Non-Invasive Hemoglobin Assessment with NIR Imaging of Blood Vessels in Transmittance Geometry: Monte Carlo and Experimental Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Monte Carlo Simulations
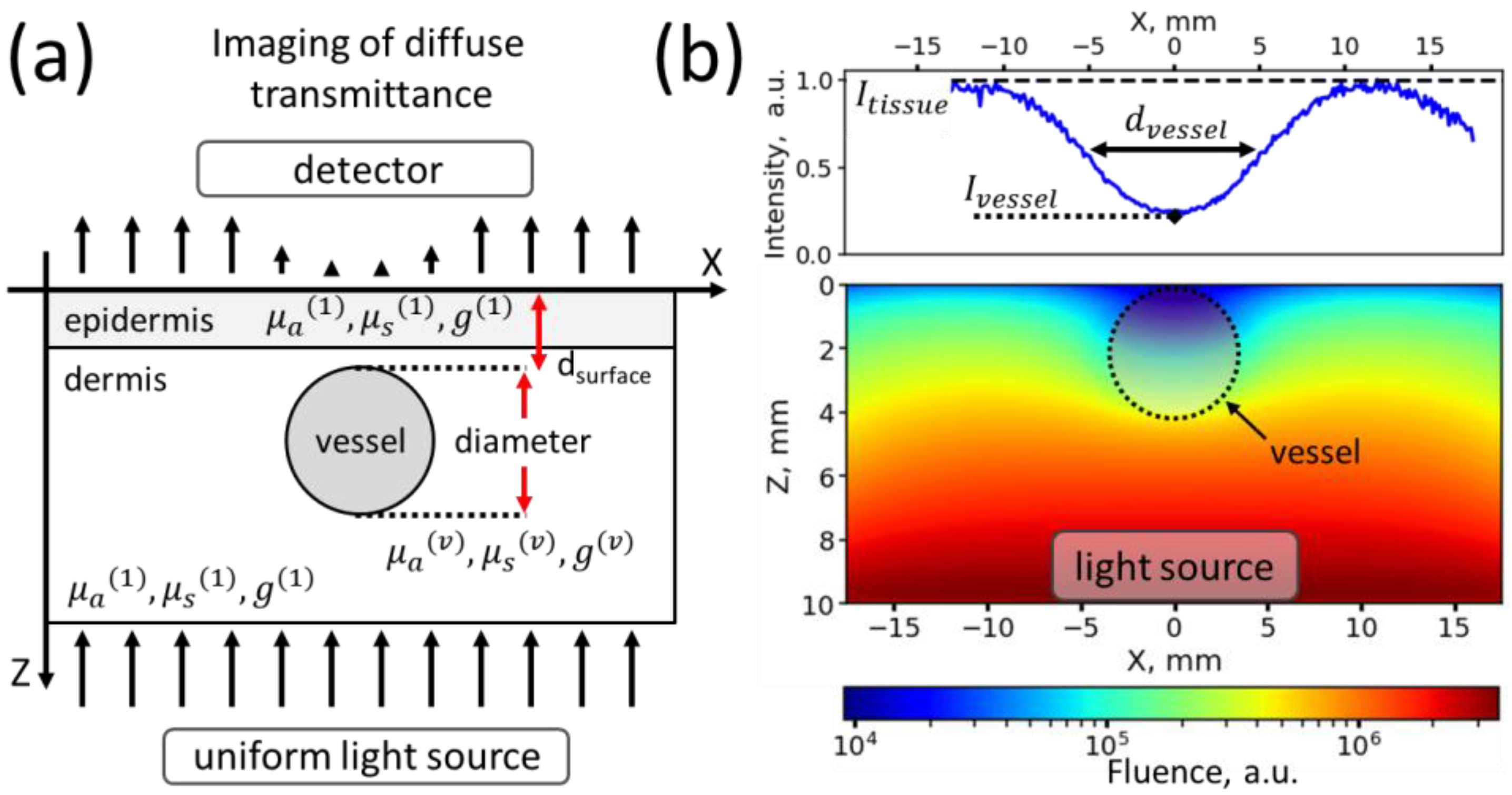
2.1.1. Simulated Object Geometrical Properties
2.1.2. General Optical Properties of Media
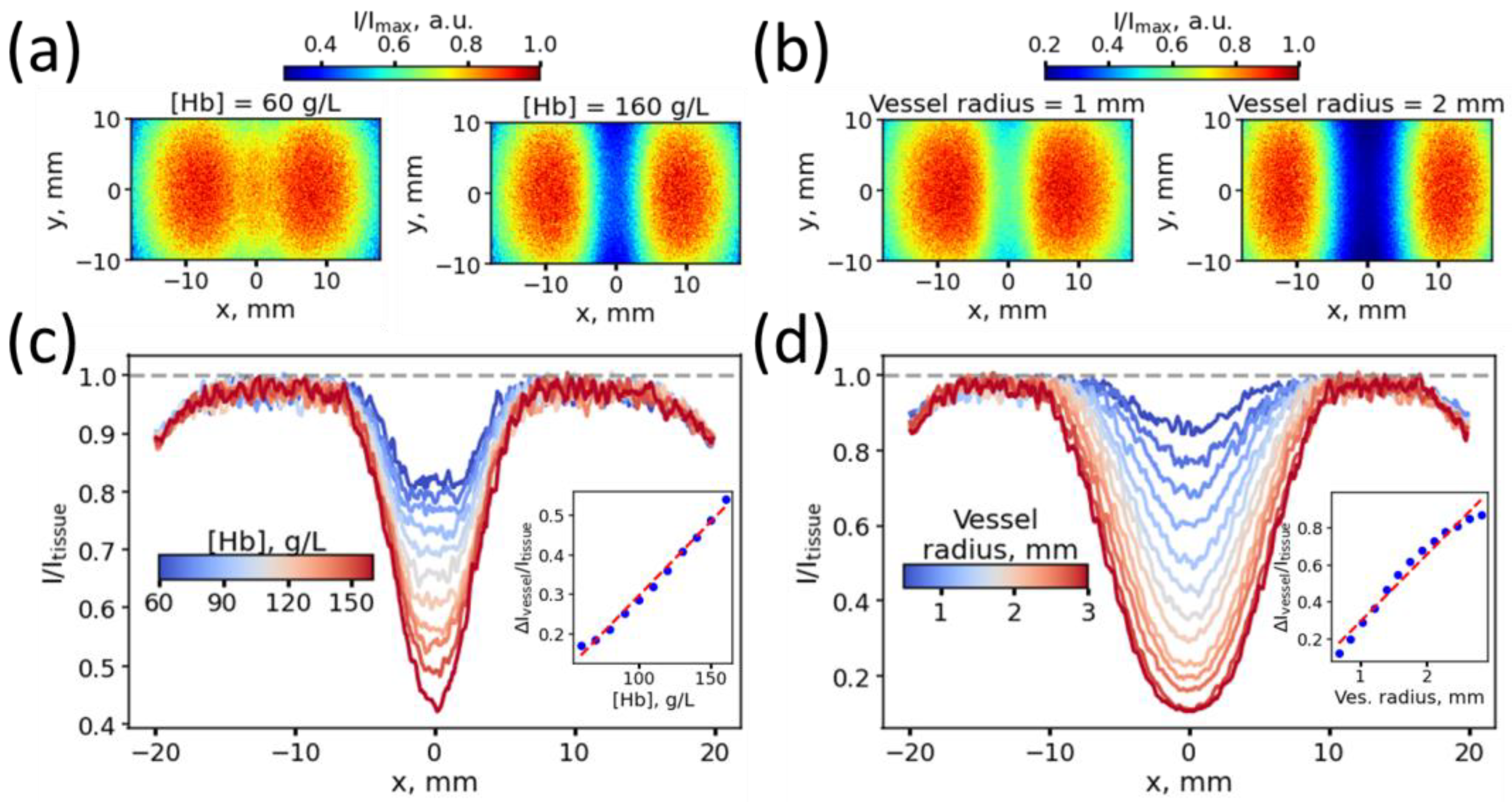
2.1.3. Experiments for Assessment of the Influence of Vessel Thickness and Blood Hb Level on the Vessel Contrast
2.1.4. Assessment of the Influence of Different Vessel Depths on the Error of Hb Level Prediction
2.1.5. Assessment of the Influence of the Scattering Coefficient, Blood Content in the Dermis and Melanin in the Epidermis on the Error of Hb Level Prediction
2.2. Data Analysis and Machine Learning Model Evaluation on Monte-Carlo Simulation Data
2.3. Volunteers
2.4. Experimental Setup
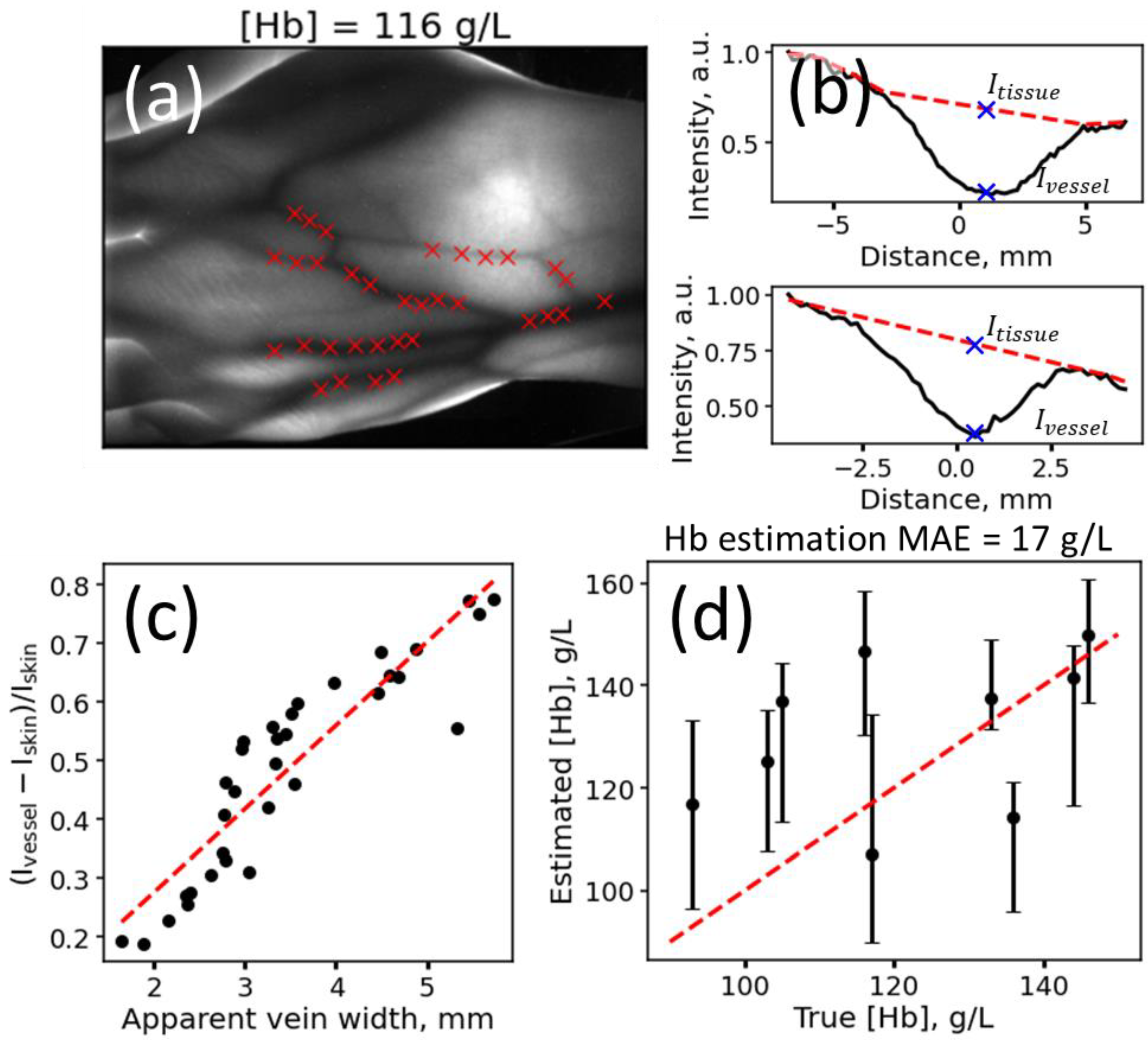
2.5. Analysis of Experimental Data
2.5.1. Feature Extraction from Images
2.5.2. Blood Hb Level Prediction for Experimental Data
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Karakochuk, C.D.; Hess, S.Y.; Moorthy, D.; Namaste, S.; Parker, M.E.; Rappaport, A.I.; Wegmüller, R.; Dary, O.; The HEmoglobin MEasurement (HEME) Working Group. Measurement and Interpretation of Hemoglobin Concentration in Clinical and Field Settings: A Narrative Review. Ann. N. Y. Acad. Sci. 2019, 1450, 126–146. [Google Scholar] [CrossRef] [PubMed]
- Causey, M.W.; Miller, S.; Foster, A.; Beekley, A.; Zenger, D.; Martin, M. Validation of Noninvasive Hemoglobin Measurements Using the Masimo Radical-7 SpHb Station. Am. J. Surg. 2011, 201, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Lychagov, V.V.; Semenov, V.M.; Volkova, E.K.; Chernakov, D.I.; Ahn, J.; Kim, J.Y. Noninvasive Hemoglobin Measurements With Photoplethysmography in Wrist. IEEE Access 2023, 11, 79636–79647. [Google Scholar] [CrossRef]
- Baulig, W.; Seifert, B.; Spahn, D.R.; Theusinger, O.M. Accuracy of Non-Invasive Continuous Total Hemoglobin Measurement by Pulse CO-Oximetry in Severe Traumatized and Surgical Bleeding Patients. J. Clin. Monit. Comput. 2017, 31, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Suner, S.; Rayner, J.; Ozturan, I.U.; Hogan, G.; Meehan, C.P.; Chambers, A.B.; Baird, J.; Jay, G.D. Prediction of Anemia and Estimation of Hemoglobin Concentration Using a Smartphone Camera. PLoS ONE 2021, 16, e0253495. [Google Scholar] [CrossRef] [PubMed]
- Appiahene, P.; Chaturvedi, K.; Asare, J.W.; Donkoh, E.T.; Prasad, M. CP-AnemiC: A Conjunctival Pallor Dataset and Benchmark for Anemia Detection in Children. Med. Nov. Technol. Devices 2023, 18, 100244. [Google Scholar] [CrossRef]
- Chen, Y.; Zhong, K.; Zhu, Y.; Sun, Q. Two-Stage Hemoglobin Prediction Based on Prior Causality. Front. Public Health 2022, 10, 1079389. [Google Scholar] [CrossRef] [PubMed]
- Dimauro, G.; Baldari, L.; Caivano, D.; Colucci, G.; Girardi, F. Automatic Segmentation of Relevant Sections of the Conjunctiva for Non-Invasive Anemia Detection. In Proceedings of the 2018 3rd International Conference on Smart and Sustainable Technologies (SpliTech), Split, Croatia, 26–29 June 2018; pp. 1–5. [Google Scholar]
- Dimauro, G.; Camporeale, M.G.; Dipalma, A.; Guarini, A.; Maglietta, R. Anaemia Detection Based on Sclera and Blood Vessel Colour Estimation. Biomed. Signal Process. Control 2023, 81, 104489. [Google Scholar] [CrossRef]
- Mitani, A.; Huang, A.; Venugopalan, S.; Corrado, G.S.; Peng, L.; Webster, D.R.; Hammel, N.; Liu, Y.; Varadarajan, A.V. Detection of Anaemia from Retinal Fundus Images via Deeplearning. Nat. Biomed. Eng. 2020, 4, 18–27. [Google Scholar] [CrossRef]
- Zhao, X.; Meng, L.; Su, H.; Lv, B.; Lv, C.; Xie, G.; Chen, Y. Deep-Learning-Based Hemoglobin Concentration Prediction and Anemia Screening Using Ultra-Wide Field Fundus Images. Front. Cell Dev. Biol. 2022, 10, 888268. [Google Scholar] [CrossRef]
- Babenko, B.; Traynis, I.; Chen, C.; Singh, P.; Uddin, A.; Cuadros, J.; Daskivich, L.P.; Maa, A.Y.; Kim, R.; Kang, E.Y.-C.; et al. A Deep Learning Model for Novel Systemic Biomarkers in Photographs of the External Eye: A Retrospective Study. Lancet Digit. Health 2023, 5, e257–e264. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kesarwani, A.; Dalui, M.; Kisku, D.R.; Sen, B.; Roy, S.; Basu, A. Smartphone-Based Non-Invasive Haemoglobin Level Estimation by Analyzing Nail Pallor. Biomed. Signal Process. Control 2023, 85, 104959. [Google Scholar] [CrossRef]
- Mannino, R.G.; Myers, D.R.; Tyburski, E.A.; Caruso, C.; Boudreaux, J.; Leong, T.; Clifford, G.D.; Lam, W.A. Smartphone App for Non-Invasive Detection of Anemia Using Only Patient-Sourced Photos. Nat. Commun. 2018, 9, 4924. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, H.; Kızılateş, B.S.; Shaaban, F.; Karataş, Z.R. A Novel Combined Deep Learning Methodology to Non-Invasively Estimate Hemoglobin Levels in Blood with High Accuracy. Med. Eng. Phys. 2022, 108, 103891. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Osea, E.A.; Martinez, G.J. Accuracy of Noninvasive Hemoglobin and Invasive Point-of-Care Hemoglobin Testing Compared with a Laboratory Analyzer. Int. J. Lab. Hematol. 2014, 36, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Honnef, G.; Auinger, D.; Eichinger, M.; Eichlseder, M.; Metnitz, P.G.H.; Rief, M.; Zajic, P.; Zoidl, P.; Bornemann-Cimenti, H. Evaluation of the Usefulness of Non-Invasive Serum Haemoglobin Measurement in a Perioperative Setting in a Prospective Observational Study. Sci. Rep. 2022, 12, 9065. [Google Scholar] [CrossRef]
- Hornedo-González, K.D.; Jacob, A.K.; Burt, J.M.; Higgins, A.A.; Engel, E.M.; Hanson, A.C.; Belch, L.; Kor, D.J.; Warner, M.A. Non-Invasive Hemoglobin Estimation for Preoperative Anemia Screening. Transfusion 2023, 63, 315–322. [Google Scholar] [CrossRef]
- Young, M.F.; Raines, K.; Jameel, F.; Sidi, M.; Oliveira-Streiff, S.; Nwajei, P.; McGlamry, K.; Ou, J.; Oladele, A.; Suchdev, P.S. Non-Invasive Hemoglobin Measurement Devices Require Refinement to Match Diagnostic Performance with Their High Level of Usability and Acceptability. PLoS ONE 2021, 16, e0254629. [Google Scholar] [CrossRef]
- Yang, S.; Hu, P.F.; Anazodo, A.; Gao, C.; Chen, H.; Wade, C.; Hartsky, L.; Miller, C.; Imle, C.; Fang, R.; et al. Trends of Hemoglobin Oximetry: Do They Help Predict Blood Transfusion During Trauma Patient Resuscitation? Anesth. Analg. 2016, 122, 115–125. [Google Scholar] [CrossRef]
- Shamah Levy, T.; Méndez-Gómez-Humarán, I.; Morales Ruán, M.D.C.; Martinez Tapia, B.; Villalpando Hernández, S.; Hernández Ávila, M. Validation of Masimo Pronto 7 and HemoCue 201 for Hemoglobin Determination in Children from 1 to 5 Years of Age. PLoS ONE 2017, 12, e0170990. [Google Scholar] [CrossRef]
- Rice, M.J.; Gravenstein, N.; Morey, T.E. Noninvasive Hemoglobin Monitoring: How Accurate Is Enough? Anesth. Analg. 2013, 117, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Juric, S.; Zalik, B. An Innovative Approach to Near-Infrared Spectroscopy Using a Standard Mobile Device and Its Clinical Application in the Real-Time Visualization of Peripheral Veins. BMC Med. Inform. Decis. Mak. 2014, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Guillon, P.; Makhloufi, M.; Baillie, S.; Roucoulet, C.; Dolimier, E.; Masquelier, A.-M. Prospective Evaluation of Venous Access Difficulty and a Near-Infrared Vein Visualizer at Four French Haemophilia Treatment Centres. Haemophilia 2015, 21, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Miyake, R.K.; Zeman, H.D.; Duarte, F.H.; Kikuchi, R.; Ramacciotti, E.; Lovhoiden, G.; Vrancken, C. Vein Imaging: A New Method of Near Infrared Imaging, Where a Processed Image Is Projected onto the Skin for the Enhancement of Vein Treatment. Dermatol. Surg. 2006, 32, 1031–1038. [Google Scholar] [CrossRef]
- Xie, S.; Fang, L.; Wang, Z.; Ma, Z.; Li, J. Review of Personal Identification Based on near Infrared Vein Imaging of Finger. In Proceedings of the 2017 2nd International Conference on Image, Vision and Computing (ICIVC), Chengdu, China, 2–4 June 2017; pp. 206–213. [Google Scholar]
- Pititheeraphab, Y.; Thongpance, N.; Aoyama, H.; Pintavirooj, C. Vein Pattern Verification and Identification Based on Local Geometric Invariants Constructed from Minutia Points and Augmented with Barcoded Local Feature. Appl. Sci. 2020, 10, 3192. [Google Scholar] [CrossRef]
- Saigo, K.; Imoto, S.; Hashimoto, M.; Mito, H.; Moriya, J.; Chinzei, T.; Kubota, Y.; Numada, S.; Ozawa, T.; Kumagai, S. Noninvasive Monitoring of Hemoglobin: The Effects of WBC Counts on Measurement. Am. J. Clin. Pathol. 2004, 121, 51–55. [Google Scholar] [CrossRef]
- Kanashima, H.; Yamane, T.; Takubo, T.; Kamitani, T.; Hino, M. Evaluation of Noninvasive Hemoglobin Monitoring for Hematological Disorders. J. Clin. Lab. Anal. 2005, 19, 1–5. [Google Scholar] [CrossRef]
- Naglič, P.; Zelinskyi, Y.; Pernuš, F.; Likar, B.; Bürmen, M. Pyxopto: An Open-Source Python Library with Utilities for Fast Light Propagation Modeling in Turbid Media. In Proceedings of the European Conferences on Biomedical Optics 2021 (ECBO), Munich, Germany, 20–24 June 2021; p. EM3C.2. [Google Scholar]
- Omlc.Org. Available online: https://omlc.org/spectra/ (accessed on 20 November 2023).
- van Beest, P.; Wietasch, G.; Scheeren, T.; Spronk, P.; Kuiper, M. Clinical Review: Use of Venous Oxygen Saturations as a Goal—A yet Unfinished Puzzle. Crit. Care 2011, 15, 232. [Google Scholar] [CrossRef]
- Bosschaart, N.; Edelman, G.J.; Aalders, M.C.G.; van Leeuwen, T.G.; Faber, D.J. A Literature Review and Novel Theoretical Approach on the Optical Properties of Whole Blood. Lasers Med. Sci. 2014, 29, 453–479. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical Properties of Biological Tissues: A Review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef]
- Salomatina, E.V.; Jiang, B.; Novak, J.; Yaroslavsky, A.N. Optical Properties of Normal and Cancerous Human Skin in the Visible and Near-Infrared Spectral Range. J. Biomed. Opt. 2006, 11, 064026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-M.; Chen, S.; Liang, Y.-Z. Baseline Correction Using Adaptive Iteratively Reweighted Penalized Least Squares. Analyst 2010, 135, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Bigio, I.J.; Fantini, S. Quantitative Biomedical Optics: Theory, Methods, and Applications; Cambridge Texts in Biomedical Engineering; Cambridge University Press: Cambridge, UK, 2016; ISBN 978-1-316-46238-6. [Google Scholar]
- Hayakawa, C.K.; Karrobi, K.; Pera, V.; Roblyer, D.; Venugopalan, V. Optical Sampling Depth in the Spatial Frequency Domain. J. Biomed. Opt. 2018, 24, 071603. [Google Scholar] [CrossRef] [PubMed]
- Tseregorodtseva, P.S.; Buiankin, K.E.; Yakimov, B.P.; Kamalov, A.A.; Budylin, G.S.; Shirshin, E.A. Single-Fiber Diffuse Reflectance Spectroscopy and Spatial Frequency Domain Imaging in Surgery Guidance: A Study on Optical Phantoms. Materials 2021, 14, 7502. [Google Scholar] [CrossRef]
- Nawaz Jadoon, R.; Shahzad, A.; Shah, S.A.; Khan, M.A.; Akram, T.; Zhou, W. Veins Depth Estimation Using Diffused Reflectance Parameter. Appl. Sci. 2020, 10, 8238. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardadin, I.; Petrov, V.; Denisenko, G.; Armaganov, A.; Rubekina, A.; Kopytina, D.; Panov, V.; Shatalov, P.; Khoronenko, V.; Shegai, P.; et al. Non-Invasive Hemoglobin Assessment with NIR Imaging of Blood Vessels in Transmittance Geometry: Monte Carlo and Experimental Evaluation. Photonics 2024, 11, 49. https://doi.org/10.3390/photonics11010049
Bardadin I, Petrov V, Denisenko G, Armaganov A, Rubekina A, Kopytina D, Panov V, Shatalov P, Khoronenko V, Shegai P, et al. Non-Invasive Hemoglobin Assessment with NIR Imaging of Blood Vessels in Transmittance Geometry: Monte Carlo and Experimental Evaluation. Photonics. 2024; 11(1):49. https://doi.org/10.3390/photonics11010049
Chicago/Turabian StyleBardadin, Ilia, Vladimir Petrov, Georgy Denisenko, Artashes Armaganov, Anna Rubekina, Daria Kopytina, Vladimir Panov, Petr Shatalov, Victoria Khoronenko, Petr Shegai, and et al. 2024. "Non-Invasive Hemoglobin Assessment with NIR Imaging of Blood Vessels in Transmittance Geometry: Monte Carlo and Experimental Evaluation" Photonics 11, no. 1: 49. https://doi.org/10.3390/photonics11010049
APA StyleBardadin, I., Petrov, V., Denisenko, G., Armaganov, A., Rubekina, A., Kopytina, D., Panov, V., Shatalov, P., Khoronenko, V., Shegai, P., Kaprin, A., Shkoda, A., & Yakimov, B. (2024). Non-Invasive Hemoglobin Assessment with NIR Imaging of Blood Vessels in Transmittance Geometry: Monte Carlo and Experimental Evaluation. Photonics, 11(1), 49. https://doi.org/10.3390/photonics11010049




