Multiwavelength Fluorescence and Diffuse Reflectance Spectroscopy for an In Situ Analysis of Kidney Stones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
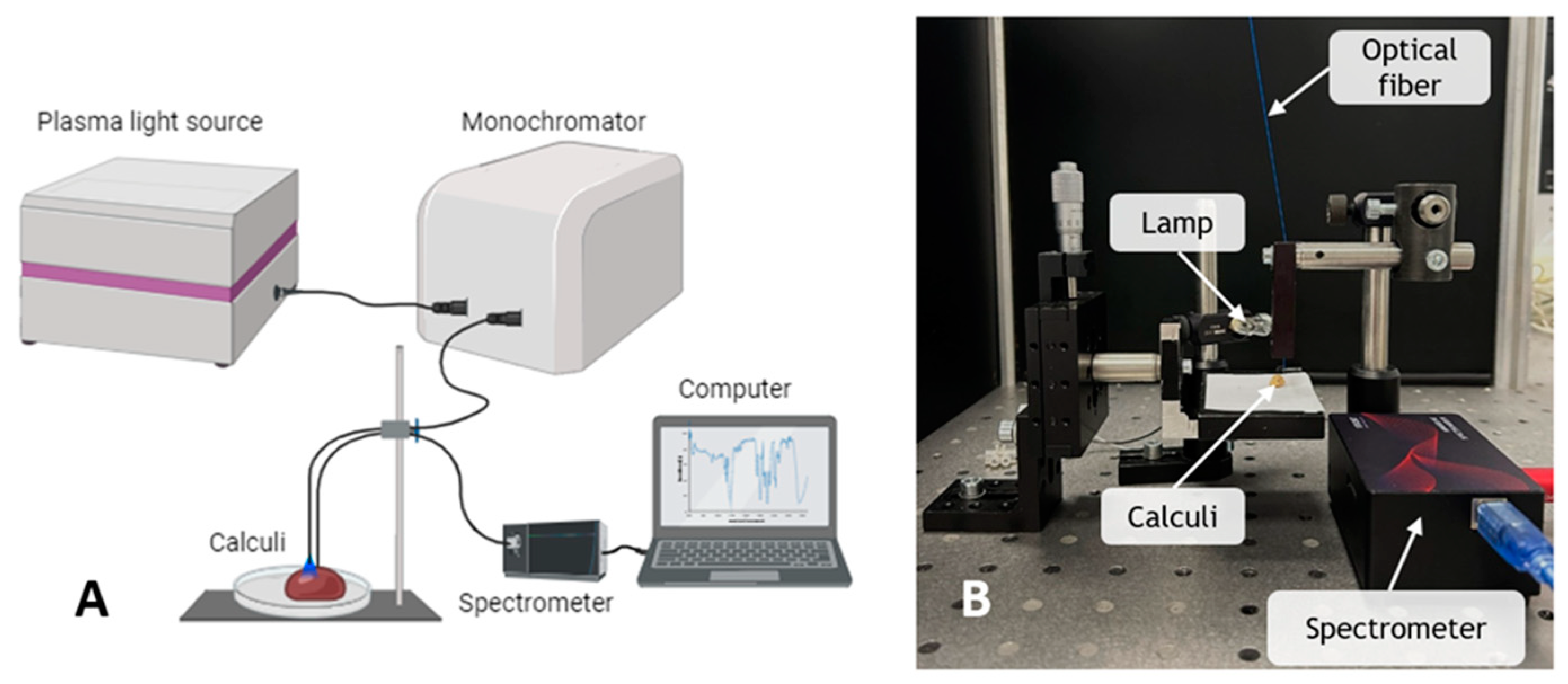
2.2. Experimental Setups
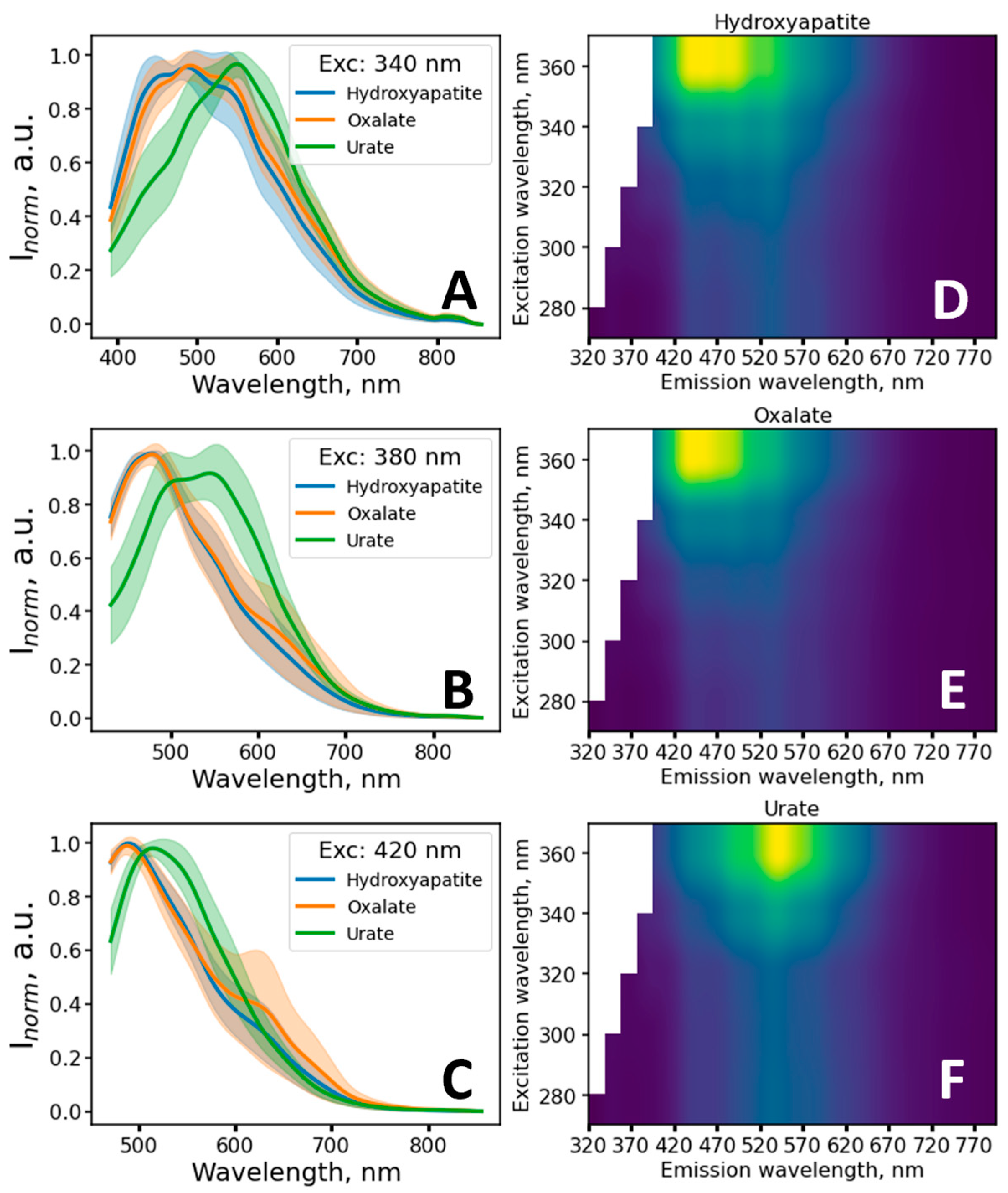
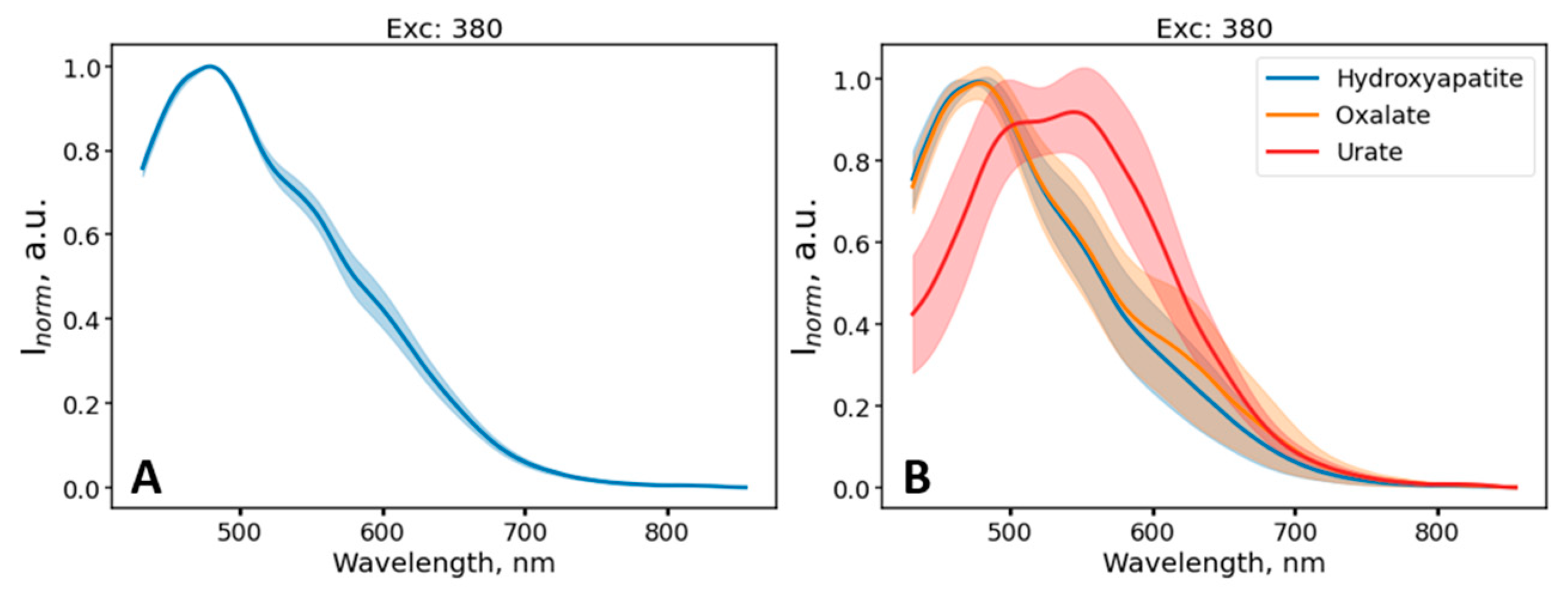
2.2.1. EEM Measurements
2.2.2. DRS Measurements
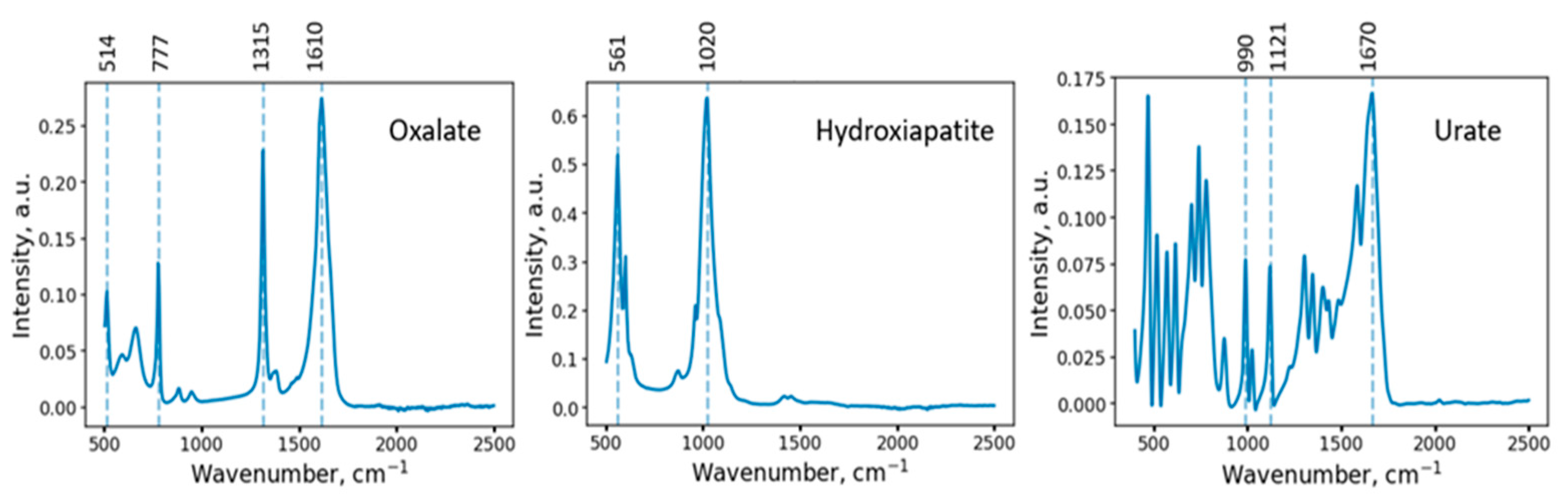
2.2.3. FTIR Spectroscopy
2.3. Data Processing
2.3.1. DRS
2.3.2. Multiwavelength Fluorescence Spectroscopy
2.3.3. FTIR Spectroscopy
2.4. Classification
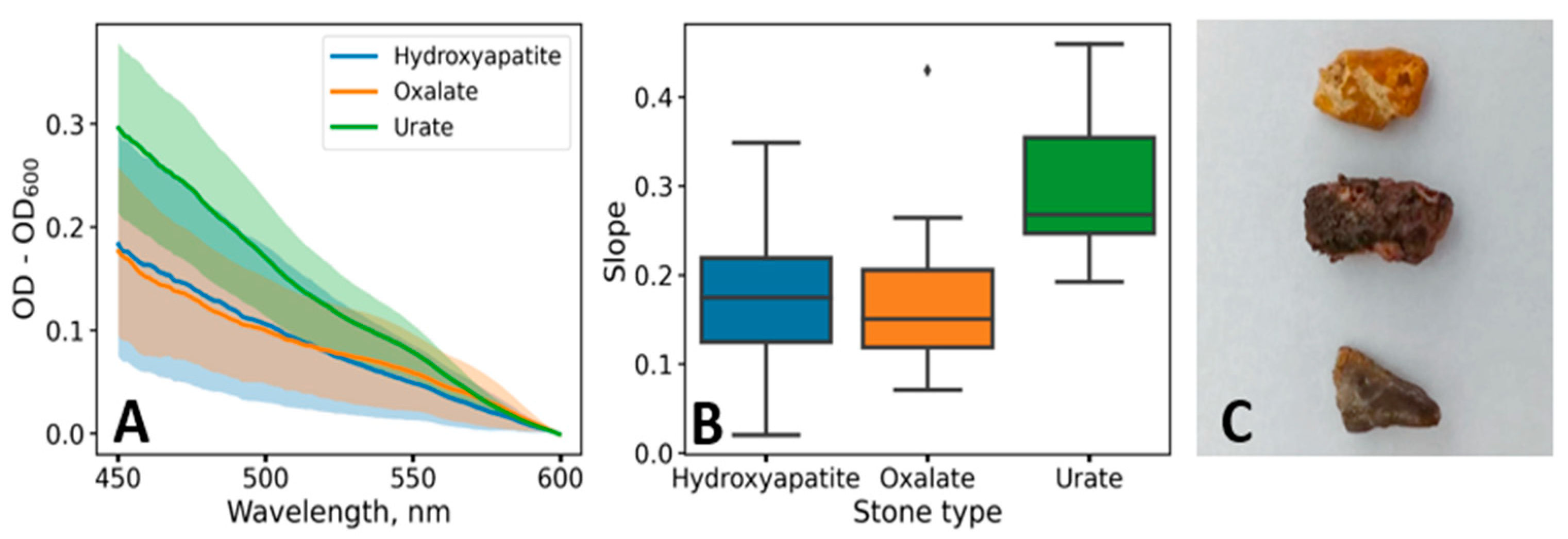
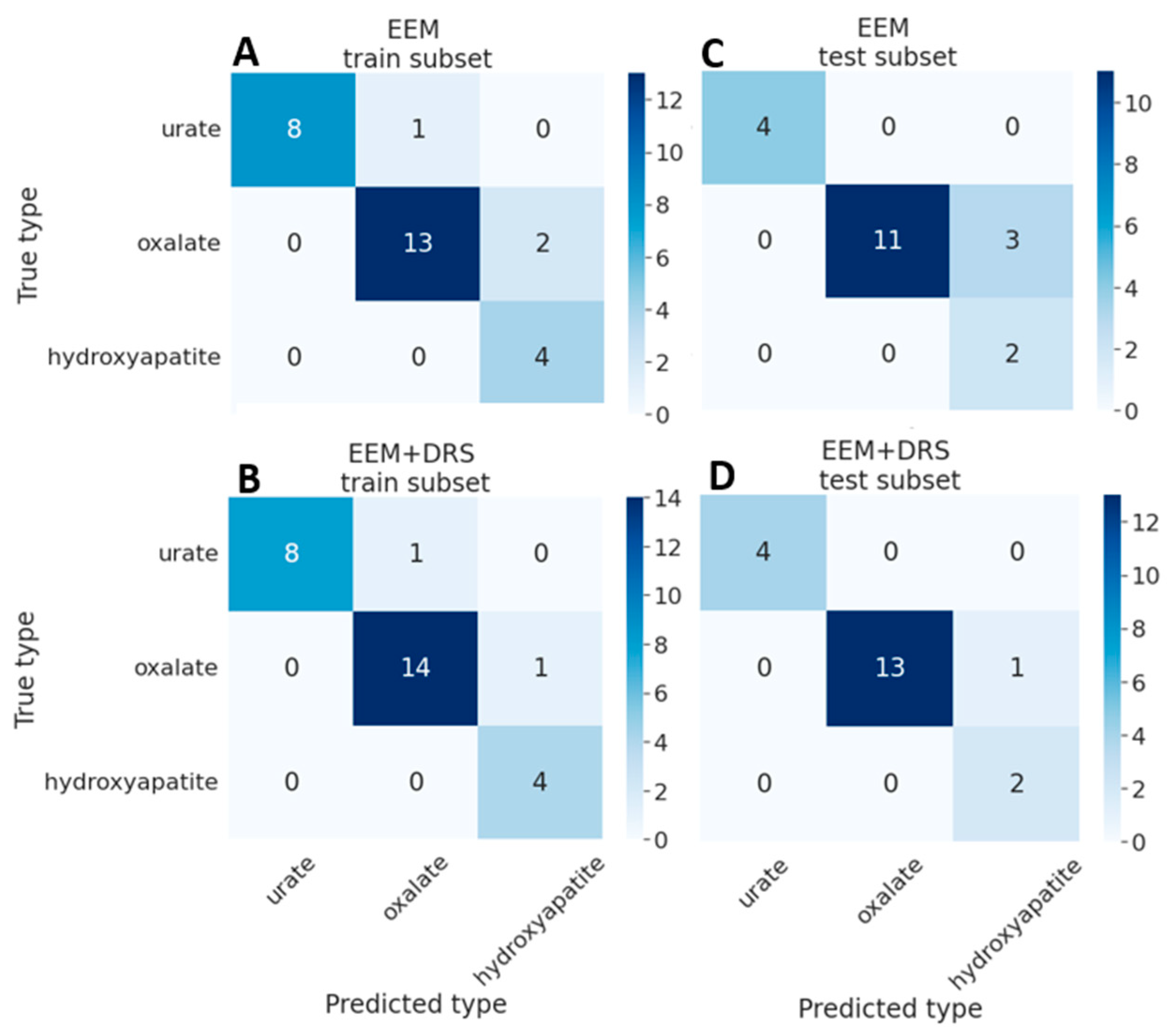
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearle, M.S.; Calhoun, E.A.; Curhan, G.C. The Urologic Diseases of America Project. Urologic Diseases in America Project: Urolithiasis. J. Urol. 2005, 173, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Tiselius, H.-G. Stone Incidence and Prevention. Braz. J. Urol. 2000, 26, 452–462. [Google Scholar]
- Fried, N.M. Recent Advances in Infrared Laser Lithotripsy. Biomed. Opt. Express 2018, 9, 4552–4568. [Google Scholar] [CrossRef] [PubMed]
- Teichman, J.M.H. Laser Lithotripsy. Curr. Opin. Urol. 2002, 12, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Corrales, M.; Doizi, S.; Barghouthy, Y.; Traxer, O.; Daudon, M. Classification of Stones According to Michel Daudon: A Narrative Review. Eur. Urol. Focus 2021, 7, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Moe, O.W. Kidney Stones: Pathophysiology and Medical Management. Lancet 2006, 367, 333–344. [Google Scholar] [CrossRef]
- Wisenbaugh, E.S.; Paden, R.G.; Silva, A.C.; Humphreys, M.R. Dual-Energy vs Conventional Computed Tomography in Determining Stone Composition. Urology 2014, 83, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Uvarov, V.; Popov, I.; Shapur, N.; Abdin, T.; Gofrit, O.N.; Pode, D.; Duvdevani, M. X-ray Diffraction and SEM Study of Kidney Stones in Israel: Quantitative Analysis, Crystallite Size Determination, and Statistical Characterization. Environ. Geochem. Health 2011, 33, 613–622. [Google Scholar] [CrossRef]
- Kravdal, G.; Helgø, D.; Moe, M.K. Infrared Spectroscopy Is the Gold Standard for Kidney Stone Analysis. Tidsskr. Den Nor. Legeforening 2015, 135, 313–314. [Google Scholar] [CrossRef]
- Tonannavar, J.; Deshpande, G.; Yenagi, J.; Patil, S.B.; Patil, N.A.; Mulimani, B.G. Identification of Mineral Compositions in Some Renal Calculi by FT Raman and IR Spectral Analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 154, 20–26. [Google Scholar] [CrossRef]
- Daudon, M.; Bazin, D.; André, G.; Jungers, P.; Cousson, A.; Chevallier, P.; Véron, E.; Matzen, G. Examination of Whewellite Kidney Stones by Scanning Electron Microscopy and Powder Neutron Diffraction Techniques. J. Appl. Crystallogr. 2009, 42, 109–115. [Google Scholar] [CrossRef]
- Orlando, M.T.D.; Kuplich, L.; De Souza, D.O.; Belich, H.; Depianti, J.B.; Orlando, C.G.P.; Medeiros, E.F.; Da Cruz, P.C.M.; Martinez, L.G.; Corrêa, H.P.S. Study of Calcium Oxalate Monohydrate of Kidney Stones by X-ray Diffraction. Powder Diffr. 2008, 23, S59–S64. [Google Scholar] [CrossRef]
- Larsson, L.; Sörbo, B.; Tiselius, H.-G.; Öhman, S. A Method for Quantitative Wet Chemical Analysis of Urinary Calculi. Clin. Chim. Acta 1984, 140, 9–20. [Google Scholar] [CrossRef] [PubMed]
- ROSE, G.A.; Woodfine, C. The Thermogravimetric Analysis of Renal Stones (in Clinical Practice). Br. J. Urol. 1976, 48, 403–412. [Google Scholar] [CrossRef]
- Štěpánková, K.; Novotný, K.; Galiová, M.V.; Kanický, V.; Kaiser, J.; Hahn, D.W. Laser Ablation Methods for Analysis of Urinary Calculi: Comparison Study Based on Calibration Pellets. Spectrochim. Acta Part B At. Spectrosc. 2013, 81, 43–49. [Google Scholar] [CrossRef]
- Ghosh, S.; Basu, S.; Chakraborty, S.; Mukherjee, A.K. Structural and Microstructural Characterization of Human Kidney Stones from Eastern India Using IR Spectroscopy, Scanning Electron Microscopy, Thermal Study and X-ray Rietveld Analysis. J. Appl. Crystallogr. 2009, 42, 629–635. [Google Scholar] [CrossRef]
- Afzal, M.; Iqbal, M.; Ahmad, H. Thermal Analysis of Renal Stones. J. Therm. Anal. 1992, 38, 1671–1682. [Google Scholar] [CrossRef]
- Tamosaityte, S.; Pucetaite, M.; Zelvys, A.; Varvuolyte, S.; Hendrixson, V.; Sablinskas, V. Raman Spectroscopy as a Non-Destructive Tool to Determine the Chemical Composition of Urinary Sediments. Comptes Rendus. Chim. 2022, 25, 73–82. [Google Scholar] [CrossRef]
- Castiglione, V.; Sacre, P.-Y.; Cavalier, E.; Hubert, P.; Gadisseur, R.; Ziemons, E. Raman Chemical Imaging, a New Tool in Kidney Stone Structure Analysis: Case-Study and Comparison to Fourier Transform Infrared Spectroscopy. PLoS ONE 2018, 13, e0201460. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, Z.; Zhang, G.; Chen, S.; Zhao, Y.; Lu, J. Analysis and Classification of Kidney Stones Based on Raman Spectroscopy. Biomed. Opt. Express 2018, 9, 4175–4183. [Google Scholar] [CrossRef]
- Muhammed Shameem, K.M.; Chawla, A.; Mallya, M.; Barik, B.K.; Unnikrishnan, V.K.; Kartha, V.B.; Santhosh, C. Laser-induced Breakdown Spectroscopy-Raman: An Effective Complementary Approach to Analyze Renal-calculi. J. Biophotonics 2018, 11, e201700271. [Google Scholar] [CrossRef] [PubMed]
- Miernik, A.; Eilers, Y.; Nuese, C.; Bolwien, C.; Lambrecht, A.; Hesse, A.; Rassweiler, J.J.; Schlager, D.; Wilhelm, K.; Wetterauer, U. Is in Vivo Analysis of Urinary Stone Composition Feasible? Evaluation of an Experimental Setup of a Raman System Coupled to Commercial Lithotripsy Laser Fibers. World J. Urol. 2015, 33, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.C.; Bottiroli, G. Autofluorescence Spectroscopy for Monitoring Metabolism in Animal Cells and Tissues. Histochem. Single Mol. Methods Protoc. 2017, 1560, 15–43. [Google Scholar]
- Bratchenko, I.A.; Artemyev, D.N.; Myakinin, O.O.; Khristoforova, Y.A.; Moryatov, A.A.; Kozlov, S.V.; Zakharov, V.P. Combined Raman and Autofluorescence Ex Vivo Diagnostics of Skin Cancer in Near-Infrared and Visible Regions. J. Biomed. Opt. 2017, 22, 27005. [Google Scholar] [CrossRef]
- Schütz, J.; Miernik, A.; Brandenburg, A.; Schlager, D. Experimental Evaluation of Human Kidney Stone Spectra for Intraoperative Stone-Tissue-Instrument Analysis Using Autofluorescence. J. Urol. 2019, 201, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, S.; Yao, J.; Liao, X.; Chen, M.; Zhai, J.; Lang, L.; Lin, C.; Zhang, N.; Yuan, C. Autofluorescence Spectral Analysis for Detecting Urinary Stone Composition in Emulated Intraoperative Ambient. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 300, 122913. [Google Scholar] [CrossRef] [PubMed]
- Database of Raman Spectroscopy, X-ray Diffraction and Chemistry of Minerals. Available online: https://rruff.info/ (accessed on 10 November 2023).
- Channa, N.A.; Ghangro, A.B.; Soomro, A.M.; Noorani, L. Analysis of Kidney Stones by FTIR Spectroscopy. Jlumhs 2007, 2, 66–73. [Google Scholar]
- Asyana, V.; Haryanto, F.; Fitri, L.A.; Ridwan, T.; Anwary, F.; Soekersi, H. Analysis of Urinary Stone Based on a Spectrum Absorption FTIR-ATR. J. Phys. Conf. Ser. 2016, 694, 012051. [Google Scholar] [CrossRef]
- Chatterjee, P.; Chakraborty, A.; Mukherjee, A.K. Phase Composition and Morphological Characterization of Human Kidney Stones Using IR Spectroscopy, Scanning Electron Microscopy and X-ray Rietveld Analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 200, 33–42. [Google Scholar] [CrossRef]
- Mandel, I.; Mandel, N. Structure and Compositional Analysis of Kidney Stones. In Urinary Stone Disease: The Practical Guide to Medical and Surgical Management; Humana Press: Totowa, NJ, USA, 2007; pp. 69–81. [Google Scholar]
- Kanchana, G.; Sundaramoorthi, P.; Jeyanthi, G.P. Bio-Chemical Analysis and FTIR-Spectral Studies of Artificially Removed Renal Stone Mineral Constituents. J. Miner. Mater. Charact. Eng. 2009, 8, 161–170. [Google Scholar] [CrossRef]
- Lange, B.; Cordes, J.; Brinkmann, R. Stone/Tissue Differentiation for Holmium Laser Lithotripsy Using Autofluorescence. Lasers Surg. Med. 2015, 47, 737–744. [Google Scholar] [CrossRef] [PubMed]
- McGrath, T.A.; Frank, R.A.; Schieda, N.; Blew, B.; Salameh, J.-P.; Bossuyt, P.M.M.; McInnes, M.D.F. Diagnostic Accuracy of Dual-Energy Computed Tomography (DECT) to Differentiate Uric Acid from Non-Uric Acid Calculi: Systematic Review and Meta-Analysis. Eur. Radiol. 2020, 30, 2791–2801. [Google Scholar] [CrossRef] [PubMed]
- Primak, A.N.; Fletcher, J.G.; Vrtiska, T.J.; Dzyubak, O.P.; Lieske, J.C.; Jackson, M.E.; Williams, J.C., Jr.; McCollough, C.H. Noninvasive Differentiation of Uric Acid versus Non–Uric Acid Kidney Stones Using Dual-Energy CT. Acad. Radiol. 2007, 14, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.N.; Yakimov, B.P.; Rubekina, A.A.; Gorin, D.A.; Drachev, V.P.; Zarubin, M.P.; Velikanov, A.N.; Lademann, J.; Fadeev, V.V.; Priezzhev, A.V.; et al. The Oxidation-Induced Autofluorescence Hypothesis: Red Edge Excitation and Implications for Metabolic Imaging. Molecules 2020, 25, 1863. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseregorodtseva, P.S.; Budylin, G.S.; Zlobina, N.V.; Gevorkyan, Z.A.; Filatova, D.A.; Tsigura, D.A.; Armaganov, A.G.; Strigunov, A.A.; Nesterova, O.Y.; Kamalov, D.M.; et al. Multiwavelength Fluorescence and Diffuse Reflectance Spectroscopy for an In Situ Analysis of Kidney Stones. Photonics 2023, 10, 1353. https://doi.org/10.3390/photonics10121353
Tseregorodtseva PS, Budylin GS, Zlobina NV, Gevorkyan ZA, Filatova DA, Tsigura DA, Armaganov AG, Strigunov AA, Nesterova OY, Kamalov DM, et al. Multiwavelength Fluorescence and Diffuse Reflectance Spectroscopy for an In Situ Analysis of Kidney Stones. Photonics. 2023; 10(12):1353. https://doi.org/10.3390/photonics10121353
Chicago/Turabian StyleTseregorodtseva, Polina S., Gleb S. Budylin, Nadezhda V. Zlobina, Zare A. Gevorkyan, Daria A. Filatova, Daria A. Tsigura, Artashes G. Armaganov, Andrey A. Strigunov, Olga Y. Nesterova, David M. Kamalov, and et al. 2023. "Multiwavelength Fluorescence and Diffuse Reflectance Spectroscopy for an In Situ Analysis of Kidney Stones" Photonics 10, no. 12: 1353. https://doi.org/10.3390/photonics10121353
APA StyleTseregorodtseva, P. S., Budylin, G. S., Zlobina, N. V., Gevorkyan, Z. A., Filatova, D. A., Tsigura, D. A., Armaganov, A. G., Strigunov, A. A., Nesterova, O. Y., Kamalov, D. M., Afanasyevskaya, E. V., Mershina, E. A., Sorokin, N. I., Sinitsyn, V. E., Kamalov, A. A., & Shirshin, E. A. (2023). Multiwavelength Fluorescence and Diffuse Reflectance Spectroscopy for an In Situ Analysis of Kidney Stones. Photonics, 10(12), 1353. https://doi.org/10.3390/photonics10121353




