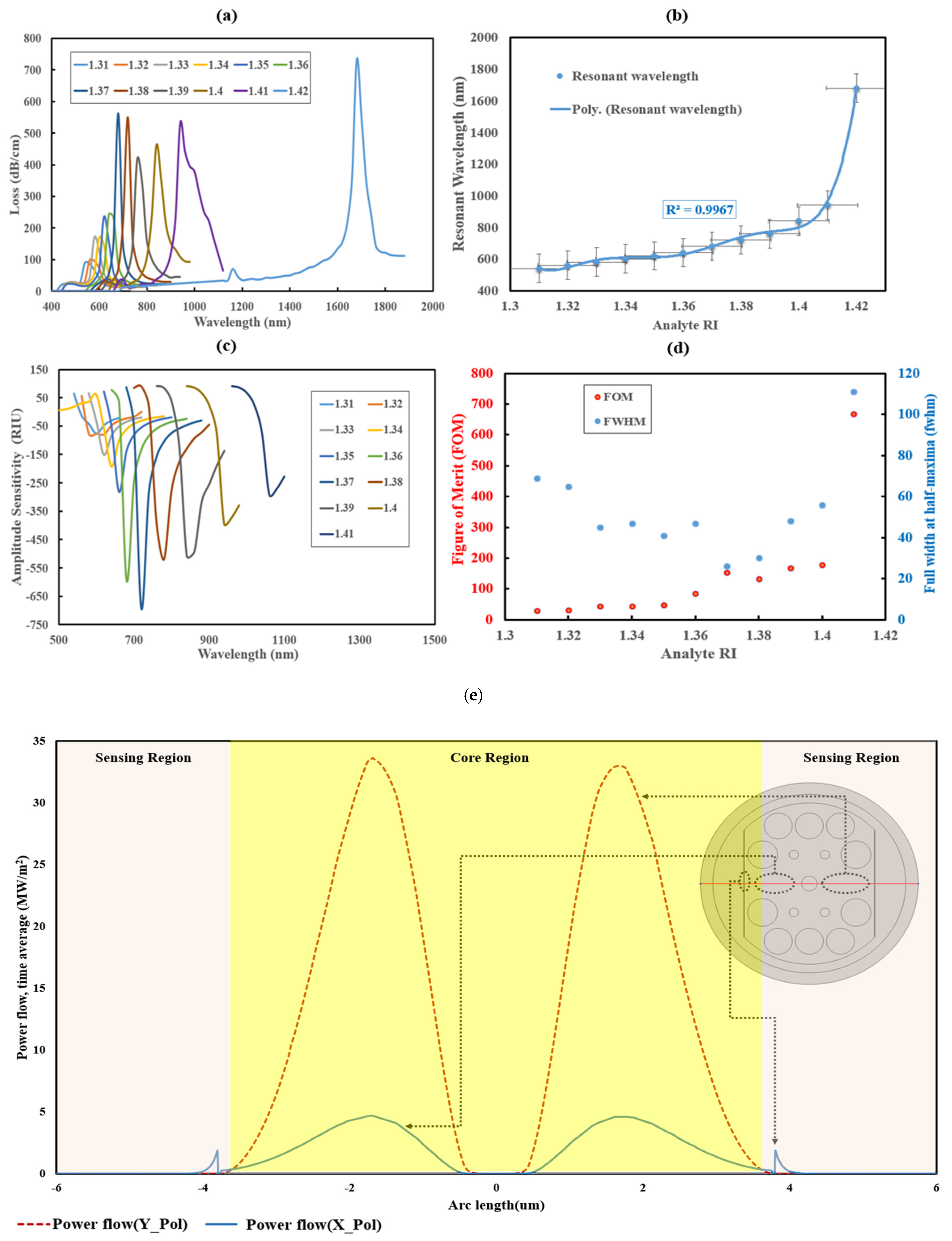
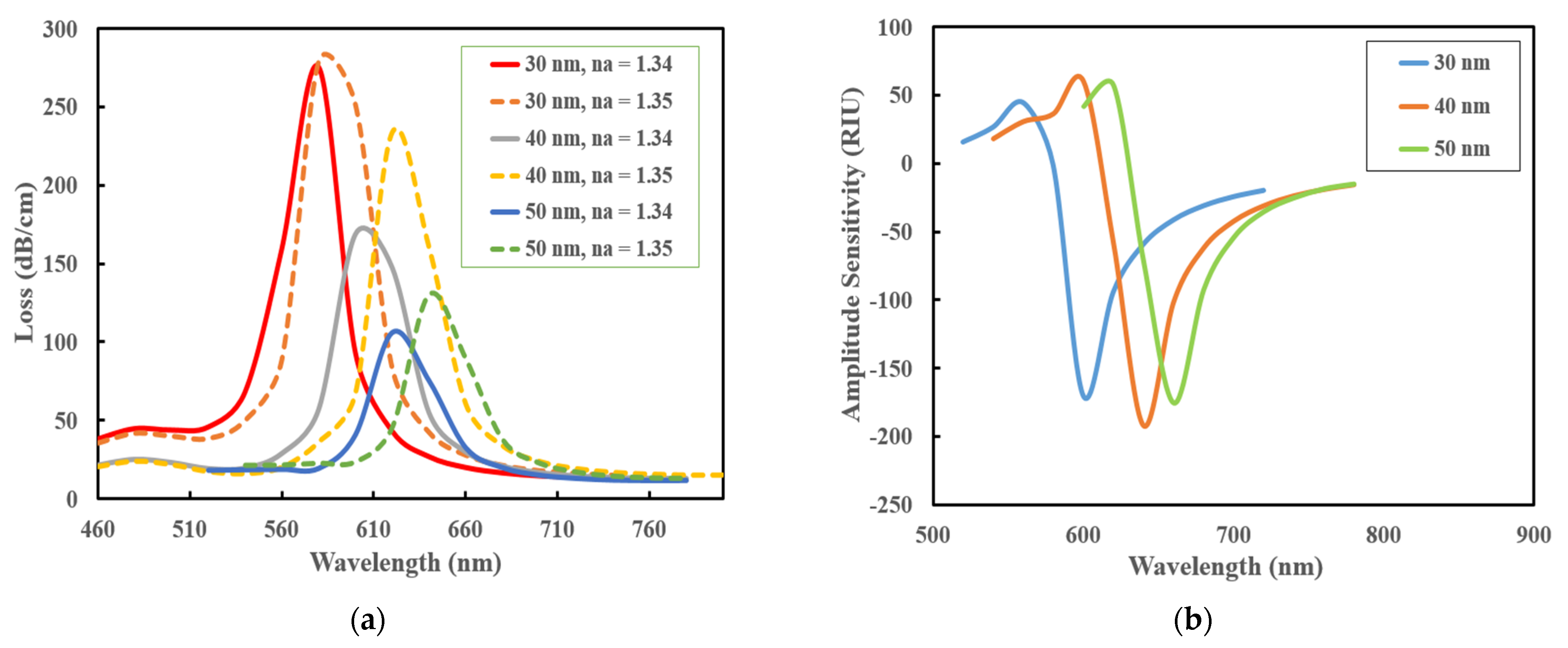
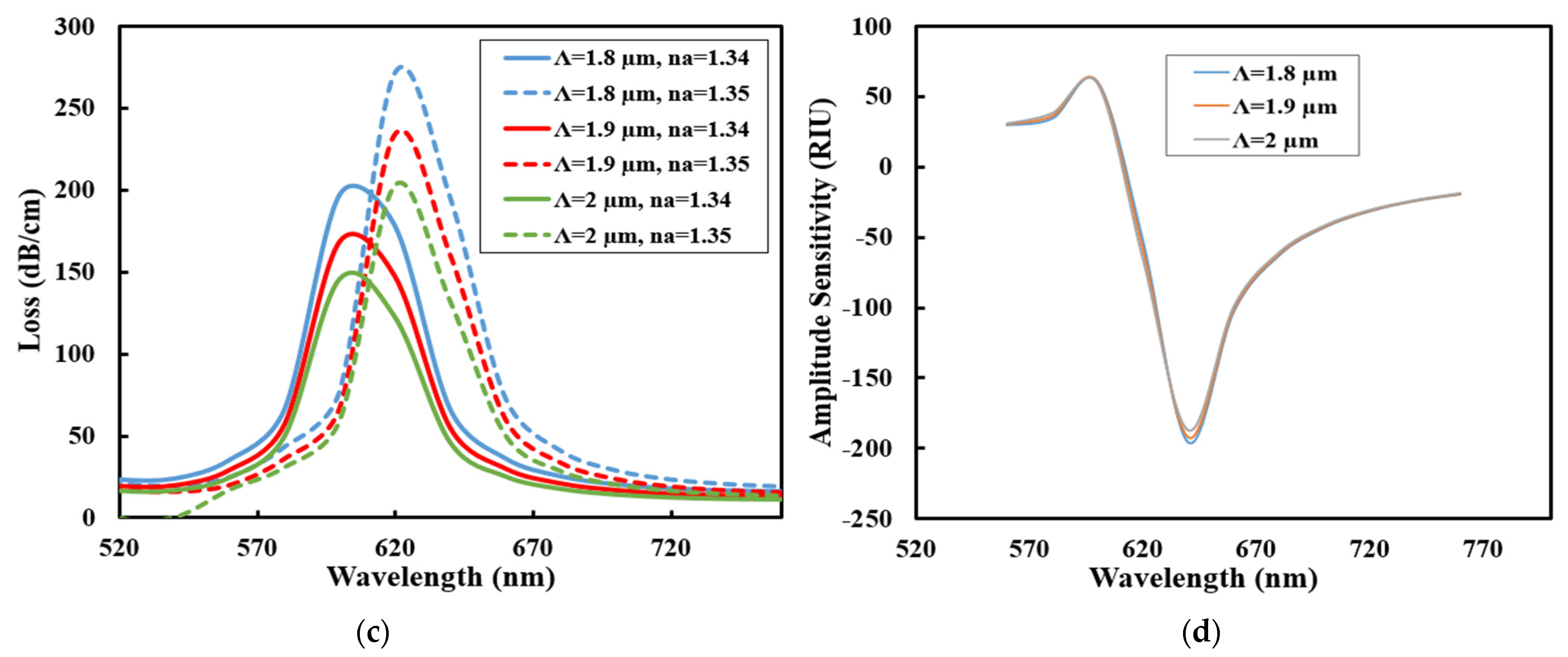
3.1. Performance Analysis
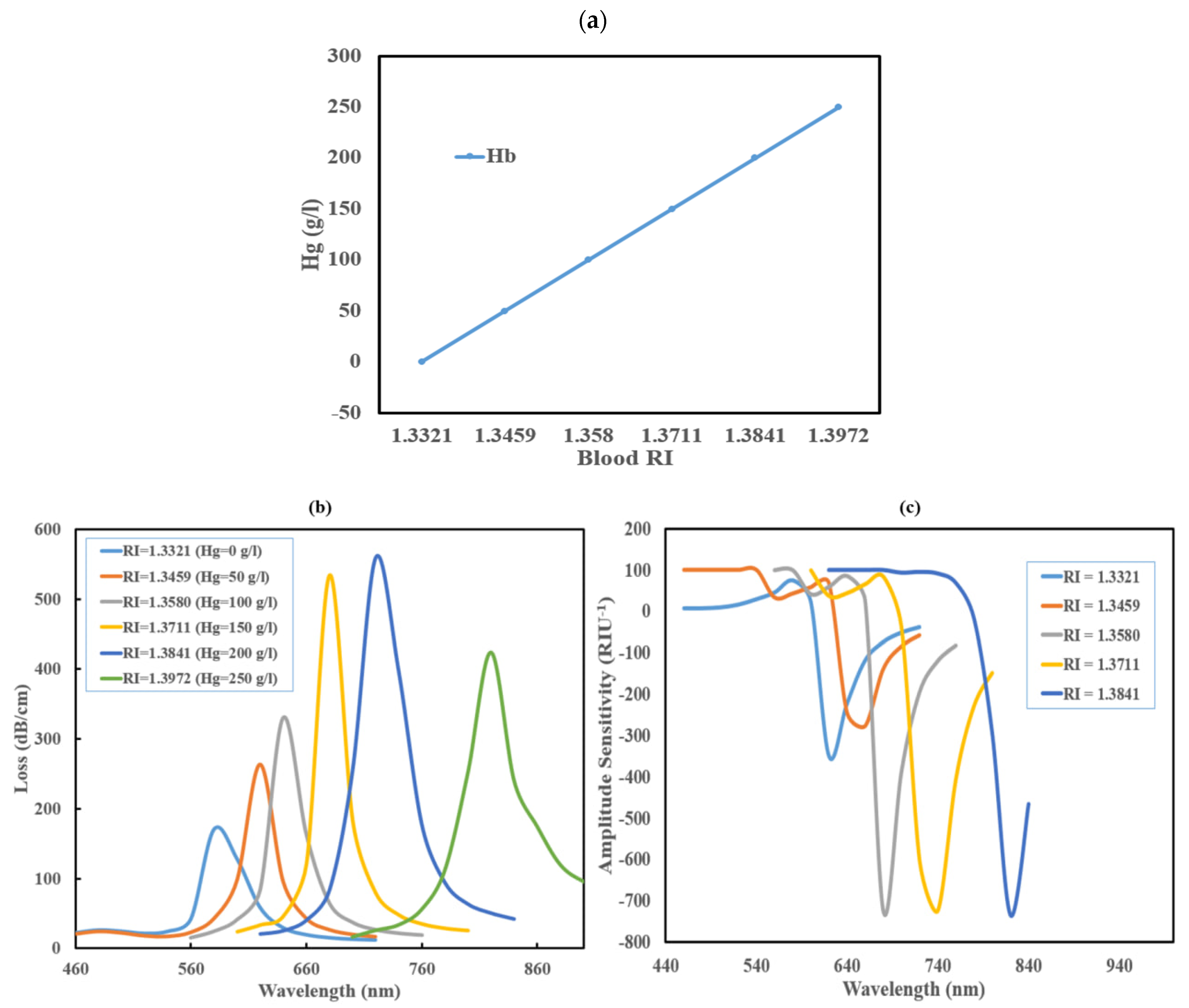
3.2. Hemoglobin (Hb) Level Detection Analysis
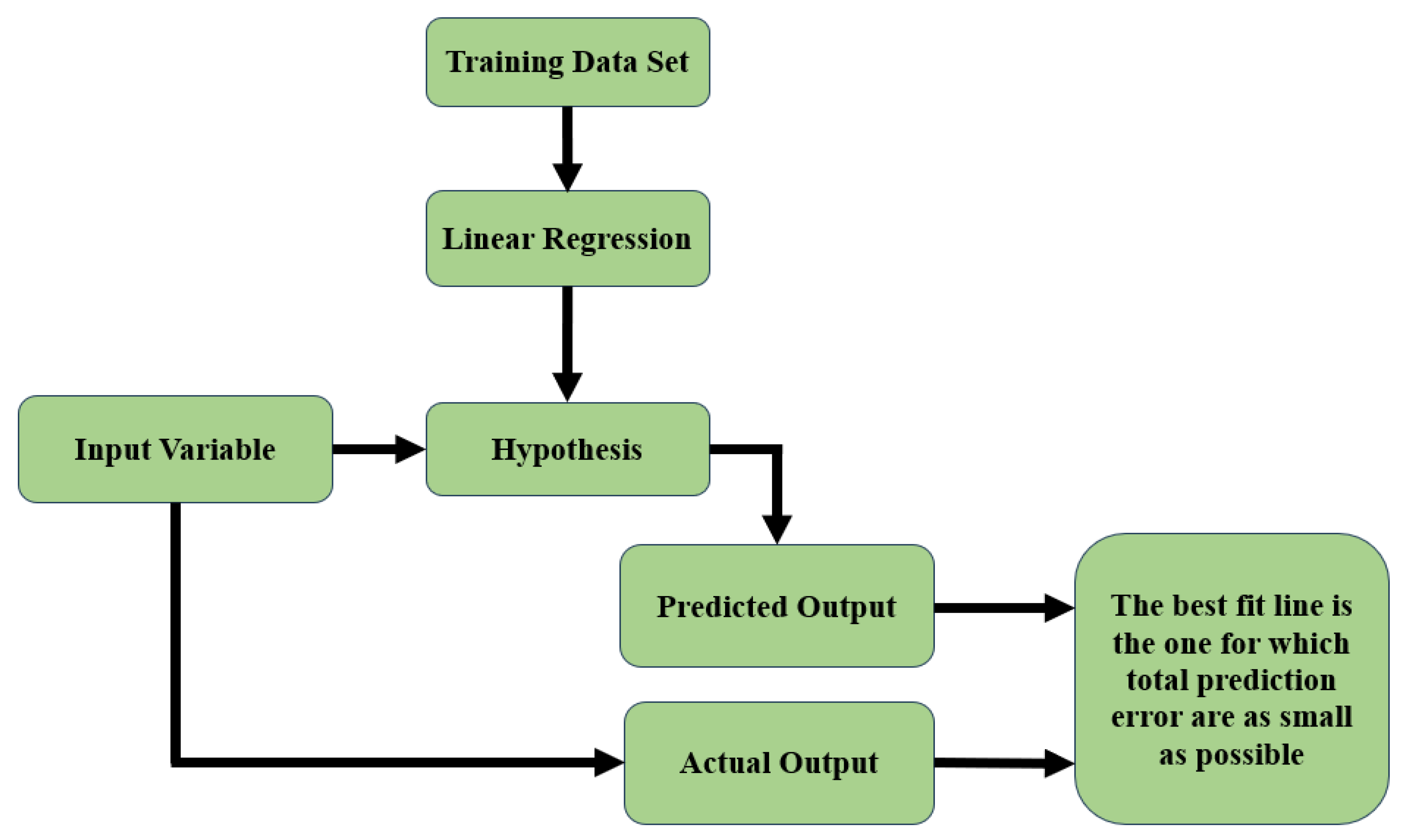
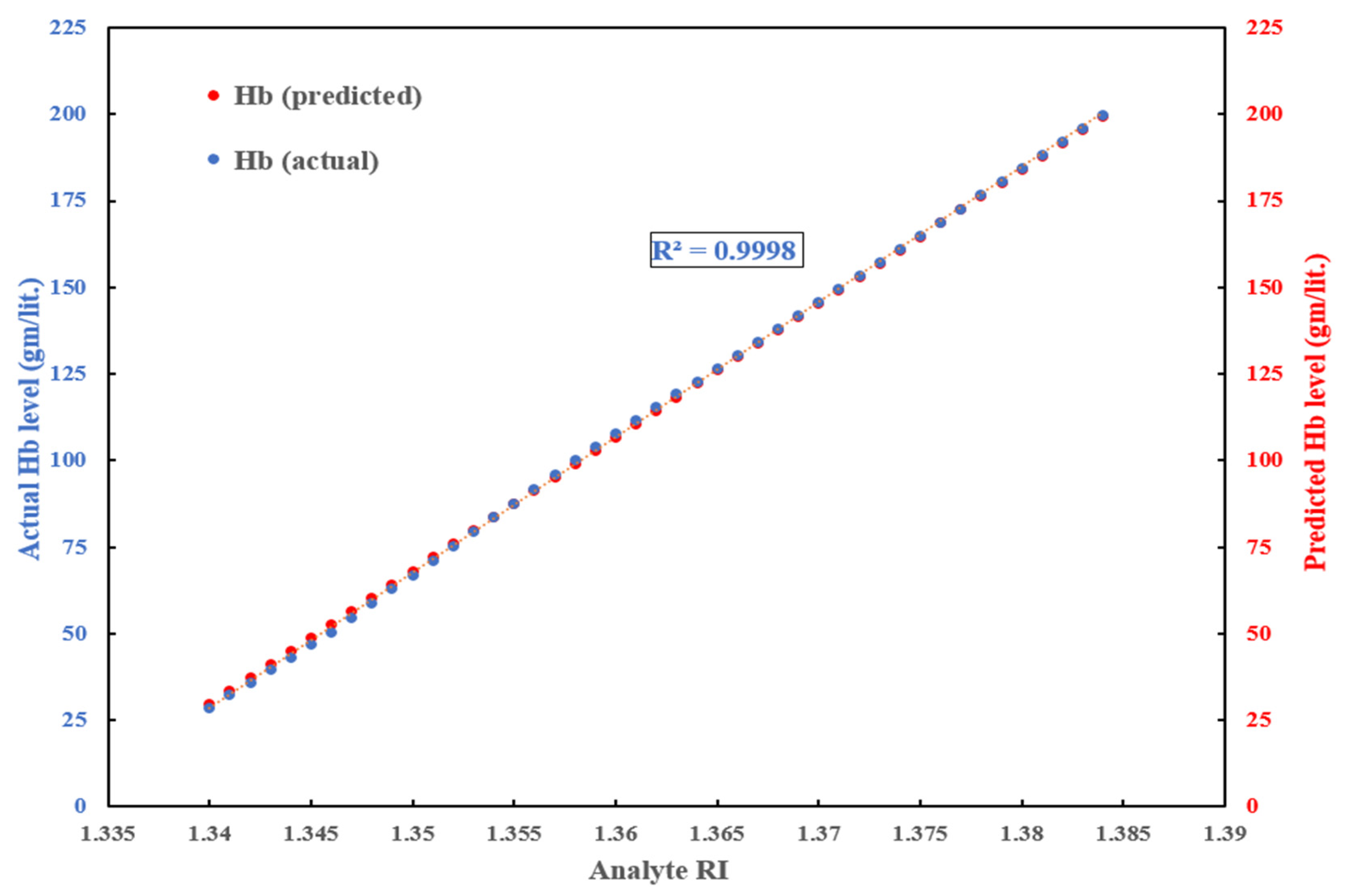
3.3. Machine Learning Model to Detect Hemoglobin
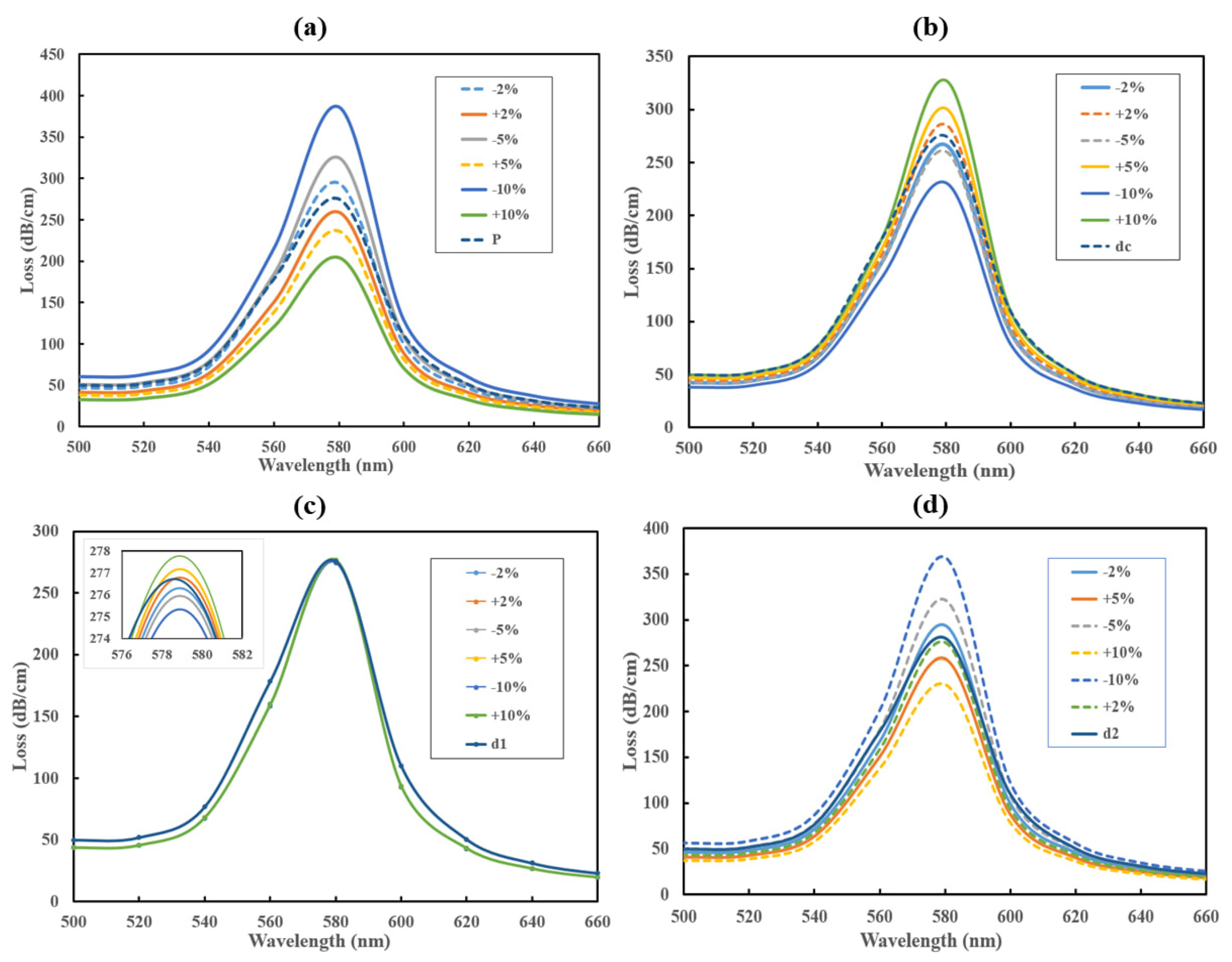
4. Optimization and Fabrication Tolerance Investigation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Homola, J. Present and future of surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef]
- Ahmed, T.; Haider, F.; Aoni, R.A.; Ahmed, R. Highly sensitive U-shaped micro-channel photonic crystal fiber-based plasmonic biosensor. Plasmonics 2021, 16, 2215–2223. [Google Scholar] [CrossRef]
- Vasimalla, Y.; Singh, L. Design and analysis of planar waveguide-based SPR sensor for formalin detection using Ag-Chloride-BP structure. IEEE Trans. Nanobiosci. 2022, 22, 365–374. [Google Scholar] [CrossRef]
- Hossain, B.; Paul, A.K.; Islam, A.; Hossain, F.; Rahman, M. Design and analysis of highly sensitive prism-based surface plasmon resonance optical salinity sensor. Results Opt. 2022, 7, 100217. [Google Scholar] [CrossRef]
- Hassani, A.; Skorobogatiy, M. Design criteria for microstructured-optical-fiber-based surface-plasmon-resonance sensors. J. Opt. Soc. Am. B 2007, 24, 1423–1429. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Z.-H.; Zhao, W.-M.; Wang, L.; Yan, X.; Zhu, A.-S.; Qiu, F.-M.; Zhang, K.-K. Research advances on surface plasmon resonance biosensors. Nanoscale 2022, 14, 564–591. [Google Scholar] [CrossRef]
- Maleki, M.J.; Soroosh, M.; Al-Shammri, F.K.; Alkhayer, A.G.; Mondal, H. Design and simulation of a compact subwavelength graphene-based switch for surface plasmon polariton transmission in integrated optoelectronic circuits. Plasmonics 2025, 20, 3605–3617. [Google Scholar] [CrossRef]
- Kaur, V.; Singh, S. A dual-channel surface plasmon resonance biosensor based on a photonic crystal fiber for multianalyte sensing. J. Comput. Electron. 2019, 18, 319–328. [Google Scholar] [CrossRef]
- Yasli, A.; Ademgil, H.; Haxha, S.; Aggoun, A. Multi-channel photonic crystal fiber-based surface plasmon resonance sensor for multi-analyte sensing. IEEE Photonics J. 2020, 12, 6800515. [Google Scholar] [CrossRef]
- Kumar, B.M.H.; Vaibhav, A.; Srikanth, P.C. Si/SiO2 based nano-cavity biosensor for detection of anemia, HIV and cholesterol using refractive index of blood sample. Indian J. Sci. Technol. 2022, 15, 899–907. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, M.; Tewari, R.K.; Kumar, A. A biosensor for the detection of anemia using metal Ag and defect multilayer photonic crystal. Plasmonics 2024, 19, 1463–1473. [Google Scholar] [CrossRef]
- Karki, B.; Vasudevan, B.; Uniyal, A.; Pal, A.; Srivastava, V. Hemoglobin detection in blood samples using a graphene-based surface plasmon resonance biosensor. Optik 2022, 270, 169947. [Google Scholar] [CrossRef]
- Rafiee, E. Diabetes and anemia prognosis by 2-D photonic crystal biosensor. Braz. J. Phys. 2025, 55, 73. [Google Scholar] [CrossRef]
- Shoshi, M.S.; Huraiya, A.; R, V.R.; Jawad, A.; Kong, C.Y.; Tabata, H.; Ramaraj, S.G.; Razzak, S.A. Revolutionary barium titanate-BlueP/TMDCs SPR sensor: Ultra-sensitive detection of urine glucose levels. Talanta Open 2025, 11, 100401. [Google Scholar] [CrossRef]
- Huraiya, A.; Ramaraj, S.G.; Hossain, S.M.S.; Chakrabarti, K.; Tabata, H.; Razzak, S.M.A. A highly optimized and sensitive bowtie shape-based SPR biosensor for different analyte detection. Nanoscale Adv. 2025, 7, 899–908. [Google Scholar] [CrossRef]
- Ashrafian, M.; Olyaee, S.; Seifouri, M. Highly sensitive cancer detection using an open D-channel PCF-based SPR biosensor. Sci. Rep. 2025, 15, 10168. [Google Scholar] [CrossRef] [PubMed]
- Hoque, A.M.T.; Islam, A.; Haider, F.; Aoni, R.A.; Ahmed, R. Point-of-care testing of hyponatremia and hypernatremia levels: An optoplasmonic biosensing approach. PLoS ONE 2025, 20, e0319559. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Haider, F.; Aoni, R.A.; Ahmed, R. Plasmonic photonic biosensor: In situ detection and quantification of SARS-CoV-2 particles. Opt. Express 2022, 30, 40277–40291. [Google Scholar] [CrossRef]
- Jain, S.; Choudhary, K.; Kumar, A.; Marques, C.; Kumar, S. PCF-based plasmonic sensor for the detection of cervical and skin cancer cells. Results Opt. 2024, 14, 100589. [Google Scholar] [CrossRef]
- Islam, A.; Haider, F.; Aoni, R.A.; Hossen, M.; Begum, F.; Ahmed, R. U-grooved dual-channel plasmonic sensor for simultaneous multi-analyte detection. J. Opt. Soc. Am. B 2021, 38, 3055–3063. [Google Scholar] [CrossRef]
- Das, A.; Huraiya, A.; R, V.R.; Tabata, H.; Ramaraj, S.G. Ultra-sensitive refractive index detection with gold-coated PCF-based SPR sensor. Talanta Open 2024, 10, 100384. [Google Scholar] [CrossRef]
- Huraiya, A.; Razzak, S.M.A.; Tabata, H.; Ramaraj, S.G. New approach for a highly sensitive V-shaped SPR biosensor for a wide range of analyte RI detection. J. Phys. Chem. C 2024, 128, 15117–15123. [Google Scholar] [CrossRef]
- Ahmed, T.; Paul, A.K.; Anower, S.; Razzak, S.M.A. Surface plasmon resonance biosensor based on hexagonal lattice dual-core photonic crystal fiber. Appl. Opt. 2019, 58, 8416–8422. [Google Scholar] [CrossRef]
- Khatun, M.R.; Islam, M.S. Design optimization of high-sensitivity PCF-SPR biosensor using machine learning and explainable AI. PLoS ONE 2025, 20, e0330944. [Google Scholar] [CrossRef]
- Divya, J.; Selvendran, S.; Itapu, S.; Borra, V. A novel dual-channel SPR-based PCF biosensor for simultaneous tuberculosis and urinary tract infection diagnosis toward SDG3. IEEE Access 2024, 12, 85484–85498. [Google Scholar] [CrossRef]
- Ehyaee, A.; Mohammadi, M.; Seifouri, M.; Olyaee, S. Design and numerical investigation of a dual-core photonic crystal fiber refractive index sensor for cancer cells detection. Eur. Phys. J. Plus 2023, 138, 129. [Google Scholar] [CrossRef]
- Mostufa, S.; Paul, A.K.; Chakrabarti, K. Detection of hemoglobin in blood and urine glucose level samples using a graphene-coated SPR based biosensor. OSA Contin. 2021, 4, 2164–2176. [Google Scholar] [CrossRef]
- Lee, H.; Hwang, S.; Kong, H.J.; Hong, K.H.; Yu, T.J. Simultaneous measurement of group refractive index dispersion and thickness of fused silica using a scanning white light interferometer. Sensors 2023, 24, 17. [Google Scholar] [CrossRef]
- Milam, D. Review and assessment of measured values of the nonlinear refractive-index coefficient of fused silica. Appl. Opt. 1998, 37, 546–550. [Google Scholar] [CrossRef]
- Sharma, P.; Sharan, P. Design of photonic crystal-based biosensor for detection of glucose concentration in urine. IEEE Sens. J. 2015, 15, 1035–1042. [Google Scholar] [CrossRef]
- Yajima, T.; Yamamoto, J.; Ishii, F.; Hirooka, T.; Yoshida, M.; Nakazawa, M. Low-loss photonic crystal fiber fabricated by a slurry casting method. Opt. Express 2013, 21, 30500–30506. [Google Scholar] [CrossRef] [PubMed]
- Boehm, J.; François, A.; Ebendorff-Heidepriem, H.; Monro, T.M. Chemical deposition of silver for the fabrication of surface plasmon microstructured optical fibre sensors. Plasmonics 2011, 6, 133–136. [Google Scholar] [CrossRef]
- Singhal, A.; Dalmiya, A.; Lynch, P.T.; Paprotny, I. 2-photon polymerized IP-DIP 3D photonic crystals for Mid IR spectroscopic applications. IEEE Photonics Technol. Lett. 2023, 35, 410–413. [Google Scholar] [CrossRef]
- Bunea, A.-I.; del Castillo Iniesta, N.; Droumpali, A.; Wetzel, A.E.; Engay, E.; Taboryski, R. Micro 3D printing by two-photon polymerization: Configurations and parameters for the Nanoscribe system. Micro 2021, 1, 164–180. [Google Scholar] [CrossRef]
- Wdowiak, E.; Ziemczonok, M.; Martinez-Carranza, J.; Kuś, A. Phase-assisted multi-material two-photon polymerization for extended refractive index range. Addit. Manuf. 2023, 73, 103666. [Google Scholar] [CrossRef]
- Dash, J.N.; Jha, R. SPR biosensor based on polymer PCF coated with conducting metal oxide. IEEE Photonics Technol. Lett. 2014, 26, 595–598. [Google Scholar] [CrossRef]
- Ma, A.; Li, Y.; Zhang, X. Coupled mode theory for surface plasmon polariton waveguides. Plasmonics 2013, 8, 769–777. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, Y.; Hu, X.G.; Yang, Y. Optical fiber quantum biosensor based on surface plasmon polaritons for the label-free measurement of protein. Sens. Actuators B Chem. 2020, 316, 128097. [Google Scholar] [CrossRef]
- Liu, C.; Yang, L.; Lu, X.; Liu, Q.; Wang, F.; Lv, J.; Chu, P.K. Mid-infrared surface plasmon resonance sensor based on photonic crystal fibers. Opt. Express 2017, 25, 14227–14237. [Google Scholar] [CrossRef]
- Dash, J.N.; Jha, R. Highly sensitive side-polished birefringent PCF-based SPR sensor in near IR. Plasmonics 2016, 11, 1505–1509. [Google Scholar] [CrossRef]
- Roni, T.A.; Hassan, R.; Faisal, M. Dual-side polished SPR biosensor with wide sensing range. In Proceedings of the 2020 11th International Conference on Electrical and Computer Engineering (ICECE), Dhaka, Bangladesh, 17–19 December 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 487–490. [Google Scholar]
- Gauvreau, B.; Hassani, A.; Fehri, M.F.; Kabashin, A.; Skorobogatiy, M.A. Photonic bandgap fiber-based surface plasmon resonance sensors. Opt. Express 2007, 15, 11413–11426. [Google Scholar] [CrossRef]
- Hautakorpi, M.; Mattinen, M.; Ludvigsen, H. Surface-plasmon-resonance sensor based on three-hole microstructured optical fiber. Opt. Express 2008, 16, 8427–8432. [Google Scholar] [CrossRef]
- Chen, N.; Chang, M.; Zhang, X.; Zhou, J.; Lu, X.; Zhuang, S. Highly sensitive plasmonic sensor based on a dual-side polished photonic crystal fiber for component content sensing applications. Nanomaterials 2019, 9, 1587. [Google Scholar] [CrossRef]
- Atiqullah, S.M.; Palit, A.; Reja, M.I.; Akhtar, J.; Fatema, S.; Absar, R. Detection of harmful food additives using highly sensitive photonic crystal fiber. Sens. Bio-Sens. Res. 2019, 23, 100275. [Google Scholar] [CrossRef]
- Bulbul, A.A.-M.; Imam, F.; Awal, A.; Mahmud, M.A.P. A novel ultra-low loss rectangle-based porous-core PCF for efficient THz waveguidance: Design and numerical analysis. Sensors 2020, 20, 6500. [Google Scholar] [CrossRef] [PubMed]
- Malitson, H. Interspecimen comparison of the refractive index of fused silica. J. Opt. Soc. Am. 1965, 55, 1205–1208. [Google Scholar] [CrossRef]
- Leviton, D.B.; Freed, C.J. Temperature-dependent absolute refractive index measurements of synthetic fused silica. In Proceedings of SPIE; SPIE: Bellingham, DC, USA, 2008; Volume 6273, 62732K. [Google Scholar]
- Reja, M.I.; Nguyen, L.V.; Peng, L.; Ebendorff-Heidepriem, H.; Warren-Smith, S.C. Temperature-compensated interferometric high-temperature pressure sensor using a pure silica microstructured optical fiber. IEEE Trans. Instrum. Meas. 2022, 71, 7002612. [Google Scholar] [CrossRef]
- Habia, M.I.; Manallah, A.; Ayadi, K. Plasmonic biosensor for the study of blood diseases by analysis of hemoglobin concentration. Opt. Quantum Electron. 2023, 55, 234. [Google Scholar] [CrossRef]
- White, M.; Fan, X. On the performance quantification of resonant refractive index sensors. Opt. Express 2008, 16, 1020–1028. [Google Scholar] [CrossRef]
- Asaduzzaman, S.; Ahmed, K. Investigation of ultra-low loss surface plasmon resonance-based PCF for biosensing application. Results Phys. 2018, 11, 358–361. [Google Scholar] [CrossRef]
- Liu, W.; Wang, F.; Liu, C.; Yang, L.; Liu, Q.; Su, W.; Lv, J.; An, S.; Li, X.; Sun, T.; et al. A hollow dual-core PCF-SPR sensor with gold layers on the inner and outer surfaces of the thin cladding. Res. Opt. 2020, 1, 100004. [Google Scholar] [CrossRef]
- Melwin, G.; Senthilnathan, K. Modelling a simple arc shaped gold coated PCF-based SPR sensor. J. Opt. 2024, 53, 117–126. [Google Scholar] [CrossRef]
- Tasnim, N.; Rahman, M.A.; Rahman, M.R.; Ahmed, T. Highly sensitive photonic crystal fiber-based surface plasmon resonance biosensor for detection of a wide range of organic solutions. Sens. Bio-Sens. Res. 2024, 43, 100623. [Google Scholar] [CrossRef]
- Das, S.; Singh, V.K. Highly sensitive PCF based plasmonic biosensor for hemoglobin concentration detection. Photonics Nanostruct.-Fundam. Appl. 2022, 51, 101040. [Google Scholar] [CrossRef]
- Azadi, M.; Seifouri, M.; Olyaee, S.; Mohammadi, M. Ultrahigh-sensitivity D-shaped PCF-SPR biosensor with TiO2–Au hybrid layers for precise detection of blood constituents. Results Phys. 2025, 77, 108463. [Google Scholar] [CrossRef]
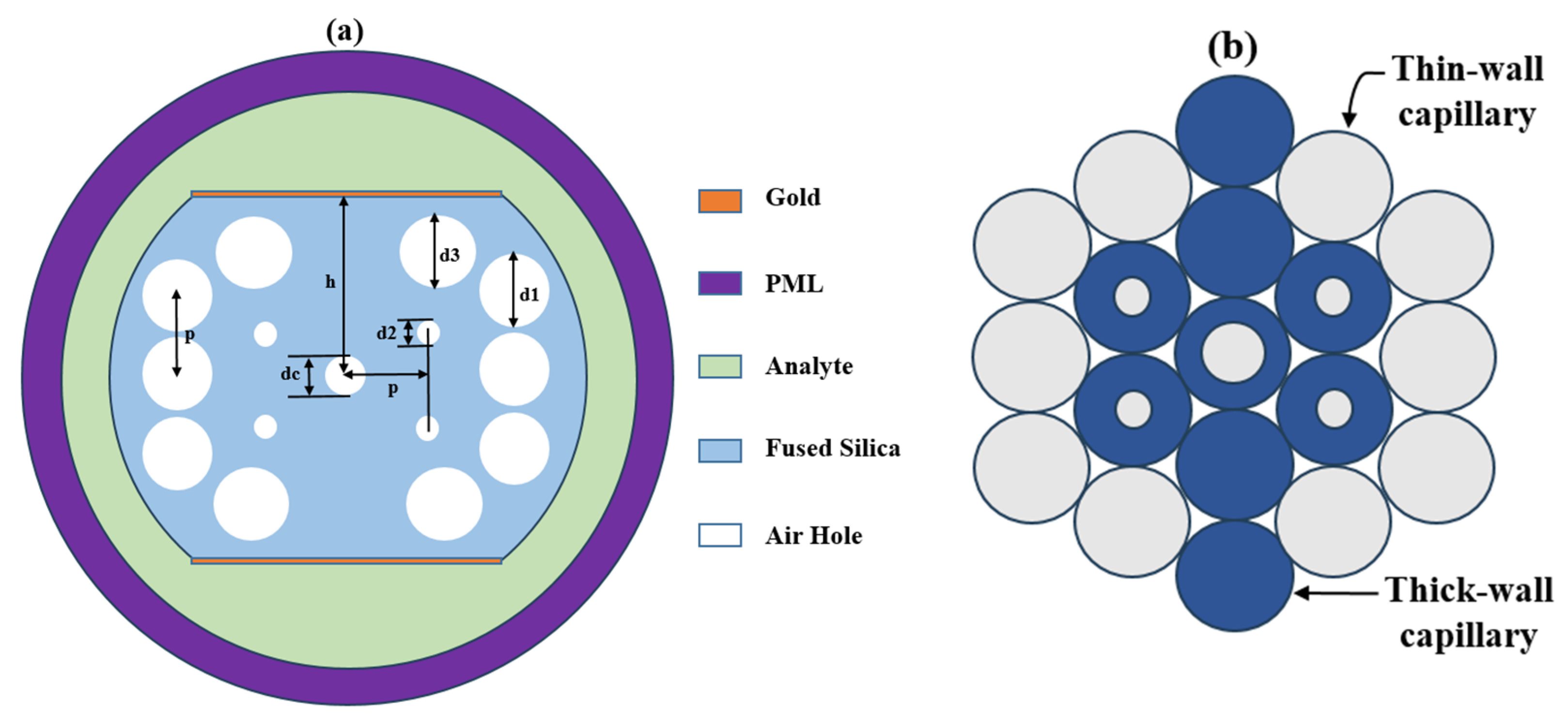
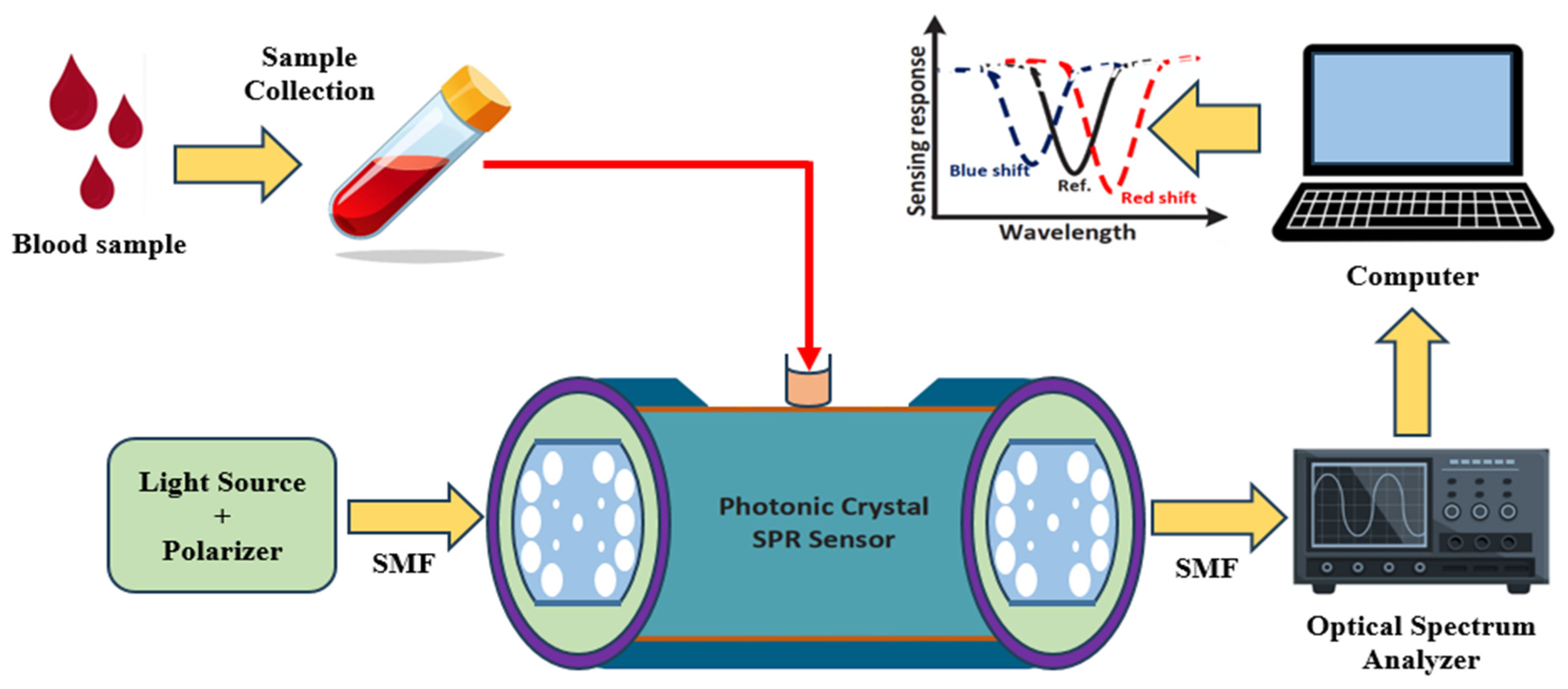
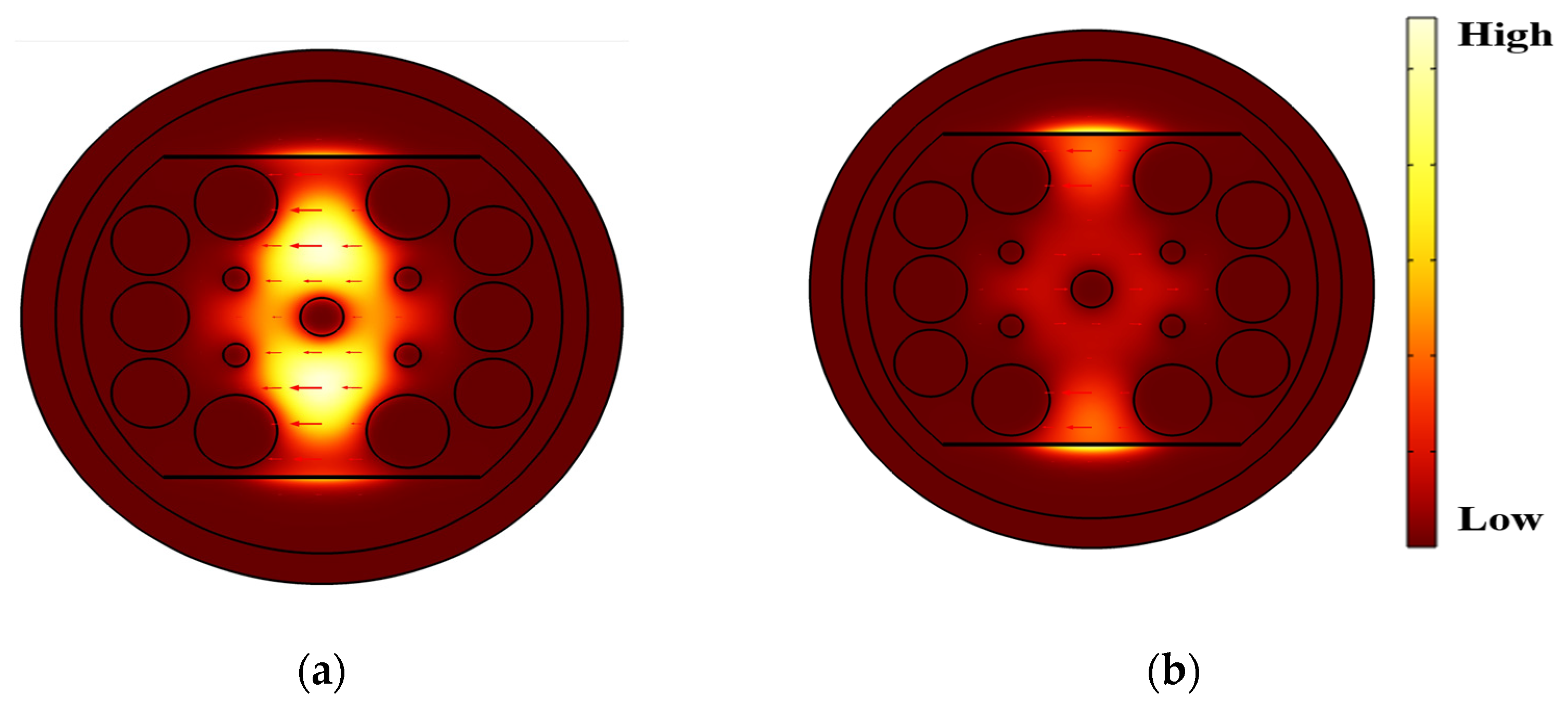
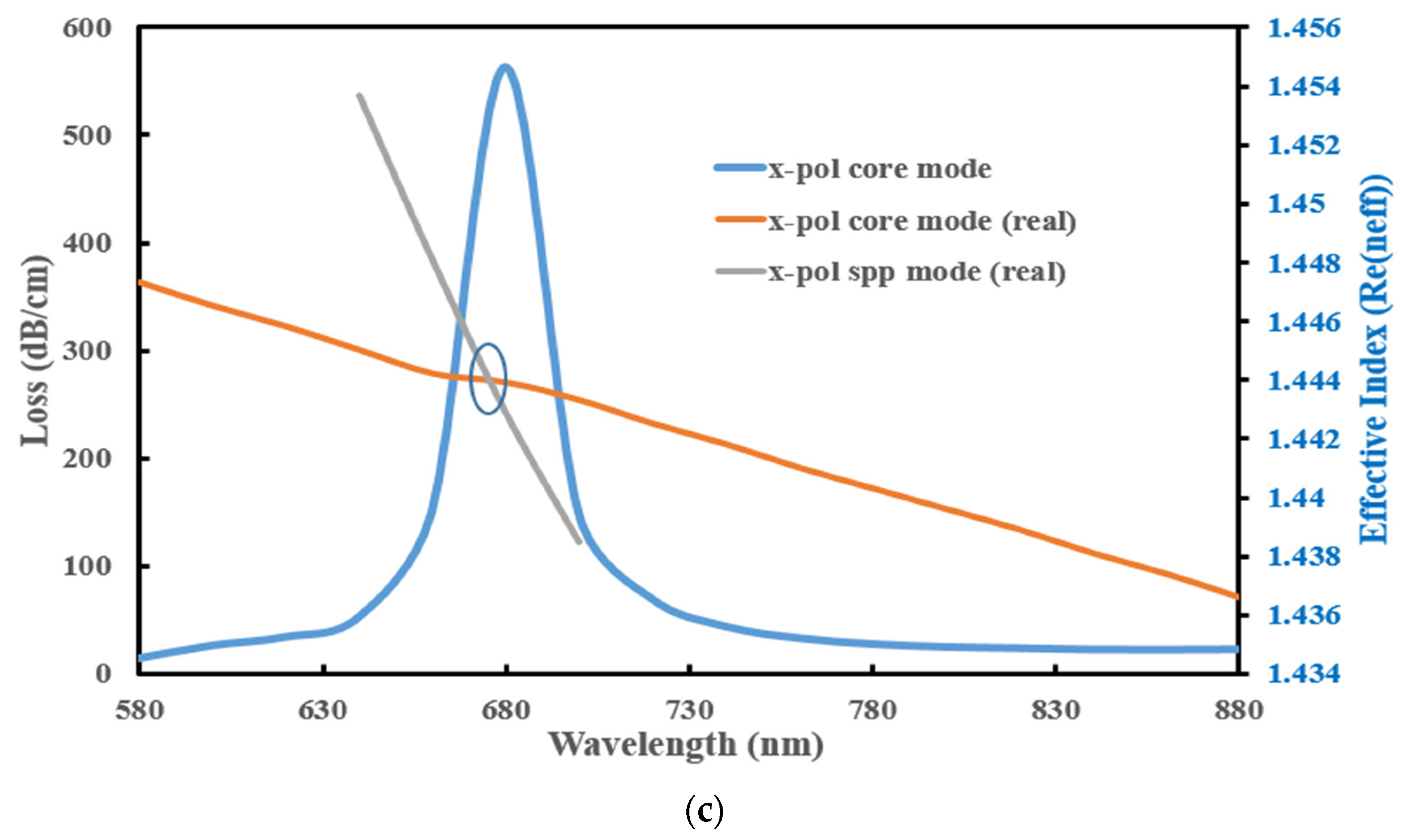
| Pitch, Λ (µm) | d1 (µm) | d2 (µm) | d3 (µm) | dc (µm) | h (µm) |
|---|---|---|---|---|---|
| 1.8 | 0.9Λ | 0.3Λ | 0.96Λ | 0.5Λ | 4.2 |
| RI | λres | Sλ (nm/RIU) | Rλ (RIU) | SA (RIU−1) | FWHM (nm) | FOM |
|---|---|---|---|---|---|---|
| 1.31 | 540 | 2000 | 5 × 10−5 | 78 | 69 | 29 |
| 1.32 | 560 | 2000 | 5 × 10−5 | 80 | 65 | 31 |
| 1.33 | 580 | 2000 | 5 × 10−5 | 151 | 45 | 44 |
| 1.34 | 600 | 2000 | 5 × 10−5 | 192 | 47 | 43 |
| 1.35 | 620 | 2000 | 5 × 10−5 | 282 | 41 | 49 |
| 1.36 | 640 | 4000 | 2.5 × 10−5 | 595 | 47 | 85 |
| 1.37 | 680 | 4000 | 2.5 × 10−5 | 694 | 26 | 154 |
| 1.38 | 720 | 4000 | 2.5 × 10−5 | 520 | 30 | 133 |
| 1.39 | 760 | 8000 | 1.25 × 10−5 | 509 | 48 | 167 |
| 1.40 | 840 | 10,000 | 1 × 10−5 | 394 | 56 | 179 |
| 1.41 | 940 | 74,000 | 1.35 × 10−6 | 292 | 111 | 667 |
| 1.42 | 1680 | N/A | N/A | N/A | N/A | N/A |
| Hb (gm/lit.) | RI |
|---|---|
| 0 | 1.3321 |
| 50 | 1.3459 |
| 100 | 1.3580 |
| 150 | 1.3711 |
| 200 | 1.3841 |
| 250 | 1.3972 |
| RI | λres | Sλ (nm/RIU) | Rλ (RIU) | SA (RIU−1) | FWHM (nm) | FOM |
|---|---|---|---|---|---|---|
| 1.3321 | 580 | 4000 | 2.5 × 10−5 | 350 | 45 | 89 |
| 1.3459 | 620 | 2000 | 5 × 10−5 | 277 | 32 | 63 |
| 1.3580 | 640 | 4000 | 2.5 × 10−5 | 726 | 32 | 125 |
| 1.3711 | 680 | 4000 | 2.5 × 10−5 | 723 | 28 | 143 |
| 1.3841 | 720 | 12,000 | 8.33 × 10−6 | 734 | 48 | 250 |
| 1.3972 | 840 | N/A | N/A | N//A | N//A | N/A |
| Ref. | Structure Model | Plasmonic Material Requirement | RI Range | Sλ (nm/RIU) | Rλ(RIU) | SA (RIU−1) | FOM |
|---|---|---|---|---|---|---|---|
| [52] | Circular-shaped | 100% | 1.34–1.37 | 8500 | 1.16 × 10−5 | 335 | N/A* |
| [53] | Hollow core | 100% | 1.36–1.41 | 30,000 | 3.33 × 10−6 | 106.23 | N/A |
| [54] | Arc-shaped, gold-coated | <50% | 1.32–1.37 | 14,100 | 7.09 × 10−6 | 109 | N/A |
| [55] | Butterfly-shaped | 100% | 1.28–1.40 | 17,000 | 5.88 × 10−6 | 253 | 298 |
| [19] | Circular-shaped, gold-coated | 100% | 1.36–1.392 | 5000 | 2 × 10−5 | 505 | N/A |
| [56] | Nanoscale gold-coated | 100% | 1.34–1.41 | 34,800 | 2.87 × 10−6 | N/A | 229 |
| [57] | D-shaped TiO2-Ag hybrid | <50% | 1.33–1.40 | 14,000 | 7.14 × 10−6 | 610 | N/A |
| This work | Dual-polished, dual-core | <50% | 1.31–1.42 | 74,000 | 1.35 × 10−6 | 734 | 667 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adib, A.; Chowdhury, A.; Chowdhury, A.; Huraiya, M.A.; Mitul, A.F.; Reja, M.I. Highly Sensitive Dual-Polished Dual-Core PCF-Based SPR Sensor for Hemoglobin Detection Using FEM and Machine Learning. Photonics 2025, 12, 1078. https://doi.org/10.3390/photonics12111078
Adib A, Chowdhury A, Chowdhury A, Huraiya MA, Mitul AF, Reja MI. Highly Sensitive Dual-Polished Dual-Core PCF-Based SPR Sensor for Hemoglobin Detection Using FEM and Machine Learning. Photonics. 2025; 12(11):1078. https://doi.org/10.3390/photonics12111078
Chicago/Turabian StyleAdib, Abrar, Anik Chowdhury, Aditta Chowdhury, Md Abu Huraiya, Abu Farzan Mitul, and Mohammad Istiaque Reja. 2025. "Highly Sensitive Dual-Polished Dual-Core PCF-Based SPR Sensor for Hemoglobin Detection Using FEM and Machine Learning" Photonics 12, no. 11: 1078. https://doi.org/10.3390/photonics12111078
APA StyleAdib, A., Chowdhury, A., Chowdhury, A., Huraiya, M. A., Mitul, A. F., & Reja, M. I. (2025). Highly Sensitive Dual-Polished Dual-Core PCF-Based SPR Sensor for Hemoglobin Detection Using FEM and Machine Learning. Photonics, 12(11), 1078. https://doi.org/10.3390/photonics12111078





