Abstract
Upconversion nanoparticles are promising for many applications. For triple-doped nanoparticles (NPs), the luminescence intensity shows a non-linear dependence on the rare-earth ion concentration, making it difficult to obtain bright phosphors with high energy output. We investigated the energy transfer processes in β-NaGdF4:Yb-Er-Tm NPs and considered strategies for increasing the thulium luminescence intensity, in particular, the use of core–shell structures. The luminescence spectra were analyzed in the short-wavelength infrared (SWIR) and visible (VIS) regions. The Er3+ and Tm3+ luminescence lifetimes in the VIS region were measured to study the energy transfer processes between the active ions. The quenching of the Tm3+ luminescence in the SWIR region was observed. However, both Er3+ and Tm3+ luminescence bands were observed in the VIS range. We attribute these effects to energy transfer between Tm3+ 3F4 → 3H6 and Er3+ 4I13/2 → 4I9/2, which occurs due to overlap of Er3+ and Tm3+ luminescence bands, and also to competition between Er3+ and Tm3+ for energy transfer from Yb3+. For core–shell NPs, when Tm3+ and Er3+ are separated into adjacent layers, quenching cannot be avoided, likely due to the mutual diffusion of ions during shell synthesis. The most optimal strategy to obtain luminescence in the SWIR range is to use an inert intermediate shell between the layers containing Tm3+ and Er3+.
1. Introduction
In recent years, upconversion nanoparticles (UCNPs), which produce higher-energy VIS or near-infrared (NIR) luminescence when excited with low-energy NIR light, have been extensively investigated. Due to their unique characteristics [1,2,3] such as very high photostability, absence of photo blinking, long luminescence lifetimes, narrow emission peaks with large shifts relative to the excitation wavelength, low toxicity, low imaging background, and functional multimodality, they have been used in a variety of applications, including bioimaging [4,5], solar cell enhancement [6,7], chemical and biosensing [8,9,10,11], contactless thermometry at the nanoscale [12,13], cancer therapy [14,15], and anti-counterfeiting [16]. UCNPs are highly attractive for biosensing and bioimaging applications due to their ability to be excited by NIR radiation, which results in lower photo-damage and auto-fluorescence, as well as deeper penetration into biological tissues due to lower absorption and scattering [17].
UCNPs typically consist of an inert matrix doped with two types of active ions: sensitizers and acceptors. The sensitizer absorbs exciting NIR excitation and non-radiatively transfers energy to an acceptor that luminesces in the visible or NIR range [18]. UCNPs doped with Yb3+-Tm3+ are especially promising because they can could convert NIR excitation to NIR luminescence [19,20,21] and operate within the biological tissue transparency window at wavelengths between 650 nm and 950 nm. However, this range is not optimal due to tissue autofluorescence and background noise, which limit the tissue penetration depth to 1–2 cm [22]. In 2003, it was demonstrated that fluorescence imaging can be significantly improved in depth and sensitivity by using fluorophores that emit light in the short-wavelength infrared (SWIR) at 1320 nm. This also provides excellent contrast and a high signal-to-noise ratio for the resulting luminescence images [23]. Nowadays, there is a growing interest in bioimaging in the second (1000–1350 nm) and third (1500–1800 nm) biological windows [24,25,26,27]. However, the lack of biocompatible fluorescent probes limits the use of this highly sensitive spectral range for in vivo imaging. One of them are UCNPs doped with rare-earth ions, which can be excited through multiple electron states and produce luminescence bands in a wide range of the visible (VIS), NIR, and SWIR spectra [28,29]. A significant number of publications are devoted to the study of nanoparticles (NPs) with triple doping, which could be used for multimodal bioimaging with subsequent control of temperature, microenvironment, or probing depth [30,31,32,33,34,35,36,37,38]. Doping with multiple active ions allows for the production of multicolor luminescence with controllable bands upon single wavelength excitation [39,40,41,42].
For biomedical applications, it is very important to achieve high intensity of upconversion luminescence. The luminescent properties of UCNPs strongly depend on many factors, including their matrix and doping ions concentration, particle size, shape, phase and morphology, nanostructure design (such as core−shell parameters), surface ligands, and excitation characteristics (including pumping power density and beam profile) [43,44,45,46,47]. The choice of host material is crucial for achieving high upconversion luminescence intensity. The hexagonal NaYF4 reported to be the most effective matrix due to its low phonon energy [48,49,50,51]. The hexagonal phase NaGdF4, which was studied in this work, possesses even lower values of phonon energy [52].
Many papers are devoted to optimizing of the composition and structure of UCNPs to achieve intense upconversion luminescence in the VIS and NIR ranges [53], but the luminescence in the SWIR range has not been thoroughly studied yet. The study of energy transfer processes in NPs with triple doping is of great importance for optimizing the dopant concentration and obtaining SWIR phosphors with high quantum efficiency. The development of SWIR-based luminescent markers with high luminescence intensity under infrared excitation is one of the urgent tasks today. This study focuses on synthesizing β-NaGdF4 NPs doped with rare-earth ions Yb3+, Er3+, and Tm3+, investigating their luminescence characteristics and effect of the shell on the luminescence intensity and energy transfer processes between the rare-earth ions.
Er3+ and Tm3+ ions are promising for obtaining multicolor luminescence in various spectral ranges because their matching energy levels enable several resonant and non-resonant energy transfers and corresponding spectral tuning. To control the luminescence spectrum, it is necessary to optimize the interactions between ions in such a way that certain transitions are stimulated or suppressed [54]. The successful modulation of the VIS Er3+ emission for the Er3+–Tm3+ ion pair has been realized. After the introduction of Tm3+, the green emission of Er3+ (2H11/2 → 4I15/2, 4S3/2 → 4I15/2) decreased, while the red emission (4F9/2 → 4I15/2) increased due to the presence of the cross-relaxation process 3F4 (Tm3+) + 4I11/2 (Er3+) → 3H6 (Tm3+) + 4F9/2 (Er3+) [55]. This hypothesis was confirmed by a kinetic model that provided microscopic insight into the energy transfer pathways leading to spectrally pure emission in multi-doped UCNPs [56,57].
To obtain intense luminescence, it is also important to reduce quenching on high energy surface oscillators [58]. This can be achieved by using core–shell or complex core–multishell structures [20,59]. Adding an intermediate shell between the core and second shell, doped with different active ions, prevents direct chemical and energy transfer interactions between the active ions. This results in multicolor luminescence with high intensity [58,60]. However, it has been shown that active ions in different shells can interact [61]; therefore, determining the optimal shell thickness and composition is usually a complex issue. Efficient energy transfer between ions in adjacent shells has also been reported in the literature [62].
In this article, we investigated how the processes of energy transfer between Er3+–Tm3+ ions in triple-doped β-NaGdF4:Yb-Er-Tm NPs affect the luminescence in the SWIR range. Various strategies to increase the intensity of thulium luminescence are also considered, including the use of core–shell structures for the separation of active ions.
2. Materials and Methods
2.1. Synthesis and Characterization of Yb, Er, and Tm Doped β-NaGdF4 NPs
The solvothermal technique was used to synthesize oleate-stabilized β-NaGdF4:Yb(30.0 mol.%):Tm(0.5 mol.%) NPs, β-NaGdF4:Yb(20.0 mol.%):Tm(2.0 mol.%) and Yb-Er-Tm tri-doped β-NaGdF4 NPs as described in [42,63]. No additional purification procedures were employed for the chemicals used. Ytterbium, thulium, and gadolinium acetates (99.99%, LANHIT, Russia) were added to a 500 mL three-necked flask with a reflux condenser. Then, oleic acid (Chimmed, purity) and octadecene-1 (90%, tech Acros Organic) were added. The solution was stirred and heated to 140 °C under argon atmosphere (4.8 purity) for 30 min to form the Ln(oleate)3 complex and to remove the water and acetic acid under vacuum. The temperature was then lowered to room temperature, and ammonium fluoride (NH4F) (purity, Chimmed, Russia) and sodium hydroxide (NaOH) (Chimmed, chemical purity) dissolved in the methanol (chemical purity, Chimmed, Russia) were added to the reaction flask with the corresponding oleates. The resulting solution was stirred at 50 °C for 1 h. The solution was then slowly heated to 70 °C to remove methanol under vacuum. After removal of methanol, the solution was heated to 300 °C under argon atmosphere and kept in such conditions for 1.5 h. The sediment of NPs was obtained by centrifugation (6500 rpm, 5 min). The resulting NPs were dispersed in chloroform and washed with alcohol. Part of the prepared NPs was dispersed in cyclohexane for core–shell particle preparation.
To synthesize core–shell NPs, lanthanide acetates were added to a 500 mL three-necked flask with a reflux condenser in appropriate proportions, followed by addition of oleic acid and octadecene-1. The solution was stirred and heated to 140 °C under argon atmosphere for 30 min to form the Ln(oleate)3 complex, and to remove the water and acetic acid under vacuum. The solution was cooled to room temperature before adding the core NPs in cyclohexane and NH4F with NaOH dissolved in methanol to the reaction flask. The resulting mixture was stirred at 50 °C for 1 h, followed by slow heating to 70 °C to remove methanol and cyclohexane under vacuum. After the removal of methanol, the solution was heated to 310 °C under argon atmosphere and kept in such conditions for 1.5 h. The sediment of NPs was obtained by centrifugation. The resulting NPs were dissolved in chloroform and washed with alcohol.
The determination of the phase composition of the synthesized samples was performed by X-ray powder diffraction (XRD) technique (D8 Bruker® Advance diffractometer with CuKα radiation, Bruker, Karlsruhe, Germany). The lattice parameters and coherent scattering region were calculated using TOPAS v.4.2 software. Particle size and morphology were analyzed by transmission electron microscopy (TEM, Jeol JEM-2100, Jeol, Tokyo, Japan) using ImageJ v.1.53e software.
2.2. Spectroscopic Study of Yb, Er, and Tm Doped β-NaGdF4 NPs in VIS and SWIR Ranges
The study involved measuring luminescence spectra in the VIS and SWIR ranges, as well as luminescence lifetime. Luminescence excitation was carried out using a 980 nm wavelength laser (Biospec, Moscow, Russia). The output power from the optical fiber was measured using a PM100D power meter equipped with an S142C integrating sphere (Thorlabs, Newton, NJ, USA). The laser irradiation was focused onto the sample in a spot with 1 cm2 area. The pumping power density was estimated to be 1 W/cm2. Scattered laser radiation and upconversion luminescence were collected by the fiber and delivered to the spectrometer.
Luminescence spectra in the VIS spectral range were measured using a fiber-optic spectrometer LESA-01 (Biospec, Moscow, Russia). To suppress radiation with wavelengths above 900 nm and to record the upconversion luminescence spectrum in the VIS range, the FESH900 interference filter (Thorlabs, USA) was used.
The luminescence spectra in the 1000–1700 nm region were measured with a fiber-optic spectrometer DWARF-Star (StellarNet, Tampa, FL, USA). To suppress radiation with wavelengths below 1050 nm and to record the luminescence spectrum in the SWIR range, the FELH1050 interference filter (Thorlabs, Newton, NJ, USA) was used.
Upconversion luminescence decay kinetics were measured using a C9300-508 streak camera with a C10627-13 streak scope (Hamamatsu, Hamamatsu-city, Japan). The samples were illuminated with pulsed laser radiation at a wavelength of 980 nm. The luminescence signal was recorded using a Hamamatsu streak camera in combination with a fiber-optic light delivery system. The pulse duration was 8 ms with a repetition rate of 100 Hz. The sweep time was 2 ms, starting 0.1 ms before the laser pulse end. The detected luminescence signal was divided into green (transitions 2H11/2, 4S3/2 → 4I15/2) and red (transitions 4F9/2 → 4I15/2 Er3+ and 1G4 → 3F4 Tm3+) bands. An exponential fit was used to calculate the luminescence lifetime for each band.
3. Results and Discussion
3.1. Synthesis and Characterization of Yb, Er, and Tm Doped β-NaGdF4 NPs
The β-NaGdF4 NPs were synthesized with tri-doping of rare earth ions Yb3+, Er3+, and Tm3+ at Yb:Er:Tm ratios of 10:1:0.5 and 10:1:1. Also during the step-by-step synthesis, a series of core–shell samples were obtained: (1) tri-doped with Yb3+, Er3+, and Tm3+ rare-earth ions core with Yb:Er:Tm ratio equal to 10:1:0.5 and 10:1:1 covered with inert NaYF4 shell; (2) NaGdF4:Yb-Er core covered with first NaGdF4:Yb-Tm shell, covered with inert NaYF4 shell; (3) NaGdF4:Yb-Tm core covered with first shell NaGdF4:Yb-Er, covered with inert shell NaYF4; and (4) NaGdF4:Yb-Tm core covered with first inert NaYF4 shell (intermediate shell), covered with NaGdF4:Yb-Er shell, covered with second inert NaYF4 shell. The doping concentration ratios of Yb:Tm and Yb:Er were chosen to be 30:0.5 and 20:2, respectively, according to the literature data and our previous study [64,65].
3.1.1. X-ray Powder Diffraction Results
The diffraction patterns of synthesized β-NaGdF4:Yb-Tm(30:0.5), β-NaGdF4:Yb-Tm(30:0.5)@NaYF4, β-NaGdF4:Yb-Tm(30:0.5)@NaYF4@NaGdF4:Yb-Er(20:2), β-NaGdF4:NaGdF4:Yb-Tm(30:0.5)@NaYF4@NaGdF4:Yb-Er(20:2)@NaYF4 samples are shown in Figure 1, Figure 2 and Figure 3.
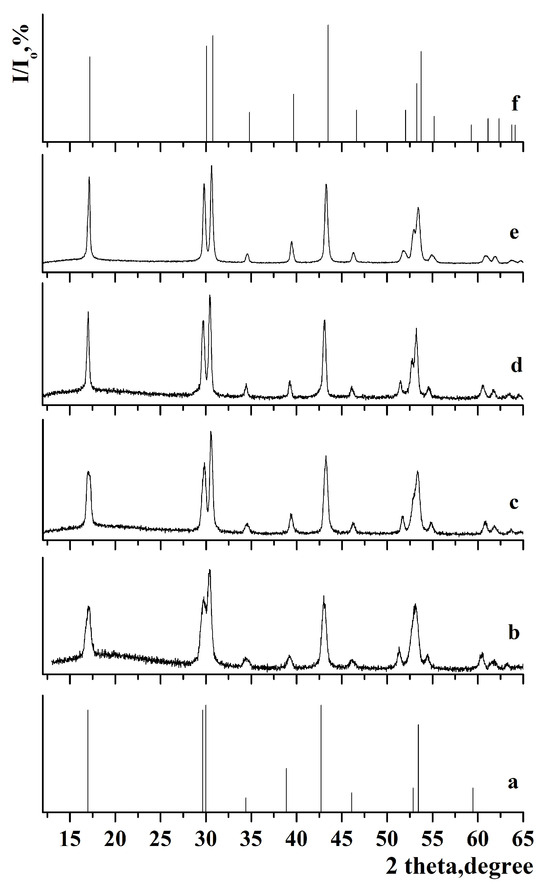
Figure 1.
X-ray diffraction pattern of the samples: (a) JCPDS 27-0699 of β-NaGdF4, (b) β-NaGdF4:Yb-Tm, (c) β-NaGdF4:Yb-Tm@NaYF4, (d) β-NaGdF4:Yb-Tm@NaYF4@NaGdF4:Yb-Er, (e) β-NaGdF4:Yb-Tm@NaYF4@NaGdF4:Yb-Er@NaYF4, and (f) JCPDS 00-016-0334 of β-NaYF4.
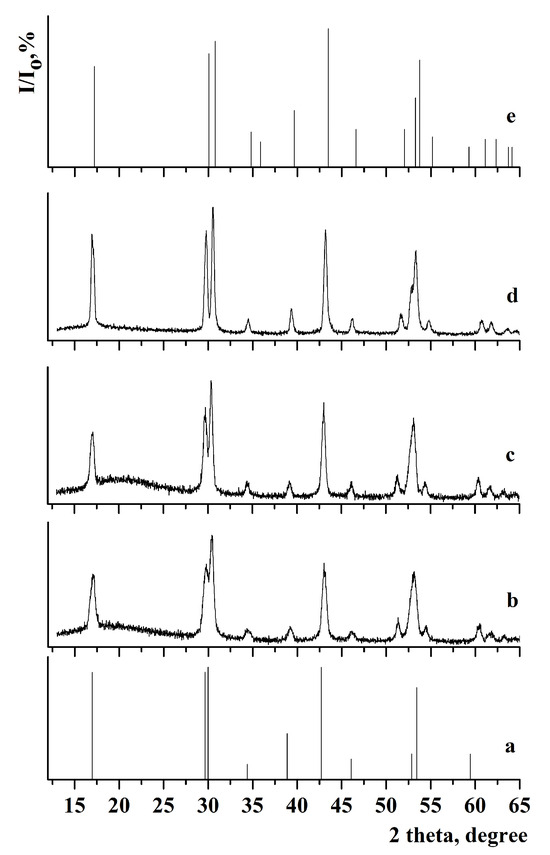
Figure 2.
X-ray diffraction pattern of the samples: (a) JCPDS 27-0699 of β-NaGdF4, (b) β-NaGdF4:Yb-Tm, (c) β-NaGdF4:Yb-Tm@NaGdF4:Yb-Er, (d) β-NaGdF4:Yb-Tm@NaGdF4:Yb-Er@NaYF4, and (e) JCPDS 00-016-0334 of β-NaYF4.
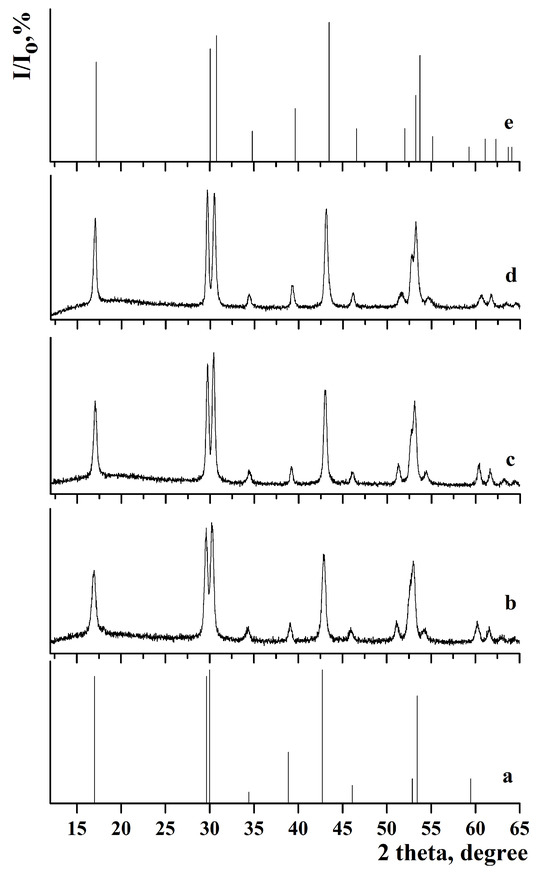
Figure 3.
X-ray diffraction pattern of the samples: (a) JCPDS 27-0699 of β-NaGdF4, (b) β-NaGdF4:Yb-Er, (c) β-NaGdF4:Yb-Er@NaGdF4:Yb-Tm, (d) β-NaGdF4:Yb-Tm@NaGdF4:Yb-Er@NaYF4, and (e) JCPDS 00-016-0334 of β-NaYF4.
Comparison of the X-ray diffraction patterns with JCPDS 27-0699 (a = 6.020 Å, c = 3.601 Å) for β-NaGdF4 and JCPDS 00-016-0334 (a = 5.96 Å, c = 3.53 Å) for β-NaYF4 did not reveal any additional peaks. This indicates that single-phase samples with low-temperature hexagonal phase were synthesized. The calculated lattice parameters are presented in Table 1.

Table 1.
Lattice parameters, unit cell volume, and coherent scattering regions (D) for samples based on β-NaGdF4: Yb-Tm and β-NaGdF4:Yb-Er core with multishell architecture. ΔD indicates the coherent scattering regions resizing after the next shell growing.
The lattice parameters for samples with the outer shell based on β-NaGdF4 are similar. After synthesizing the NaYF4 shell, a decrease in lattice parameters was observed in the following samples: β-NaGdF4:Yb-Tm(30:0.5)@NaGdF4:Yb-Er(20:2)@NaYF4, β-NaGdF4:Yb-Tm(30:0.5)@NaYF4, β-NaGdF4:Yb-Tm(30:0.5)@NaYF4@NaGdF4:Yb-Er(20:2)@NaYF4, and β-NaGdF4:Yb-Er(20:2)@NaGdF4:Yb-Tm(30:0.5)@NaYF4. This effect could be attributed to the smaller size of yttrium cations compared to gadolinium cations [66]. The analysis of lattice parameters shows that the chemical composition of the outer shell has a strong effect on cell parameters. Despite the different particle size, the lattice parameters for the β-NaGdF4:Yb-Tm(30:0.5)@NaGdF4:Yb-Er(20:2)@NaYF4 and β-NaGdF4:Yb-Tm(30:0.5)@NaYF4 samples are similar due to the same chemical composition of the outer shell.
The analysis of the samples with NaYF4 shell has shown that the coherent scattering regions resizing after the next shell growth were 6.4 and 6.7 nm in the case of shell growing on β-NaGdF4:Yb-Er (for β-NaGdF4:Yb-Tm(30:0.5)@NaGdF4:Yb-Er(20:2)@NaYF4 and β-NaGdF4:Yb-Tm(30:0.5)@NaYF4@NaGdF4:Yb-Er(20:2)@NaYF4 samples). For NaYF4 grown on β-NaGdF4:Yb-Tm, the sizes of the scattering regions were smaller after the next shell growing, measuring 2.8 and 4.7 nm for the β-NaGdF4:Yb-Tm(30:0.5)@NaYF4 and β-NaGdF4:Yb-Er(20:2)@NaGdF4:Yb-Tm(30:0.5)@NaYF4 samples, respectively.
The coherent scattering region increased in size after the synthesis of each subsequent shell, reaching a maximum value for the NaGdF4:Yb-Tm(30:0.5)@ NaYF4@NaGdF4:Yb-Er(20:2)@NaYF4 sample. This suggests that during the shell synthesis, particle growth occurred through classical crystallization mechanism, where the initial core reacted with appropriate oleates, NaOH and NH4F from the liquid medium. Finally, the defectiveness of the core surface decreases, the crystallinity of the nanoparticle improves, and the coherent scattering region increases.
3.1.2. Transmission Electron Microscopy Results
The size of the particles was also determined by TEM images, as shown in Figure 4.
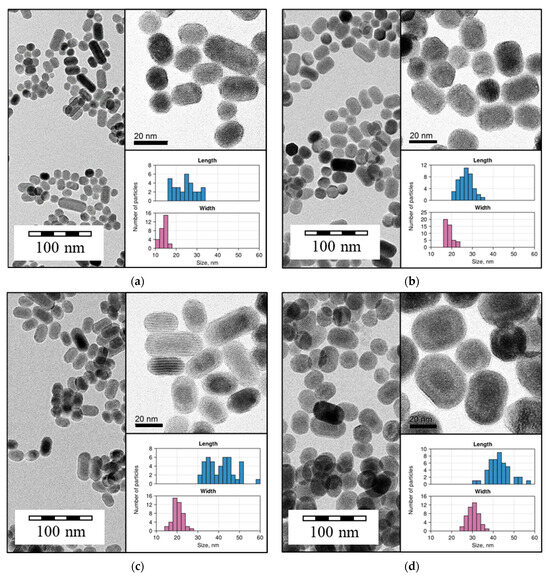
Figure 4.
Micrographs obtained by TEM for (a) β-NaGdF4:Yb-Tm(30:0.5); (b) β-NaGdF4:Yb-Tm(30:0.5)@NaGdF4:Yb-Er(20:2); (c) β-NaGdF4:Yb-Tm(30:0.5)@NaYF4; and (d) β-NaGdF4:Yb-Tm(30:0.5)@ NaYF4@NaGdF4:Yb-Er(20:2). Inset: magnified TEM images with adjusted contrast; size distribution histograms for length and width.
According to the TEM data, the samples consisted of particles with semi-spherical morphology. The core-only β-NaGdF4:Yb-Tm(30:0.5) NPs ranged in size from 15 to 40 nm. The increase in particle size was observed after shell synthesis for both NaGdF4:Yb-Er(20:2) and NaYF4 shell; the shell thickness was about 5 nm.
The NaYF4 shell was clearly distinguishable by TEM, in contrast to NaGdF4:Yb-Er, which may be due to the difference in atomic number of yttrium and gadolinium. TEM images revealed the core–shell architecture of NaGdF4:Yb-Tm(30:0.5)@NaYF4@NaGdF4:Yb-Er(20:2) NPs.
3.2. Spectroscopic Study Results
3.2.1. Luminescence Spectra in VIS and SWIR Ranges Analysis
The luminescence spectra, measured in the SWIR range for Yb-Er-Tm tri-doped β-NaGdF4, are presented in Figure 5a. The synthesized tri-doped NPs exhibited 1050 nm (2F5/2 → 2F7/2 Yb3+) and 1540 nm (4I15/2 → 4I13/2 Er3+) luminescence bands in the SWIR range.
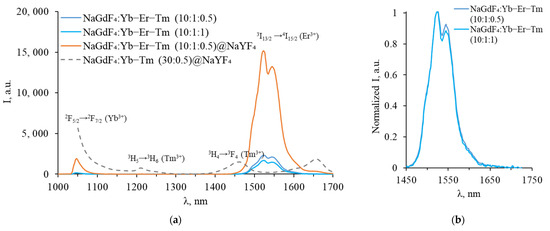
Figure 5.
(a) Luminescence spectra of Yb-Er-Tm tri-doped β-NaGdF4 in the SWIR range; (b) same spectra normalized to maximum.
As can be seen from the obtained spectra, no characteristic Tm3+ luminescence peaks were observed in the SWIR range for tri-doped NPs. The luminescence bands corresponding to 3H4 → 3H6 and 3F4 → 3H6 Tm3+ transitions (near 1450 nm, and 1650–2000 nm) are absent in the spectra of tri-doped NPs. A two-fold increase in the Tm3+ concentration (up to 1%) did not significantly change the shape of the luminescence spectrum. As shown in Figure 4b, only a small decrease in the long-wavelength part of the Er3+ emission peak was observed, which could be attributed to energy transfer from Er3+ 4I13/2 to Tm3+ 3F4 or reabsorption of part of the Er3+ luminescence by Tm3+ ions. The integral Er3+ luminescence intensity at 1540 nm wavelength also decreases due to competition between Er3+ and Tm3+ for energy transfer from Yb3+. After the synthesis of the inert NaYF4 shell, the emission intensity increases dramatically, indicating that the shell separates activators from quenchers and defects on the NPs surface.
To confirm the presence of Tm3+ in the NPs composition, we measured the upconversion luminescence spectra of tri-doped NPs in the VIS range, as shown in Figure 6.
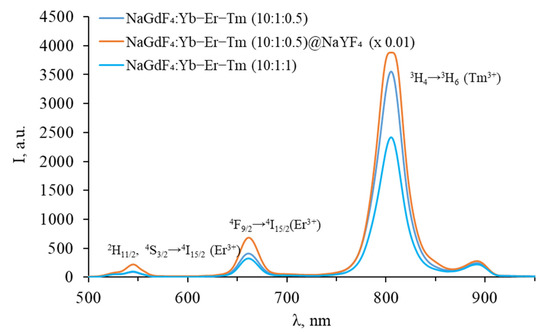
Figure 6.
Luminescence spectra of Yb-Er-Tm tri-doped β-NaGdF4 in the VIS range.
The spectra in the VIS range exhibit bands characteristic of both Er3+ and Tm3+, confirming the successful doping of the studied NPs with Tm3+. Since Yb3+-Er3+ energy transfer is resonant, while Yb3+-Tm3+ energy transfer is non-resonant, the transfer to Er3+ is more likely. This is the reason for the absence of blue thulium luminescence, which requires at least three energy transfers. However, energy transfer to Tm3+ does occur, as evidenced by the presence of a luminescence peak at 800 nm, corresponding to the 3H4 → 3H6 transition of thulium. The intensity of thulium luminescence at 800 nm wavelength decreases with an increase in the thulium concentration from 0.5 to 1.0%, which may be due to the cross-relaxation process Tm3+-Tm3+: 3H4 → 3F4 and 3H6 → 3F4, resulting in a decrease in the population of the 3H4 state, as shown in Figure 7.
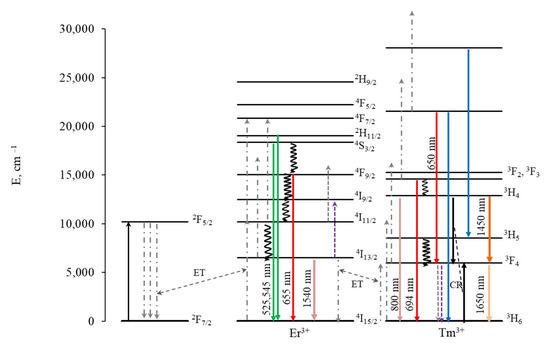
Figure 7.
Energy level diagram for Yb-Er-Tm with schematic representation of energy transfer processes.
In contrast to Er-Tm doping, an increase in the thulium concentration leads to a decrease in the red luminescence intensity of erbium for triple doping with Yb-Er-Tm. The increase in red luminescence for Er-Tm doping has been explained in the literature by the 3F4 (Tm3+) + 4I11/2 (Er3+) → 3H6 (Tm3+) + 4F9/2 (Er3+) cross-relaxation process [55,56,57]. We assume that in our case there is a cross-relaxation between Tm3+ 3F4 → 3H6 and Er3+ 4I13/2 → 4I9/2, resulting in a decrease in the population of the 4I13/2 level. Energy transfer from Yb3+ to 4I13/2 leads to the population of 4F9/2, which causes the red luminescence of erbium. The population of thulium 3F4, which participates in the population of 3H4 during energy transfer from Yb3+, also decreases, which can lead to a decrease in the intensity of luminescence from 3H4 along with cross-relaxation Tm3+-Tm3+ 3H4 → 3F4 and 3H6 → 3F4, and a decrease in the luminescence of thulium in the SWIR range from the 3H4 level, which occurs between 1600 and 2000 nm. Luminescence in the SWIR range during the 3H4 → 3F4 transition is less likely than luminescence at 800 nm during the 3H4 → 3H6 transition and is therefore not observed at low concentrations.
Increasing the thulium concentration does not lead to an increase in this peak due to Tm3+-Tm3+: 3H4 → 3F4 and 3H6 → 3F4 cross-relaxation. As a result, Tm3+ demonstrated weak luminescence in the SWIR range, making it difficult to distinguish between Tm3+ and Er3+ luminescence peaks. To prevent energy transfer between Tm3+ and Er3+, core–shell NPs were synthesized, in which Er3+ and Tm3+ are located in different layers. Spectra in the SWIR and in the VIS ranges recorded for NPs with an Er3+ doped core coated with a Tm3+ doped shell are shown in Figure 8 and Figure 9.
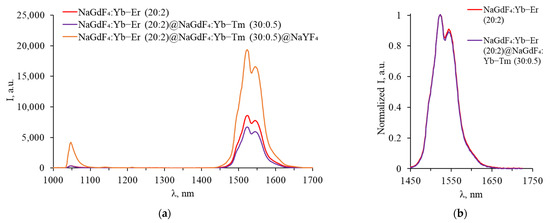
Figure 8.
(a) Luminescence spectra of core–shell β-NaGdF4 (NaGdF4:Yb-Er core covered with first NaGdF4:Yb-Tm shell, covered with inert NaYF4 shell) in SWIR range; (b) same spectra normalized to maximum.
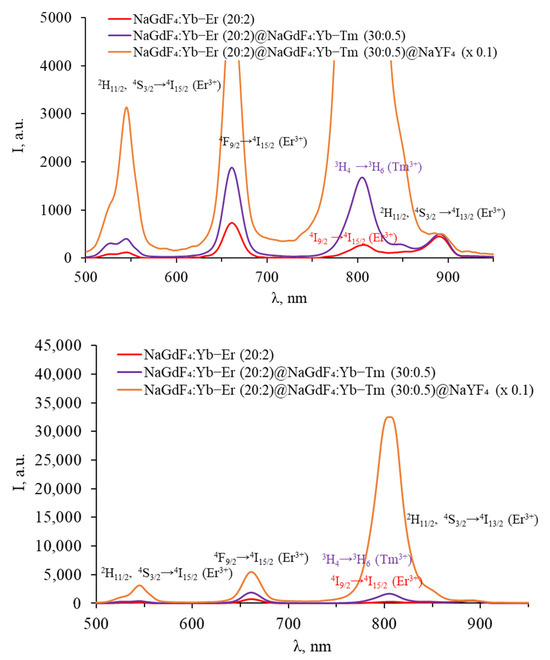
Figure 9.
Luminescence spectra of core–shell β-NaGdF4 (NaGdF4:Yb-Er core covered with first NaGdF4:Yb-Tm shell, covered with inert NaYF4 shell) in the VIS range.
In the luminescence spectra of particles with an erbium-doped core in the SWIR, a characteristic band at 1540 nm was observed. After coating with a thulium-doped shell, the integral intensity of the band decreases, as in the case of triple-doped NPs with an increase in the Tm3+ concentration, which is due to the competition between Er3+ and Tm3+ for energy transfer from Yb3+. There is almost no change in the shape of the spectrum after the growth of the shell with thulium; the luminescence peaks of thulium are still not observed in the SWIR spectra, indicating the energy transfer between the Er3+ and Tm3+ ions located in adjacent shells.
The core-only NPs in the VIS range exhibited characteristic erbium luminescence peaks in the green (2H11/2, 4S3/2 → 4I15/2), red (4F9/2 → 4I15/2), and NIR (4I9/2 → 4I15/2 and 2H11/2, 4S3/2 → 4I13/2) parts of the spectrum. After coating with a thulium shell, an increase in the intensity of the erbium bands is observed in the spectrum of the particles, presumably due to the additional shell protecting erbium ions from surface quenching. A thulium luminescence peak at about 800 nm (3H4 → 3H6) was also present. The intensity of all peaks was increased by coating the NPs with an inert shell.
Spectra recorded for NPs with a Tm3+ doped core covered by an Er3+ doped shell, in the SWIR and in the VIS range, are shown in Figure 10 and Figure 11, respectively.
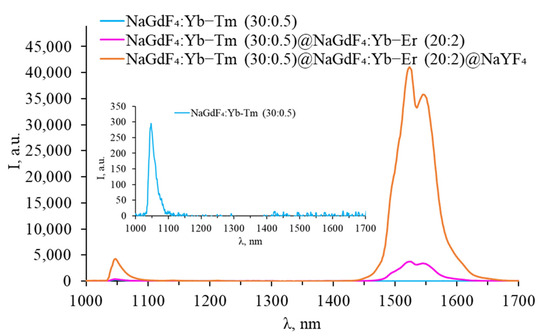
Figure 10.
Luminescence spectra of core–shell β-NaGdF4 (NaGdF4:Yb-Tm core covered with first NaGdF4:Yb-Er shell, covered with inert NaYF4 shell) in the SWIR range.
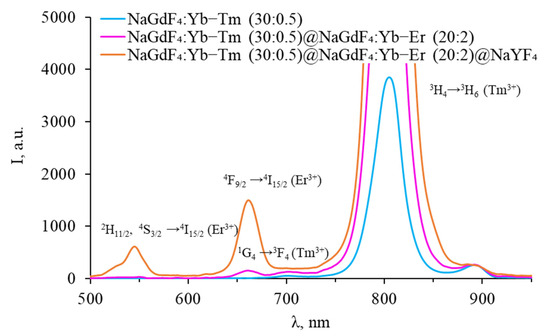
Figure 11.
Luminescence spectra of core–shell β-NaGdF4 (NaGdF4:Yb-Tm core covered with first NaGdF4:Yb-Er shell, covered with inert NaYF4 shell) in the VIS range.
No signal was observed in the SWIR spectra of core-only β-NaGdF4:Yb-Tm NPs, but a peak corresponding to the 3H4 → 3H6 transition was observed in the VIS range. After coating the particles with an erbium-doped shell, the intensity of this peak increases, while the typical peaks of thulium in the SWIR range are still not observed. The erbium-doped shell prevents the quenching of the thulium luminescence, while the erbium ions themselves remained optically inactive in the VIS range. An erbium luminescence band was observed in the SWIR range at a wavelength of 1540 nm. After coating the particles with an inert shell, erbium luminescence peaks appear in the green and red parts of the spectrum. Thus, the absence of thulium luminescence in the SWIR range can be attributed to the interaction of ions in adjacent shells and/or penetration of rare-earth ions into adjacent shells during the NPs synthesis.
To separate Er3+ and Tm3+ ions and prevent energy transfer between them, particles with an inert intermediate shell were synthesized: β-NaGdF4:Yb-Tm core covered with a first inert NaYF4 shell (intermediate shell), covered with a NaGdF4:Yb-Er shell, and finally covered with a second inert NaYF4 shell. Spectra in the SWIR and VIS ranges are shown in Figure 12 and Figure 13, respectively.
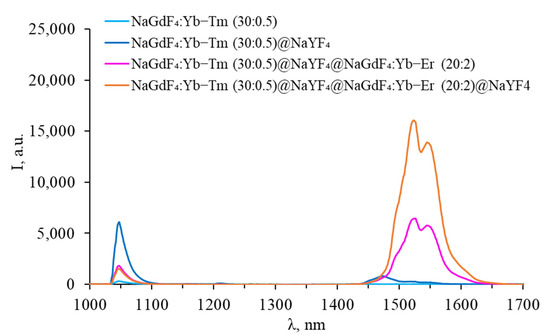
Figure 12.
Luminescence spectra of core–shell β-NaGdF4 (NaGdF4:Yb-Tm core covered with first inert NaYF4 shell (intermediate shell), covered with NaGdF4:Yb-Er shell, and covered with second inert NaYF4 shell) in the SWIR range.
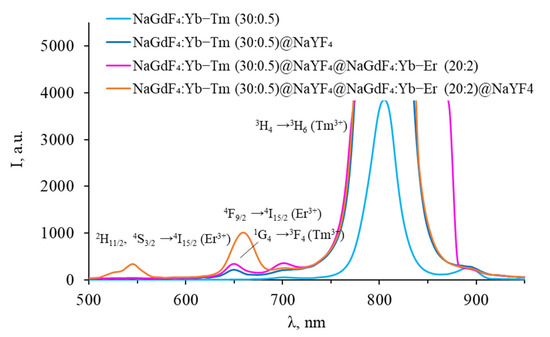
Figure 13.
Luminescence spectra of core–shell β-NaGdF4 (NaGdF4:Yb-Tm core covered with first inert NaYF4 shell (intermediate shell), covered with NaGdF4:Yb-Er shell, and covered with second inert NaYF4 shell) in the VIS range.
After coating thulium-doped NPs with an inert shell, the intensity of thulium luminescence in the VIS region increased, and an additional peak corresponding to the 1G4 → 3F4 transition appeared. A characteristic peak at 1450 nm appeared in the SWIR range, corresponding to the 3H4 → 3F4 transition. This indicates that surface quenching was observed for the luminescence from 3H4 level for uncoated particles. After coating with erbium-doped shell, the peak at 1450 nm disappears, an increase in the intensity of the peaks in the VIS range was observed, while there are no erbium peaks in the VIS range. The erbium shell prevents surface quenching of thulium, while the erbium ions themselves remain optically inactive in the VIS range. However, the thulium luminescence peak in the SWIR range disappeared completely, leaving only the characteristic erbium luminescence peak at 1540 nm. Coating NPs with an inert shell leads to the appearance of additional erbium peaks in the VIS range, an increase in the intensity of erbium luminescence in the SWIR range, and a decrease in the Tm3+ luminescence intensity at 800 nm. This suggests that after coating NPs with a final inert shell, erbium ions become optically active, while the role of energy transfer between Tm3+ 3F4 → 3H6 and Er3+ 4I13/2 → 4I9/2 increases. The upconversion luminescence efficiency for all studied Yb-Er-Tm tri-doped β-NaGdF4 nanoparticles, calculated for the visible range, is presented in Table S1, Supplementary Materials.
3.2.2. Luminescence Lifetime in VIS Range Analysis
To confirm the presence of energy transfer between Tm3+ and Er3+ active ions, we studied the luminescence lifetime for synthesized samples. The measured decay curves for Er3+ 2H11/2, 4S3/2 → 4I15/2 (510–550 nm), 4F9/2 → 4I15/2 (650–670 nm) transitions and Tm3+ 1G4 → 3F4 (640–650 nm) transition are shown in Figure 14.
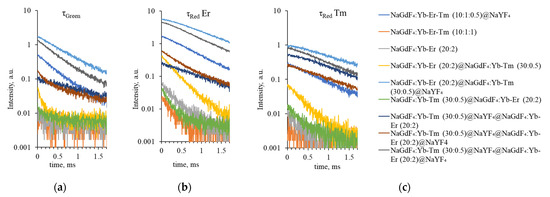
Figure 14.
Upconversion luminescence decay curves for (a) Er3+ 2H11/2, 4S3/2 → 4I15/2 (τgreen, 510–550 nm), (b) 4F9/2 → 4I15/2 (τred Er, 650–670 nm), and (c) Tm3+ 1G4 → 3F4 (τred Tm, 640–650 nm) transitions.
The calculated luminescence lifetime values are presented in Table 2.

Table 2.
Calculated luminescence lifetime for Er3+ 2H11/2, 4S3/2 → 4I15/2 (τgreen, 510–550 nm), 4F9/2 → 4I15/2 (τred Er, 650–670 nm) transitions and Tm3+ 1G4 → 3F4 (τred Tm, 640–650 nm) transition. NA indicates that no single peak was observed in this range; the value in brackets shows the lifetime calculated for this wavelength range.
For tri-doped NPs, no single peak was observed in the Tm3+ luminescence region; the lifetime calculated for the 640–650 nm range corresponds to Er3+ red luminescence lifetime (0.210 ± 0.037 ms and 0.221 ± 0.009 ms, respectively). After inert NaYF4 shell coating, the luminescence lifetimes for green and red emission increased up to four times, indicating that the surface quenching is reduced by the NaYF4 shell because the inert shell inhibited surface quenching.
For NPs with Tm3+ and Er3+ ions separated in the core and the shell, respectively, we have observed the decrease of Tm3+ luminescence intensity in the SWIR range and the reduction of luminescence lifetime upon Er3+ doping in the shell.
For the β-NaGdF₄:Yb-Tm (30:0.5)@NaGdF₄:Yb-Er (20:2)@NaYF₄ sample without an inert shell, the thulium luminescence lifetime was estimated to be 0.953 ± 0.020 ms and that of erbium was 0.449 ± 0.003 ms and 0.835 ± 0.011 ms for the green and red regions, respectively. When an intermediate shell is present, the lifetime of thulium increases to 1.078 ± 0.036 ms, while the lifetime of erbium decreases to 0.324 ± 0.007 in the green range and to 0.498 ± 0.005 ms in the red range. However, the lifetime of thulium does not reach the 1.139 ± 0.047 ms registered for β-NaGdF₄:Yb-Tm (30:0.5)@NaYF₄@NaGdF₄:Yb-Er (20:2) composition. We assume that this is because when the shell with Yb-Er is covered with an inert shell, the erbium ions become active and participate in the energy transfer. If there is no inert shell and the erbium shell is exposed, the erbium ions are quenched as a result of interaction with the environment and surface defects (confirmed by the absence of erbium luminescence peaks for β-NaGdF₄:Yb-Tm (30:0.5)@NaYF₄@NaGdF₄:Yb-Er (20:2)).
4. Conclusions
The tri-doped and core–shell single low-temperature hexagonal phase β-NaGdF4 NPs with rare earth ions Yb3+, Er3+, and Tm3+ were synthesized. According to TEM data, the samples consisted of particles with semi-spherical morphology. The size of core-only β-NaGdF4:Yb-Tm(30:0.5) NPs ranged from 15 to 40 nm. After shell synthesis, an increase in particle size was observed for both β-NaGdF4:Yb-Er(20:2) and NaYF4 shell, with a shell thickness of about 5 nm.
The lattice parameters of the samples are strongly influenced by the chemical composition of the outer shell. Samples with a NaGdF4-based outer shell have similar lattice parameters. Upon synthesis of a NaYF4 shell, a decrease in lattice parameters was observed, which can be attributed to the smaller size of yttrium cations compared to gadolinium cations.
The coherent scattering region increased in size after the synthesis of each subsequent shell and reached its maximum value for the β-NaGdF4:Yb-Tm(30:0.5)@ NaYF4@NaGdF4:Yb-Er(20:2)@NaYF4 sample. This suggests that particle growth occurred through a classical crystallization mechanism, where the initial core reacted with appropriate oleates, NaOH and NH4F from the liquid medium. Finally, defectiveness of the core surface decreases, the crystallinity of the nanoparticle improves, and the coherent scattering region increases.
The energy transfer processes in β-NaGdF4:Yb-Er-Tm NPs and core–shell structures were analyzed. No characteristic Tm3+ luminescence peaks were observed in the SWIR range for tri-doped NPs. However, the spectra in the VIS range exhibit bands characteristic of both Er3+ and Tm3+, which confirms the successful doping of the studied NPs with Tm3+. Since Yb3+-Er3+ energy transfer is resonant, while Yb3+-Tm3+ energy transfer is non-resonant, transfer to Er3+ is more likely.
For core–shell NPs, when Tm3+ and Er3+ are separated into adjacent layers, quenching cannot be avoided, which is probably due to the mutual diffusion of ions during shell synthesis. For β-NaGdF₄:Yb-Er(20:2)@NaGdF₄:Yb-Tm(30:0.5)@NaYF₄ and β-NaGdF₄:Yb-Tm(30:0.5)@NaGdF₄:Yb-Er(20:2)@NaYF₄, luminescence of thulium could not be registered in the SWIR region.
The use of an inert intermediate NaYF4 shell between the layers containing Tm3+ and Er3+ was necessary to clearly detect the Tm3+ SWIR luminescence in the β-NaGdF₄:Yb-Tm (30:0.5)@NaYF₄ sample and to partially prevent energy transfer. Without an inert shell for the β-NaGdF₄:Yb-Tm(30:0.5)@NaGdF₄:Yb-Er(20:2)@NaYF₄ sample, the thulium luminescence lifetime was estimated to be 0.953 ± 0.020 ms, while erbium luminescence lifetime was estimated to be 0.449 ± 0.003 ms and 0.835 ± 0.011 ms for the green and red ranges, respectively. When using an intermediate shell, the lifetime of thulium increased to 1.078 ± 0.036 ms, while the lifetime of erbium decreased to 0.324 ± 0.007 ms in the green region and to 0.498 ± 0.005 ms in the red region. However, the lifetime of thulium does not reach the 1.139 ± 0.047 ms registered for β-NaGdF₄:Yb-Tm(30:0.5)@NaYF₄@NaGdF₄:Yb-Er(20:2) composition. After coating the β-NaGdF₄:Yb-Tm(30:0.5)@NaYF₄ particles with an erbium-doped shell, the thulium luminescence in the SWIR range becomes indistinguishable.
Thus, the use of the core–shell structure does not prevent the energy transfer between ions in adjacent shells. The use of an inert intermediate shell prevents interaction, but only partially, which is probably due to the mutual diffusion of ions during shell synthesis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/photonics11010038/s1, Table S1: upconversion luminescence efficiency for Yb-Er-Tm tri-doped β-NaGdF4 nanoparticles, calculated for the visible range. Ref. [67] has been cited in Supplementary Materials.
Author Contributions
Conceptualization, D.P., S.K. and V.P.; methodology, A.R., I.R., D.P., V.P., N.T., S.K. and K.L.; synthesis, V.P.; validation, S.K., V.P., I.R., A.R. and D.P.; formal analysis, D.P., V.P., S.K., N.T. and I.R.; investigation, D.P., V.P. and I.R.; resources, S.K., N.T., D.P. and K.L.; software data processing, I.R., V.P. and K.L.; writing—original draft preparation, D.P.; writing—review and editing, A.R., I.R., S.K., V.P. and D.P.; project administration and funding acquisition, D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the President of the Russian Federation MK-3098.2022.1.2.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mandl, G.A.; Cooper, D.R.; Hirsch, T.; Seuntjens, J.; Capobianco, J.A. Perspective: Lanthanide-Doped Upconverting Nanoparticles. Methods Appl. Fluoresc. 2019, 7, 012004. [Google Scholar] [CrossRef]
- Chen, B.; Wang, F. Emerging Frontiers of Upconversion Nanoparticles. Trends Chem. 2020, 2, 427–439. [Google Scholar] [CrossRef]
- Liang, G.; Wang, H.; Shi, H.; Wang, H.; Zhu, M.; Jing, A.; Li, J.; Li, G. Recent Progress in the Development of Upconversion Nanomaterials in Bioimaging and Disease Treatment. J. Nanobiotechnol. 2020, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Mettenbrink, E.M.; Yang, W.; Wilhelm, S. Bioimaging with Upconversion Nanoparticles. Adv. Photonics Res. 2022, 3, 2200098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jin, D.; Schartner, E.P.; Lu, Y.; Liu, Y.; Zvyagin, A.V.; Zhang, L.; Dawes, J.M.; Xi, P.; Piper, J.A.; et al. Single-Nanocrystal Sensitivity Achieved by Enhanced Upconversion Luminescence. Nat. Nanotechnol. 2013, 8, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Sun, Y.; Gao, H.; Jin, S.; Zhang, Z.; Zhang, H.; Pan, G.; Kang, M.; Ma, X.; Mao, Y. High-Performance Perovskite Solar Cells Based on NaCsWO3@ NaYF 4@NaYF4:Yb,Er Upconversion Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Madirov, E.; Busko, D.; Hossain, I.M.; Konyushkin, V.A.; Nakladov, A.N.; Kuznetsov, S.V.; Farooq, A.; Gharibzadeh, S.; Paetzold, U.W.; et al. Harvesting Sub-Bandgap Photons via Upconversion for Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 54874–54883. [Google Scholar] [CrossRef] [PubMed]
- Del Barrio, M.; Cases, R.; Cebolla, V.; Hirsch, T.; De Marcos, S.; Wilhelm, S.; Galbán, J. A Reagentless Enzymatic Fluorescent Biosensor for Glucose Based on Upconverting Glasses, as Excitation Source, and Chemically Modified Glucose Oxidase. Talanta 2016, 160, 586–591. [Google Scholar] [CrossRef]
- Del Barrio, M.; De Marcos, S.; Cebolla, V.; Heiland, J.; Wilhelm, S.; Hirsch, T.; Galbán, J. Enzyme-Induced Modulation of the Emission of Upconverting Nanoparticles: Towards a New Sensing Scheme for Glucose. Biosens. Bioelectron. 2014, 59, 14–20. [Google Scholar] [CrossRef]
- Radunz, S.; Andresen, E.; Würth, C.; Koerdt, A.; Tschiche, H.R.; Resch-Genger, U. Simple Self-Referenced Luminescent pH Sensors Based on Upconversion Nanocrystals and pH-Sensitive Fluorescent BODIPY Dyes. Anal. Chem. 2019, 91, 7756–7764. [Google Scholar] [CrossRef]
- Wilhelm, S.; Del Barrio, M.; Heiland, J.; Himmelstoß, S.F.; Galbán, J.; Wolfbeis, O.S.; Hirsch, T. Spectrally Matched Upconverting Luminescent Nanoparticles for Monitoring Enzymatic Reactions. ACS Appl. Mater. Interfaces 2014, 6, 15427–15433. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zou, X.; Su, Q.; Yuan, W.; Cao, C.; Wang, Q.; Zhu, X.; Feng, W.; Li, F. Ratiometric Nanothermometer In Vivo Based on Triplet Sensitized Upconversion. Nat. Commun. 2018, 9, 2698. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Vetrone, F. Luminescence Nanothermometry. Nanoscale 2012, 4, 4301. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Tu, L.; Li, Q.; Feng, Y.; Que, I.; Zhang, Y.; Liu, X.; Xue, B.; Cruz, L.J.; Chang, Y.; et al. Near Infrared Light Sensitive Ultraviolet–Blue Nanophotoswitch for Imaging-Guided “Off–On” Therapy. ACS Nano 2018, 12, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, G. Upconversion Nanoparticles for Cancer Therapy. Adv. NanoBiomed Res. 2022, 2, 2200092. [Google Scholar] [CrossRef]
- Suo, H.; Zhu, Q.; Zhang, X.; Chen, B.; Chen, J.; Wang, F. High-Security Anti-Counterfeiting through Upconversion Luminescence. Mater. Today Phys. 2021, 21, 100520. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Liu, F.; Liu, J. Engineered Lanthanide-Doped Upconversion Nanoparticles for Biosensing and Bioimaging Application. Microchim. Acta 2022, 189, 109. [Google Scholar] [CrossRef]
- Auzel, F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 2004, 104, 139–174. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Yang, T.; Feng, W.; Li, C.; Li, F. Sub-10 Nm Hexagonal Lanthanide-Doped NaLuF4 Upconversion Nanocrystals for Sensitive Bioimaging In Vivo. J. Am. Chem. Soc. 2011, 133, 17122–17125. [Google Scholar] [CrossRef]
- Chen, G.; Shen, J.; Ohulchanskyy, T.Y.; Patel, N.J.; Kutikov, A.; Li, Z.; Song, J.; Pandey, R.K.; Ågren, H.; Prasad, P.N.; et al. (α-NaYbF4:Tm3+)/CaF2 Core/Shell Nanoparticles with Efficient Near-Infrared to Near-Infrared Upconversion for High-Contrast Deep Tissue Bioimaging. ACS Nano 2012, 6, 8280–8287. [Google Scholar] [CrossRef]
- Tessitore, G.; Mandl, G.A.; Brik, M.G.; Park, W.; Capobianco, J.A. Recent Insights into Upconverting Nanoparticles: Spectroscopy, Modeling, and Routes to Improved Luminescence. Nanoscale 2019, 11, 12015–12029. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In Vivo Cancer Targeting and Imaging with Semiconductor Quantum Dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.T.; Kim, S.; Nakayama, A.; Stott, N.E.; Bawendi, M.G.; Frangioni, J.V. Selection of Quantum Dot Wavelengths for Biomedical Assays and Imaging. Mol. Imaging 2003, 2, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, E.; Venkatachalam, N.; Hyodo, H.; Hattori, A.; Ebina, Y.; Kishimoto, H.; Soga, K. Upconverting and NIR Emitting Rare Earth Based Nanostructures for NIR-Bioimaging. Nanoscale 2013, 5, 11339. [Google Scholar] [CrossRef] [PubMed]
- Skripka, A.; Benayas, A.; Marin, R.; Canton, P.; Hemmer, E.; Vetrone, F. Double Rare-Earth Nanothermometer in Aqueous Media: Opening the Third Optical Transparency Window to Temperature Sensing. Nanoscale 2017, 9, 3079–3085. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, E.; Benayas, A.; Légaré, F.; Vetrone, F. Exploiting the Biological Windows: Current Perspectives on Fluorescent Bioprobes Emitting above 1000 Nm. Nanoscale Horiz. 2016, 1, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Mancini, M.C.; Nie, S. Second Window for In Vivo Imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef]
- Ma, D.; Xu, X.; Hu, M.; Wang, J.; Zhang, Z.; Yang, J.; Meng, L. Rare-Earth-Based Nanoparticles with Simultaneously Enhanced Near-Infrared (NIR)-Visible (Vis) and NIR-NIR Dual-Conversion Luminescence for Multimodal Imaging. Chem. Asian J. 2016, 11, 1050–1058. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, H.; Xie, X.; Wan, Y.; Li, Q.; Wu, F.; Yang, R.; Wang, W.; Kong, X. Bright Tm3+-Based Downshifting Luminescence Nanoprobe Operating around 1800 Nm for NIR-IIb and c Bioimaging. Nat. Commun. 2023, 14, 1079. [Google Scholar] [CrossRef]
- Wang, L.; Yan, R.; Huo, Z.; Wang, L.; Zeng, J.; Bao, J.; Wang, X.; Peng, Q.; Li, Y. Fluorescence Resonant Energy Transfer Biosensor Based on Upconversion-Luminescent Nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 6054–6057. [Google Scholar] [CrossRef]
- Kamimura, M.; Matsumoto, T.; Suyari, S.; Umezawa, M.; Soga, K. Ratiometric Near-Infrared Fluorescence Nanothermometry in the OTN-NIR (NIR II/III) Biological Window Based on Rare-Earth Doped β-NaYF4 Nanoparticles. J. Mater. Chem. B 2017, 5, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Naczynski, D.J.; Tan, M.C.; Zevon, M.; Wall, B.; Kohl, J.; Kulesa, A.; Chen, S.; Roth, C.M.; Riman, R.E.; Moghe, P.V. Rare-Earth-Doped Biological Composites as In Vivo Shortwave Infrared Reporters. Nat. Commun. 2013, 4, 2199. [Google Scholar] [CrossRef] [PubMed]
- Sekiyama, S.; Umezawa, M.; Kuraoka, S.; Ube, T.; Kamimura, M.; Soga, K. Temperature Sensing of Deep Abdominal Region in Mice by Using over-1000 Nm Near-Infrared Luminescence of Rare-Earth-Doped NaYF4 Nanothermometer. Sci. Rep. 2018, 8, 16979. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, L.; Suyari, S.; Ube, T.; Kamimura, M.; Soga, K. Tuning the Thermal Sensitivity of β-NaYF4: Yb3+, Ho3+, Er3+ Nanothermometers for Optimal Temperature Sensing in OTN-NIR (NIR II/III) Biological Window. J. Lumin. 2018, 198, 236–242. [Google Scholar] [CrossRef]
- Ximendes, E.C.; Rocha, U.; Sales, T.O.; Fernández, N.; Sanz-Rodríguez, F.; Martín, I.R.; Jacinto, C.; Jaque, D. In Vivo Subcutaneous Thermal Video Recording by Supersensitive Infrared Nanothermometers. Adv. Funct. Mater. 2017, 27, 1702249. [Google Scholar] [CrossRef]
- Cortelletti, P.; Skripka, A.; Facciotti, C.; Pedroni, M.; Caputo, G.; Pinna, N.; Quintanilla, M.; Benayas, A.; Vetrone, F.; Speghini, A. Tuning the Sensitivity of Lanthanide-Activated NIR Nanothermometers in the Biological Windows. Nanoscale 2018, 10, 2568–2576. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Lei, L.; Xia, J.; Hua, Y.; Deng, D.; Xu, S. Yb/Er/Tm Tri-Doped Na3ZrF7 Upconversion Nanocrystals for High Performance Temperature Sensing. J. Lumin. 2019, 209, 8–13. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhang, X.; Li, L.; Hao, H. Up-Converting Luminescence and Temperature Sensing of Er3+/Tm3+/Yb3+ Co-Doped NaYF4 Phosphors Operating in Visible and the First Biological Window Range. Nanomaterials 2021, 11, 2660. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, C.; Peng, C.; Li, C.; Wang, L.; Chai, R.; Lin, J. Controllable Red, Green, Blue (RGB) and Bright White Upconversion Luminescence of Lu2 O3:Yb3+/Er3+/Tm3+ Nanocrystals through Single Laser Excitation at 980 Nm. Chem. Eur. J. 2009, 15, 4649–4655. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, C.; Yang, Q. White Upconversion of Rare-Earth Doped ZnO Nanocrystals and Its Dependence on Size of Crystal Particles and Content of Yb3+ and Tm3+. J. Appl. Phys. 2009, 105, 084701. [Google Scholar] [CrossRef]
- Ray, S.K.; Joshi, B.; Hur, J. White-Light Emission in Yb3+/Er3+/Tm3+- and Yb3+/Er3+/Tm3+ /Ho3+-Doped α-NiMoO4 Nanoparticles. Nanotechnology 2022, 33, 395705. [Google Scholar] [CrossRef] [PubMed]
- Pominova, D.; Proydakova, V.; Romanishkin, I.; Ryabova, A.; Kuznetsov, S.; Uvarov, O.; Fedorov, P.; Loschenov, V. Temperature Sensing in the Short-Wave Infrared Spectral Region Using Core-Shell NaGdF4:Yb3+, Ho3+, Er3+@NaYF4 Nanothermometers. Nanomaterials 2020, 10, 1992. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion Luminescent Materials: Advances and Applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ågren, H.; Ohulchanskyy, T.Y.; Prasad, P.N. Light Upconverting Core–Shell Nanostructures: Nanophotonic Control for Emerging Applications. Chem. Soc. Rev. 2015, 44, 1680–1713. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Deng, R.; Xie, X.; Liu, X. Enhancing Luminescence in Lanthanide-Doped Upconversion Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 11702–11715. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Zhao, B.; Lin, C.; Berry, M.; May, P.S.; Smith, S. Spectroscopic Imaging and Power Dependence of Near-Infrared to Visible Upconversion Luminescence from NaYF4:Yb3+, Er3+ Nanoparticles on Nanocavity Arrays. J. Phys. Chem. C 2015, 119, 24976–24982. [Google Scholar] [CrossRef]
- Noculak, A.; Podhorodecki, A. Size and Shape Effects in β-NaGdF4: Yb3+, Er3+ Nanocrystals. Nanotechnology 2017, 28, 175706. [Google Scholar] [CrossRef]
- Krämer, K.W.; Biner, D.; Frei, G.; Güdel, H.U.; Hehlen, M.P.; Lüthi, S.R. Hexagonal Sodium Yttrium Fluoride Based Green and Blue Emitting Upconversion Phosphors. Chem. Mater. 2004, 16, 1244–1251. [Google Scholar] [CrossRef]
- Suyver, J.F.; Aebischer, A.; Biner, D.; Gerner, P.; Grimm, J.; Heer, S.; Krämer, K.W.; Reinhard, C.; Güdel, H.U. Novel Materials Doped with Trivalent Lanthanides and Transition Metal Ions Showing Near-Infrared to Visible Photon Upconversion. Opt. Mater. 2005, 27, 1111–1130. [Google Scholar] [CrossRef]
- Niu, N.; He, F.; Gai, S.; Li, C.; Zhang, X.; Huang, S.; Yang, P. Rapid Microwave Reflux Process for the Synthesis of Pure Hexagonal NaYF4:Yb3+,Ln3+,Bi3+ (Ln3+ = Er3+, Tm3+, Ho3+) and Its Enhanced UC Luminescence. J. Mater. Chem. 2012, 22, 21613. [Google Scholar] [CrossRef]
- Kumar, V.; Som, S.; Dutta, S.; Das, S.; Swart, H.C. Influence of Ho3+ Doping on the Temperature Sensing Behavior of Er3+-Yb3+ Doped La2CaZnO5 Phosphor. RSC Adv. 2016, 6, 84914–84925. [Google Scholar] [CrossRef]
- Banski, M.; Podhorodecki, A.; Misiewicz, J.; Afzaal, M.; Abdelhady, A.L.; O’Brien, P. Selective Excitation of Eu3+ in the Core of Small β-NaGdF 4 Nanocrystals. J. Mater. Chem. C 2013, 1, 801–807. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.-D.; Yan, C.-H. Energy Transfer in Lanthanide Upconversion Studies for Extended Optical Applications. Chem. Soc. Rev. 2015, 44, 1608–1634. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.M.; Levy, E.S.; Cohen, B.E. Rationally Designed Energy Transfer in Upconverting Nanoparticles. Adv. Mater. 2015, 27, 5753–5761. [Google Scholar] [CrossRef]
- Huang, L.; Wang, L.; Xue, X.; Zhao, D.; Qin, G.; Qin, W. Enhanced Red Upconversion Luminescence in Er–Tm Codoped NaYF4 Phosphor. J. Nanosci. Nanotechnol. 2011, 11, 9498–9501. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.M.; Han, G.; Goldberg, J.D.; Gargas, D.J.; Ostrowski, A.D.; Schuck, P.J.; Cohen, B.E.; Milliron, D.J. Combinatorial Discovery of Lanthanide-Doped Nanocrystals with Spectrally Pure Upconverted Emission. Nano Lett. 2012, 12, 3839–3845. [Google Scholar] [CrossRef]
- Chan, E.M.; Gargas, D.J.; Schuck, P.J.; Milliron, D.J. Concentrating and Recycling Energy in Lanthanide Codopants for Efficient and Spectrally Pure Emission: The Case of NaYF4:Er 3+ /Tm 3+ Upconverting Nanocrystals. J. Phys. Chem. B 2012, 116, 10561–10570. [Google Scholar] [CrossRef]
- Prorok, K.; Pawlyta, M.; Stręk, W.; Bednarkiewicz, A. Energy Migration Up-Conversion of Tb3+ in Yb3+ and Nd3+ Codoped Active-Core/Active-Shell Colloidal Nanoparticles. Chem. Mater. 2016, 28, 2295–2300. [Google Scholar] [CrossRef]
- Chen, B.; Wang, F. Combating Concentration Quenching in Upconversion Nanoparticles. Acc. Chem. Res. 2020, 53, 358–367. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, R.; Hu, J.; Guan, D.; Qiu, X.; Zhang, Y.; Kohane, D.S.; Liu, Q. Enhancement of Single Upconversion Nanoparticle Imaging by Topologically Segregated Core-Shell Structure with Inward Energy Migration. Nat. Commun. 2022, 13, 5927. [Google Scholar] [CrossRef]
- Shen, B.; Cheng, S.; Gu, Y.; Ni, D.; Gao, Y.; Su, Q.; Feng, W.; Li, F. Revisiting the Optimized Doping Ratio in Core/Shell Nanostructured Upconversion Particles. Nanoscale 2017, 9, 1964–1971. [Google Scholar] [CrossRef]
- Zhou, B.; Tang, B.; Zhang, C.; Qin, C.; Gu, Z.; Ma, Y.; Zhai, T.; Yao, J. Enhancing Multiphoton Upconversion through Interfacial Energy Transfer in Multilayered Nanoparticles. Nat. Commun. 2020, 11, 1174. [Google Scholar] [CrossRef]
- Pominova, D.V.; Romanishkin, I.D.; Proydakova, V.Y.; Grachev, P.V.; Moskalev, A.S.; Ryabova, A.V.; Makarov, V.I.; Linkov, K.G.; Kuznetsov, S.V.; Voronov, V.V.; et al. Optimization of Upconversion Luminescence Excitation Mode for Deeper In Vivo Bioimaging without Contrast Loss or Overheating. Methods Appl. Fluoresc. 2020, 8, 025006. [Google Scholar] [CrossRef]
- Pominova, D.; Romanishkin, I.; Proydakova, V.; Kuznetsov, S.; Grachev, P.; Ryabova, A.; Tabachkova, N.; Fedorov, P.; Loschenov, V. Study of Synthesis Temperature Effect on β-NaGdF4: Yb3+, Er3+ Upconversion Luminescence Efficiency and Decay Time Using Maximum Entropy Method. Methods Appl. Fluoresc. 2022, 10, 024005. [Google Scholar] [CrossRef]
- Pominova, D.V.; Proydakova, V.Y.; Romanishkin, I.D.; Ryabova, A.V.; Grachev, P.V.; Makarov, V.I.; Kuznetsov, S.V.; Uvarov, O.V.; Voronov, V.V.; Yapryntsev, A.D.; et al. Achieving High NIR-to-NIR Conversion Efficiency by Optimization of Tm3+ Content in Na(Gd,Yb)F4: Tm Upconversion Luminophores. Laser Phys. Lett. 2020, 17, 125701. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Ryabova, A.V.; Pominova, D.V.; Krutko, V.A.; Komova, M.G.; Loschenov, V.B. Spectroscopic research of upconversion nanomaterials based on complex oxide compounds doped with rare-earth ion pairs: Benefit for cancer diagnostics by upconversion fluorescence and radio sensitive methods. Photon. Lasers Med. 2013, 2, 117–128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).