High-Dynamic-Range Absorption Spectroscopy by Generating a Wide Path-Length Distribution with Scatterers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
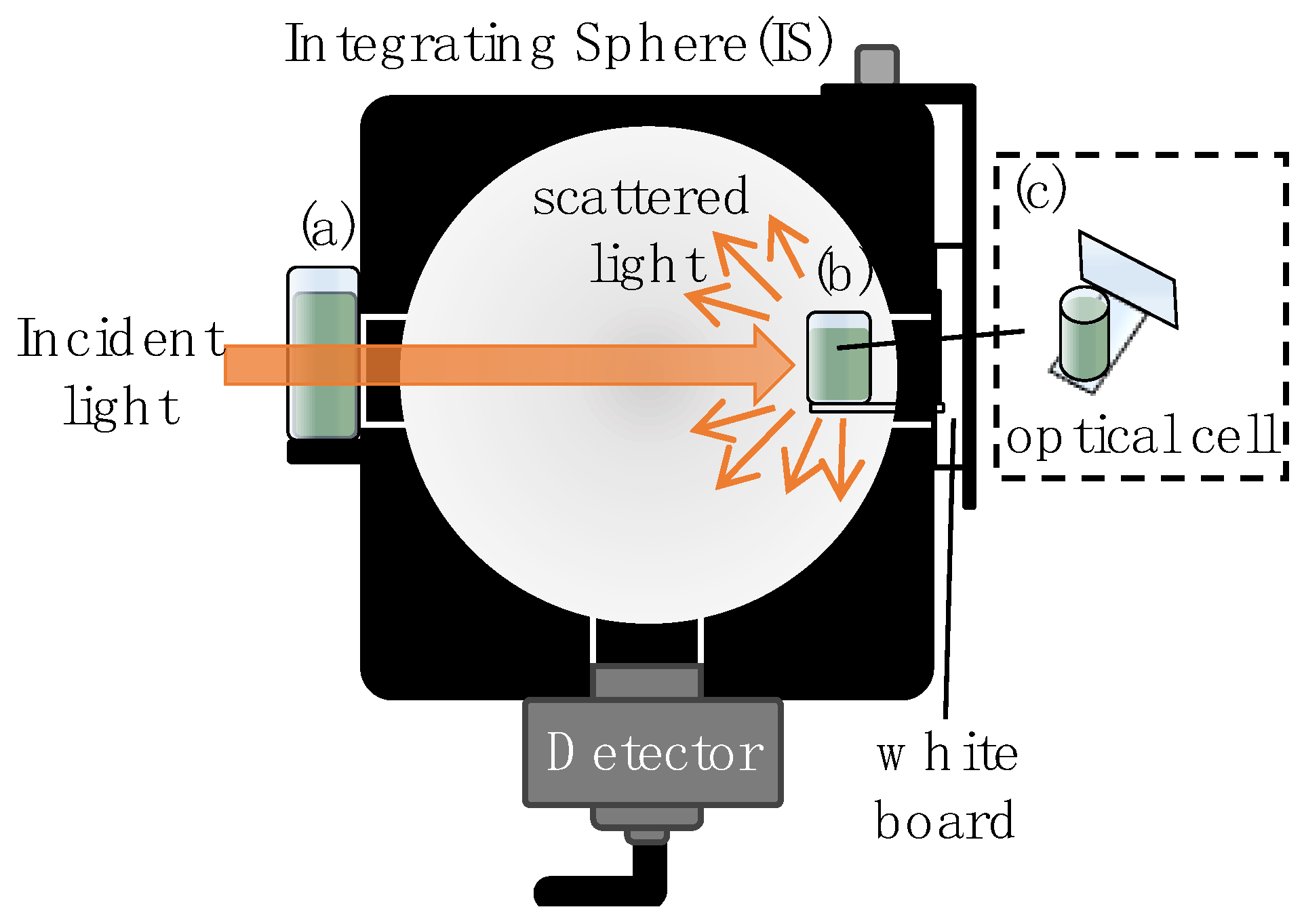
2.2. Setup
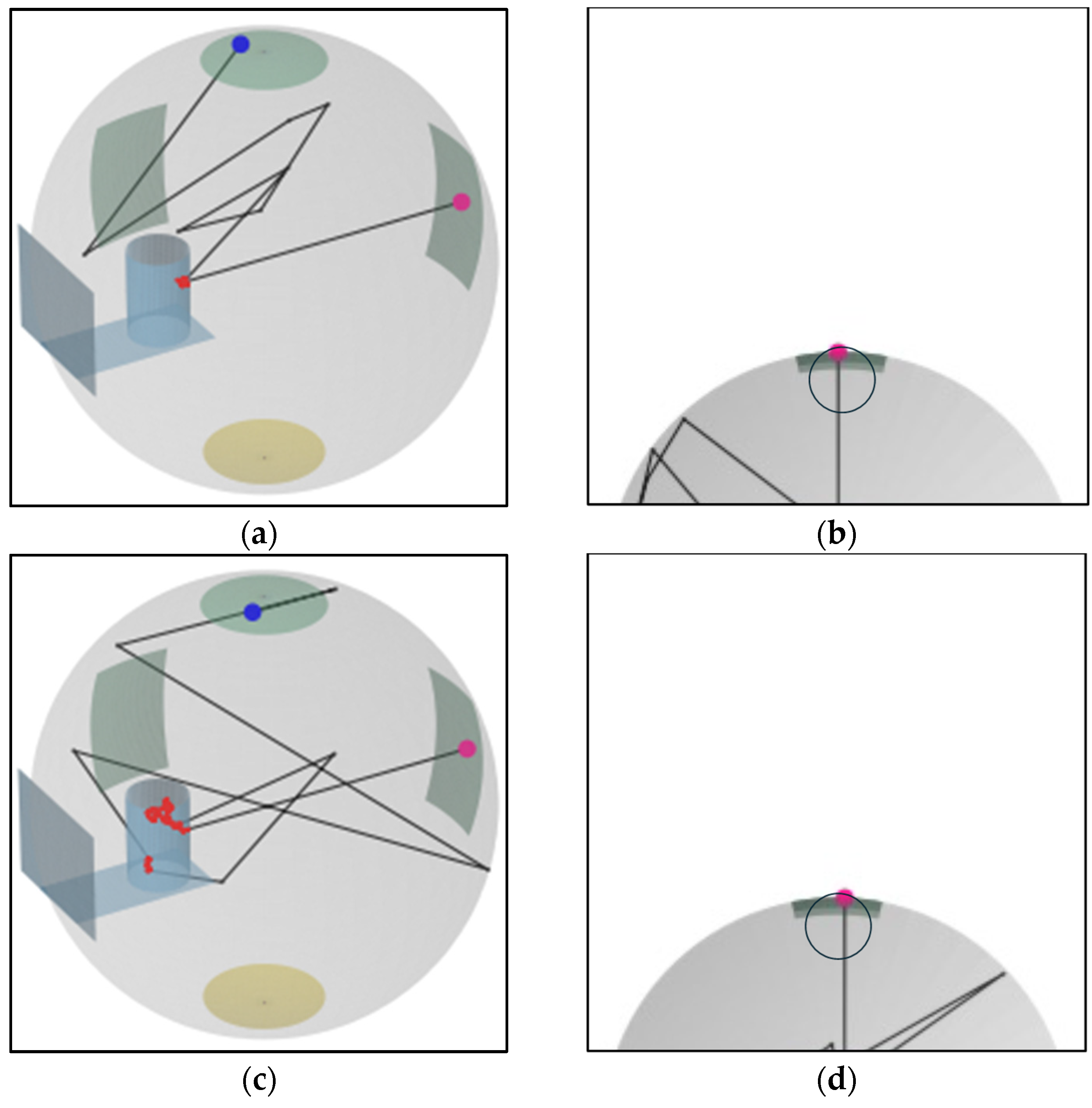
2.3. Monte Carlo Simulation
2.3.1. Estimation of µa for Scatterer-Added Samples by MC
- Estimation of A for each λ and µa by MC: MC simulations were performed under a total of 4680 conditions, corresponding to 36 different wavelengths λ and 130 different absorption coefficients µa, as shown in Table 1. For each combination of λ and µa, AMC was calculated as the estimation of the absorbance A that would be measured in the setup shown in Figure 1b. The remaining input parameters, the scattering coefficient µs and anisotropy factor g, were calculated for each λ based on Mie scattering theory for PSs 500 nm in diameter [Appendix A]. Calculations based on Mie scattering theory were performed using a Fortran code downloaded from reference [45]. The refractive index of PSs and the refractive index of the medium, as input parameters for these calculations, were determined using the first-term Sellmeier equation cited in reference [46] for PSs [Figure A1a] and set at 1.33 for the medium, corresponding to the refractive index of water. The Monte Carlo (MC) ray-tracing simulations were conducted 50,000 times for each condition, and the average detected intensity from these simulations was used as the measured intensity. In processing the MC results, the baseline AMC(λ, 0), which represents the results for samples containing only PSs (corresponding to cdye = 0, µa = 0), was subtracted from AMC(λ, µa). This approach follows the processing of measured data.
- 2.
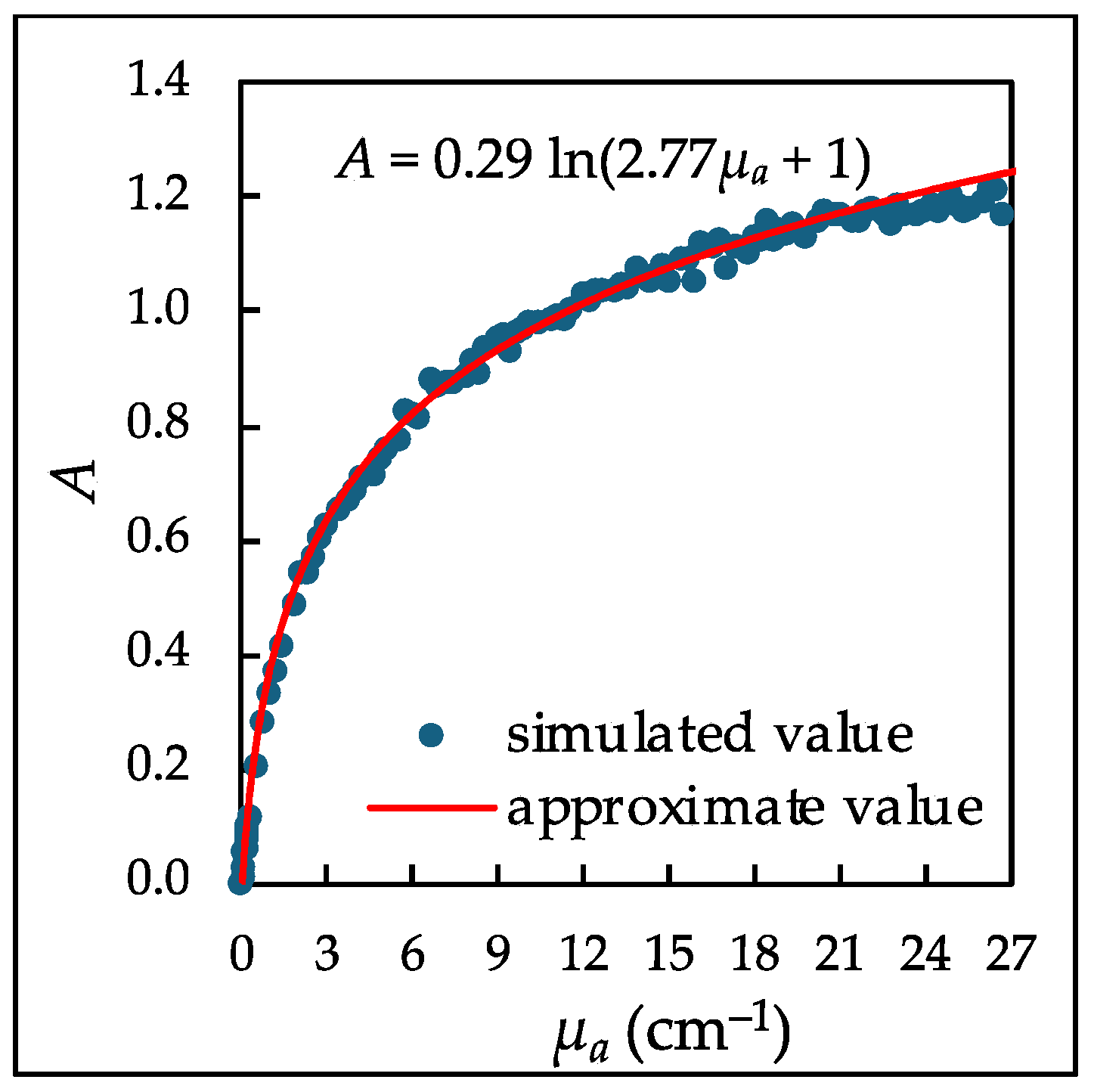
- Defining the relation between A and µa: The relation between A and µa was defined by fitting the simulation results to a correction function. As an example, the MC results at an incident-light wavelength of 426 nm are shown in Figure 2. The MC results, indicated by blue dots, were fitted with the following logarithmic function, shown as the red line:
2.3.2. Estimation of PL Distribution
- Initialize the count for each PL in {d1, …, d500} to zero.
- During each simulation run, if a photon reaches the detector, determine which of the PLs {d1, …, d500} the total distance traveled by the photon within the sample falls into and increment that PL’s count by one.
- After all ray-tracing simulations are complete, calculate the percentage that the count of each PL represents out of the total counts for all PLs.
3. Results and Discussion
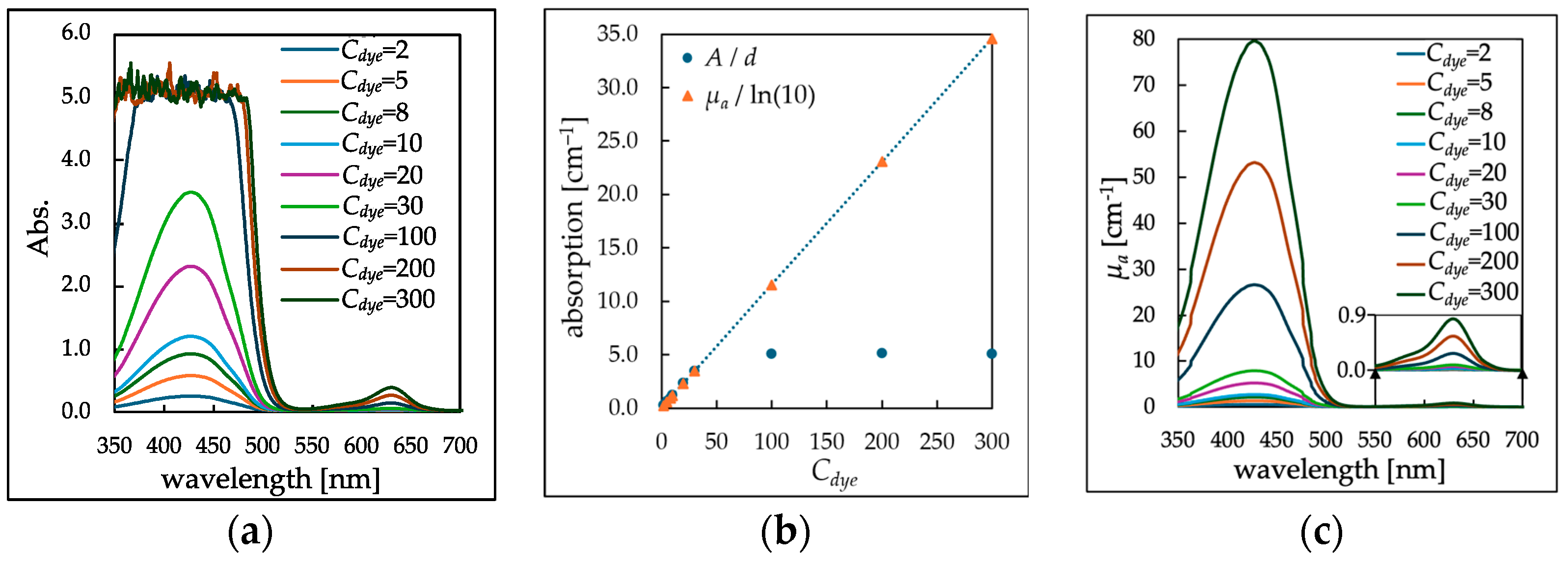
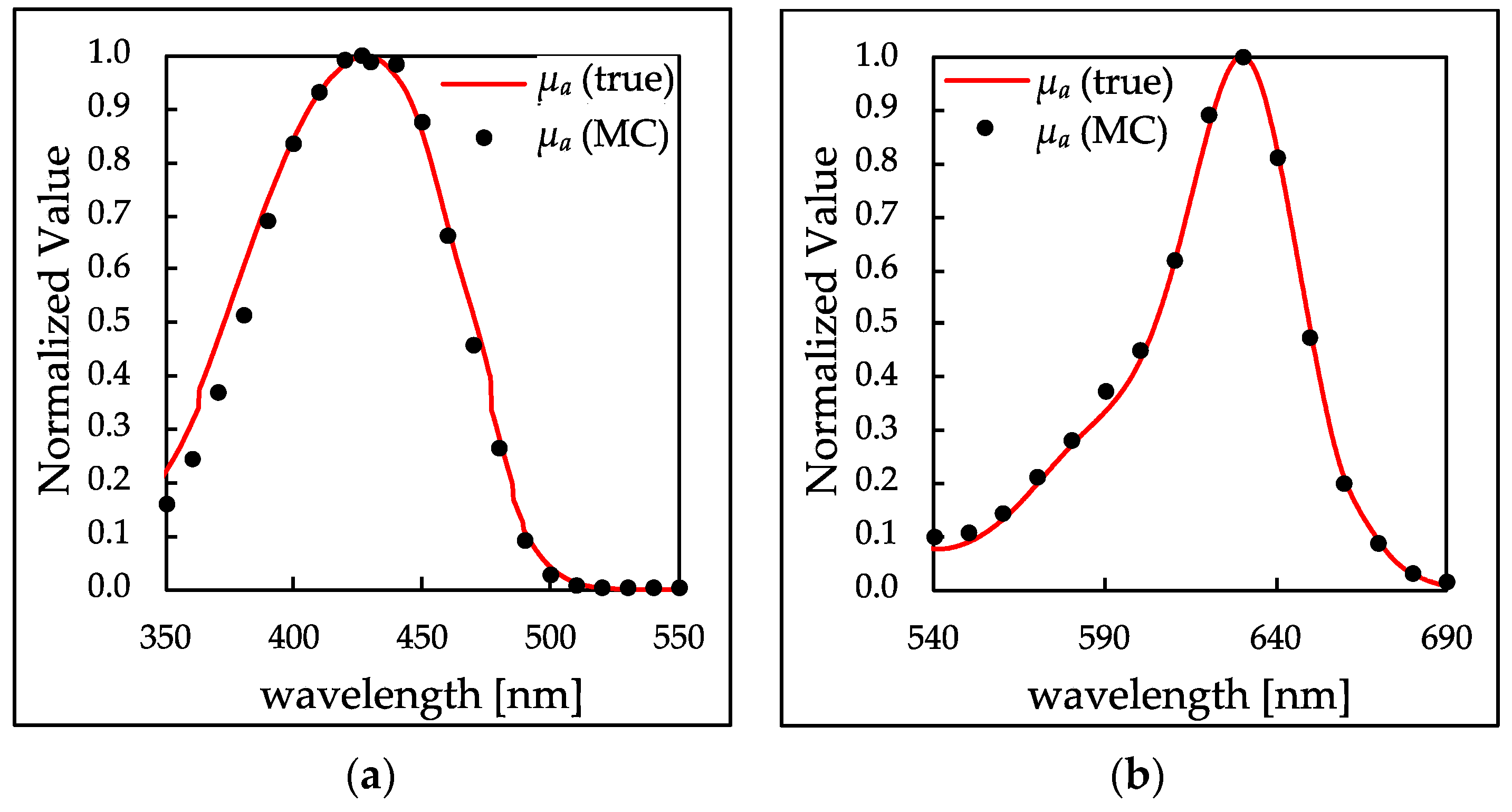
3.1. Determination of μa of Dye Solutions
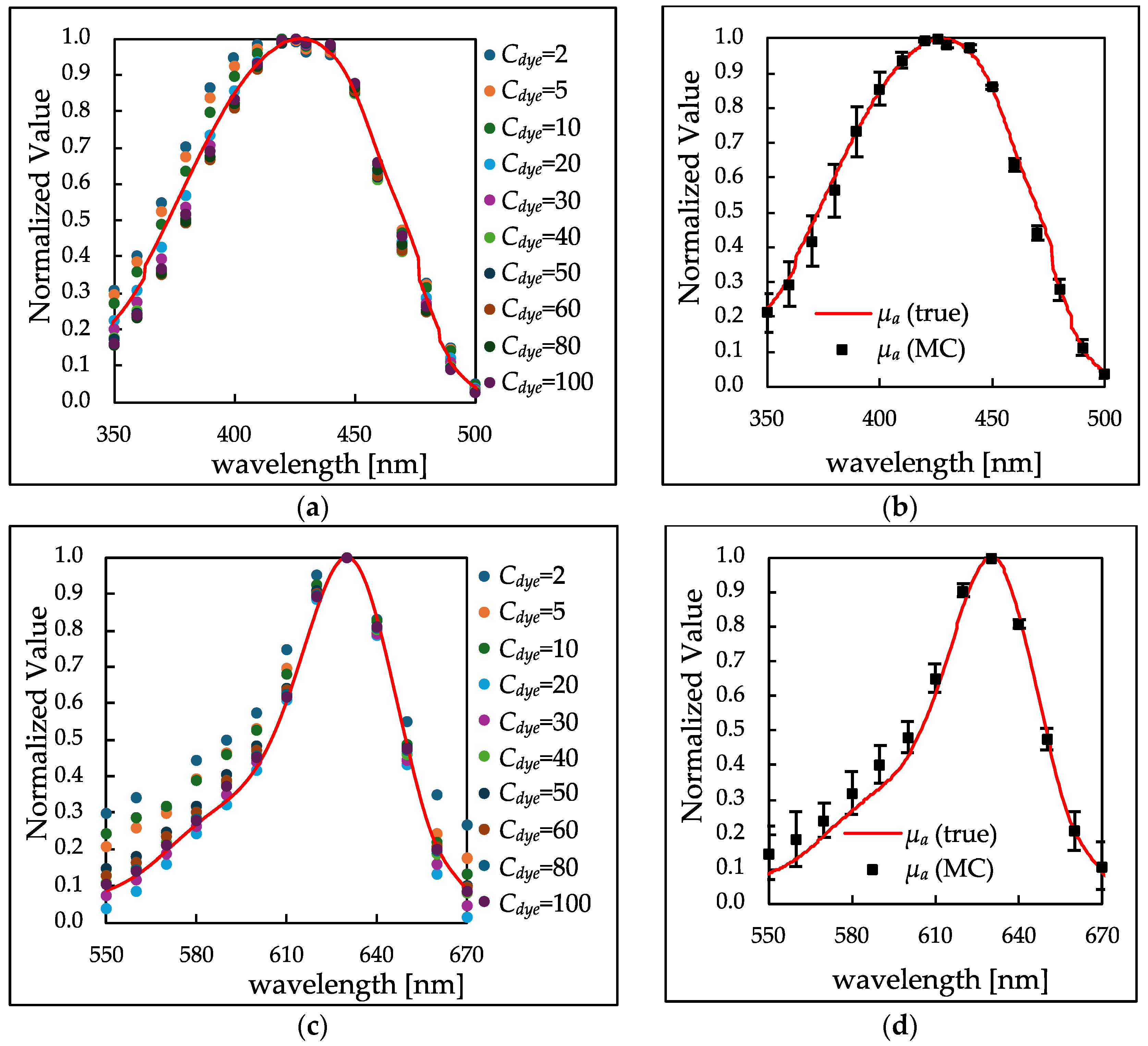
3.2. Simultaneous Detection of High and Low Absorption Peaks by Adding Scatterers to Samples
3.2.1. Absorbance Spectrum of Scatterer-Added Samples
3.2.2. Comparison of Peak Shapes
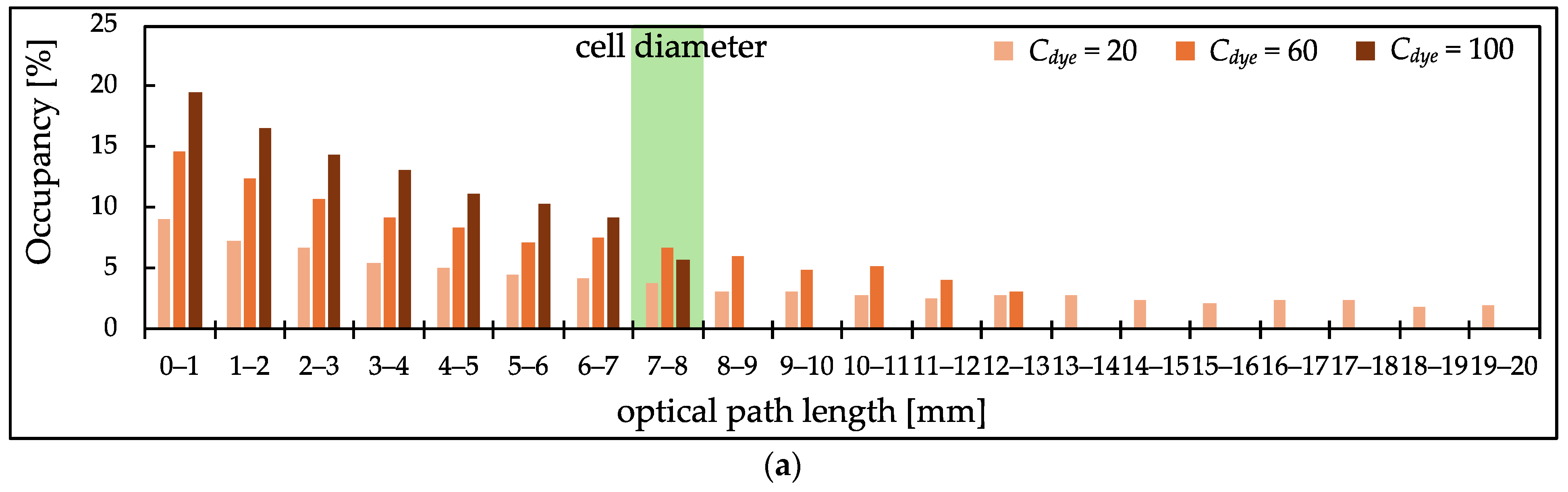
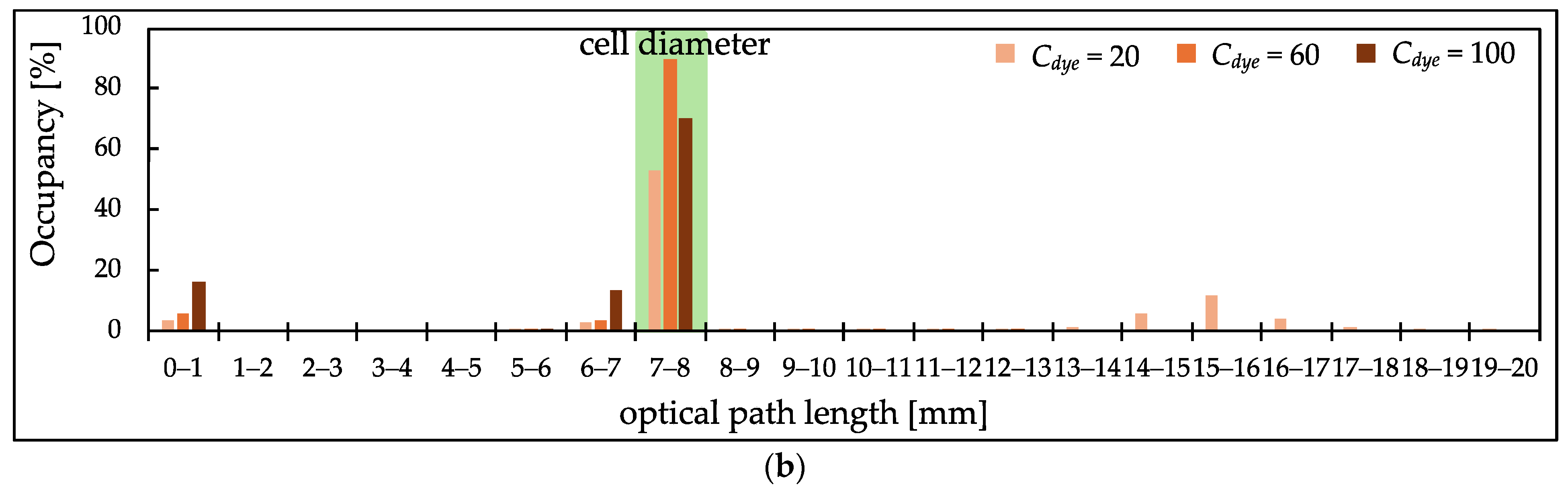
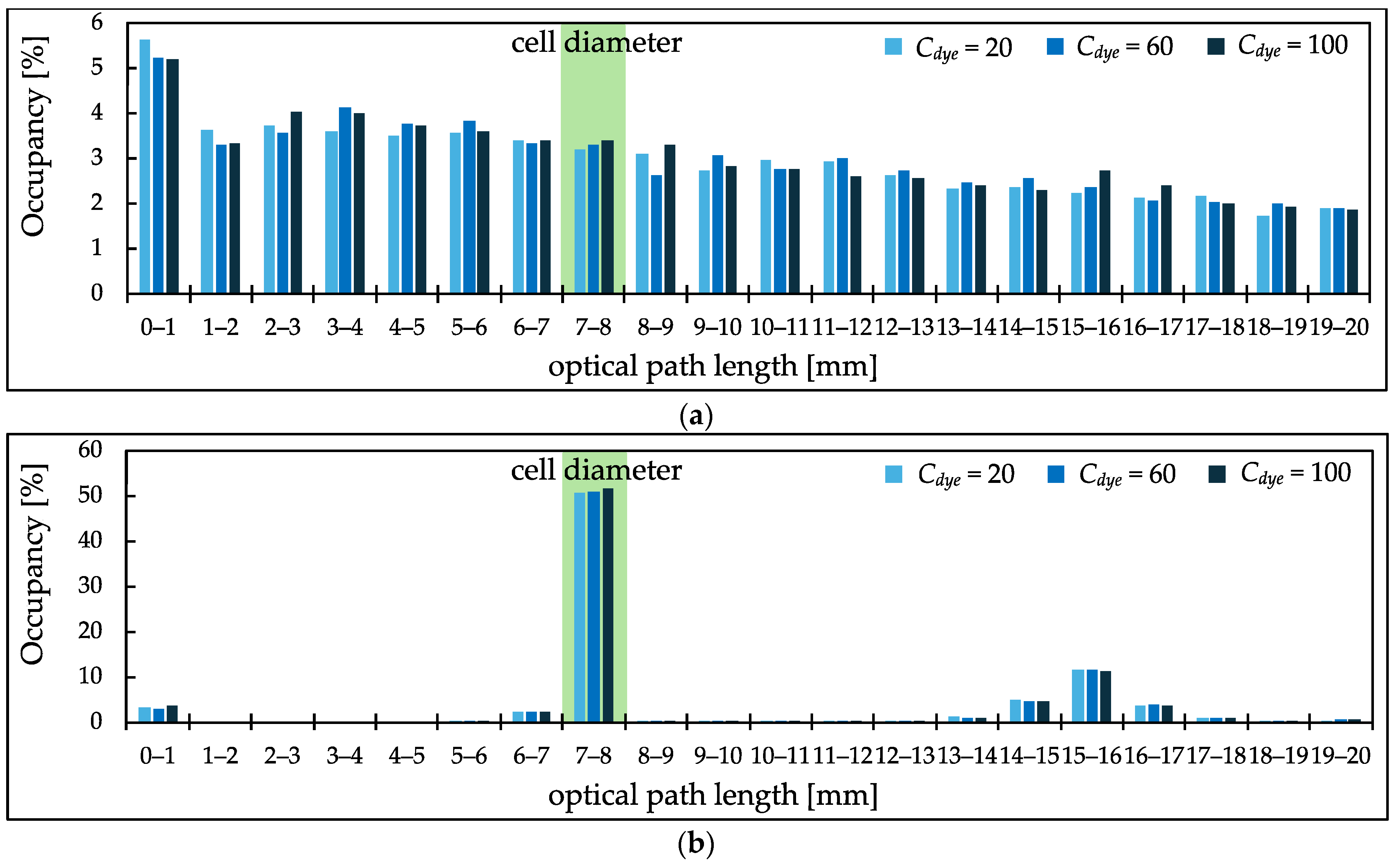
3.3. PL Distribution of Scatterer-Added Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B

Appendix C
References
- Adimule, V.; Suryavanshi, A.; Bc, Y.; Nandi, S.S. A Facile Synthesis of Poly(3-Octyl Thiophene):Ni0.4Sr0.6TiO3 Hybrid Nanocomposites for Solar Cell Applications. Macromol. Symp. 2020, 392, 2000001. [Google Scholar] [CrossRef]
- Drieschner, T.; Ostertag, E.; Boldrini, B.; Lorenz, A.; Brecht, M.; Rebner, K. Direct Optical Detection of Cell Density and Viability of Mammalian Cells by Means of UV/VIS Spectroscopy. Anal. Bioanal. Chem. 2020, 412, 3359–3371. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre-Tudo, J.L.; Buica, A.; Nieuwoudt, H.; Aleixandre, J.L.; du Toit, W. Spectrophotometric Analysis of Phenolic Compounds in Grapes and Wines. J. Agric. Food Chem. 2017, 65, 4009–4026. [Google Scholar] [CrossRef] [PubMed]
- McKechnie, W.S.; Tugcu, N.; Kandula, S. Accurate and Rapid Protein Concentration Measurement of In-Process, High Concentration Protein Pools. Biotechnol. Prog. 2018, 34, 1234–1241. [Google Scholar] [CrossRef]
- Shi, Z.; Chow, C.W.K.; Fabris, R.; Liu, J.; Jin, B. Applications of Online UV-Vis Spectrophotometer for Drinking Water Quality Monitoring and Process Control: A Review. Sensors 2022, 22, 2987. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, C.; Ye, R.; Duan, Q. Advances on Water Quality Detection by UV-Vis Spectroscopy. Appl. Sci. 2020, 10, 6874. [Google Scholar] [CrossRef]
- Hossain, S.; Chow, C.W.K.; Hewa, G.A.; Cook, D.; Harris, M. Spectrophotometric Online Detection of Drinking Water Disinfectant: A Machine Learning Approach. Sensors 2020, 20, 6671. [Google Scholar] [CrossRef]
- Du, Y.; Peng, Z.; Ding, Y. Wavelength Modulation Spectroscopy for Recovering Absolute Absorbance. Opt. Express 2018, 26, 9263–9272. [Google Scholar] [CrossRef] [PubMed]
- Probst, J.; Howes, P.; Arosio, P.; Stavrakis, S.; deMello, A. Broad-Band Spectrum, High-Sensitivity Absorbance Spectroscopy in Picoliter Volumes. Anal. Chem. 2021, 93, 7673–7681. [Google Scholar] [CrossRef]
- Li, Y.; Nesterenko, P.N.; Stanley, R.; Paull, B.; Macka, M. High Sensitivity Deep-UV LED-Based z-Cell Photometric Detector for Capillary Liquid Chromatography. Anal. Chim. Acta 2018, 1032, 197–202. [Google Scholar] [CrossRef]
- ul Rehman, A.; Ahmad, I.; Qureshi, S.A. Biomedical Applications of Integrating Sphere: A Review. Photodiagn. Photodyn. Ther. 2020, 31, 101712. [Google Scholar] [CrossRef] [PubMed]
- Dazzi, A.; Deniset-Besseau, A.; Lasch, P. Minimising Contributions from Scattering in Infrared Spectra by Means of an Integrating Sphere. Analyst 2013, 138, 4191–4201. [Google Scholar] [CrossRef]
- Gaigalas, A.K.; He, H.-J.; Wang, L. Measurement of Absorption and Scattering with an Integrating Sphere Detector: Application to Microalgae. J. Res. Natl. Inst. Stand. Technol. 2009, 114, 69–81. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Razi Naqvi, K. On Recording the True Absorption Spectrum and the Scattering Spectrum of a Turbid Sample: Application to Cell Suspensions of the Cyanobacterium Anabaena Variabilis. J. Photochem. Photobiol. B Biol. 2000, 58, 123–129. [Google Scholar] [CrossRef]
- Röttgers, R.; Gehnke, S. Measurement of Light Absorption by Aquatic Particles: Improvement of the Quantitative Filter Technique by Use of an Integrating Sphere Approach. Appl. Opt. 2012, 51, 1336–1351. [Google Scholar] [CrossRef] [PubMed]
- Chess, D.J.; Billings, E.; Covian, R.; Glancy, B.; French, S.; Taylor, J.; de Bari, H.; Murphy, E.; Balaban, R.S. Optical Spectroscopy in Turbid Media Using an Integrating Sphere: Mitochondrial Chromophore Analysis during Metabolic Transitions. Anal. Biochem. 2013, 439, 161–172. [Google Scholar] [CrossRef]
- Villanueva, Y.; Veenstra, C.; Steenbergen, W. Measuring Absorption Coefficient of Scattering Liquids Using a Tube inside an Integrating Sphere. Appl. Opt. 2016, 55, 3030–3038. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Yamashita, K.; Tokunaga, E. Reproduction of Visible Absorbance Spectra of Highly Scattering Suspensions within an Integrating Sphere by Monte Carlo Simulation. Photonics 2023, 10, 754. [Google Scholar] [CrossRef]
- Mori, A.; Yamashita, K.; Tabata, Y.; Seto, K.; Tokunaga, E. Absorbance Spectroscopy of Light Scattering Samples Placed inside an Integrating Sphere for Wide Dynamic Range Absorbance Measurement. Rev. Sci. Instrum. 2021, 92, 123103. [Google Scholar] [CrossRef]
- Nelson, N.B.; Prézelin, B.B. Calibration of an Integrating Sphere for Determining the Absorption Coefficient of Scattering Suspensions. Appl. Opt. 1993, 32, 6710–6717. [Google Scholar] [CrossRef]
- Numata, Y.; Nair, S.V.; Nakagawa, K.; Ishino, H.; Kobayashi, T.; Tokunaga, E. Optical Size Effect of Organic Nanocrystals Studied by Absorption Spectroscopy within an Integrating Sphere. Chem. Phys. Lett. 2014, 601, 128–133. [Google Scholar] [CrossRef]
- Leyre, S.; Coutino-Gonzalez, E.; Joos, J.J.; Ryckaert, J.; Meuret, Y.; Poelman, D.; Smet, P.F.; Durinck, G.; Hofkens, J.; Deconinck, G.; et al. Absolute Determination of Photoluminescence Quantum Efficiency Using an Integrating Sphere Setup. Rev. Sci. Instrum. 2014, 85, 123115. [Google Scholar] [CrossRef] [PubMed]
- Würth, C.; Pauli, J.; Lochmann, C.; Spieles, M.; Resch-Genger, U. Integrating Sphere Setup for the Traceable Measurement of Absolute Photoluminescence Quantum Yields in the Near Infrared. Anal. Chem. 2012, 84, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Yust, B.G.; Mimun, L.C.; Sardar, D.K. Optical Absorption and Scattering of Bovine Cornea, Lens, and Retina in the near-Infrared Region. Lasers Med. Sci. 2012, 27, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, O.; Mohammed, H.S. Investigating the Transmission Profiles of 808 Nm Laser through Different Regions of the Rat’s Head. Lasers Med. Sci. 2021, 36, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Y. Monte-Carlo Optical Model Coupled with Inverse Adding-Doubling for Building Integrated Photovoltaic Smart Window Design and Characterisation. Sol. Energy Mater. Sol. Cells 2021, 223, 110972. [Google Scholar] [CrossRef]
- Vincely, V.D.; Vishwanath, K. Accuracy of Retrieving Optical Properties from Liquid Tissue Phantoms Using a Single Integrating Sphere. Appl. Opt. 2022, 61, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Friebel, M.; Helfmann, J.; Netz, U.J.; Meinke, M.C. Influence of Oxygen Saturation on the Optical Scattering Properties of Human Red Blood Cells in the Spectral Range 250 to 2000 Nm. J. Biomed. Opt. 2009, 14, 034001. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, F.; Foschum, F.; Zuber, R.; Kienle, A. Precise Determination of the Optical Properties of Turbid Media Using an Optimized Integrating Sphere and Advanced Monte Carlo Simulations. Part 2: Experiments. Appl. Opt. 2020, 59, 3216–3226. [Google Scholar] [CrossRef]
- Foschum, F.; Bergmann, F.; Kienle, A. Precise Determination of the Optical Properties of Turbid Media Using an Optimized Integrating Sphere and Advanced Monte Carlo Simulations. Part 1: Theory. Appl. Opt. 2020, 59, 3203–3215. [Google Scholar] [CrossRef]
- Salomatina, E.V.; Jiang, B.; Novak, J.; Yaroslavsky, A.N. Optical Properties of Normal and Cancerous Human Skin in the Visible and Near-Infrared Spectral Range. J. Biomed. Opt. 2006, 11, 064026. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.R.; Kohl, M.; Essenpreis, M.; Cope, M. Near-Infrared Optical Properties Ofex Vivohuman Skin and Subcutaneous Tissues Measured Using the Monte Carlo Inversion Technique. Phys. Med. Biol. 1998, 43, 2465–2478. [Google Scholar] [CrossRef]
- Nishimura, T.; Takai, Y.; Shimojo, Y.; Hazama, H.; Awazu, K. Determination of Optical Properties in Double Integrating Sphere Measurement by Artificial Neural Network Based Method. Opt. Rev. 2021, 28, 42–47. [Google Scholar] [CrossRef]
- Smith, J.H.C.; Shibata, K.; Hart, R.W. A Spectrophotometer Accessory for Measuring Absorption Spectra of Light-Scattering Samples: Spectra of Dark-Grown Albino Leaves and of Adsorbed Chlorophylls. Arch. Biochem. Biophys. 1957, 72, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Benson, A.A.; Calvin, M. The Absorption Spectra of Suspensions of Living Micro-Organisms. Biochim. Biophys. Acta 1954, 15, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Hervey, J.R.D.; Bombelli, P.; Lea-Smith, D.J.; Hulme, A.K.; Hulme, N.R.; Rullay, A.K.; Keighley, R.; Howe, C.J. A Dual Compartment Cuvette System for Correcting Scattering in Whole-Cell Absorbance Spectroscopy of Photosynthetic Microorganisms. Photosynth. Res. 2022, 151, 61–69. [Google Scholar] [CrossRef]
- Koman, V.B.; Santschi, C.; Martin, O.J.F. Multiscattering-Enhanced Absorption Spectroscopy. Anal. Chem. 2015, 87, 1536–1543. [Google Scholar] [CrossRef]
- Oh, J.; Lee, K.; Park, Y. Enhancing Sensitivity in Absorption Spectroscopy Using a Scattering Cavity. Sci. Rep. 2021, 11, 14916. [Google Scholar] [CrossRef]
- Leulescu, M.; Rotaru, A.; Pălărie, I.; Moanţă, A.; Cioateră, N.; Popescu, M.; Morîntale, E.; Bubulică, M.V.; Florian, G.; Hărăbor, A.; et al. Tartrazine: Physical, Thermal and Biophysical Properties of the Most Widely Employed Synthetic Yellow Food-Colouring Azo Dye. J. Therm. Anal. Calorim. 2018, 134, 209–231. [Google Scholar] [CrossRef]
- Rovina, K.; Siddiquee, S.; Shaarani, S.M. A Review of Extraction and Analytical Methods for the Determination of Tartrazine (E 102) in Foodstuffs. Crit. Rev. Anal. Chem. 2017, 47, 309–324. [Google Scholar] [CrossRef]
- Avazpour, M.; Shiri, S.; Delpisheh, A.; Abbasi, A. mohamad Simultaneous Determination of Brilliant Blue FCF and Carmoisine in Food Samples by Aqueous Two-Phase System and Spectrophometric Detection. J. Basic Res. Med. Sci. 2014, 1, 56–65. [Google Scholar]
- Chebotarev, A.N.; Bevziuk, K.V.; Snigur, D.V.; Bazel, Y.R. The Brilliant Blue FCF Ion-Molecular Forms in Solutions According to the Spectrophotometry Data. Russ. J. Phys. Chem. A 2017, 91, 1907–1912. [Google Scholar] [CrossRef]
- Bhat, V.T.; James, N.R.; Jayakrishnan, A. A Photochemical Method for Immobilization of Azidated Dextran onto Aminated Poly(Ethylene Terephthalate) Surfaces. Polym. Int. 2008, 57, 124–132. [Google Scholar] [CrossRef]
- Welch, A.J.; Gemert, M.J.C. Optical-Thermal Response of Laser-Irradiated Tissue; Plenum Press: New York, NY, USA, 1995. [Google Scholar]
- Mie Scattering. Available online: https://omlc.org/software/mie/ (accessed on 16 April 2023).
- Gienger, J.; Bär, M.; Neukammer, J. Extinction Spectra of Suspensions of Microspheres: Determination of the Spectral Refractive Index and Particle Size Distribution with Nanometer Accuracy. Appl. Opt. 2018, 57, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, M.I.; Yang, P. Far-Field Lorenz–Mie Scattering in an Absorbing Host Medium: Theoretical Formalism and FORTRAN Program. J. Quant. Spectrosc. Radiat. Transf. 2018, 205, 241–252. [Google Scholar] [CrossRef]
- Yurkin, M.A.; Moskalensky, A.E. Open-Source Implementation of the Discrete-Dipole Approximation for a Scatterer in an Absorbing Host Medium. J. Phys. Conf. Ser. 2021, 2015, 012167. [Google Scholar] [CrossRef]


| Input Parameter | Values |
|---|---|
| µa [cm−1] | Ranges from 0.0 to 0.09β in increments of 0.01β and from 0.1β to 12.0β in increments of 0.1β |
| λ [nm] | Ranges from 350 to 700 in increments of 10 and includes 426 |
| µs [cm−1] and g | Calculated for each λ based on Mie scattering theory |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, A.; Yamashita, K.; Tokunaga, E. High-Dynamic-Range Absorption Spectroscopy by Generating a Wide Path-Length Distribution with Scatterers. Photonics 2024, 11, 556. https://doi.org/10.3390/photonics11060556
Mori A, Yamashita K, Tokunaga E. High-Dynamic-Range Absorption Spectroscopy by Generating a Wide Path-Length Distribution with Scatterers. Photonics. 2024; 11(6):556. https://doi.org/10.3390/photonics11060556
Chicago/Turabian StyleMori, Ayaka, Kyohei Yamashita, and Eiji Tokunaga. 2024. "High-Dynamic-Range Absorption Spectroscopy by Generating a Wide Path-Length Distribution with Scatterers" Photonics 11, no. 6: 556. https://doi.org/10.3390/photonics11060556
APA StyleMori, A., Yamashita, K., & Tokunaga, E. (2024). High-Dynamic-Range Absorption Spectroscopy by Generating a Wide Path-Length Distribution with Scatterers. Photonics, 11(6), 556. https://doi.org/10.3390/photonics11060556






