Transcranial Photobiomodulation for Executive Function in Bipolar Disorder (TPEB): Study Protocol
Abstract
1. Introduction
1.1. Bipolar Disorder
1.2. Transcranial Photobiomodulation (t-PBM)
1.3. Preliminary Data
1.3.1. Within Our Institution
1.3.2. Within Our Collaborators in the Region
1.3.3. Within Our International Collaborators
1.4. Innovation
2. Aims
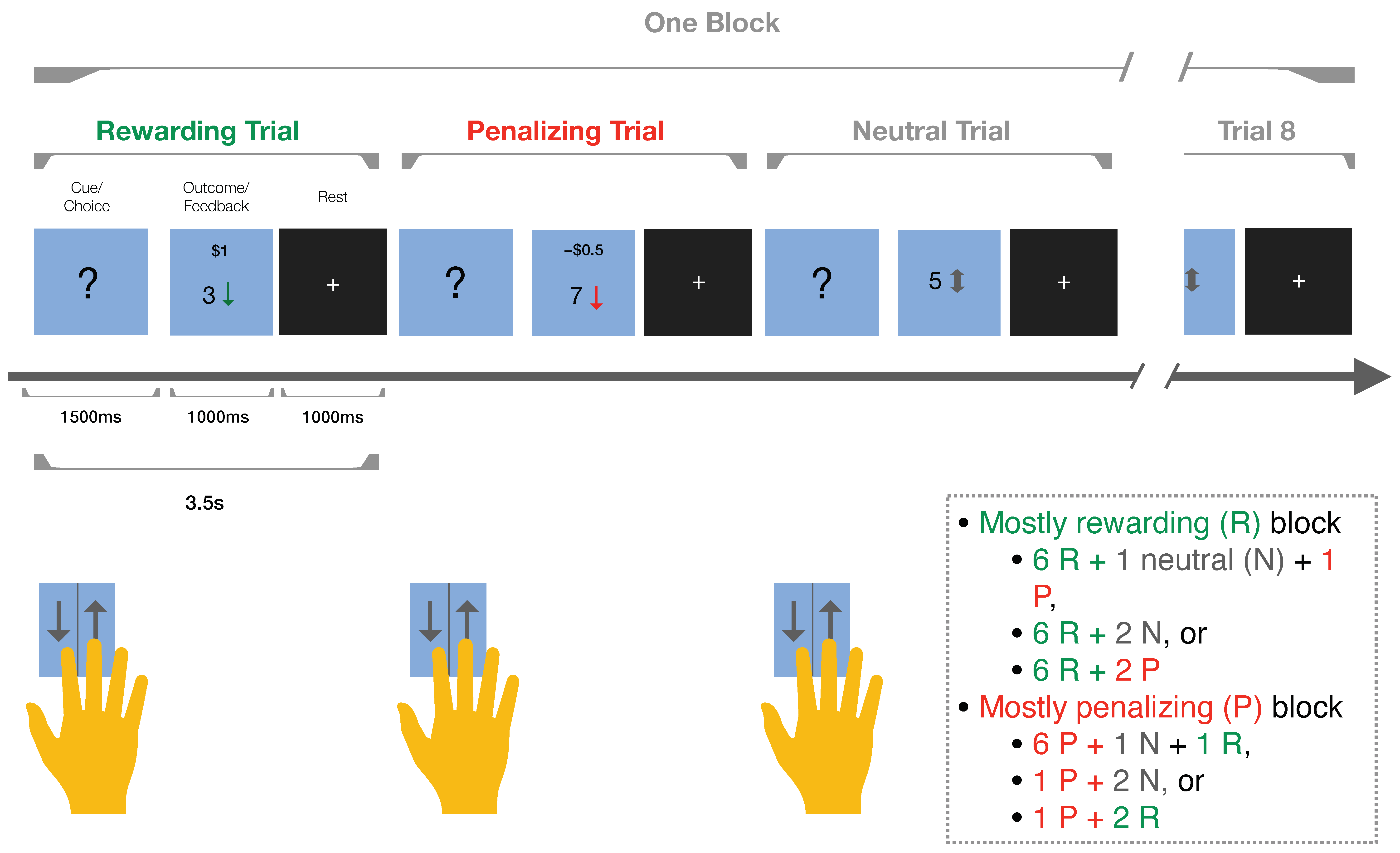
3. Gambling Tasks
3.1. Iowa Gambling Task
3.2. Reward Gambling Task
4. Study Design, Schedule, and Assessments
4.1. Stimulation Sessions
4.2. Outcomes
5. Subjects
6. Transcranial Photobiomodulation Administration
7. MRI Scanning and Imagining Processing
8. Statistical Analysis
9. Demographic Data on Existing Participants
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Merikangas, K.R.; Jin, R.; He, J.-P.; Kessler, R.C.; Lee, S.; Sampson, N.A.; Viana, M.C.; Andrade, L.H.; Hu, C.; Karam, E.G.; et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch. Gen. Psychiatry 2011, 68, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Nowrouzi, B.; McIntyre, R.S.; MacQueen, G.; Kennedy, S.H.; Kennedy, J.L.; Ravindran, A.; Yatham, L.; De Luca, V. Admixture analysis of age at onset in first episode bipolar disorder. J. Affect. Disord. 2016, 201, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Laje, G.; Blanco, C.; Jiang, H.; Schmidt, A.B.; Olfson, M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch. Gen. Psychiatry 2007, 64, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Rowland, T.A.; Marwaha, S. Epidemiology and risk factors for bipolar disorder. Ther. Adv. Psychopharmacol. 2018, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Kroon, J.S.; Wohlfarth, T.D.; Dieleman, J.; Sutterland, A.L.; Storosum, J.G.; Denys, D.; de Haan, L.; Sturkenboom, M.C. Incidence rates and risk factors of bipolar disorder in the general population: A population-based cohort study. Bipolar Disord. 2013, 15, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.; Petukhova, M.; Vilagut, G.; Chatterji, S.; Heeringa, S.; Üstün, T.B.; Alhamzawi, A.O.; Viana, M.C.; Angermeyer, M.; Bromet, E.; et al. Days out of role due to common physical and mental conditions: Results from the WHO World Mental Health surveys. Mol. Psychiatry 2011, 16, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Dome, P.; Rihmer, Z.; Gonda, X. Suicide Risk in Bipolar Disorder: A Brief Review. Medicina 2019, 55, 403. [Google Scholar] [CrossRef]
- Strakowski, S.M.; Fleck, D.E.; DelBello, M.P.; Adler, C.M.; Shear, P.K.; Kotwal, R.; Arndt, S. Impulsivity across the course of bipolar disorder. Bipolar Disord. 2010, 12, 285–297. [Google Scholar] [CrossRef]
- Newman, A.L.; Meyer, T.D. Impulsivity: Present during euthymia in bipolar disorder?—A systematic review. Int. J. Bipolar Disord. 2014, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Sylvia, L.G.; Montana, R.E.; Deckersbach, T.; Thase, M.E.; Tohen, M.; Reilly-Harrington, N.; McInnis, M.G.; Kocsis, J.H.; Bowden, C.; Calabrese, J.; et al. Poor quality of life and functioning in bipolar disorder. Int. J. Bipolar Disord. 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Perugi, G.; Medda, P.; Toni, C.; Mariani, M.G.; Socci, C.; Mauri, M. The Role of Electroconvulsive Therapy (ECT) in Bipolar Disorder: Effectiveness in 522 Patients with Bipolar Depression, Mixed-state, Mania and Catatonic Features. Curr. Neuropharmacol. 2017, 15, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.B.; Segger, F.; Fischer, K.; Obermeier, M.; Musil, R. Update on weight-gain caused by antipsychotics: A systematic review and meta-analysis. Expert. Opin. Drug Saf. 2020, 19, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.S.; Hawken, E.R.; Baldessarini, R.J.; Vázquez, G.H. Maintenance Pharmacological Treatment of Juvenile Bipolar Disorder: Review and Meta-Analyses. Int. J. Neuropsychopharmacol. 2019, 22, 531–540. [Google Scholar] [CrossRef] [PubMed]
- García-Blanco, A.; García-Portilla, M.P.; de la Fuente-Tomás, L.; Batalla, M.; Sánchez-Autet, M.; Arranz, B.; Safont, G.; Arqués, S.; Livianos, L.; Sierra, P. Sexual Dysfunction and Mood Stabilizers in Long-Term Stable Patients with Bipolar Disorder. J. Sex. Med. 2020, 17, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Bahji, A.; Ermacora, D.; Stephenson, C.; Hawken, E.R.; Vazquez, G. Comparative efficacy and tolerability of pharmacological treatments for the treatment of acute bipolar depression: A systematic review and network meta-analysis. J. Affect. Disord. 2020, 269, 154–184. [Google Scholar] [CrossRef]
- Parker, G.; Ricciardi, T.; Tavella, G.; Hadzi-Pavlovic, D. A Single-Blind Randomized Comparison of Lithium and Lamotrigine as Maintenance Treatments for Managing Bipolar II Disorder. J. Clin. Psychopharmacol. 2021, 41, 381–388. [Google Scholar] [CrossRef]
- Sajatovic, M.; Valenstein, M.; Blow, F.; Ganoczy, D.; Ignacio, R. Treatment adherence with lithium and anticonvulsant medications among patients with bipolar disorder. Psychiatr. Serv. 2007, 58, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Jawad, I.; Watson, S.; Haddad, P.M.; Talbot, P.S.; McAllister-Williams, R.H. Medication nonadherence in bipolar disorder: A narrative review. Ther. Adv. Psychopharmacol. 2018, 8, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Semahegn, A.; Torpey, K.; Manu, A.; Assefa, N.; Tesfaye, G.; Ankomah, A. Psychotropic medication non-adherence and its associated factors among patients with major psychiatric disorders: A systematic review and meta-analysis. Syst. Rev. 2020, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Lintunen, J.; Lähteenvuo, M.; Tanskanen, A.; Tiihonen, J.; Taipale, H. Non-adherence to mood stabilizers and antipsychotics among persons with bipolar disorder—A nationwide cohort study. J. Affect. Disord. 2023, 333, 403–408. [Google Scholar] [CrossRef]
- Abdullah-Koolmees, H.; Nawzad, S.; Egberts, T.C.G.; Vuyk, J.; Gardarsdottir, H.; Heerdink, E.R. The effect of non-adherence to antipsychotic treatment on rehospitalization in patients with psychotic disorders. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211027449. [Google Scholar] [CrossRef]
- Prajapati, A.R.; Dima, A.L.; Clark, A.B.; Gant, C.; Gibbons, C.; Gorrod, R.; Mosa, G.; Scott, S.; Song, F.; Teague, B.; et al. Mapping of modifiable barriers and facilitators of medication adherence in bipolar disorder to the Theoretical Domains Framework: A systematic review protocol. BMJ Open 2019, 9, e026980. [Google Scholar] [CrossRef] [PubMed]
- Yerevanian, B.I.; Koek, R.J.; Mintz, J. Bipolar pharmacotherapy and suicidal behavior. Part I: Lithium, divalproex and carbamazepine. J. Affect. Disord. 2007, 103, 5–11. [Google Scholar] [CrossRef]
- Lew, K.H.; Chang, E.Y.; Rajagopalan, K.; Knoth, R.L. The effect of medication adherence on health care utilization in bipolar disorder. Manag. Care Interface 2006, 19, 41–46. [Google Scholar] [PubMed]
- Powers, R.L.; Russo, M.; Mahon, K.; Brand, J.; Braga, R.J.; Malhotra, A.K.; Burdick, K.E. Impulsivity in bipolar disorder: Relationships with neurocognitive dysfunction and substance use history. Bipolar Disord. 2013, 15, 876–884. [Google Scholar] [CrossRef]
- Madero, S.; Anmella, G.; Sagué-Vilavella, M.; Pons, M.T.; Giménez, A.; Murru, A.; Gómez-Ramiro, M.; Gil-Badenes, J.; Rios, J.; Bioque, M.; et al. Evaluating maintenance electroconvulsive therapy in Bipolar Disorders: 3-year mirror-image study. J. Affect. Disord. 2022, 298, 58–64. [Google Scholar] [CrossRef]
- Mutz, J. Brain stimulation treatment for bipolar disorder. Bipolar Disord. 2023, 25, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Agbese, E.; Leslie, D.L.; Ba, D.M.; Rosenheck, R. Does Electroconvulsive Therapy for Patients with Mood Disorders Extend Hospital Length of Stays and Increase Inpatient Costs? Adm. Policy Ment. Health 2022, 49, 71–78. [Google Scholar] [CrossRef]
- Paris, J. Why electroconvulsive therapy still carries a stigma. Br. J. Psychiatry 2022, 220, 113–114. [Google Scholar] [CrossRef]
- Bahji, A. The Rise, Fall, and Resurgence of Electroconvulsive Therapy. J. Psychiatr. Pract. 2022, 28, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Mutz, J.; Edgcumbe, D.R.; Brunoni, A.R.; Fu, C.H.Y. Efficacy and acceptability of non-invasive brain stimulation for the treatment of adult unipolar and bipolar depression: A systematic review and meta-analysis of randomised sham-controlled trials. Neurosci. Biobehav. Rev. 2018, 92, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, E.; Cerioli, M.; Castiglioni, M.; Larini, L.; Scarpa, C.; Dell’Osso, B. Recent innovations in non-invasive brain stimulation (NIBS) for the treatment of unipolar and bipolar depression: A narrative review. Int. Rev. Psychiatry 2022, 34, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L.; Carver, C.S.; Mulé, S.; Joormann, J. Impulsivity and risk for mania: Towards greater specificity. Psychol. Psychother. 2013, 86, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Burdick, K.E.; Millett, C.E.; Yocum, A.K.; Altimus, C.M.; Andreassen, O.A.; Aubin, V.; Belzeaux, R.; Berk, M.; Biernacka, J.M.; Blumberg, H.P.; et al. Predictors of functional impairment in bipolar disorder: Results from 13 cohorts from seven countries by the global bipolar cohort collaborative. Bipolar Disord. 2022, 24, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.C.; Alter, S.; Hazlett, E.A.; Shafritz, K.M.; Yehuda, R.; Goodman, M.; Haznedar, M.M.; Szeszko, P.R. Neural correlates of impulsivity in bipolar disorder: A systematic review and clinical implications. Neurosci. Biobehav. Rev. 2023, 147, 105109. [Google Scholar] [CrossRef] [PubMed]
- Santana, R.P.; Kerr-Gaffney, J.; Ancane, A.; Young, A.H. Impulsivity in Bipolar Disorder: State or Trait? Brain Sci. 2022, 12, 1351. [Google Scholar] [CrossRef] [PubMed]
- Nusslock, R.; Almeida, J.R.; Forbes, E.E.; Versace, A.; Frank, E.; LaBarbara, E.J.; Klein, C.R.; Phillips, M.L. Waiting to win: Elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord. 2012, 14, 249–260. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, N.; Szczepanowski, R.; El-Deredy, W.; Mason, L.; Bentall, R.P. fMRI evidence of a relationship between hypomania and both increased goal-sensitivity and positive outcome-expectancy bias. Neuropsychologia 2011, 49, 2825–2835. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Koshimori, Y.; Aminian, K.; Obeso, I.; Rusjan, P.; Lang, A.E.; Daskalakis, Z.J.; Houle, S.; Strafella, A.P. Investing in the Future: Stimulation of the Medial Prefrontal Cortex Reduces Discounting of Delayed Rewards. Neuropsychopharmacology 2015, 40, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Brevet-Aeby, C.; Brunelin, J.; Iceta, S.; Padovan, C.; Poulet, E. Prefrontal cortex and impulsivity: Interest of noninvasive brain stimulation. Neurosci. Biobehav. Rev. 2016, 71, 112–134. [Google Scholar] [CrossRef]
- Hsu, T.-Y.; Tseng, L.-Y.; Yu, J.-X.; Kuo, W.-J.; Hung, D.L.; Tzeng, O.J.; Walsh, V.; Muggleton, N.G.; Juan, C.-H. Modulating inhibitory control with direct current stimulation of the superior medial frontal cortex. Neuroimage 2011, 56, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, M.; Lindenberg, R.; Antonenko, D.; Flaisch, T.; Flöel, A. Anodal transcranial direct current stimulation temporarily reverses age-associated cognitive decline and functional brain activity changes. J. Neurosci. 2013, 33, 12470–12478. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef]
- Pohar, R.; Farrah, K. Repetitive Transcranial Magnetic Stimulation for Patients with Depression: A Review of Clinical Effectiveness, Cost-Effectiveness and Guidelines—An Update; CADTH Rapid Response Reports; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019. Available online: http://www.ncbi.nlm.nih.gov/books/NBK545105/ (accessed on 28 January 2024).
- Sauvaget, A.; Tostivint, A.; Etcheverrigaray, F.; Pichot, A.; Dert, C.; Schirr-Bonnais, S.; Clouet, J.; Sellal, O.; Mauduit, N.; Leux, C.; et al. Hospital production cost of transcranial direct current stimulation (tDCS) in the treatment of depression. Neurophysiol. Clin. 2019, 49, 11–18. [Google Scholar] [CrossRef]
- Hamblin, M.R. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef]
- Gavish, L.; Houreld, N.N. Therapeutic Efficacy of Home-Use Photobiomodulation Devices: A Systematic Literature Review. Photobiomodulation. Photomed. Laser Surg. 2019, 37, 4–16. [Google Scholar] [CrossRef]
- Cassano, P.; Caldieraro, M.A.; Norton, R.; Mischoulon, D.; Trinh, N.H.; Nyer, M.; Dording, C.; Hamblin, M.R.; Campbell, B.; Iosifescu, D.V. Reported Side Effects, Weight and Blood Pressure, After Repeated Sessions of Transcranial Photobiomodulation. Photobiomodul. Photomed. Laser Surg. 2019, 37, 651–656. [Google Scholar] [CrossRef]
- Cassano, P.; Norton, R.; Caldieraro, M.A.; Vahedifard, F.; Vizcaino, F.; McEachern, K.M.; Iosifescu, D. Tolerability and Safety of Transcranial Photobiomodulation for Mood and Anxiety Disorders. Photonics 2022, 9, 507. [Google Scholar] [CrossRef]
- Konstantinović, L.M.; Jelić, M.B.; Jeremić, A.; Stevanović, V.B.; Milanović, S.D.; Filipović, S.R. Transcranial application of near-infrared low-level laser can modulate cortical excitability. Lasers Surg. Med. 2013, 45, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, L.; Antal, A.; Masurat, F.; Paulus, W. Neuroplastic effects of transcranial near-infrared stimulation (tNIRS) on the motor cortex. Front. Behav. Neurosci. 2015, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.A.; Morries, L.D. SPECT Perfusion Imaging Demonstrates Improvement of Traumatic Brain Injury with Transcranial Near-infrared Laser Phototherapy. Adv. Mind Body Med. 2015, 29, 27–33. [Google Scholar] [PubMed]
- Nawashiro, H.; Wada, K.; Nakai, K.; Sato, S. Focal increase in cerebral blood flow after treatment with near-infrared light to the forehead in a patient in a persistent vegetative state. Photomed. Laser Surg. 2012, 30, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.S.I.; Zângaro, R.A.; Parreira, R.B.; Kerppers, I.I. The effects of transcranial LED therapy (TCLT) on cerebral blood flow in the elderly women. Lasers Med. Sci. 2015, 30, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Hase, S.N.; Gonzalez-Lima, F.; Liu, H. Transcranial laser stimulation improves human cerebral oxygenation. Lasers Surg. Med. 2016, 48, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Hacke, W.; Schellinger, P.D.; Albers, G.W.; Bornstein, N.M.; Dahlof, B.L.; Fulton, R.; Kasner, S.E.; Shuaib, A.; Richieri, S.P.; Dilly, S.G.; et al. Transcranial laser therapy in acute stroke treatment: Results of neurothera effectiveness and safety trial 3, a phase III clinical end point device trial. Stroke 2014, 45, 3187–3193. [Google Scholar] [CrossRef] [PubMed]
- Lampl, Y.; Zivin, J.A.; Fisher, M.; Lew, R.; Welin, L.; Dahlof, B.; Borenstein, P.; Andersson, B.; Perez, J.; Caparo, C.; et al. Infrared laser therapy for ischemic stroke: A new treatment strategy: Results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1). Stroke 2007, 38, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, K.; Farhadi, M.; Fekrazad, R.; Akbarnejad, Z.; Chaibakhsh, S.; Mahmoudian, S. Transcranial photobiomodulation in the management of brain disorders. J. Photochem. Photobiol. B Biol. 2021, 221, 112207. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.W.; Gonzalez-Lima, F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 2013, 230, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Blanco, N.J.; Saucedo, C.L.; Gonzalez-Lima, F. Transcranial infrared laser stimulation improves rule-based, but not information-integration, category learning in humans. Neurobiol. Learn. Mem. 2017, 139, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Castelli, D.M.; Gonzalez-Lima, F. Cognitive enhancement by transcranial laser stimulation and acute aerobic exercise. Lasers Med. Sci. 2016, 31, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Morries, L.D.; Cassano, P.; Henderson, T.A. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr. Dis. Treat. 2015, 11, 2159–2175. [Google Scholar] [PubMed]
- Naeser, M.A.; Saltmarche, A.; Krengel, M.H.; Hamblin, M.R.; Knight, J.A. Improved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: Two case reports. Photomed. Laser Surg. 2011, 29, 351–358. [Google Scholar] [CrossRef]
- Naeser, M.A.; Zafonte, R.; Krengel, M.H.; Martin, P.I.; Frazier, J.; Hamblin, M.R.; Knight, J.A.; Meehan, W.P.; Baker, E.H. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: Open-protocol study. J. Neurotrauma 2014, 31, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Saltmarche, A.E.; Naeser, M.A.; Ho, K.F.; Hamblin, M.R.; Lim, L. Significant Improvement in Cognition in Mild to Moderately Severe Dementia Cases Treated with Transcranial Plus Intranasal Photobiomodulation: Case Series Report. Photomed. Laser Surg. 2017, 35, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Disner, S.G.; Beevers, C.G.; Gonzalez-Lima, F. Transcranial Laser Stimulation as Neuroenhancement for Attention Bias Modification in Adults with Elevated Depression Symptoms. Brain Stimul. 2016, 9, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Coelho, D.R.A.; Gersten, M.; Jimenez, A.S.; Fregni, F.; Cassano, P.; Vieira, W.F. Treating neuropathic pain and comorbid affective disorders: Preclinical and clinical evidence. Pain. Pract. 2024, 00, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Coelho, D.R.A.; Salvi, J.D.; Vieira, W.F.; Cassano, P. Inflammation in obsessive-compulsive disorder: A literature review and hypothesis-based potential of transcranial photobiomodulation. J. Neurosci. Res. 2024, 102, e25317. [Google Scholar] [CrossRef] [PubMed]
- Cassano, P.; Petrie, S.R.; Mischoulon, D.; Cusin, C.; Katnani, H.; Yeung, A.; De Taboada, L.; Archibald, A.; Bui, E.; Baer, L.; et al. Transcranial Photobiomodulation for the Treatment of Major Depressive Disorder. The ELATED-2 Pilot Trial. Photomed. Laser Surg. 2018, 36, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Fradkin, Y.; De Taboada, L.; Naeser, M.; Saltmarche, A.; Snyder, W.; Steingold, E. Transcranial photobiomodulation in children aged 2–6 years: A randomized sham-controlled clinical trial assessing safety, efficacy, and impact on autism spectrum disorder symptoms and brain electrophysiology. Front. Neurol. 2024, 15, 1221193. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, F.; Khan, A.; Bolger, E.; Flynn, E.; Seltzer, W.P.; Teicher, M.H. An Effective and Safe Novel Treatment of Opioid Use Disorder: Unilateral Transcranial Photobiomodulation. Front. Psychiatry 2021, 12, 713686. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Li, L.; Zhou, X.; Dong, Q.; Liu, H.; Liu, H.; Yang, Q.; Han, Y.; Niu, H. Repeated transcranial photobiomodulation improves working memory of healthy older adults: Behavioral outcomes of poststimulation including a three-week follow-up. Neurophotonics 2022, 9, 035005. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.M.; Barrett, D.W.; Fink, L.H.; Garcia-Pittman, E.C.; Gonzalez-Lima, F. Transcranial Infrared Laser Stimulation Improves Cognition in Older Bipolar Patients: Proof of Concept Study. J. Geriatr. Psychiatry Neurol. 2022, 35, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Uozumi, Y.; Nawashiro, H.; Sato, S.; Kawauchi, S.; Shima, K.; Kikuchi, M. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg. Med. 2010, 42, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Hipskind, S.G.; Grover, F.L., Jr.; Fort, T.R.; Helffenstein, D.; Burke, T.J.; Quint, S.A.; Bussiere, G.; Stone, M.; Hurtado, T. Pulsed Transcranial Red/Near-Infrared Light Therapy Using Light-Emitting Diodes Improves Cerebral Blood Flow and Cognitive Function in Veterans with Chronic Traumatic Brain Injury: A Case Series. Photomed. Laser Surg. 2018, 37, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Urquhart, E.L.; Wanniarachchi, H.; Wang, X.; Gonzalez-Lima, F.; Alexandrakis, G.; Liu, H. Transcranial photobiomodulation-induced changes in human brain functional connectivity and network metrics mapped by whole-head functional near-infrared spectroscopy in vivo. Biomed. Opt. Express 2020, 11, 5783–5799. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.C.D.; Wang, X.; Wanniarachchi, H.; Liu, H. Enhancement of Frequency-Specific Hemodynamic Power and Functional Connectivity by Transcranial Photobiomodulation in Healthy Humans. Front. Neurosci. 2022, 16, 896502. [Google Scholar] [CrossRef] [PubMed]
- Jog, M.; Jann, K.; Yan, L.; Huang, Y.; Parra, L.; Narr, K.; Bikson, M.; Wang, D.J.J. Concurrent Imaging of Markers of Current Flow and Neurophysiological Changes During tDCS. Front. Neurosci. 2020, 14, 374. [Google Scholar] [CrossRef] [PubMed]
- Jog, M.S.; Kim, E.; Anderson, C.; Kubicki, A.; Kayathi, R.; Jann, K.; Yan, L.; Leaver, A.; Hellemann, G.; Iacoboni, M.; et al. In-vivo imaging of targeting and modulation of depression-relevant circuitry by transcranial direct current stimulation: A randomized clinical trial. Transl. Psychiatry 2021, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.; Barrett, D.W.; Saucedo, C.L.; Huang, L.-D.; Abraham, J.A.; Tanaka, H.; Haley, A.P.; Gonzalez-Lima, F. Beneficial neurocognitive effects of transcranial laser in older adults. Lasers Med. Sci. 2017, 32, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Dmochowski, G.M.; Dmochowski, J.P. Increased Blood Flow and Oxidative Metabolism in the Human Brain by Transcranial Laser Stimulation. bioRxiv 2018. Available online: https://www.biorxiv.org/content/10.1101/459883v1 (accessed on 11 March 2024). [CrossRef]
- Lebon, V.; Carlier, P.G.; Brillault-Salvat, C.; Leroy-Willig, A. Simultaneous measurement of perfusion and oxygenation changes using a multiple gradient-echo sequence: Application to human muscle study. Magn. Reson. Imaging 1998, 16, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Posse, S.; Wiese, S.; Gembris, D.; Mathiak, K.; Kessler, C.; Grosse-Ruyken, M.-L.; Elghahwagi, B.; Richards, T.; Dager, S.R.; Kiselev, V.G. Enhancement of BOLD-contrast sensitivity by single-shot multi-echo functional MR imaging. Magn. Reson. Med. 1999, 42, 87–97. [Google Scholar] [CrossRef]
- Mannu, P.; Saccaro, L.F.; Spera, V.; Cassano, P. Transcranial Photobiomodulation to Augment Lithium in Bipolar-I Disorder. Photobiomodul Photomed. Laser Surg. 2019, 37, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Sanches, M.; Bauer, I.E.; Galvez, J.F.; Zunta-Soares, G.B.; Soares, J.C. The management of cognitive impairment in bipolar disorder: Current status and perspectives. Am. J. Ther. 2015, 22, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, G.; Hui, J.; Ortiz, A.; Kaster, T.S.; Downar, J.; Blumberger, D.M.; Daskalakis, Z.J. Repetitive transcranial magnetic stimulation (rTMS) in bipolar disorder: A systematic review. Bipolar Disord. 2022, 24, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Dondé, C.; Neufeld, N.H.; Geoffroy, P.A. The Impact of Transcranial Direct Current Stimulation (tDCS) on Bipolar Depression, Mania, and Euthymia: A Systematic Review of Preliminary Data. Psychiatr. Q. 2018, 89, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A. Iowa Gambling Task. Professional Manual; Psychological Assessment Resources, Inc.: Lake Magdalene, FL, USA, 2007. [Google Scholar]
- Delgado, M.R.; Nystrom, L.E.; Fissell, C.; Noll, D.C.; Fiez, J.A. Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol. 2000, 84, 3072–3077. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Z.L.; D’Argembeau, A.; Ng, M.; Bechara, A. The Iowa Gambling Task in fMRI images. Hum. Brain Mapp. 2010, 31, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Bull, P.N.; Tippett, L.J.; Addis, D.R. Decision making in healthy participants on the Iowa Gambling Task: New insights from an operant approach. Front. Psychol. 2015, 6, 391. [Google Scholar] [CrossRef]
- Brevers, D.; Bechara, A.; Cleeremans, A.; Noël, X. Iowa Gambling Task (IGT): Twenty years after—Gambling disorder and IGT. Front. Psychol. 2013, 4, 665. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Janakiprasad Kumar, K.; Benegal, V. Underlying decision-making processes on Iowa Gambling Task. Asian J. Psychiatry 2019, 39, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Edge, M.D.; Johnson, S.L.; Ng, T.; Carver, C.S. Iowa Gambling Task performance in euthymic bipolar I disorder: A meta-analysis and empirical study. J. Affect. Disord. 2013, 150, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Gorlyn, M.; Keilp, J.G.; Oquendo, M.A.; Burke, A.K.; John Mann, J. Iowa gambling task performance in currently depressed suicide attempters. Psychiatry Res. 2013, 207, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Nejtek, V.A.; Kaiser, K.A.; Zhang, B.; Djokovic, M. Iowa Gambling Task scores predict future drug use in bipolar disorder outpatients with stimulant dependence. Psychiatry Res. 2013, 210, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Kikuchi, M.; Hirosawa, T.; Hino, S.; Nagasawa, T.; Hashimoto, T.; Munesue, T.; Minabe, Y. Reduced prefrontal activation during performance of the Iowa Gambling Task in patients with bipolar disorder. Psychiatry Res. 2015, 233, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stanford, M.S.; Mathias, C.W.; Dougherty, D.M.; Lake, S.L.; Anderson, N.E.; Patton, J.H. Fifty years of the Barratt Impulsiveness Scale: An update and review. Personal. Individ. Differ. 2009, 47, 385–395. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Berk, M.; Brietzke, E.; Goldstein, B.I.; López-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A.; et al. Bipolar disorders. Lancet 2020, 396, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Mir-Moghtadaei, A.; Siddiqi, S.H.; Mir-Moghtadaei, K.; Blumberger, D.M.; Vila-Rodriguez, F.; Daskalakis, Z.J.; Fox, M.D.; Downar, J. Updated scalp heuristics for localizing the dorsolateral prefrontal cortex based on convergent evidence of lesion and brain stimulation studies in depression. Brain Stimul. 2022, 15, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.; Yacoub, E.; Olman, C.A.; Auerbach, E.; Strupp, J.; Harel, N.; Uğurbil, K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med. 2010, 63, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Glasser, M.F.; Smith, S.M.; Marcus, D.S.; Andersson, J.L.R.; Auerbach, E.J.; Behrens, T.E.J.; Coalson, T.S.; Harms, M.P.; Jenkinson, M.; Moeller, S.; et al. The Human Connectome Project’s Neuroimaging Approach. Nat. Neurosci. 2016, 19, 1175–1187. [Google Scholar] [CrossRef]
- Elam, J.S.; Glasser, M.F.; Harms, M.P.; Sotiropoulos, S.N.; Andersson, J.L.; Burgess, G.C.; Curtiss, S.W.; Oostenveld, R.; Larson-Prior, L.J.; Schoffelen, J.-M.; et al. The Human Connectome Project: A retrospective. Neuroimage 2021, 244, 118543. [Google Scholar] [CrossRef] [PubMed]



| Measure | Pre-Screen | Screen | Baseline | Tx 1 | Tx 2 | Tx 3 | Tx 4 | Tx 5 | Check-In | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Screen Form | X | |||||||||
| ASRM | X | X | ||||||||
| PHQ-9 | X | X | ||||||||
| Consent Form | X | |||||||||
| Demographic Form | X | |||||||||
| Concomitant Treatments Log | X | X | X | X | X | X | X | X | X | |
| AE | X | X | X | X | X | X | X | X | ||
| Prior Treatment Log | X | |||||||||
| Medical History | X | |||||||||
| Drug Screen | X | |||||||||
| Pregnancy Test | X | |||||||||
| MINI | X | |||||||||
| C-SSRS | X | X | X | X | ||||||
| MOODS-SR Lifetime | X | |||||||||
| YMRS | X | |||||||||
| Screening Note | X | |||||||||
| NIS-SCS | X | |||||||||
| MOODS-SR Last Week | X | X | X | |||||||
| BIS | X | X | X | X | ||||||
| I-7 | X | X | X | |||||||
| BRIEF | X | X | X | |||||||
| t-PBM | X * | X | X | X | X * | |||||
| Reward Gambling Task | X | X * | X * | X | ||||||
| lowa Gambling Task | X | X | X | X | ||||||
| Vitals | X | X | ||||||||
| MRI Safety Form | X | X | X | |||||||
| SAFTEE | X | X | X | |||||||
| CGI-S | X | X | X | |||||||
| CGI-I | X | X | ||||||||
| PBQ | X | |||||||||
| TSRQ | X | X | X | X | X | |||||
| CAST-IRR | X | X | X | X | X | X | ||||
| Intervention Tracking | X | X | X | X | X | |||||
| Adverse Event Log | X | X | X | X | X | X | X | X |
| Measure (Unit) | Value |
|---|---|
| Wavelength (nm) | 808 |
| Exposure time (s) | 333 |
| Area of exposure (cm2) | 24 |
| Irradiance, Power Density (mW/cm2) | 300 |
| Fluence (J/cm2) | 100 |
| Total Energy (kJ) | 2.4 |
| NIR Source | Laser |
| Laser Output (W) | 3.5 per fiber (7 total) |
| Wave Mode | Continuous |
| Anatomical Targets | F4 & Fp2 |
| Device | Litecure’s LightForce® EXPi Deep Tissue Laser Therapy TM System, Transcranial PhotoBioModulation-1000 (t-PBM-2.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, D.R.A.; Puerto, A.M.H.; Vieira, W.F.; Lohmann, C.A.; Shahab, M.H.; Gersten, M.B.; Vahedifard, F.; McEachern, K.M.; Clancy, J.A.; Cassano, P. Transcranial Photobiomodulation for Executive Function in Bipolar Disorder (TPEB): Study Protocol. Photonics 2024, 11, 761. https://doi.org/10.3390/photonics11080761
Coelho DRA, Puerto AMH, Vieira WF, Lohmann CA, Shahab MH, Gersten MB, Vahedifard F, McEachern KM, Clancy JA, Cassano P. Transcranial Photobiomodulation for Executive Function in Bipolar Disorder (TPEB): Study Protocol. Photonics. 2024; 11(8):761. https://doi.org/10.3390/photonics11080761
Chicago/Turabian StyleCoelho, David Richer Araujo, Aura Maria Hurtado Puerto, Willians Fernando Vieira, Carlos Alberto Lohmann, Muhammad Hamza Shahab, Maia Beth Gersten, Farzan Vahedifard, Kayla Marie McEachern, Julie A. Clancy, and Paolo Cassano. 2024. "Transcranial Photobiomodulation for Executive Function in Bipolar Disorder (TPEB): Study Protocol" Photonics 11, no. 8: 761. https://doi.org/10.3390/photonics11080761
APA StyleCoelho, D. R. A., Puerto, A. M. H., Vieira, W. F., Lohmann, C. A., Shahab, M. H., Gersten, M. B., Vahedifard, F., McEachern, K. M., Clancy, J. A., & Cassano, P. (2024). Transcranial Photobiomodulation for Executive Function in Bipolar Disorder (TPEB): Study Protocol. Photonics, 11(8), 761. https://doi.org/10.3390/photonics11080761






