Bypassing the Heat Risk and Efficacy Limitations of Pulsed 630 nm LED Photobiomodulation Therapy for Anti-Primary Dysmenorrhea: A Prospective Randomized Cross-Over Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participant Selection
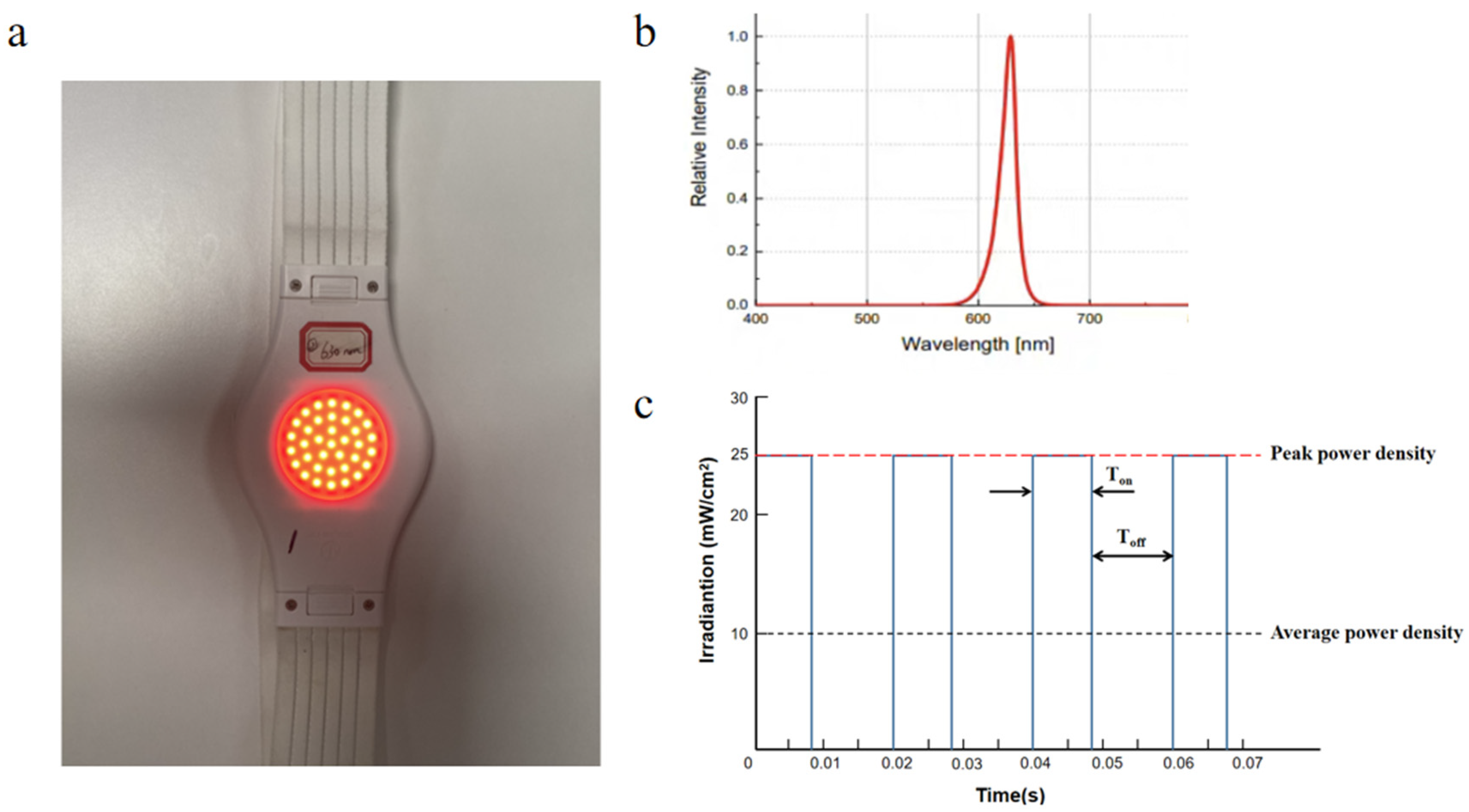
2.3. Study Intervention
2.4. Randomization Procedures
2.5. Pain Assessment
2.5.1. VAS for Pain
2.5.2. Quality of Life
2.5.3. Global Evaluation Assessment
2.6. Evaluation of Safety
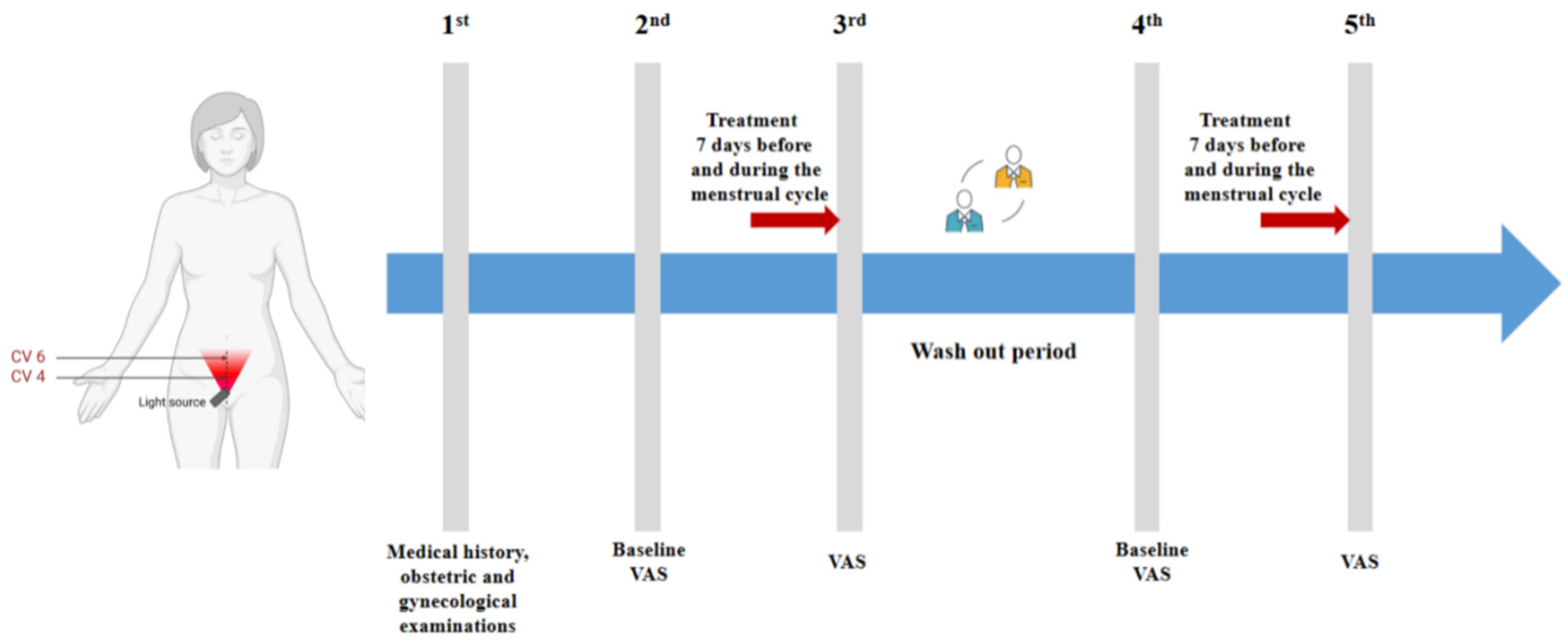
2.7. Study Procedure
2.7.1. Screening Phase
2.7.2. Baseline VAS Scores
2.7.3. Treatment Phase 1
2.7.4. Baseline VAS Scores after a Wash-Out Period
2.7.5. Treatment Phase 2
2.8. Statistical Analysis
3. Results
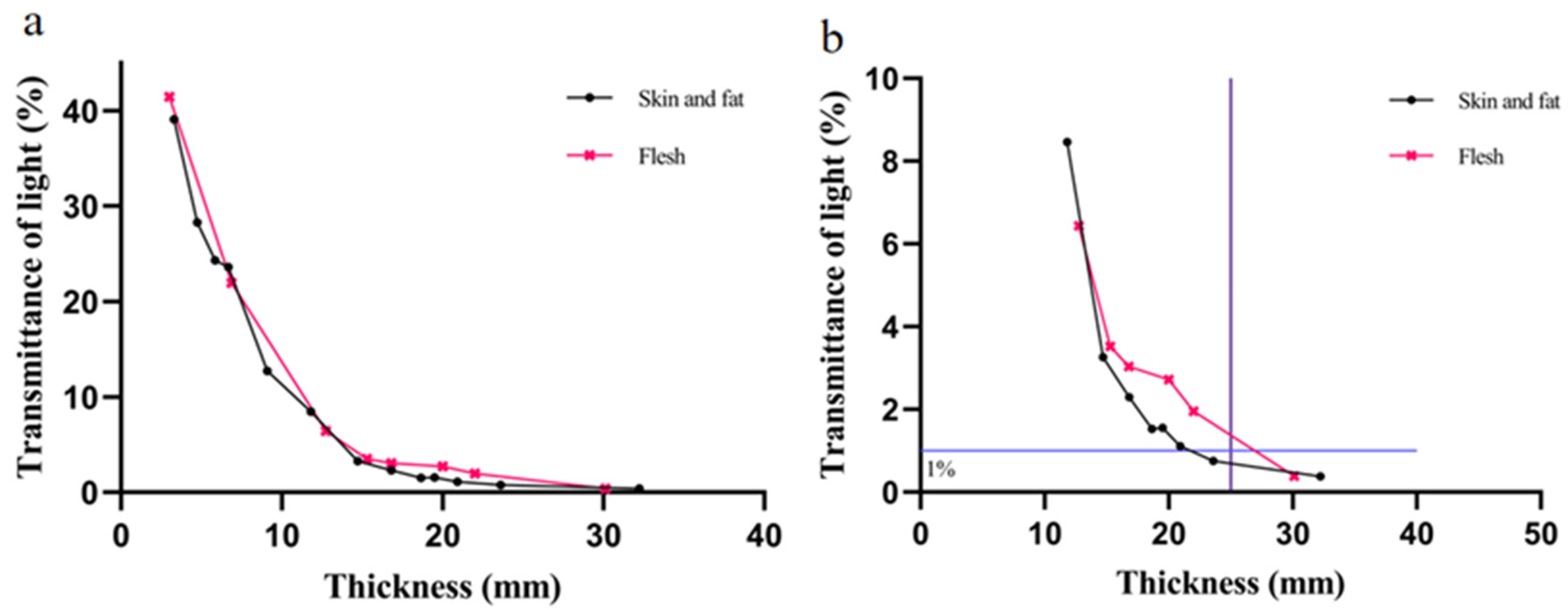
3.1. Light Transmittance Assay and PBM Intervention Parameters
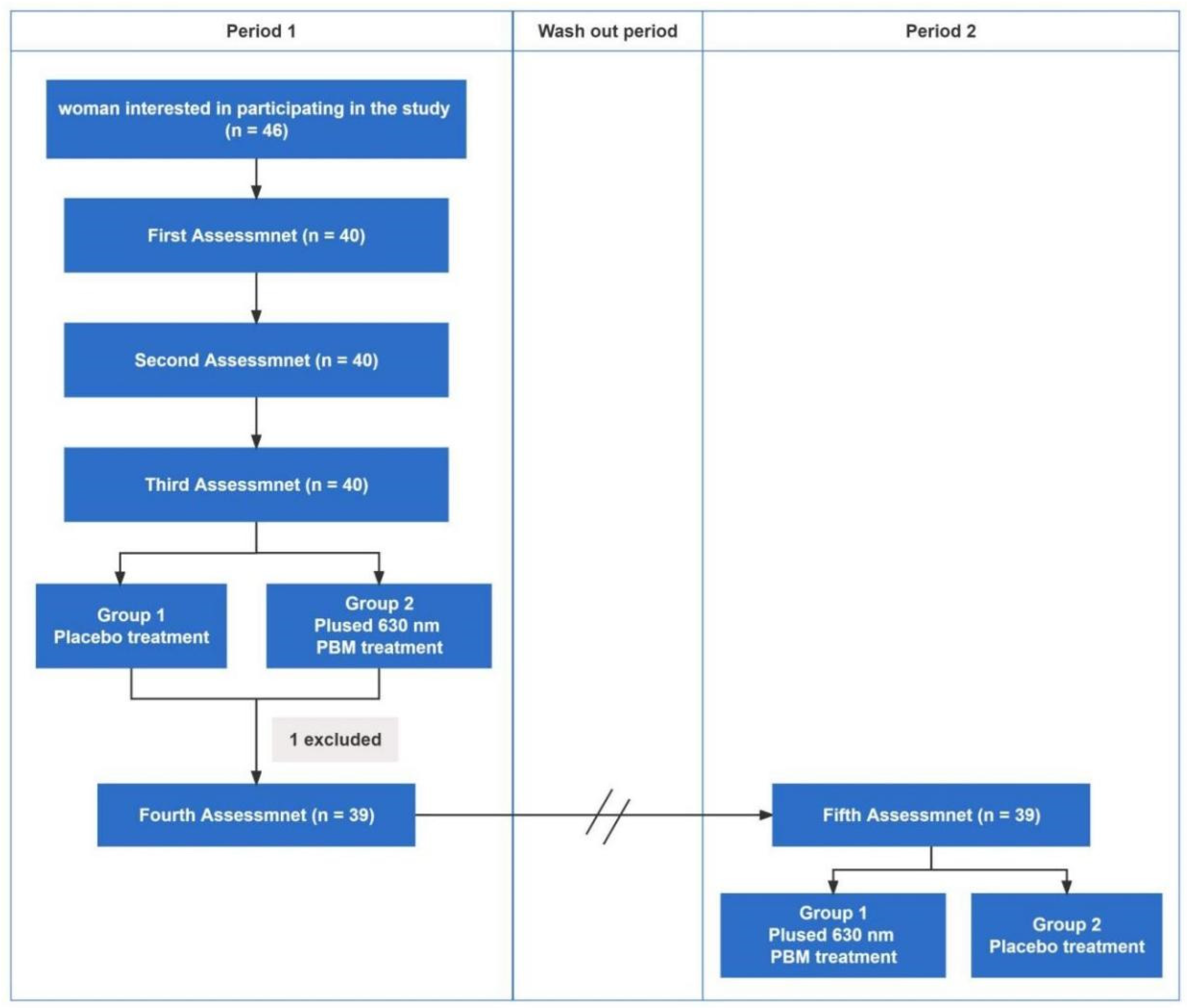
3.2. Screening of Samples
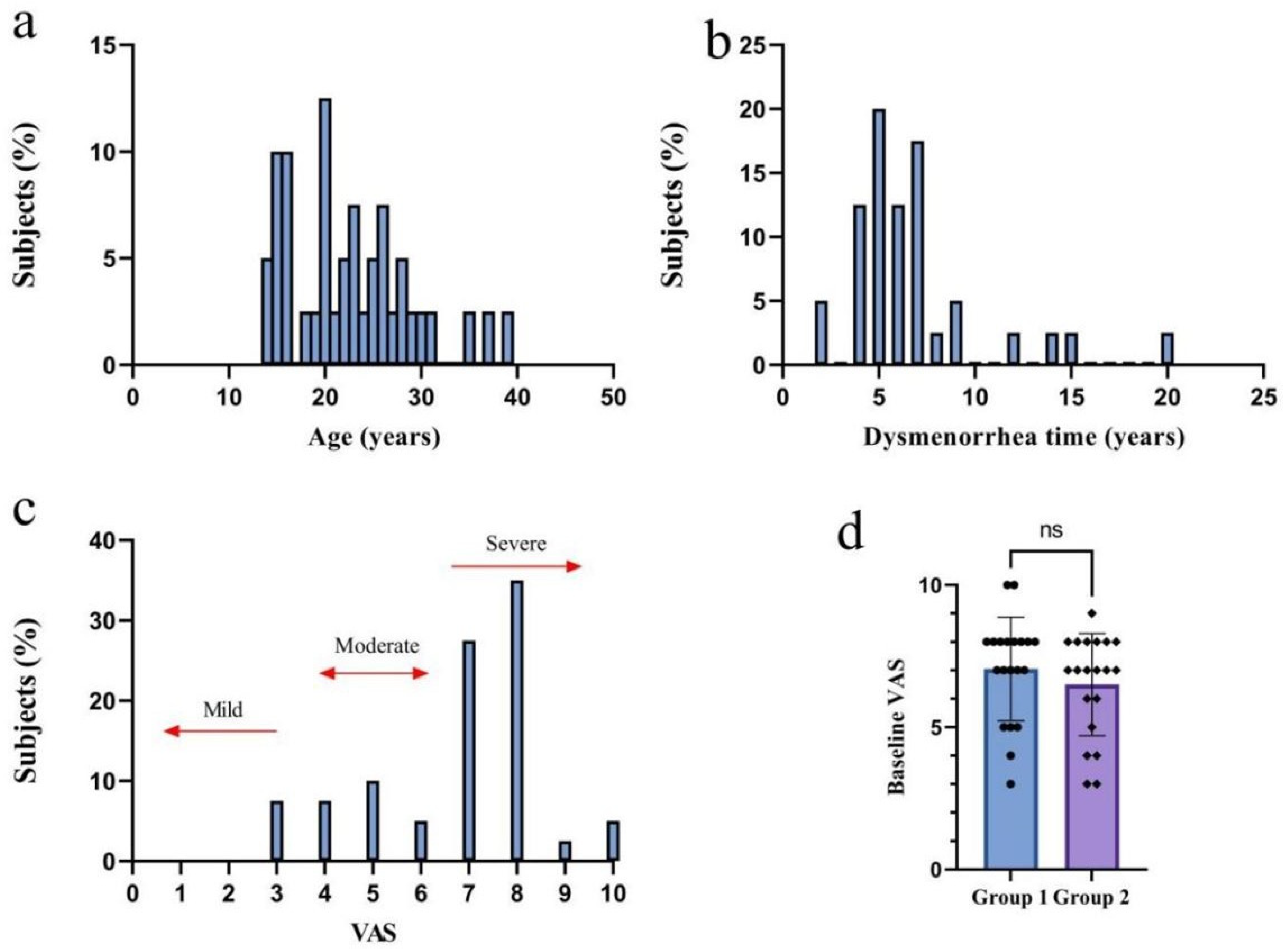
3.3. Baseline Characteristics
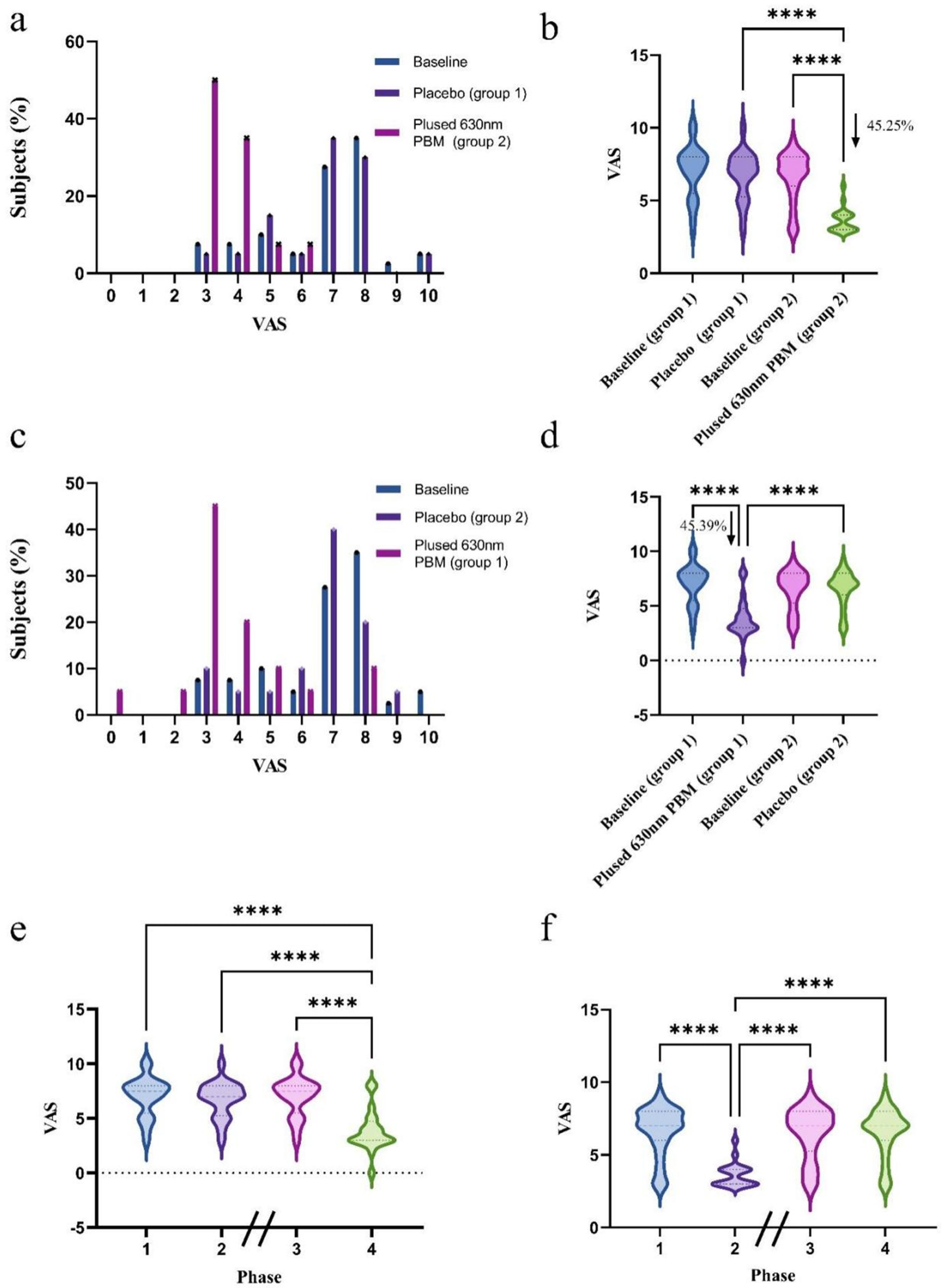
3.4. Pain Severity
3.5. Quality of Life
3.6. Global Evaluation Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elizabeth, F.; Elizabeth, C.; Johanna, S. Primary Dysmenorrhea: Diagnosis and Therapy. Obstet. Gynecol. 2020, 136, 1047–1058. [Google Scholar] [CrossRef]
- Dawood, M. Primary dysmenorrhea: Advances in pathogenesis and management. Obstet. Gynecol. 2006, 108, 428–441. [Google Scholar] [CrossRef]
- Ma, B.; Yang, S.; Tan, T.; Li, J.; Zhang, X.; Ouyang, H.; He, M.; Feng, Y. An integrated study of metabolomics and transcriptomics to reveal the anti-primary dysmenorrhea mechanism of Akebiae Fructus. J. Ethnopharmacol. 2021, 270, 113763. [Google Scholar] [CrossRef] [PubMed]
- Iacovides, S.; Avidon, I.; Baker, F.C. What we know about primary dysmenorrhea today: A critical review. Hum. Reprod. Update 2015, 21, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Zhou, J.; Zhao, L.; Wang, C.; Wu, W.; Zhang, L.; Ji, B.; Zhang, N.; Zhou, F. Preventive effect of different citrus essential oils on primary dysmenorrhea: In vivo and in vitro study. Food Biosci. 2021, 42, 101135. [Google Scholar] [CrossRef]
- Hong, G.; Shin, B.; Park, S.; Gu, Y.; Kim, N.; Park, K.; Kim, S.; Shin, Y. Randomized controlled trial of the efficacy and safety of self-adhesive low-level light therapy in women with primary dysmenorrhea. Int. J. Gynaecol. Obstet. 2016, 133, 37–42. [Google Scholar] [CrossRef]
- Chiang, Y.-F.; Hung, H.-C.; Chen, H.-Y.; Huang, K.-C.; Lin, P.-H.; Chang, J.-Y.; Huang, T.-C.; Hsia, S.-M. The Inhibitory Effect of Extra Virgin Olive Oil and Its Active Compound Oleocanthal on Prostaglandin-Induced Uterine Hypercontraction and Pain-Ex Vivo and In Vivo Study. Nutrients 2020, 12, 3012. [Google Scholar] [CrossRef]
- Jo, J.; Lee, S. Heat therapy for primary dysmenorrhea: A systematic review and meta-analysis of its effects on pain relief and quality of life. Sci. Rep. 2018, 8, 16252. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fu, Q.; Jiang, H.; Li, Y.; Liu, M. Progress of phototherapy for osteosarcoma and application prospect of blue light photobiomodulation therapy. Front. Oncol. 2022, 12, 1022973. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Martin, L.F.; Slepian, M.J.; Patwardhan, A.M.; Ibrahim, M.M. Mechanisms and Pathways of Pain Photobiomodulation: A Narrative Review. J. Pain 2021, 22, 763–777. [Google Scholar] [CrossRef]
- Hu, D.; Zhu, S.; Potas, J.R. Red LED photobiomodulation reduces pain hypersensitivity and improves sensorimotor function following mild T10 hemicontusion spinal cord injury. J. Neuroinflamm. 2016, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Patwardhan, A.; Yang, X.; Li, W.; Cai, S.; Ji, Y.; Chew, L.A.; Dorame, A.; Bellampalli, S.S.; Schmoll, R.W.; et al. Development and Characterization of An Injury-free Model of Functional Pain in Rats by Exposure to Red Light. J. Pain 2019, 20, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, K.; Fekrazad, R.; Raoufi, Z. The Effects of Photobiomodulation Therapy on Post-Surgical Pain. J. Lasers Med. Sci. 2019, 10, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.-Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Ma, X.; Ding, X.; Gan, X.; Deng, Y.; Wang, Y.; Sun, A. Comparative evaluation of low-level light therapy and ethinyl estradiol and desogestrel combined oral contraceptive for clinical efficacy and regulation of serum biochemical parameters in primary dysmenorrhoea: A prospective randomised multicentre trial. Lasers Med. Sci. 2022, 37, 2239–2248. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Kim, N.; Park, K.; Kim, D.; Hong, G.; Shin, B. Skin adhesive low-level light therapy for dysmenorrhoea: A randomized, double-blind, placebo-controlled, pilot trial. Arch. Gynecol. Obstet. 2012, 286, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.; Daniel, G.; Trelles, M. Optimising the design of a broad-band light source for the treatment of skin. J. Cosmet. Laser Ther. 2005, 7, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, W.; Hu, X.; Liu, M. Irradiance plays a significant role in photobiomodulation of B16F10 melanoma cells by increasing reactive oxygen species and inhibiting mitochondrial function. Biomed. Opt. Express 2020, 11, 27–39. [Google Scholar] [CrossRef]
- Agarwal, D.; Chaudhary, P. Effect of Turmeric-Boswellia-Sesame Formulation in Menstrual Cramp Pain Associated with Primary Dysmenorrhea-A Double-Blind, Randomized, Placebo-Controlled Study. J. Clin. Med. 2023, 12, 3968. [Google Scholar] [CrossRef]
- Shaw, C.J.; Civale, J.; Botting, K.J.; Niu, Y.; Ter Haar, G.; Rivens, I.; Giussani, D.A.; Lees, C.C. Noninvasive high-intensity focused ultrasound treatment of twin-twin transfusion syndrome: A preliminary in vivo study. Sci. Transl. Med. 2016, 8, 347ra95. [Google Scholar] [CrossRef]
- Aksoy, A.N.; Laloglu, E.; Ozkaya, A.L.; Yilmaz, E.P.T. Serum heme oxygenase-1 levels in patients with primary dysmenorrhea. Arch. Gynecol. Obstet. 2017, 295, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Chiang, Y.-F.; Shih, Y.-H.; Chiu, C.-H.; Chen, H.-Y.; Shieh, T.-M.; Wang, K.-L.; Huang, T.-C.; Hong, Y.-H.; Hsia, S.-M. Salvia sclarea L. Essential Oil Extract and Its Antioxidative Phytochemical Sclareol Inhibit Oxytocin-Induced Uterine Hypercontraction Dysmenorrhea Model by Inhibiting the Ca2+-MLCK-MLC20 Signaling Cascade: An Ex Vivo and In Vivo Study. Antioxidants 2020, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Ou, M.; Ho, C.; Lin, Y.; Liu, H.; Chang, W. Effects of somatothermal far-infrared ray on primary dysmenorrhea: A pilot study. Evid.-Based Complement. Altern. Med. 2012, 2012, 240314. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bi, J.; Lv, B.; Zheng, W.; Wang, Z.; Xiao, W.; Sun, Y.; Li, E. An experimental study of the anti-dysmenorrhea effect of Chinese herbal medicines used in Jin Gui Yao Lue. J. Ethnopharmacol. 2019, 245, 112181. [Google Scholar] [CrossRef] [PubMed]
- Oladosu, F.A.; Tu, F.F.; Hellman, K.M. Nonsteroidal antiinflammatory drug resistance in dysmenorrhea: Epidemiology, causes, and treatment. Am. J. Obstet. Gynecol. 2018, 218, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Correyero-León, M.; Llamas-Ramos, R.; Calvo-Rodrigo, J.; Alvarado-Omenat, J.J.; Llamas-Ramos, I. Transcutaneous Tibial Nerve Stimulation for Primary Dysmenorrhea: A Protocol for a Randomized Controlled Trial. Healthcare 2023, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Gøtzsche, P.C. Niels Finsen’s treatment for lupus vulgaris. J. R. Soc. Med. 2011, 104, 41–42. [Google Scholar] [CrossRef]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar]
- Busanello-Costa, M.; Renno, A.C.M.; de Goes Santos, C.P.; Quintana, H.T.; Martignago, C.C.S.; Tim, C.R.; Assis, L. Red LED light therapy associated with epidermal growth factor on wound repair process in rats. Lasers Med. Sci. 2023, 38, 36. [Google Scholar] [CrossRef]
- Barolet, A.C.; Litvinov, I.V.; Barolet, D. Light-induced nitric oxide release in the skin beyond UVA and blue light: Red & near-infrared wavelengths. Nitric Oxide 2021, 117, 16–25. [Google Scholar] [CrossRef]
- Chen, A.C.-H.; Huang, Y.-Y.; Sharma, S.K.; Hamblin, M.R. Effects of 810-nm laser on murine bone-marrow-derived dendritic cells. Photomed. Laser Surg. 2011, 29, 383–389. [Google Scholar] [CrossRef]
- Zdagkas, A.; McDonnell, C.; Deng, J.; Shen, Y.; Li, G.; Ellenbogen, T.; Papasimakis, N.; Zheludev, N.I. Observation of toroidal pulses of light. Nat. Photon. 2022, 16, 523–528. [Google Scholar] [CrossRef]
- Piccolo, D.; Crisman, G.; Kostaki, D.; Cannarozzo, G.; Sannino, M.; Chimenti, S. Rhodamine intense pulsed light versus conventional intense pulsed light for facial telangiectasias. J. Cosmet. Laser Ther. 2016, 18, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Jiang, H.; Sun, M.; Liu, M. Pulsed transcranial photobiomodulation generates distinct beneficial neurocognitive effects compared with continuous wave transcranial light. Lasers Med. Sci. 2023, 38, 203. [Google Scholar] [CrossRef] [PubMed]
- Poem, E.; Hiemstra, T.; Eckstein, A.; Jin, X.-M.; Walmsley, I.A. Free-space spectro-temporal and spatio-temporal conversion for pulsed light. Opt. Lett. 2016, 41, 4328–4331. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-R.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B 2017, 170, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.; Gendelman, R.; Noofi, N. Photobiomodulation of human osteoblast-like cells in vitro by low-intensity-pulsed LED light. FEBS Open Bio 2020, 10, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Chaudary, S.; Karner, L.; Weidinger, A.; Meixner, B.; Rieger, S.; Metzger, M.; Zipperle, J.; Dungel, P. In vitro effects of 635 nm photobiomodulation under hypoxia/reoxygenation culture conditions. J. Photochem. Photobiol. B 2020, 209, 111935. [Google Scholar] [CrossRef]
- Yu, S.; Xu, J.; Shen, Z.; Wang, Y.; Wei, W.; Guo, X.; Tian, J.; Liu, L.; Yang, Y.; Zeng, F.; et al. Frequency-Specific Alterations in Brain Function in Patients with Primary Dysmenorrhea. Pain Med. 2022, 23, 902–911. [Google Scholar] [CrossRef]
- Assaf, L.; Eid, A.A.; Nassif, J. Role of AMPK/mTOR, mitochondria, and ROS in the pathogenesis of endometriosis. Life Sci. 2022, 306, 120805. [Google Scholar] [CrossRef]
- Li, J.-M.; Liao, C.-C.; Huang, H.-C.; Lin, C.-L.; Lo, H.-Y.; Hsiang, C.-Y.; Ho, T.-Y. Regulation effect and mechanism of Sheng-Hua-Tang on female reproductive system: From experimental transcriptomic analysis to clinical applications. J. Ethnopharmacol. 2020, 249, 112431. [Google Scholar] [CrossRef]
- Li, N.; Cui, X.; Ma, C.; Yu, Y.; Li, Z.; Zhao, L.; Xiong, H. Uncovering the effects and mechanism of Danggui Shaoyao San intervention on primary dysmenorrhea by serum metabolomics approach. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2022, 1209, 123434. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Jiang, M.; Xu, Y.; Wang, C.; Hu, Y.; Yang, S.; Gao, J.; Wang, W.; Wang, T. Wenjing Zhitong recipe exhibits potential anti-primary dysmenorrhea properties by inhibiting COX2 and PKC signaling pathway in rats induced by estradiol benzoate and oxytocin. J. Tradit. Chin. Med. Sci. 2023, 10, 296–309. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Chen, Y.-C.; Chen, H.-Y.; Chiang, Y.-F.; Ali, M.; Chiang, W.; Chung, C.-P.; Hsia, S.-M. Ethanolic Extracts of Adlay Testa and Hull and Their Active Biomolecules Exert Relaxing Effect on Uterine Muscle Contraction through Blocking Extracellular Calcium Influx in Ex Vivo and In Vivo Studies. Biomolecules 2021, 11, 887. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, S.; Ding, X.; Deng, Y.; Ma, X.; Gan, J.; Wang, Y.; Sun, A. Metabolomic study combined with the low-level light therapy of Chinese acupuncture points and combined oral contraceptives in treatment of primary dysmenorrhea: A prospective, multicenter, randomized controlled study. Heliyon 2023, 9, e13821. [Google Scholar] [CrossRef]
- Kho, K.A.; Shields, J.K. Diagnosis and Management of Primary Dysmenorrhea. JAMA 2020, 323, 268–269. [Google Scholar] [CrossRef]
- Kaplan, Ö.; Nazıroğlu, M.; Güney, M.; Aykur, M. Non-steroidal anti-inflammatory drug modulates oxidative stress and calcium ion levels in the neutrophils of patients with primary dysmenorrhea. J. Reprod. Immunol. 2013, 100, 87–92. [Google Scholar] [CrossRef]
- de Marchi, T.; Schmitt, V.M.; Da Danúbia Silva Fabro, C.; Da Silva, L.L.; Sene, J.; Tairova, O.; Salvador, M. Phototherapy for Improvement of Performance and Exercise Recovery: Comparison of 3 Commercially Available Devices. J. Athl. Train. 2017, 52, 429–438. [Google Scholar] [CrossRef]
| Mode | Pulsed |
|---|---|
| Wavelength (nm) | 630 |
| Duration (s) | 1200 |
| Average irradiance (mW/cm2) | 10 |
| Dose (J/cm2) | 12 |
| Duty cycle | 40% |
| Peak irradiance (mW/cm2) | 25 |
| Frequency (Hz) | 50 |
| Category | Placebo, n (%) | Pulsed 630 nm PBM, n (%) |
|---|---|---|
| <30 | 40 (100.00%) | 10 (25.00%) |
| 30–49 | 0 | 8 (20.00%) |
| 50–69 | 0 | 19 (47.50%) |
| ≥70 | 0 | 3 (7.50%) |
| Symptom | Numbers of Improved Volunteers | Numbers of Adverse Reactions | Corresponding Number |
|---|---|---|---|
| Period regularity | 12 | 0 | / |
| Blood clotting | 23 | 0 | / |
| Muscle soreness | 24 | 0 | / |
| Abdominal distension and pain | 16 | 0 | / |
| Breast tenderness | 12 | 0 | / |
| Joint pain | 3 | 0 | / |
| Lumbago | 13 | 1 | 28 |
| Diarrhea | 6 | 0 | / |
| Constipation | 1 | 0 | / |
| Appetite | 2 | 0 | / |
| Diet | 11 | 0 | / |
| Headache | 12 | 1 | 28 |
| Agitation | 14 | 0 | / |
| Insomnia | 0 | 0 | / |
| Nausea sensation | 0 | 0 | / |
| Placebo, n (%) | Pulsed 630 nm PBM, n (%) | |
|---|---|---|
| Poor (=0) | 34 (85.00%) | 0 |
| Fair (=1) | 4 (10.00%) | 2 (5.00%) |
| Good (=2) | 2 (5.00%) | 5 (12.50%) |
| Very good (=3) | 0 | 7 (17.50%) |
| Excellent (=4) | 0 | 26 (65.00%) |
| Mean ± SD | 0.20 ± 1.80 | 3.43 ± 1.43 |
| Mean difference ± SE | 3.23 ± 0.16 | |
| p-Value | <0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Q.; Jiang, H.; Yang, J.; Li, Y.; Fei, H.; Huang, J.; Li, Y.; Liu, M. Bypassing the Heat Risk and Efficacy Limitations of Pulsed 630 nm LED Photobiomodulation Therapy for Anti-Primary Dysmenorrhea: A Prospective Randomized Cross-Over Trial. Photonics 2024, 11, 136. https://doi.org/10.3390/photonics11020136
Fu Q, Jiang H, Yang J, Li Y, Fei H, Huang J, Li Y, Liu M. Bypassing the Heat Risk and Efficacy Limitations of Pulsed 630 nm LED Photobiomodulation Therapy for Anti-Primary Dysmenorrhea: A Prospective Randomized Cross-Over Trial. Photonics. 2024; 11(2):136. https://doi.org/10.3390/photonics11020136
Chicago/Turabian StyleFu, Qiqi, Hui Jiang, Jiali Yang, Yafei Li, He Fei, Jianlong Huang, Yinghua Li, and Muqing Liu. 2024. "Bypassing the Heat Risk and Efficacy Limitations of Pulsed 630 nm LED Photobiomodulation Therapy for Anti-Primary Dysmenorrhea: A Prospective Randomized Cross-Over Trial" Photonics 11, no. 2: 136. https://doi.org/10.3390/photonics11020136
APA StyleFu, Q., Jiang, H., Yang, J., Li, Y., Fei, H., Huang, J., Li, Y., & Liu, M. (2024). Bypassing the Heat Risk and Efficacy Limitations of Pulsed 630 nm LED Photobiomodulation Therapy for Anti-Primary Dysmenorrhea: A Prospective Randomized Cross-Over Trial. Photonics, 11(2), 136. https://doi.org/10.3390/photonics11020136





