Thermoacoustic Imaging Using Single-Channel Data Acquisition System for Non-Invasive Assessment of Liver Microwave Ablation: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
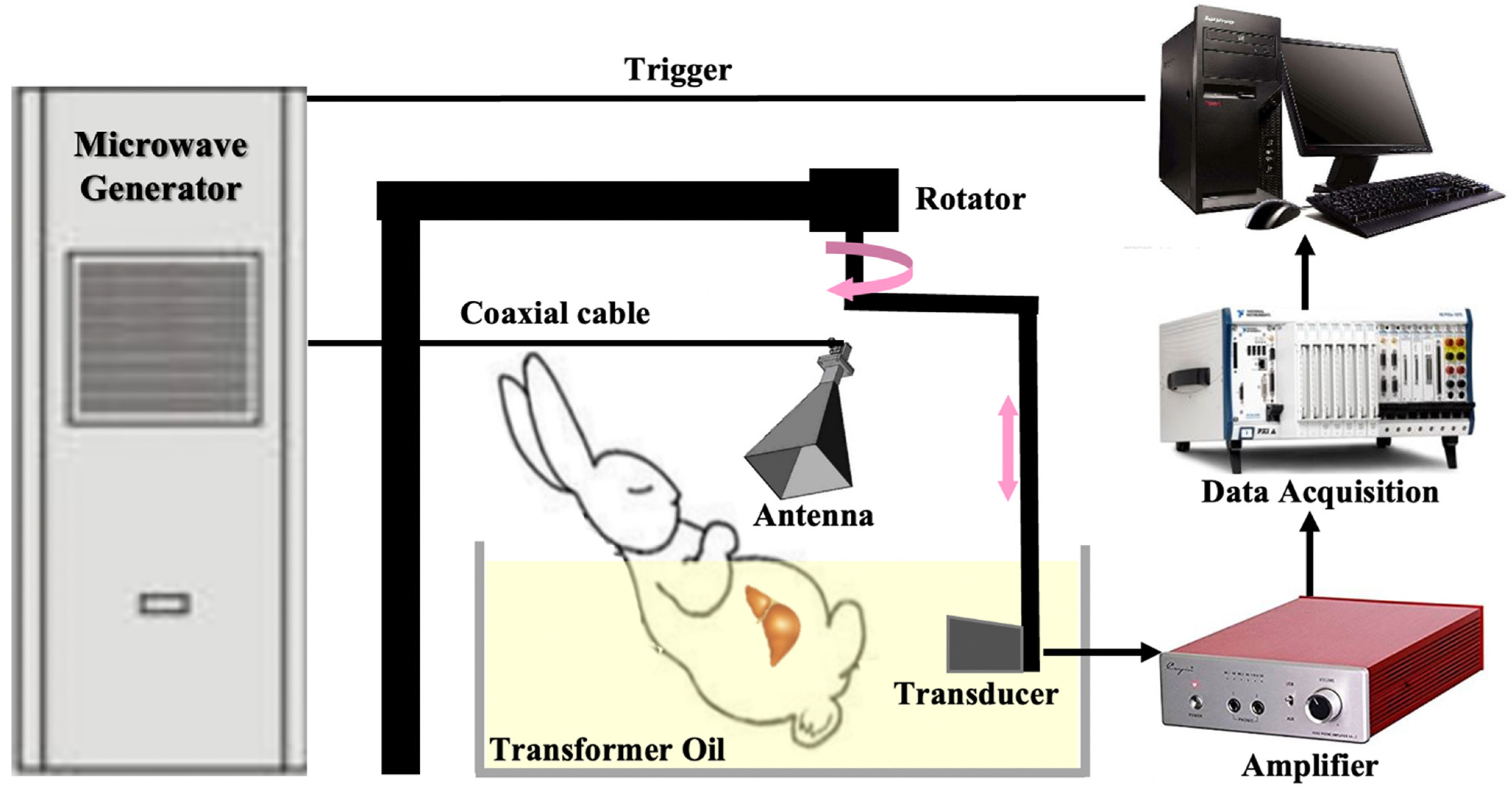
2.1. The Circular Scanned Single-Channel Data Acquisition TAI System
2.2. TAI of Porcine Liver Ex Vivo
2.3. TAI Imaging Depth Investigation
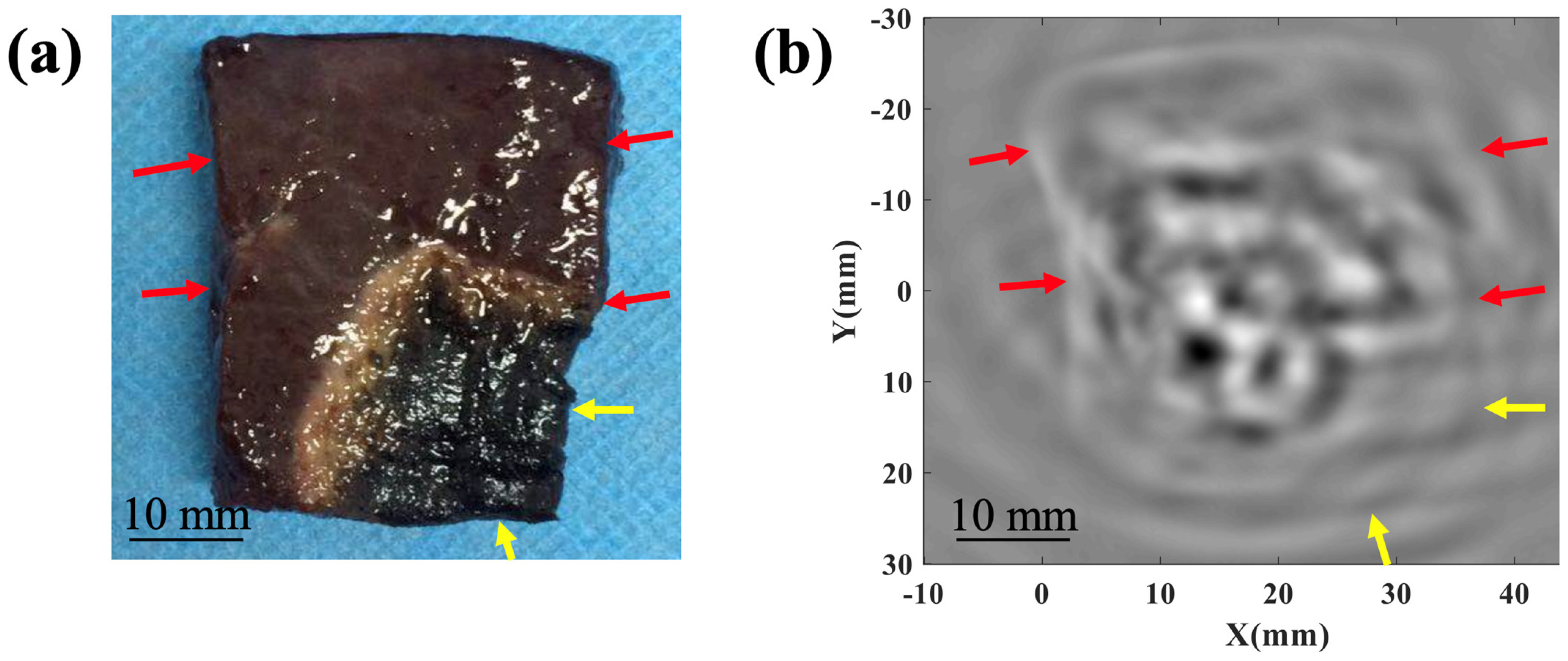
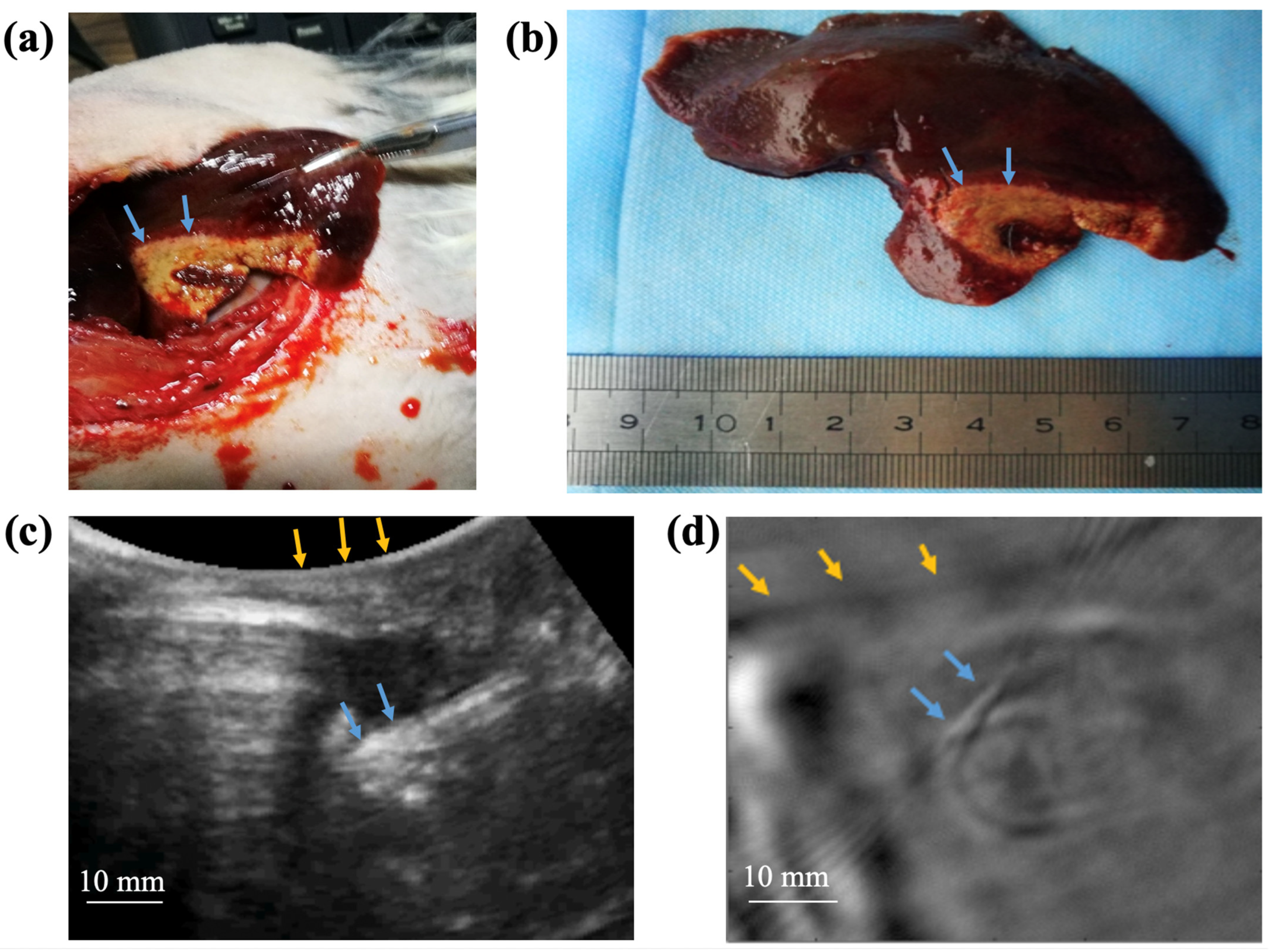
2.4. TAI of Rabbit Liver In Vivo
3. Result and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global Burden of Primary Liver Cancer in 2020 and Predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Agarwal, P.; Villanueva, A.; Rao, S.; Dawson, L.A.; Karasic, T.; Llovet, J.M.; Finn, R.S.; Groopman, J.D.; El-Serag, H.B.; et al. Recent Developments and Therapeutic Strategies against Hepatocellular Carcinoma. Cancer Res. 2019, 79, 4326–4330. [Google Scholar] [CrossRef]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef]
- Chakraborty, E.; Sarkar, D. Emerging Therapies for Hepatocellular Carcinoma (HCC). Cancers 2022, 14, 2798. [Google Scholar] [CrossRef]
- Xie, D.-Y.; Ren, Z.-G.; Zhou, J.; Fan, J.; Gao, Q. 2019 Chinese Clinical Guidelines for the Management of Hepatocellular Carcinoma: Updates and Insights. Hepatobiliary Surg. Nutr. 2020, 9, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grüll, H.; Ter Haar, G.; Wust, P.; Crezee, J. Heating Technology for Malignant Tumors: A Review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef]
- Wu, H.; Wilkins, L.R.; Ziats, N.P.; Haaga, J.R.; Exner, A.A. Real-Time Monitoring of Radiofrequency Ablation and Postablation Assessment: Accuracy of Contrast-Enhanced US in Experimental Rat Liver Model. Radiology 2014, 270, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Puijk, R.S.; Ruarus, A.H.; Scheffer, H.J.; Vroomen, L.G.P.H.; Van Tilborg, A.A.J.M.; De Vries, J.J.J.; Berger, F.H.; Van Den Tol, P.M.P.; Meijerink, M.R. Percutaneous Liver Tumour Ablation: Image Guidance, Endpoint Assessment, and Quality Control. Can. Assoc. Radiol. J. 2018, 69, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, X.; Nie, G.; Wang, Z.; Hu, J.; Hu, J.; Qin, H. Quantitative Tracing of Bioprobes by Simultaneously Monitoring Radiative and Nonradiative Relaxations. J. Innov. Opt. Health Sci. 2023, 16, 2243002. [Google Scholar] [CrossRef]
- Etoz, S.; Brace, C.L. Computed Tomography-Based Modeling of Water Vapor-Induced Changes in Permittivity During Microwave Ablation. IEEE Trans. Biomed. Eng. 2020, 67, 2427–2433. [Google Scholar] [CrossRef]
- Lopresto, V.; Pinto, R.; Lovisolo, G.A.; Cavagnaro, M. Changes in the Dielectric Properties of Ex Vivo Bovine Liver during Microwave Thermal Ablation at 2.45 GHz. Phys. Med. Biol. 2012, 57, 2309–2327. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ji, Z.; Qin, B.; Xing, D. A Thermoacoustic Imaging System with Variable Curvature and Multi-Dimensional Detection Adapted to Breast Tumor Screening. J. Appl. Phys. 2018, 124, 144902. [Google Scholar] [CrossRef]
- Saraswat, S.; Tak, J.; Liang, M.; Lyu, C.; Witte, R.S.; Xin, H. Towards Study on Thermoacoustic Imaging Guided Focused Microwave Therapy for Breast Cancer Treatment. In Proceedings of the 2018 IEEE/MTT-S International Microwave Symposium—IMS, Philadelphia, PA, USA, 10–15 June 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 953–956. [Google Scholar]
- Huang, G.; Li, Y.; Ren, M.; Zhang, H.; Qin, H. Real-Time Thermoacoustic Imaging for Breast Tumor Biomarker Biopsy Navigation Basing on a Semi-Ring Ultrasonic Transducer. Appl. Phys. Lett. 2023, 123, 183702. [Google Scholar] [CrossRef]
- Cao, C.; Nie, L.; Lou, C.; Xing, D. The Feasibility of Using Microwave-Induced Thermoacoustic Tomography for Detection and Evaluation of Renal Calculi. Phys. Med. Biol. 2010, 55, 5203–5212. [Google Scholar] [CrossRef]
- Patch, S.K.; Hanson, E.; Thomas, M.; Kelly, H.; Jacobsohn, K.; See, W.A. Thermoacoustic Imaging of Fresh Prostates up to 6-cm Diameter; Oraevsky, A.A., Wang, L.V., Eds.; SPIE: San Francisco, CA, USA, 2013; p. 85812K. [Google Scholar]
- Chi, Z.; Huang, L.; Ge, S.; Jiang, H. Technical Note: Anti-phase Microwave Illumination-based Thermoacoustic Tomography of in Vivo Human Finger Joints. Med. Phys. 2019, 46, 2363–2369. [Google Scholar] [CrossRef]
- Chi, Z.; Zhao, Y.; Huang, L.; Zheng, Z.; Jiang, H. Thermoacoustic Imaging of Rabbit Knee Joints. Med. Phys. 2016, 43, 6226. [Google Scholar] [CrossRef]
- Zhao, Y.; Shan, T.; Chi, Z.; Jiang, H. Thermoacoustic Tomography of Germinal Matrix Hemorrhage in Neonatal Mouse Cerebrum. J. X-ray Sci. Technol. 2020, 28, 83–93. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Z.; Peng, W.; Song, L.; Luo, Y.; Zhao, Z.; Huang, L. RPCA-Based Thermoacoustic Imaging for Microwave Ablation Monitoring. Photoacoustics 2024, 38, 100622. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zheng, Z.; Huang, L.; Qiu, T.; Luo, Y.; Jiang, H. In Vivo Liver Thermoacoustic Imaging and Demonstration Based on Localization Wire. Med. Phys. 2021, 48, 1608–1615. [Google Scholar] [CrossRef]
- Huang, L.; Li, T.; Jiang, H. Technical Note: Thermoacoustic Imaging of Hemorrhagic Stroke: A Feasibility Study with a Human Skull. Med. Phys. 2017, 44, 1494–1499. [Google Scholar] [CrossRef]
- Huang, L.; Yao, L.; Liu, L.; Rong, J.; Jiang, H. Quantitative Thermoacoustic Tomography: Recovery of Conductivity Maps of Heterogeneous Media. Appl. Phys. Lett. 2012, 101, 244106. [Google Scholar] [CrossRef]
- Hoelen, C.G.A.; De Mul, F.F.M. Image Reconstruction for Photoacoustic Scanning of Tissue Structures. Appl. Opt. 2000, 39, 5872. [Google Scholar] [CrossRef] [PubMed]
- McKeighen, R.E.; Buchin, M.P. New Techniques for Dynamically Variable Electronic Delays for Real Time Ultrasonic Imaging. In Proceedings of the 1977 Ultrasonics Symposium, Phoenix, AZ, USA, 26–28 October 1977; IEEE: Piscataway, NJ, USA, 1977; pp. 250–254. [Google Scholar]
- Jin, X.; Xu, Y.; Wang, L.V.; Fang, Y.R.; Zanelli, C.I.; Howard, S.M. Imaging of High-Intensity Focused Ultrasound-Induced Lesions in Soft Biological Tissue Using Thermoacoustic Tomography: Thermoacoustic Tomography of HIFU-Induced Lesions. Med. Phys. 2004, 32, 5–11. [Google Scholar] [CrossRef]
- Cavagnaro, M.; Pinto, R.; Lopresto, V. Numerical Models to Evaluate the Temperature Increase Induced by Ex Vivo Microwave Thermal Ablation. Phys. Med. Biol. 2015, 60, 3287–3311. [Google Scholar] [CrossRef] [PubMed]
- Bucci, O.M.; Cavagnaro, M.; Crocco, L.; Lopresto, V.; Scapaticci, R. Microwave Ablation Monitoring via Microwave Tomography: A Numerical Feasibility Assessment. In Proceedings of the 2016 10th European Conference on Antennas and Propagation (EuCAP), Davos, Switzerland, 10–15 April 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1–5. [Google Scholar]
- Evans, A.L.; Liu, R.L.; Ma, C.; Hagness, S.C. The Evolution of Microwave-Induced Thermoacoustic Signals Generated During Pulsed Microwave Ablation in Bovine Liver. IEEE J. Electromagn. RF Microw. Med. Biol. 2023, 7, 273–280. [Google Scholar] [CrossRef]
- Pramanik, M.; Wang, L.V. Thermoacoustic and Photoacoustic Sensing of Temperature. J. Biomed. Opt. 2009, 14, 054024. [Google Scholar] [CrossRef]
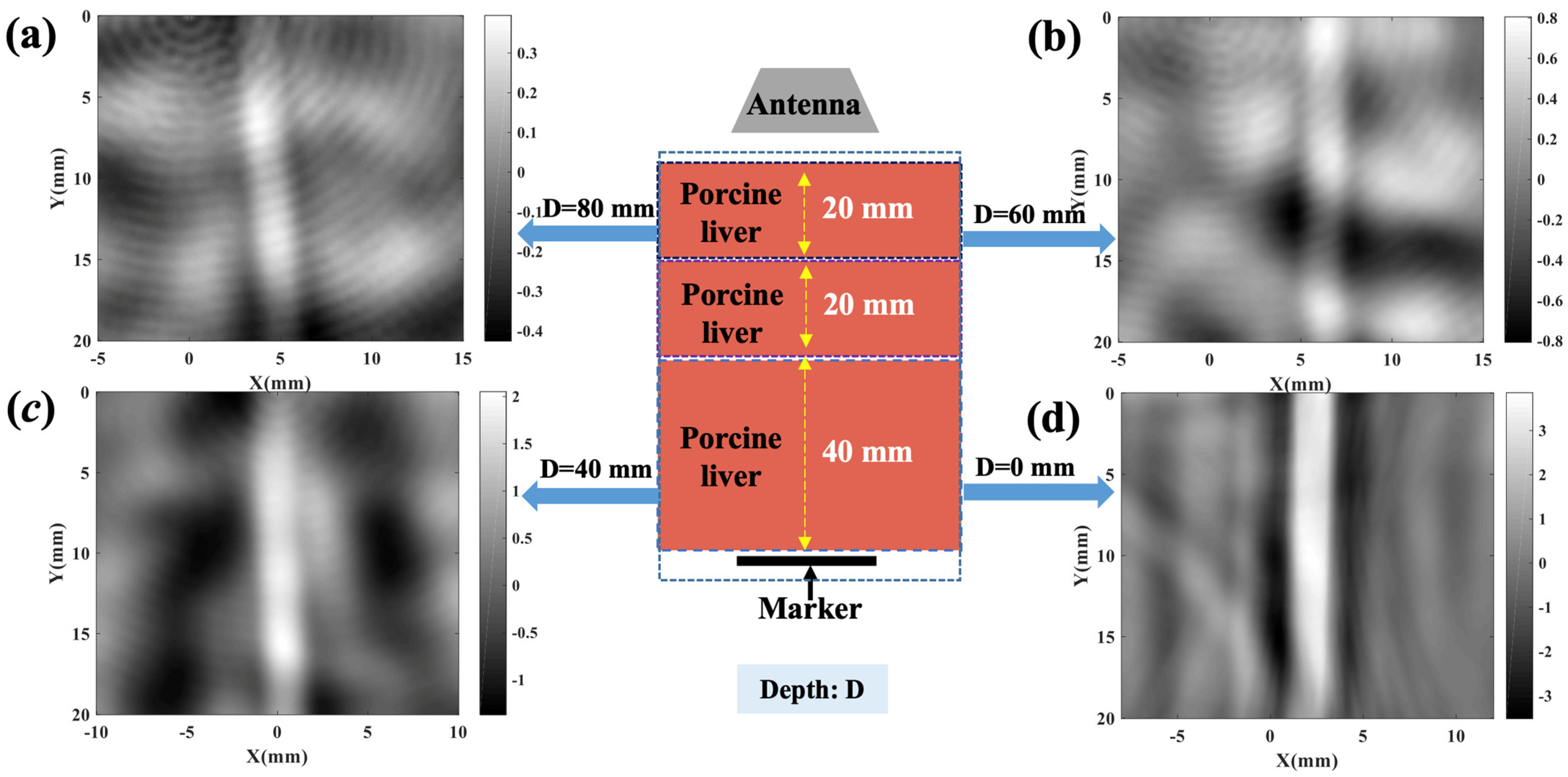
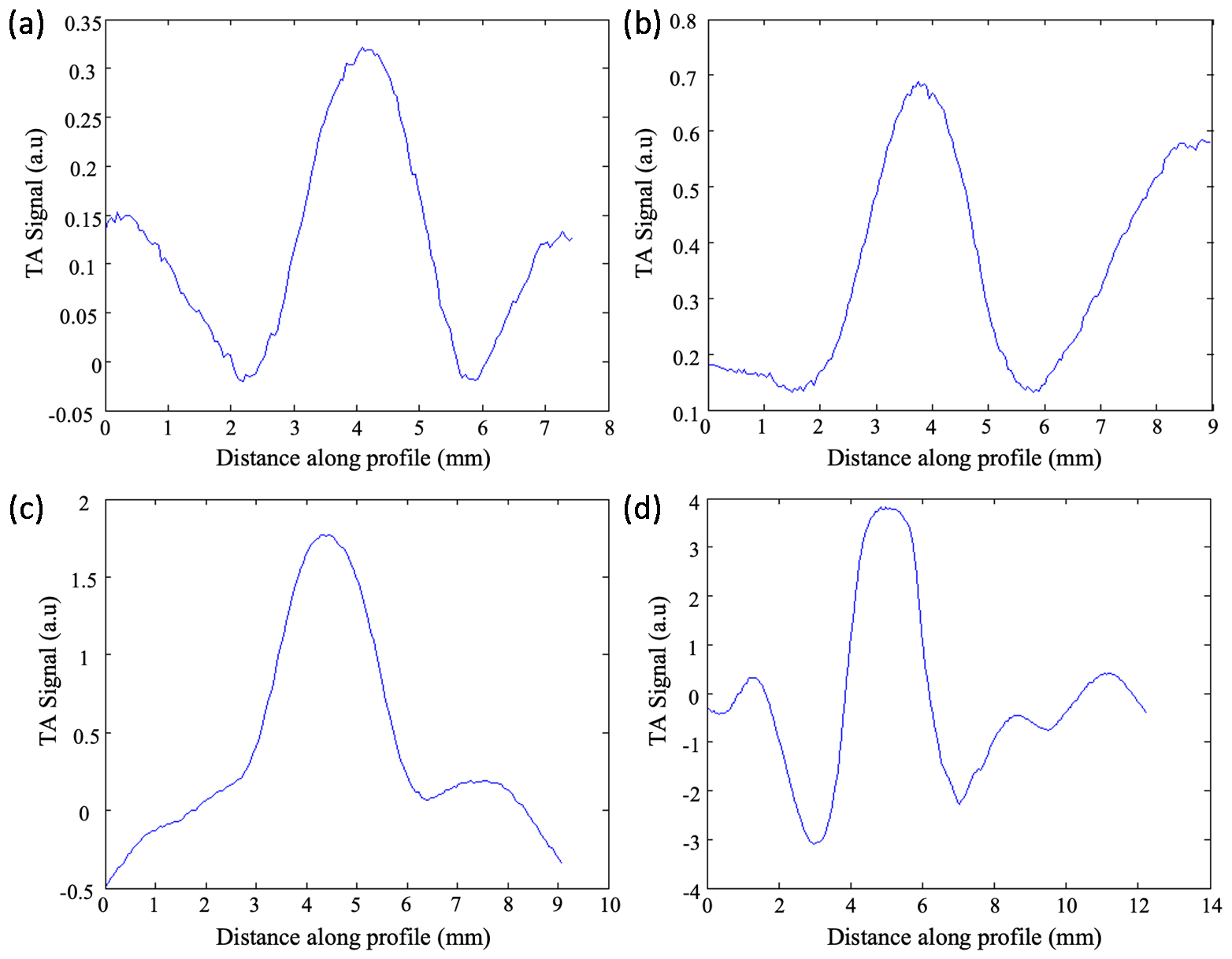
| Center Frequency (GHz) | Imaging Depth (mm) | SNR (dB) |
|---|---|---|
| 3.0 | 0 | 36 |
| 40 | 33 | |
| 60 | 23 | |
| 80 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Peng, W.; Lu, Q.; Feng, L.; Yang, Z.; Huang, L.; Luo, Y. Thermoacoustic Imaging Using Single-Channel Data Acquisition System for Non-Invasive Assessment of Liver Microwave Ablation: A Feasibility Study. Photonics 2024, 11, 807. https://doi.org/10.3390/photonics11090807
Song L, Peng W, Lu Q, Feng L, Yang Z, Huang L, Luo Y. Thermoacoustic Imaging Using Single-Channel Data Acquisition System for Non-Invasive Assessment of Liver Microwave Ablation: A Feasibility Study. Photonics. 2024; 11(9):807. https://doi.org/10.3390/photonics11090807
Chicago/Turabian StyleSong, Ling, Wanting Peng, Qiang Lu, Lian Feng, Zeqi Yang, Lin Huang, and Yan Luo. 2024. "Thermoacoustic Imaging Using Single-Channel Data Acquisition System for Non-Invasive Assessment of Liver Microwave Ablation: A Feasibility Study" Photonics 11, no. 9: 807. https://doi.org/10.3390/photonics11090807
APA StyleSong, L., Peng, W., Lu, Q., Feng, L., Yang, Z., Huang, L., & Luo, Y. (2024). Thermoacoustic Imaging Using Single-Channel Data Acquisition System for Non-Invasive Assessment of Liver Microwave Ablation: A Feasibility Study. Photonics, 11(9), 807. https://doi.org/10.3390/photonics11090807






