A Refined Model for Ablation Through Cavitation Bubbles with Ultrashort Pulse Lasers
Abstract
1. Introduction
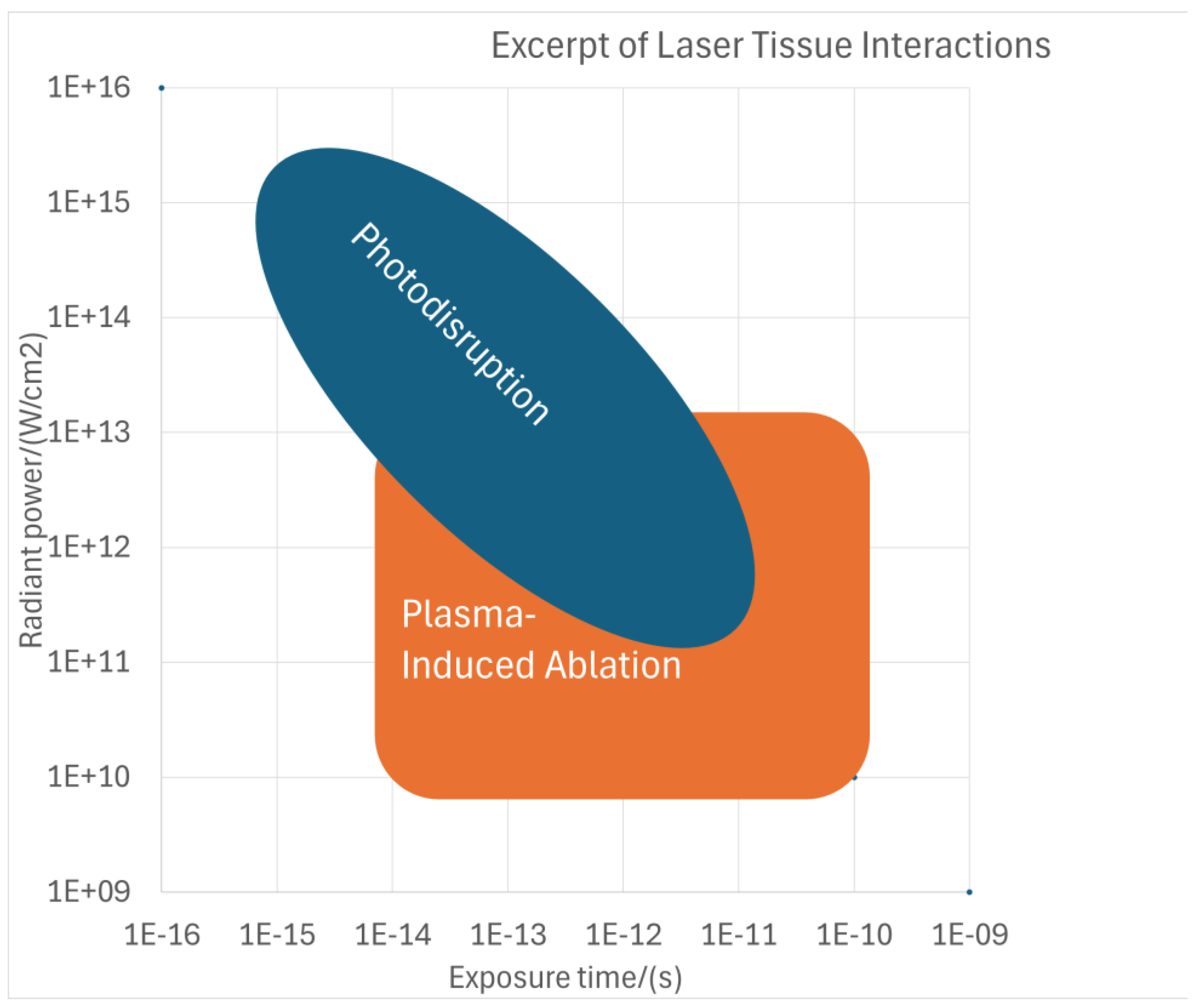
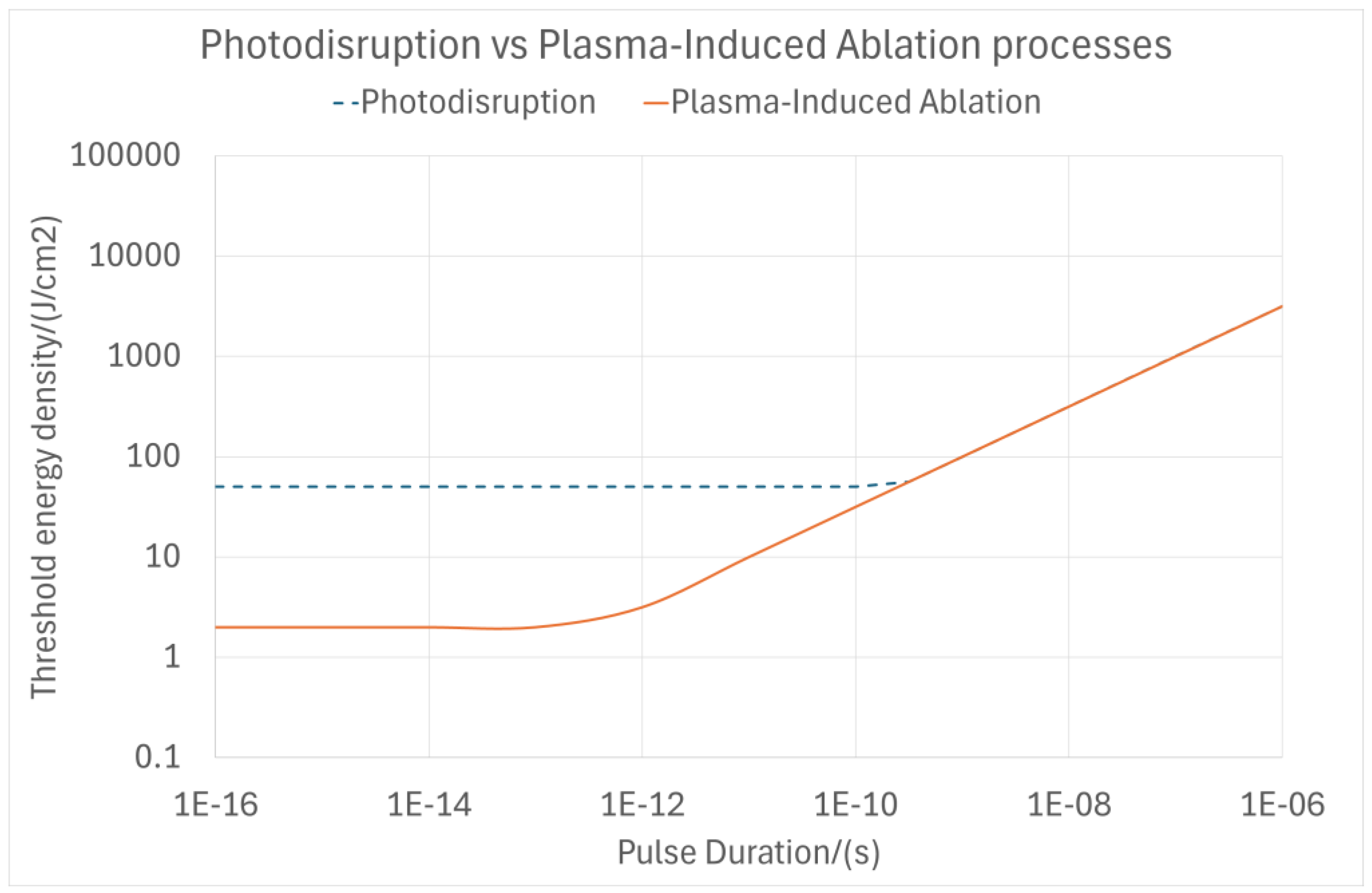
1.1. Theoretical Background of Laser–Tissue Interaction Mechanisms—Plasma-Induced Ablation and Photodisruption
1.2. Empirical Background of Laser–Tissue Interaction Mechanisms—Plasma-Induced Ablation and Photodisruption
1.3. Aims of This Work
2. Materials and Methods
2.1. Cavitation Bubble Model for Photodisruption (Photodisruption Model)
2.2. Cavitation Bubble Model for Plasma-Induced Ablation (PlasmaSQRT Model)
2.3. Analysis Methodology
3. Results
4. Discussion
5. Conclusions
- Below the optical breakdown threshold, none of the effects take place.
- Above the optical breakdown threshold, plasma-induced ablation always occurs. The models (PlasmaSQRT but further refined in the PlasmaCRT model) represent the expanding plasma region that initially grows rapidly but reaches its limit as the energy increases, leading to plasma-induced intratissue ablation (plasma melting).
- Above the optical breakdown threshold, photodisruption also occurs.
- Just above the optical breakdown threshold, despite its occurrence, photodisruption cannot be detected because the gas does not expand beyond the plasma volume, meaning that any photodisruption would be restricted within the ablation volume (plasma volume).
- At pulse energies several times above the optical breakdown threshold, photodisruption (represented by the photodisruption model) dominates because the gas largely expands beyond the plasma volume. The cavitation bubble for expanding gas leads to photo-mechanical disruption, where the tissue is primarily split by mechanical forces, with shock wave and cavitation effects propagating into adjacent tissue.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aron-Rosa, D.; Aron, J.J.; Griesemann, M.; Thyzel, R. Use of the neodymium-YAG laser to open the posterior capsule after lens implant surgery: A preliminary report. J. Am. Intraocul. Implant. Soc. 1980, 6, 352–354. [Google Scholar] [CrossRef]
- Boulnois, J.-L. Photophysical processes in recent medical laser developments: A review. Laser Med. Sci. 1986, 1, 47–66. [Google Scholar] [CrossRef]
- Markolf, H.N. Laser-Tissue Interactions: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Juhasz, T.; Hu, X.H.; Turi, L.; Bor, Z. Dynamics of shock waves and cavitation bubbles generated by picosecond laser pulses in corneal tissue and water. Lasers Surg. Med. 1994, 15, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.S.; Blinder, P.; Migliori, B.J.; Neev, J.; Jin, Y.; Squier, J.A.; Kleinfeld, D. Plasma-mediated ablation: An optical tool for submicrometer surgery on neuronal and vascular systems. Curr. Opin. Biotechnol. 2009, 20, 90–99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, D.C.; Haught, A.F. Energy-Loss Processes in Optical-Frequency Gas Breakdown. Phys. Rev. Lett. 1966, 16, 1085–1088. [Google Scholar] [CrossRef]
- Teng, P.; Nishilka, N.S.; Anderson, R.R.; Deutsch, T.F. Acoustic studies of the role of immersion in plasma mediated laser ablation. IEEE J. Q. Electron. 1987, 23, 1845–1852. [Google Scholar] [CrossRef]
- Stern, D.; Schoenlein, R.W.; Puliafito, C.A.; Dobi, E.T.; Birnngruber, R.; Fujimote, J.G. Corneal ablation by nanosecond, picosend and femtosecond lasers at 532 and 625 nm. Arch. Ophthalmol. 1989, 107, 587–592. [Google Scholar] [CrossRef]
- Niemz, M.H.; Klancnik, E.G.; Bille, J.F. Plasma mediated ablation of corneal tissue at 1053 nm using a Nd:YLF oscillator/regenerative amplifier laser. Lasers Surg. Med. 1991, 11, 426–431. [Google Scholar] [CrossRef]
- Niemz, M.H. Investigation and spectral analysis of the plasma-induced ablation mechanism of dental hydroxyapatite. Appl. Phys. B 1994, 58, 273–281. [Google Scholar] [CrossRef]
- Krasnov, M.M. Laserpuncture of anterior chamber angle in glaucoma. Am. J. Ophthalmol. 1973, 75, 674–678. [Google Scholar] [CrossRef]
- Fankhauser, F.; Roussel, P.; Steffen, J.; van der Zypen, E.; Chrenkova, A. Clinical studies on the efficiency of high power laser radiation upon some structures of the anterior segment of the eye. Int. Ophthalmol. 1981, 3, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Niemz, M.K. Laser-Tissue Interactions: Fundamentals and Applications, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Arba-Mosquera, S.; Naubereit, P.; Sobutas, S.; Verma, S. Analytical optimization of the cutting efficiency for generic cavitation bubbles. Biomed. Opt. Express. 2021, 12, 3819–3835. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vogel, A.; Linz, N.; Freidank, S.; Paltauf, G. Femtosecond-laser-induced nanocavitation in water: Implications for optical breakdown threshold and cell surgery. Phys. Rev. Lett. 2008, 100, 038102. [Google Scholar] [CrossRef] [PubMed]
- Zysset, B.; Fujimoto, J.G.; Deutsch, T.F. Time-resolved measurements of picosecond optical breakdown. Appl. Phys. B 1989, 48, 139–147. [Google Scholar] [CrossRef]
- Vogel, A.; Capon, M.R.; Asiyo-Vogel, M.N.; Birngruber, R. Intraocular photodisruption with picosecond and nanosecond laser pulses: Tissue effects in cornea, lens, and retina. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3032–3044. [Google Scholar]
- Juhasz, T.; Kastis, G.A.; Suárez, C.; Bor, Z.; Bron, W.E. Time-resolved observations of shock waves and cavitation bubbles generated by femtosecond laser pulses in corneal tissue and water. Lasers Surg. Med. 1996, 19, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Aglyamov, S.R.; Karpiouk, A.B.; Bourgeois, F.; Ben-Yakar, A.; Emelianov, S.Y. Ultrasound measurements of cavitation bubble radius for femtosecond laser-induced breakdown in water. Opt. Lett. 2008, 33, 1357–1359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vogel, A.; Busch, S.; Jungnickel, K.; Birngruber, R. Mechanisms of intraocular photodisruption with picosecond and nanosecond laser pulses. Lasers Surg. Med. 1994, 15, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, C.B.; Brodeur, A.; García, J.F.; Mazur, E. Micromachining bulk glass by use of femtosecond laser pulses with nanojoule energy. Opt. Lett. 2001, 26, 93–95. [Google Scholar] [CrossRef]
- Arnold, C.L.; Heisterkamp, A.; Ertmer, W.; Lubatschowski, H. Computational model for nonlinear plasma formation in high NA micromachining of transparent materials and biological cells. Opt. Express 2007, 15, 10303–10317. [Google Scholar] [CrossRef]
- Vogel, A.; Nahen, K.; Theisen, D.; Birngruber, R.; Thomas, R.J.; Rockwell, B.A. Influence of optical aberrations on laser-induced plasma formation in water and their consequences for intraocular photodisruption. Appl. Opt. 1999, 38, 3636–3643. [Google Scholar] [CrossRef] [PubMed]
- Giguère, D.; Olivié, G.; Vidal, F.; Toetsch, S.; Girard, G.; Ozaki, T.; Kieffer, J.C.; Nada, O.; Brunette, I. Laser ablation threshold dependence on pulse duration for fused silica and corneal tissues: Experiments and modeling. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2007, 24, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Amann, H.; Mosquera, S.A. Optimization of the Spot Spacings for Reducing Roughness in Laser-Induced Optical Breakdown Processes for Corneal Laser Vision Correction. Photonics 2024, 11, 114. [Google Scholar] [CrossRef]
- Garcia-Lechuga, M.; El Reaidy, G.G.; Ning, H.; Delaporte, P.; Grojo, D. Assessing the limits of determinism and precision in ultrafast laser ablation. Appl. Phys. Lett. 2020, 117, 171604. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M. Accuracy and reproducibility of cap thickness in small incision lenticule extraction. J. Refract. Surg. 2013, 29, 810–818. [Google Scholar] [CrossRef]
- Tinne, N.; Schumacher, S.; Nuzzo, V.; Arnold, C.L.; Lubatschowski, H.; Ripken, T. Interaction dynamics of spatially separated cavitation bubbles in water. J. Biomed. Opt. 2010, 15, 068003. [Google Scholar] [CrossRef]
- Tinne, N.; Lübking, E.; Lubatschowski, H.; Krüger, A.; Ripken, T. The influence of a spatial and temporal pulse-overlap on the laser-tissue-interaction of modern ophthalmic laser systems. Biomed. Eng./Biomed. Tech. 2012, 57, 302–305. [Google Scholar] [CrossRef]
- Sinibaldi, G.; Occhicone, A.; Pereira, F.; Caprini, D.; Marino, L.; Michelotti, F.; Casciola, C. Laser induced cavitation: Plasma generation and breakdown shockwave. Phys. Fluids 2019, 31, 103302. [Google Scholar] [CrossRef]
- Noack, J.; Vogel, A. Laser-induced plasma formation in water at nanosecond to femtosecond time scales: Calculation of thresholds, absorption coefficients, and energy density. IEEE J. Quantum Electron. 1999, 35, 1156–1167. [Google Scholar] [CrossRef]
- Liu, J.M. Simple technique for measurements of pulsed Gaussian-beam spot sizes. Opt. Lett. 1982, 7, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Tinne, N.; Kaune, B.; Krüger, A.; Ripken, T. Interaction mechanisms of cavitation bubbles induced by spatially and temporally separated fs-laser pulses. PLoS ONE 2014, 9, e114437. [Google Scholar] [CrossRef] [PubMed]
- Senegačnik, M.; Kunimoto, K.; Yamaguchi, S.; Kimura, K.; Sakka, T.; Gregorčič, P. Dynamics of laser-induced cavitation bubble during expansion over sharp-edge geometry submerged in liquid—An inside view by diffuse illumination. Ultrason. Sonochem. 2021, 73, 105460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vogel, A.; Noack, J.; Hüttman, G.; Paltauf, G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl. Phys. B 2005, 81, 1015–1047. [Google Scholar] [CrossRef]
- Vogel, A. Laserdisruption: Plasma-Mediated Surgery. Lecture 2020. Slides 33–36. Available online: https://www.bmo.uni-luebeck.de/fileadmin/files/Verschiedenes/Lehre/Lasermedizin/Scripte_2012/08_Laserdisruption.pdf (accessed on 17 November 2020).
- Wang, J.; Schuele, G.; Palanker, D. Finesse of transparent tissue cutting by ultrafast lasers at various wavelengths. J. Biomed. Opt. 2015, 20, 125004. [Google Scholar] [CrossRef]
- Genc, S.L.; Ma, H.; Venugopalan, V. Low-density plasma formation in aqueous biological media using sub-nanosecond laser pulses. Appl. Phys. Lett. 2014, 105, 063701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Coefficient | K (Photodisruption Model) | B (PlasmaSQRT Model) |
|---|---|---|
| Global Optimum Value | ~0.6 | 3.433 |
| Coefficient | K (Photodisruption Model) | B (PlasmaSQRT Model) | C (PlasmaCRT Model) |
|---|---|---|---|
| Global Optimum Value | ~0.6 | 3.433 | 3.735 |
| Threshold Energy (nJ) | Estimated Pulse Duration (fs) | Estimated Beam Waist (µm) | Cross-Over Energy (nJ) | Peak Energy Density (J/cm2) | Peak Power Density (W/cm2) |
|---|---|---|---|---|---|
| 30 | 190 | ~2.6 | 1623 | ~15 | ~8.0E+13 |
| 40 | 200 | ~2.7 | 1316 | ~11 | ~5.7E+13 |
| 50 | 210 | ~2.8 | 1110 | ~9 | ~4.3E+13 |
| 70 | 270 | ~3.0 | 768 | ~5 | ~2.0E+13 |
| 300 | 800 | ~4.2 | No cross-over | ~2 | ~2.2E+12 |
| 113 | 300 | ~3.6 | No cross-over | ~1 | ~4.3E+12 |
| 63 | 250 | ~2.9 | 895 | ~7 | ~2.7E+13 |
| Model | Photodisruption Model | PlasmaSQRT Model | PlasmaCRT Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | minOpt (nJ) | maxOpt (nJ) | properOpt (nJ) | minOpt (nJ) | maxOpt (nJ) | properOpt (nJ) | minOpt (nJ) | maxOpt (nJ) | properOpt (nJ) |
| Analytical Expression | 3/2 × ETh | 3 × ETh | 2 × ETh | × ETh | e × ETh | 2 × ETh | e(1/3) × ETh | e(2/3) × ETh | e(1/2) × ETh |
| Threshold Energy (nJ) | |||||||||
| 30 | 45 | 90 | 60 | 49 | 82 | 60 | 42 | 58 | 49 |
| 40 | 60 | 120 | 80 | 66 | 109 | 80 | 56 | 78 | 66 |
| 50 | 75 | 150 | 100 | 82 | 136 | 100 | 70 | 97 | 82 |
| 70 | 105 | 210 | 140 | 115 | 190 | 140 | 98 | 136 | 115 |
| 300 | 450 | 900 | 600 | 495 | 815 | 600 | 419 | 584 | 495 |
| 113 | 170 | 339 | 226 | 186 | 307 | 226 | 158 | 220 | 186 |
| 63 | 95 | 189 | 126 | 104 | 171 | 126 | 88 | 123 | 104 |
| Threshold Energy (nJ) | Estimate of Pulse Duration | Estimate of Beam Waist (µm) | Cross-Over Energy | Peak Energy Density (J/cm2) | Peak Power Density (W/cm2) |
|---|---|---|---|---|---|
| ~120 | 500 ps | ~0.4 | 400 nJ | ~175 | ~3.5E+11 |
| ~220 | 1 ns | ~0.4 | 800 nJ | ~350 | ~3.5E+11 |
| ~1000 | 7–11 ns | ~0.4 | 60 µJ | ~26,000 | ~2.9E+12 |
| ~6000 | 7–11 ns | ~0.4 | 100 µJ | ~19,000 | ~2.1E+12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, S.; Arba Mosquera, S. A Refined Model for Ablation Through Cavitation Bubbles with Ultrashort Pulse Lasers. Photonics 2024, 11, 1047. https://doi.org/10.3390/photonics11111047
Verma S, Arba Mosquera S. A Refined Model for Ablation Through Cavitation Bubbles with Ultrashort Pulse Lasers. Photonics. 2024; 11(11):1047. https://doi.org/10.3390/photonics11111047
Chicago/Turabian StyleVerma, Shwetabh, and Samuel Arba Mosquera. 2024. "A Refined Model for Ablation Through Cavitation Bubbles with Ultrashort Pulse Lasers" Photonics 11, no. 11: 1047. https://doi.org/10.3390/photonics11111047
APA StyleVerma, S., & Arba Mosquera, S. (2024). A Refined Model for Ablation Through Cavitation Bubbles with Ultrashort Pulse Lasers. Photonics, 11(11), 1047. https://doi.org/10.3390/photonics11111047





