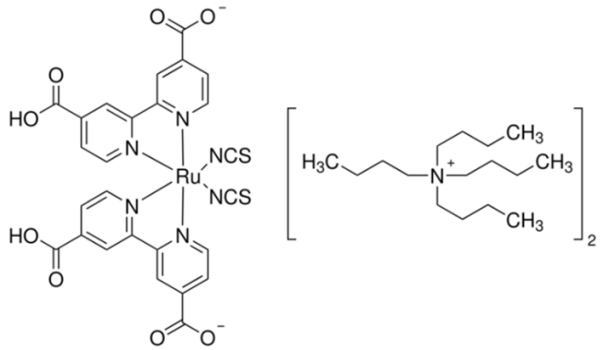
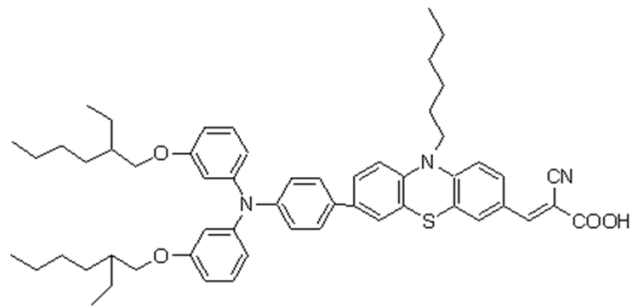
3.2. J–V Characteristics of DSSCs with Z907, N719, and Phenothiazine Dye and with and without PVDF-HFP with Different Molecular Weights in DMPII Electrolyte
Table 2 summarizes the main photovoltaic parameters at day 1 (day of cell assembly), day 2 (after 24 h), and day 3 (after 48 h) for the DSSCs based on the Z907, N719, and organic phenothiazine dyes with DMPII liquid electrolytes in the presence and in the absence of PVDF-HFP polymers with different molecular weights; the corresponding
J–
V curves are reported in
Figure 1.
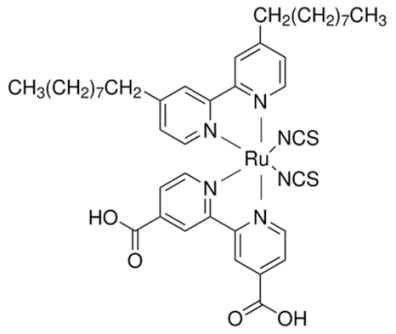
Cells R1–R4: Z907 dye
On day 1, the DSSCs using the Z907 dye exhibited diverse performance metrics. Sample R1, employing a liquid electrolyte, showed a Voc of 0.65 V, a Jsc of 10.80 mA/cm2, an FF of 0.64, and efficiency (PCE) of 4.53%. Comparatively, sample R2 with the PVH70 electrolyte exhibited a slightly lower Voc of 0.63 V but a higher Jsc of 11.85 mA/cm2 and a similar FF of 0.64, resulting in higher PCE of 4.74%. Sample R3 with the PVH80 electrolyte presented a Voc of 0.65 V, a Jsc of 10.96 mA/cm2, an improved FF of 0.67, and PCE of 4.77%. Finally, sample R4 with the PVDF-HFP electrolyte achieved a lower Voc of 0.59 V but the highest Jsc of 13.03 mA/cm2, an FF of 0.61, and PCE of 4.68%. The average values with the relative uncertainty of the photovoltaic parameters of the Z907 DSSCs evaluated on day 1 are therefore equal to Voc = (0.63 ± 0.03) V, Jsc = (11.66 ± 0.98) mA/cm2, FF = 0.64 ± 0.03, and PCE = (4.68 ± 0.10)%. Over three days, the performance metrics showed slight variations. By day 3, R1 showed PCE of 4.51%, R2 of 4.71%, R3 of 4.62%, and R4 showed a decrease to 4.35%. The primary observation is that the PVDF-HFP electrolyte, despite its high Jsc, suffers from a lower Voc and FF, leading to less stable efficiency compared to the other electrolytes.
Cells R5–R8: N719 dye
The N719 dye-based DSSCs displayed a different performance profile. On day 1, sample R5 with a liquid electrolyte recorded a Voc of 0.71 V, a Jsc of 9.23 mA/cm2, an FF of 0.69, and PCE of 4.47%. Sample R6 using the PVH70 electrolyte improved the Jsc to 10.67 mA/cm2 but had a lower FF of 0.61, yielding PCE of 4.48%. Sample R7 with PVH80 showed a similar Voc of 0.71 V, a Jsc of 10.51 mA/cm2, an improved FF of 0.63, and higher PCE of 4.72%. Sample R8 using the PVDF-HFP electrolyte displayed a Voc of 0.63 V, a Jsc of 11.36 mA/cm2, an FF of 0.62, and PCE of 4.45%. The average values with the relative uncertainty of the photovoltaic parameters of the N719 DSSCs evaluated on day 1 are therefore equal to Voc = (0.69 ± 0.03) V, Jsc = (10.44 ± 0.88) mA/cm2, FF = 0.64 ± 0.03, and PCE = (4.53 ± 0.12)%. Over three days, R5’s efficiency increased to 4.67%, indicating good stability. R6 and R7 showed improved PCE of 4.76 and 5.12%, respectively, by day 3. However, R8 experienced a significant drop in PCE to 3.83% by day 3, suggesting that while PVDF-HFP provided a higher initial Jsc, it suffered from stability issues over time.
Cells R9–R12: Phenothiazine dye
The phenothiazine dye-based DSSCs highlighted promising initial results. On day 1, sample R9 with a liquid electrolyte showed a Voc of 0.76 V, a Jsc of 10.12 mA/cm2, an FF of 0.60, and PCE of 4.59%. Sample R10 with the PVH70 electrolyte exhibited a Voc of 0.73 V, a Jsc of 10.65 mA/cm2, an FF of 0.59, and PCE of 4.48%. Sample R11 using PVH80 achieved a Voc of 0.75 V, a Jsc of 10.72 mA/cm2, an FF of 0.58, and PCE of 4.69%. Finally, sample R12 with the PVDF-HFP electrolyte recorded a Voc of 0.68 V, a Jsc of 11.03 mA/cm2, an FF of 0.56, and PCE of 4.20%. The average values with the relative uncertainty of the photovoltaic parameters of the phenothiazine DSSCs evaluated on day 1 are therefore equal to Voc = (0.73 ± 0.04) V, Jsc = (10.63 ± 0.38) mA/cm2, FF = 0.58 ± 0.02, and PCE = (4.49 ± 0.18)%. By day 3, sample R9’s efficiency slightly increased to 4.74%, indicating good stability. Samples R10 and R11 showed improved PCE of 4.54 and 5.01%, respectively, with R11 showing the best stability and efficiency among the phenothiazine dye group. However, sample R12’s PCE dropped significantly to 3.42%, mirroring the trend observed with other PVDF-HFP electrolyte-based cells.
Across all three groups, the choice of electrolyte had a significant impact on the performance and stability of the DSSCs. The PVDF-HFP electrolyte, while providing a high initial Jsc, generally resulted in lower stability and efficiency over time compared to the PVH70 and PVH80 electrolytes. This comparative study highlights the critical role of electrolyte selection in optimizing the performance and durability of DSSCs.
The performance of QsDSSCs can be significantly impacted also by the choice of photosensitizer dye, such as Z907, N719, and organic phenothiazine 2-LBH-92; below, some aspects revealing how these dyes influence the DSSC performance are reported.
Each dye displays a characteristic absorption spectrum. For instance, N719 is a ruthenium-based complex known for its broad and strong absorption in the visible spectrum and good performance in both liquid and QsDSSCs. Z907 is also a ruthenium complex but with slightly different ligands, which might absorb light differently from N719, potentially impacting the cell’s light-harvesting efficiency. Organic phenothiazine dyes, such as the one mentioned, absorb light based on their conjugated systems and can be tuned to enhance their light absorption over specific wavelength ranges. The molecular architectures of these dyes determine their ability to anchor to the TiO2 surface, resist aggregation, and avoid steric hindrance, all of which can affect the electron injection efficiency and charge recombination rates.
The dyes’ different energy levels might exhibit varying alignment with the conduction band of TiO
2. The energy level alignment determines the efficiency and the driving force for the transfer of electrons from the dye to the semiconductor upon photoexcitation. Ruthenium dyes such as N719 and Z907 generally show good energy level alignment, yielding efficient electron injection [
18]. Organic phenothiazine dyes might have different energy level alignment, which can also result in efficient electron injection, but they might require careful design to optimize their performance within a QsDSSC.
Some dyes are more stable than others when exposed to light and the DSSC operating environment. Organic phenothiazine dyes may offer higher molar extinction coefficients but can be less stable than ruthenium-based dyes under prolonged exposure to lighting conditions. Efficient charge separation in DSSCs requires that the oxidized dye molecule is effectively reduced by the redox mediator in the quasi-solid-state electrolyte. The redox potential of the dye in its oxidized state should match well with the potential of the redox couple used in the electrolyte. Ruthenium dyes like N719 and Z907 are well matched with the conventional iodide/triiodide redox couple, facilitating effective dye regeneration without much energy loss. For organic phenothiazine dyes, their redox potentials might be different, and the match with the redox mediator needs to be good to ensure efficient regeneration and to prevent potential losses that could reduce the cell’s voltage.
The interaction between the dye and the TiO2 surface influences the electron lifetime and recombination dynamics. Dyes that adsorb strongly to TiO2 and form an optimal monolayer can inhibit charge recombination and extend electron lifetimes. In the case of N719 and Z907, the strong anchoring groups and larger molecules might provide good coverage and passivation on the TiO2 surface. The molecular design of phenothiazine dyes usually includes anchoring groups like the cyanoacrylic acid moiety, which can provide similar benefits, but might require optimization for the specific quasi-solid-state system.
Overall, the dye choice for QsDSSCs should consider factors like light absorption, the energy levels, the stability under operational conditions, and the interfacial electron dynamics to maximize the photovoltaic performance and longevity. Each dye brings a unique set of advantages and challenges in terms of efficiency, stability, and fabrication requirements.
3.3. DSSCs with Z907 Dye and with and without PVDF-HFP with Different Molecular Weights in DMPII Electrolyte
3.3.1. UV–Vis Absorption and IPCE Spectra
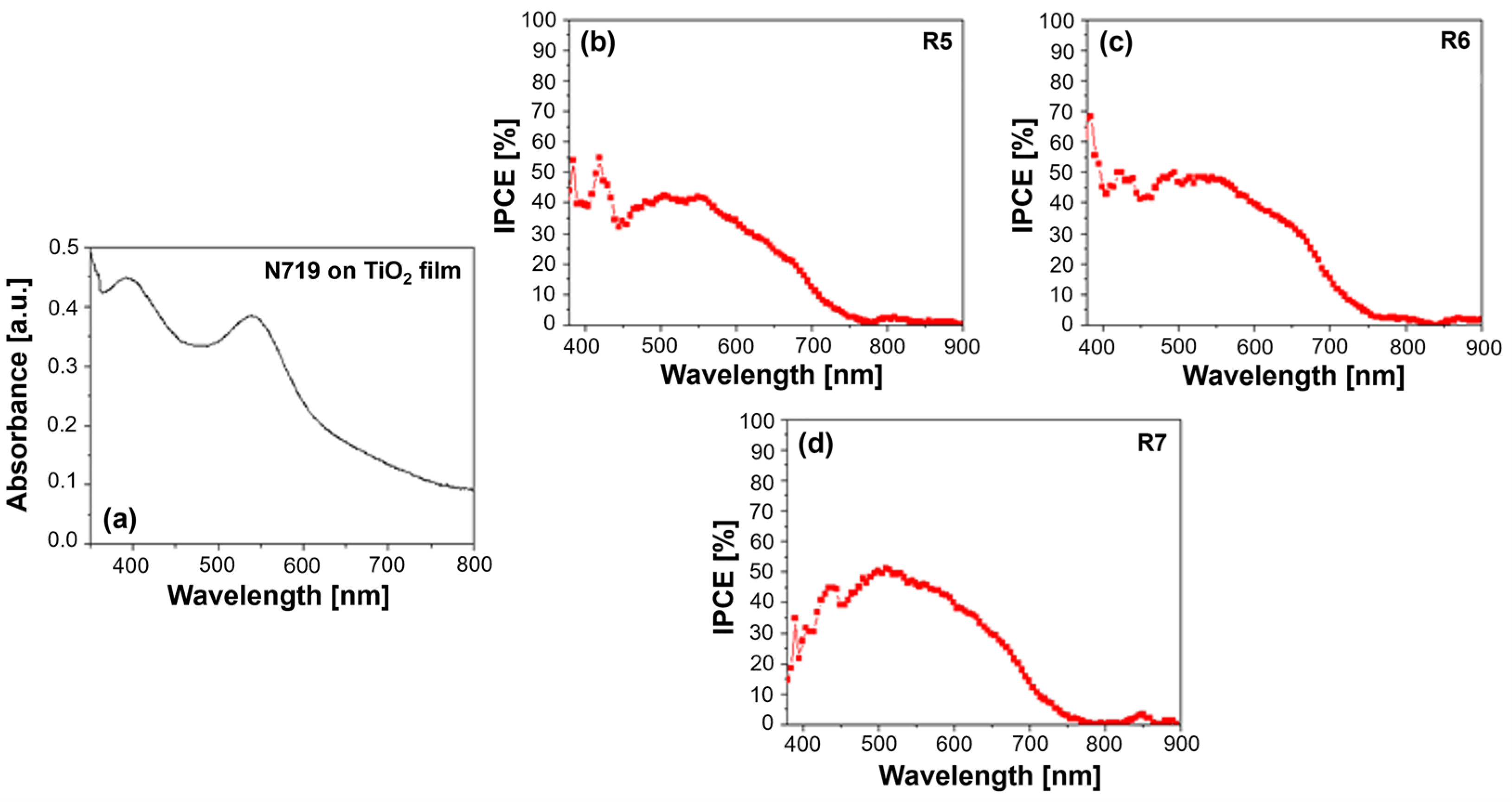
The UV–Vis absorption spectrum of the Z907 dye typically exhibits two characteristic bands: a strong absorption peak in the UV region at around 300 nm, attributed to π–π* transitions within the bipyridine ligands, and a broader absorption band in the visible region, peaking around 530 nm, arising from metal-to-ligand charge transfer (MLCT) transitions (
Figure 2a). Incorporating PVDF-HFP into the electrolyte, which comprises 0.1 M I
2, 0.5 M NMBI, and 0.6 M IL-DMPII, can influence the dye’s absorption behavior. For instance, interactions between the dye molecules and the polymer chains might lead to slight shifts in the absorption peaks or changes in peak intensity. These spectral variations can provide insights into the degree of dye aggregation, which can negatively impact device performance by increasing charge recombination.
The IPCE spectra offer a more direct measure of the DSSC’s ability to convert incident photons into an electrical current. Z907 typically exhibits a broad IPCE spectrum, mirroring its absorption profile, with the maximum efficiency often observed at around 530 nm. The presence of PVDF-HFP in the electrolyte can significantly influence the IPCE spectrum (see
Figure 2b–e). Different molecular weights of the polymer can lead to variations in the electrolyte’s viscosity and ionic conductivity, ultimately affecting the charge transport within the device. For instance, a high-molecular-weight PVDF-HFP might result in a more viscous electrolyte, hindering ion diffusion and potentially reducing the IPCE, particularly at longer wavelengths, where the charge collection efficiency becomes more critical. Conversely, a lower-molecular-weight PVDF-HFP might lead to a less viscous electrolyte, facilitating ion transport and potentially enhancing the IPCE. However, the optimal molecular weight represents a balance, as excessively low molecular weights might compromise the quasi-solid-state nature of the electrolyte, impacting the long-term stability [
19].
Furthermore, the interaction between the Z907 dye and the PVDF-HFP polymer can influence the electron injection and dye regeneration processes. For example, if the polymer interacts favorably with the dye’s excited state, it could facilitate electron injection into the TiO2 conduction band, leading to an increased IPCE. Conversely, unfavorable interactions could hinder these processes, negatively impacting the device’s performance. In conclusion, analyzing the UV–Vis absorption and IPCE spectra of Z907-sensitized QsDSSCs with varying molecular weights of PVDF-HFP in the electrolyte provides valuable information about the dye–electrolyte interactions, charge transport properties, and overall device performance. Understanding these relationships is crucial in optimizing the electrolyte composition and device architecture to achieve high efficiency and long-term stability in DSSC applications.
Several factors could have contributed to the observation of the unusually high IPCE below 400 nm as shown in
Figure 2e; they were actively investigated to try to provide a conclusive explanation. One possible factor is the scattering effects caused by the TiO
2 nanoparticles used in the photoanode. These nanoparticles can scatter light, especially in the UV region, leading to the overestimation of the IPCE in this wavelength range. Scattered photons might be detected multiple times or contribute to the photocurrent through indirect pathways, thus inflating the IPCE values. Another potential factor is measurement artifacts. Despite taking precautions to ensure accurate IPCE measurements, instrumental artifacts or limitations in the calibration of the light source and detector in the UV region could have contributed to the observed high values. Our measurement setup and protocols were thoroughly verified to rule out any systematic errors. Additionally, although less likely, the possibility of dye aggregation on the TiO
2 surface cannot be entirely ruled out. If dye aggregates are present, they could exhibit altered optical properties, potentially leading to enhanced absorption and IPCE in the UV region.
3.3.2. Stability Study
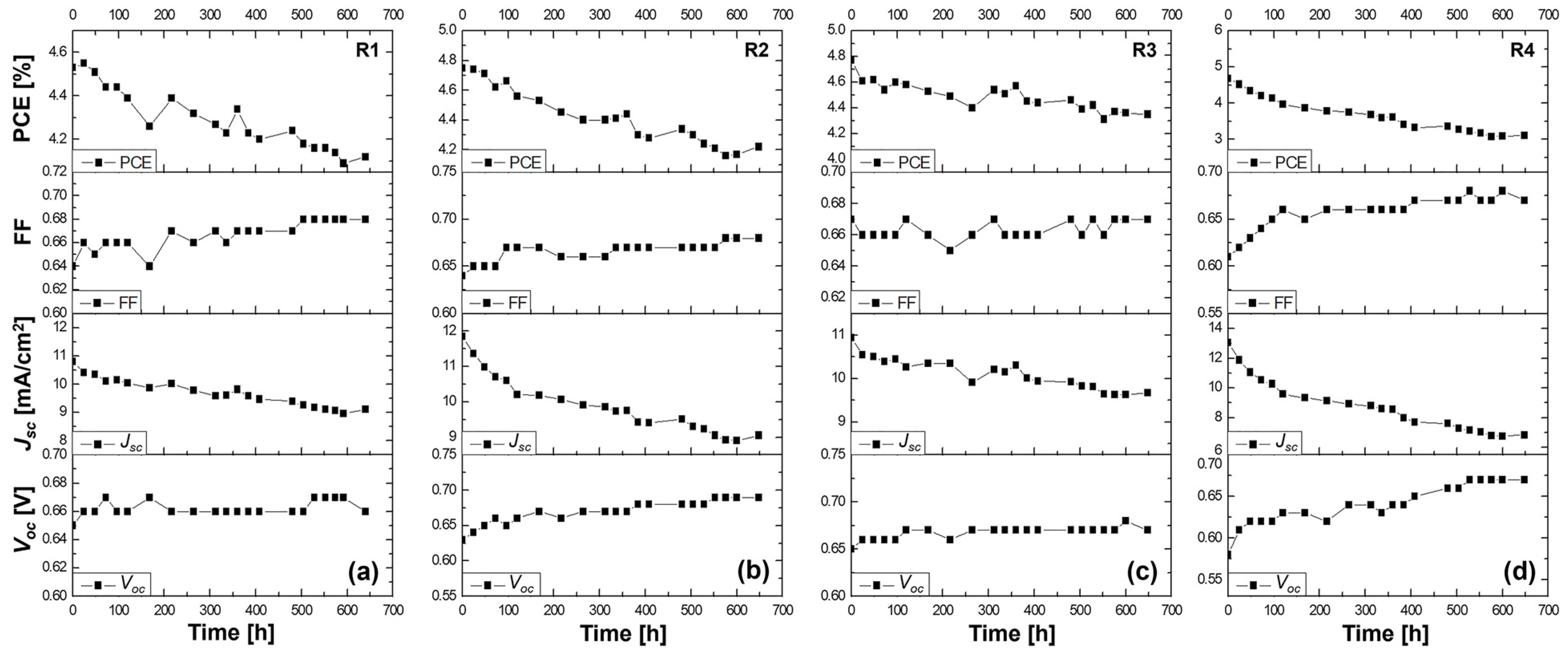
The stability study of the Z907-sensitized solar cells with the liquid DMPII electrolyte and with gel polymer electrolytes with different molecular weights is shown in
Figure 3. In this context, the stability study of QsDSSCs employing the Z907 dye and electrolytes with varying molecular weights of the PVDF-HFP polymer can reveal how these factors influence the durability and overall performance over time. In our research setup, cells R1, R2, R3, and R4 exhibited different trajectories after over 600 h of operation, indicating the complex influence of the PVDF-HFP polymer’s molecular weight within the context of a QsDSSC.
R1 cell (liquid DMPII electrolyte)
The R1 cell, utilizing a liquid DMPII electrolyte, exhibited a 9% decrease in overall efficiency after 600 h. This decline primarily stemmed from a 15% reduction in the Jsc, suggesting potential issues with electron injection, dye regeneration, or charge transport within the device. However, the Voc and FF showed slight improvements of 1.5 and 1%, respectively. This Voc increase could indicate reduced recombination losses, possibly due to passivation effects at the TiO2/dye/electrolyte interface over time. The slight FF improvement might be attributed to changes in the electrolyte composition or interface properties that enhance charge collection.
R2 cell (PVDF-HFP electrolyte with M.W. of 86,000 g/mol)
Incorporating PVDF-HFP polymers into the electrolyte significantly influenced the devices’ stability. The R2 cell, with a polymer molecular weight of 86,000 g/mol, displayed an 11% efficiency reduction after 600 h. While this decrease is comparable to that of the R1 cell, the individual parameter changes differed. The R2 cell exhibited a more pronounced Voc increase of 10%, suggesting a more substantial reduction in recombination losses, potentially due to the polymer’s ability to suppress interfacial charge recombination. However, the Jsc experienced a more significant decline of 24%, indicating that the polymer might hinder charge transport or negatively impact dye regeneration. The FF showed a notable improvement of 7%, likely due to the polymer’s influence on the electrolyte’s ionic conductivity and interfacial properties.
R3 cell (PVDF-HFP electrolyte with M.W. of 90,000 g/mol)
The R3 cell, employing a slightly higher molecular weight of PVDF-HFP (90,000 g/mol), demonstrated a 9% efficiency reduction after 600 h, similar to the R1 cell. This cell exhibited a moderate Voc increase of 4%, a slight FF improvement of 1%, and a Jsc decrease of 13%. These results suggest that this specific polymer molecular weight might offer a balance between suppressing recombination and maintaining reasonable charge transport properties.
R4 cell (PVDF-HFP electrolyte with M.W. of 455,000 g/mol)
The R4 cell, incorporating the highest molecular weight of PVDF-HFP (455,000 g/mol), suffered the most significant efficiency loss, plummeting by 33% after 600 h. This dramatic decline primarily resulted from the substantial 50% drop in the Jsc, indicating severe limitations in charge transport within the device. The high-molecular-weight polymer likely formed a dense network within the electrolyte, hindering ion diffusion and significantly impeding charge collection. Despite the substantial Voc increase of 14%, likely due to suppressed recombination, and a notable FF improvement of 9%, the severe Jsc limitation ultimately dominated the device’s performance degradation.
This stability study highlights the complex interplay between the electrolyte composition, polymer molecular weight, and device performance in QsDSSCs. While liquid electrolytes might suffer from limitations related to long-term stability and potential leakage, incorporating PVDF-HFP polymers introduces a trade-off between enhancing the stability and maintaining efficient charge transport. Lower-molecular-weight polymers appear to offer a better balance, preserving reasonable charge transport while mitigating some of the drawbacks associated with liquid electrolytes. However, excessively high molecular weights can severely hinder ion diffusion, leading to significant performance losses despite potential improvements in other parameters. Optimizing the polymer molecular weight and electrolyte composition is crucial in achieving efficient QsDSSCs that are stable in the long term.
3.4. DSSCs with N719 Dye and with and without PVDF-HFP with Different Molecular Weights in DMPII Electrolyte
3.4.1. UV–Vis Absorption and IPCE Spectra
In the field of DSSCs, evaluating the UV–Vis absorption and IPCE spectra is crucial to optimize the device performance (see
Figure 4). Analyzing these spectra for the N719 dye incorporated into both quasi-solid-state and liquid DSSCs, i.e., with and without PVDF-HFP polymers of varying molecular weights in the electrolyte (0.1 M I
2, 0.5 M NMBI, and 0.6 M IL-DMPII), can elucidate the impact of the polymer on the dye’s light-harvesting capabilities and charge generation processes [
20].
N719, a ruthenium-based dye, typically exhibits two prominent absorption bands in its UV–Vis spectrum (see
Figure 4a). A strong absorption peak at around 390 nm arises from MLCT transitions within the dye molecule. A broader absorption band, peaking at around 530 nm, also originates from MLCT transitions but involves a different energy level within the dye’s molecular orbital structure. Incorporating PVDF-HFP into the electrolyte can influence the dye’s absorption behavior. Interactions between the dye molecules and the polymer chains might induce slight shifts in the absorption peaks or alter the peak intensities. These spectral variations can provide insights into the degree of dye aggregation, which can negatively impact device performance by increasing charge recombination [
2,
9].
The IPCE spectra offer a more direct measure of the DSSC’s ability to convert incident photons into an electrical current. N719 typically exhibits a broad IPCE spectrum, mirroring its absorption profile, with the maximum efficiency observed around the wavelengths corresponding to its MLCT absorption bands. The presence of PVDF-HFP in the electrolyte can significantly influence the IPCE spectrum (see
Figure 4b–d). Different molecular weights of the polymer can lead to variations in the electrolyte’s viscosity and ionic conductivity, ultimately affecting the charge transport within the device.
For instance, a high molecular weight of PVDF-HFP might result in a more viscous electrolyte, hindering ion diffusion and potentially reducing the IPCE, particularly at longer wavelengths, where the charge collection efficiency becomes more critical. Conversely, a lower molecular weight of PVDF-HFP might lead to a less viscous electrolyte, facilitating ion transport and potentially enhancing the IPCE. However, the optimal molecular weight represents a balance, as excessively low molecular weights might compromise the quasi-solid-state nature of the electrolyte, impacting the long-term stability [
21,
22,
23].
Furthermore, the interaction between the N719 dye and the PVDF-HFP polymer can influence the electron injection and dye regeneration processes. For example, if the polymer interacts favorably with the dye’s excited state, it could facilitate electron injection into the TiO2 conduction band, leading to increased IPCE. Conversely, unfavorable interactions could hinder these processes, negatively impacting the device’s performance.
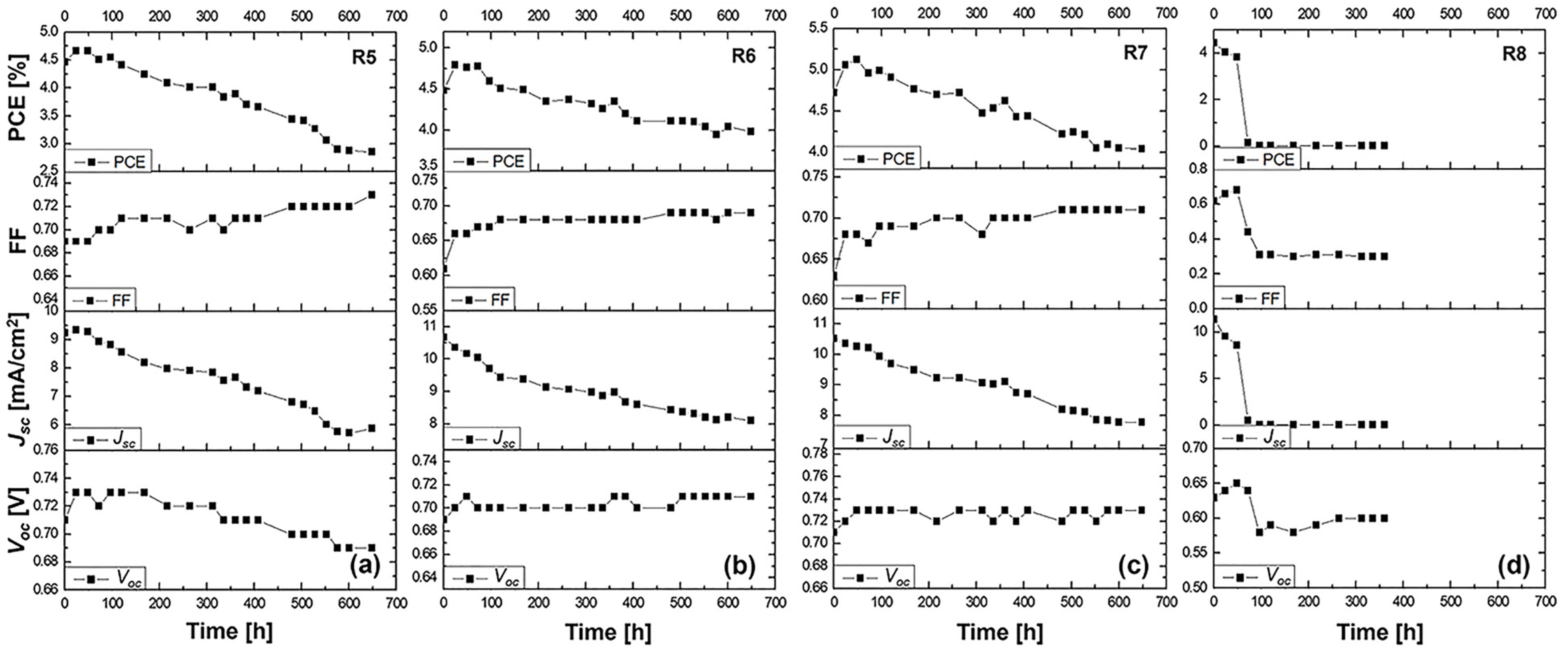
3.4.2. Stability Study
This stability study focuses on understanding the long-term performance of DSSCs utilizing the N719 dye and various electrolytes. The changes in the Voc, FF, Jsc, and overall efficiency after a 600 h aging period will be thoroughly analyzed. Our analysis encompasses a control cell (R5) with a liquid DMPII electrolyte and cells incorporating PVDF-HFP polymers of different molecular weights (R6: 86,000 g/mol, R7: 90,000 g/mol, R8: 455,000 g/mol) in the electrolyte.
The long-term stability data for the N719-sensitized solar cells with the liquid DMPII electrolyte and various molecular weights of the PVDF-HFP polymer are shown in
Figure 5.
R5 cell (liquid DMPII electrolyte)
The R5 cell, employing a liquid DMPII electrolyte, exhibited a significant 35% efficiency reduction after 600 h. This decline primarily stemmed from the substantial 36% decrease in the Jsc, indicating a significant loss in the cell’s ability to generate a current from incident light. This could be attributed to several factors, including dye degradation, electrolyte decomposition, or increased charge recombination at the TiO2/dye/electrolyte interface. The 6% decrease in the Voc suggests increased recombination losses, further supporting this hypothesis. However, the 6% increase in the FF implies improvements in charge transport and collection within the device, possibly due to changes in the electrolyte’s conductivity or interfacial properties over time.
R6 cell (PVDF-HFP electrolyte with M.W. of 86,000 g/mol)
Incorporating PVDF-HFP polymers into the electrolyte significantly influenced the devices’ stability. The R6 cell, with a polymer molecular weight of 86,000 g/mol, displayed a much smaller efficiency reduction of 11% after 600 h compared to the R5 cell. This improved stability is promising and can be attributed to the polymer’s ability to mitigate some degradation pathways present in the liquid electrolyte system. The 3% increase in the Voc suggests a slight reduction in recombination losses, potentially due to the polymer’s passivation effect at the TiO2/dye/electrolyte interface. The 10% increase in the FF indicates improved charge transport and collection, likely facilitated by the polymer’s influence on the electrolyte’s ionic conductivity and interfacial properties. However, the 25% decrease in the Jsc, while less severe than in the R5 cell, still points to some degree of dye degradation or hindered charge injection/regeneration processes.
R7 cell (PVDF-HFP electrolyte with M.W. of 90,000 g/mol)
The R7 cell, employing a slightly higher molecular weight of PVDF-HFP (90,000 g/mol), demonstrated a 15% efficiency reduction after 600 h, slightly higher compared to the R6 cell. This suggests that this specific molecular weight might not offer significant advantages over the 86,000 g/mol polymer in terms of long-term stability. The 3% increase in the Voc and 13% increase in the FF show similar trends to the R6 cell, indicating potential benefits in reducing recombination and improving charge transport. However, the 25% decrease in the Jsc remains a concern, suggesting that the further optimization of the electrolyte composition or device architecture is necessary.
R8 cell (PVDF-HFP electrolyte with M.W. of 455,000 g/mol)
The R8 cell, incorporating the highest molecular weight of PVDF-HFP (455,000 g/mol), suffered a catastrophic failure, with both the efficiency and Jsc dropping to zero after 600 h. This dramatic decline clearly demonstrates the detrimental effects of using an excessively high molecular weight of the polymer in the electrolyte. The high molecular weight likely leads to a highly viscous electrolyte, severely hindering ion transport and effectively shutting down charge collection within the device. This is further evidenced by the substantial 54% decrease in the FF, indicating extremely poor charge transport and collection. The 9% decrease in the Voc, despite the complete loss of current generation, suggests that the recombination processes are not the primary cause of the failure in this case.
3.5. DSSCs with Organic Phenothiazine Dye 2-LBH-92 and with and without PVDF-HFP with Different Molecular Weights in DMPII Electrolyte
3.5.1. UV–Vis Absorption and IPCE Spectra
The phenothiazine dye in question, characterized by a long alkoxy side chain and cyanoacrylic acid anchoring group, was designed for efficient light harvesting and electron transfer in DSSCs. The UV–Vis absorption spectrum shown in
Figure 6a exhibits a characteristic broad absorption band in the visible region, given the extended π-conjugation and donor–acceptor nature of the molecule, translating into significant photon capture within the spectral range where the solar irradiance is high. This band arises from the intramolecular charge transfer (ICT) between the phenothiazine donor and the cyanoacrylic acid acceptor. The position and intensity of this ICT band are sensitive to the surrounding medium’s polarity and can be influenced by the presence of the PVDF-HFP polymer [
24]. Additionally, 2-LBH-92 shows a prominent absorption band in the UV region, attributed to π–π* transitions within the conjugated phenothiazine and phenyl rings.
Incorporating PVDF-HFP into the DMPII electrolyte can induce changes in the UV–Vis spectra. For instance, interactions between the dye molecules and the polymer chains might lead to slight shifts in the absorption peaks, reflecting changes in the energy levels within the dye. Additionally, variations in peak intensity can occur, potentially indicating alterations in the dye’s molar extinction coefficient or aggregation state. Higher-molecular-weight PVDF-HFP might lead to increased viscosity and reduced dye aggregation, potentially enhancing the light absorption. The IPCE spectra provide a direct measure of the DSSCs’ ability to convert absorbed photons into a current. Meanwhile, 2-LBH-92, with its broad absorption profile, is expected to show a correspondingly broad IPCE spectrum, with peaks aligning with its UV and visible absorption bands. The presence of different molecular weights of PVDF-HFP in the electrolyte can significantly influence the IPCE spectra (see
Figure 6b–e). Lower-molecular-weight PVDF-HFP, due to its lower viscosity, might facilitate faster ion transport within the electrolyte, leading to more efficient charge collection and potentially higher IPCE values, especially in the longer wavelength regions, where charge recombination becomes more prominent. However, excessively low molecular weights might compromise the quasi-solid-state nature of the electrolyte, negatively impacting its long-term stability.
Conversely, higher-molecular-weight PVDF-HFP, while potentially enhancing the stability, might hinder ion transport due to increased viscosity. This could lead to lower IPCE values, particularly at longer wavelengths, as charge recombination outcompetes charge collection. However, the reduced dye aggregation potentially offered by higher-molecular-weight PVDF-HFP could partially offset this effect by improving the light harvesting. Furthermore, the interaction between the 2-LBH-92 dye and the PVDF-HFP polymer can influence the electron injection and dye regeneration processes. Favorable interactions could facilitate electron injection from the dye’s excited state into the TiO2 conduction band, leading to increased IPCE values. Conversely, unfavorable interactions could hinder these processes, negatively impacting the device performance.
3.5.2. Stability Study
The stability study of the DSSCs using the phenothiazine organic dye 2-LBH-92 with the liquid DMPII electrolyte and PVDF-HFP polymer electrolytes at different molecular weights is shown in
Figure 7.
R9 cell (liquid DMPII electrolyte)
The R9 cell, employing a liquid DMPII electrolyte, exhibited a 12% efficiency reduction after 600 h. This decline primarily stemmed from the 21% decrease in the Jsc, indicating a significant loss in the cell’s ability to generate a current from incident light. This could be attributed to several factors, including dye degradation, electrolyte decomposition, or increased charge recombination at the TiO2/dye/electrolyte interface. The 3.8% decrease in the Voc further supports this hypothesis, suggesting increased recombination losses. However, the 9% increase in the FF implies improvements in charge transport and collection within the device, possibly due to changes in the electrolyte’s conductivity or interfacial properties over time.
R10 cell (PVDF-HFP electrolyte with M.W. of 86,000 g/mol)
Incorporating PVDF-HFP polymers into the electrolyte significantly influenced the devices’ stability. The R10 cell, with a polymer molecular weight of 86,000 g/mol, displayed an 11% efficiency reduction after 600 h, comparable to the R9 cell. This suggests that this specific molecular weight might not offer significant advantages in overall efficiency retention compared to the liquid electrolyte. However, a closer look at the individual parameters reveals a more nuanced picture. The 4% increase in the Voc suggests a reduction in recombination losses, potentially due to the polymer’s passivation effect at the TiO2/dye/electrolyte interface. The substantial 33% increase in the FF indicates significantly improved charge transport and collection, likely facilitated by the polymer’s influence on the electrolyte’s ionic conductivity and interfacial properties. This improvement in the FF partially compensates for the 16% decrease in the Jsc, which, while still present, is less severe than in the R9 cell. This suggests that the polymer might be mitigating some degradation pathways affecting Jsc, even if not completely eliminating them.
R11 cell (PVDF-HFP electrolyte with M.W. of 90,000 g/mol)
The R11 cell, employing a slightly higher molecular weight of PVDF-HFP (90,000 g/mol), demonstrated the most promising stability results, with only a 4% efficiency reduction after 600 h. This suggests that this specific molecular weight might offer a trade-off in balancing the benefits of polymer incorporation with minimal adverse effects on performance. The 5% decrease in the Voc, while indicating some increase in recombination, is less pronounced than in the R9 cell. The 8% increase in the FF, while not as dramatic as in the R10 cell, still points to improved charge transport and collection. The 15% decrease in the Jsc, comparable to the R10 cell, suggests that the polymer’s influence in terms of mitigating the Jsc degradation pathways might be similar for both molecular weights.
R12 cell (PVDF-HFP electrolyte with M.W. of 455,000 g/mol)
The R12 cell, incorporating the highest molecular weight of PVDF-HFP (455,000 g/mol), suffered a significant 50% efficiency reduction after 600 h, the most severe among all tested cells. This clearly demonstrates the detrimental effects of using a polymer with an excessively high molecular weight in the electrolyte. The high molecular weight likely leads to a highly viscous electrolyte, severely hindering ion transport and negatively impacting charge collection within the device. This is further evidenced by the relatively small 3.5% increase in the FF, indicating a limited improvement in charge transport compared to the lower-molecular-weight polymers. The 8% decrease in the Voc and the substantial 46% decrease in the Jsc further highlight the negative impact of the high-molecular-weight polymer on both the recombination losses and charge generation/collection processes.
In summary, the alterations of the Voc, Jsc, FF, and overall efficiency in these cells over time provided insights into how the molecular weight of the PVDF-HFP polymer electrolytes could severely influence the long-term performance of DSSCs. The changes observed in the solar cell parameters suggested a trade-off between the structural and transport properties of the polymers used. Lower molecular weights seemed to favor a more stable interface and better transport properties, leading to a smaller decrease in efficiency, whereas very high-molecular-weight polymers may impede ion movement and increase recombination, significantly reducing the cell’s performance.