Development of Taper-in-Taper-Based Optical Fiber Sensors for Chemical and Biological Sensing
Abstract
1. Introduction
2. Materials and Methods
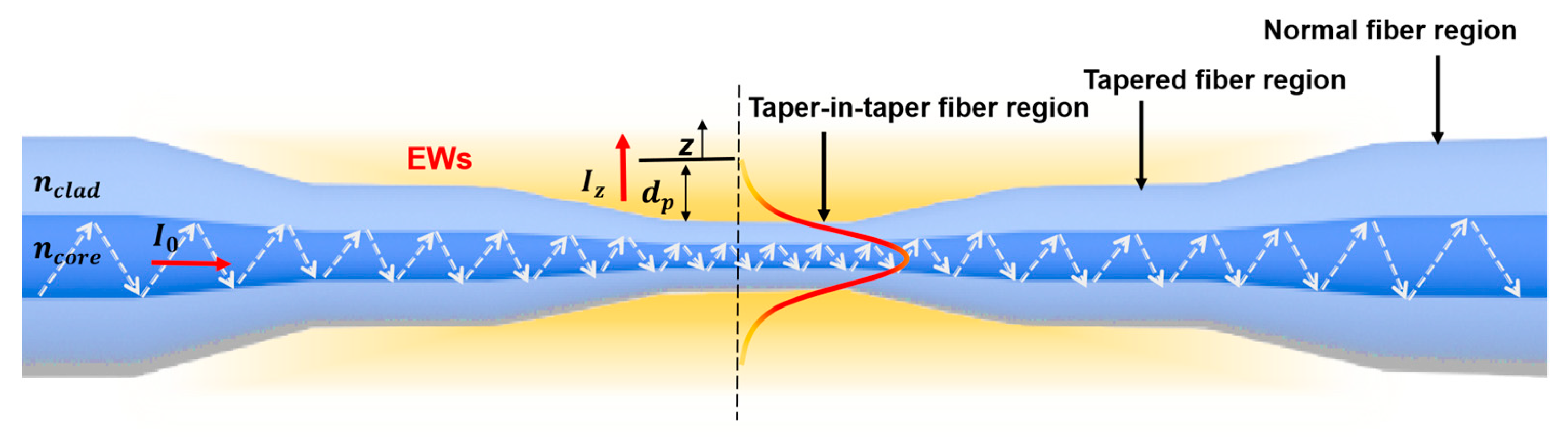
2.1. Working Principle
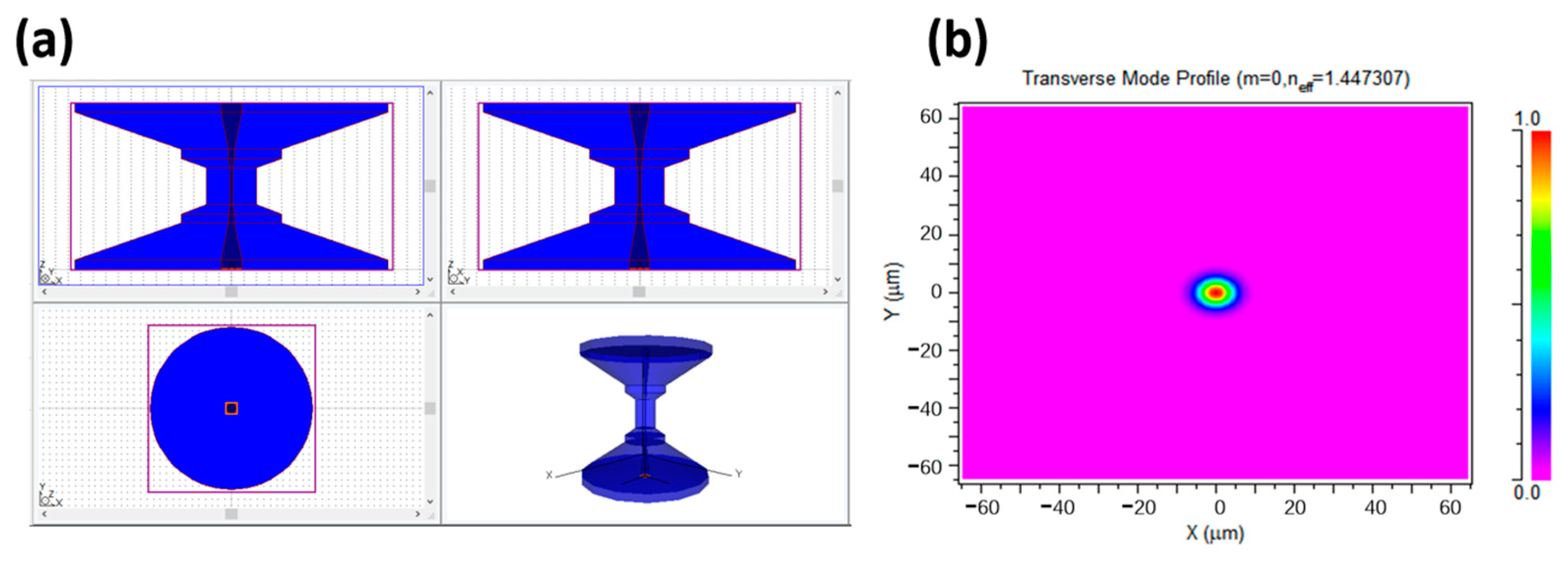
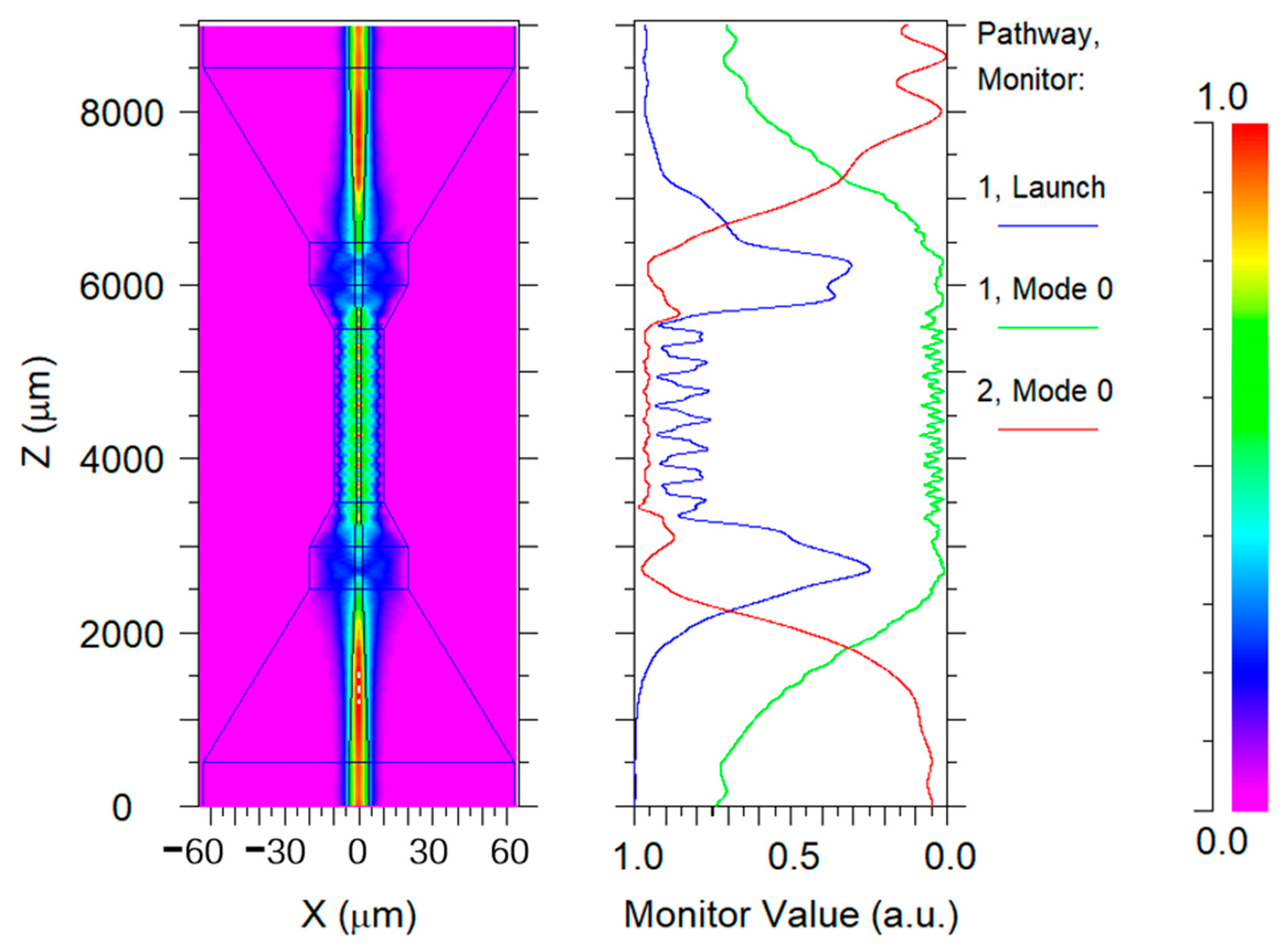
2.2. Simulation of Taper-in-Taper Fiber Structure
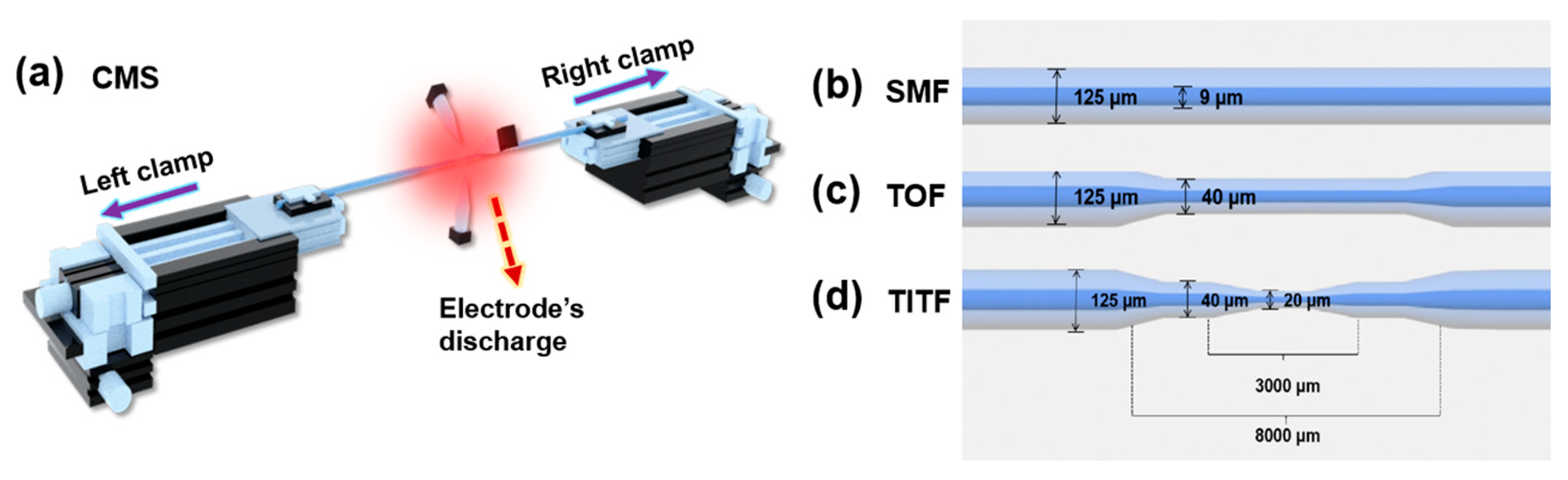
2.3. Experimental Instruments
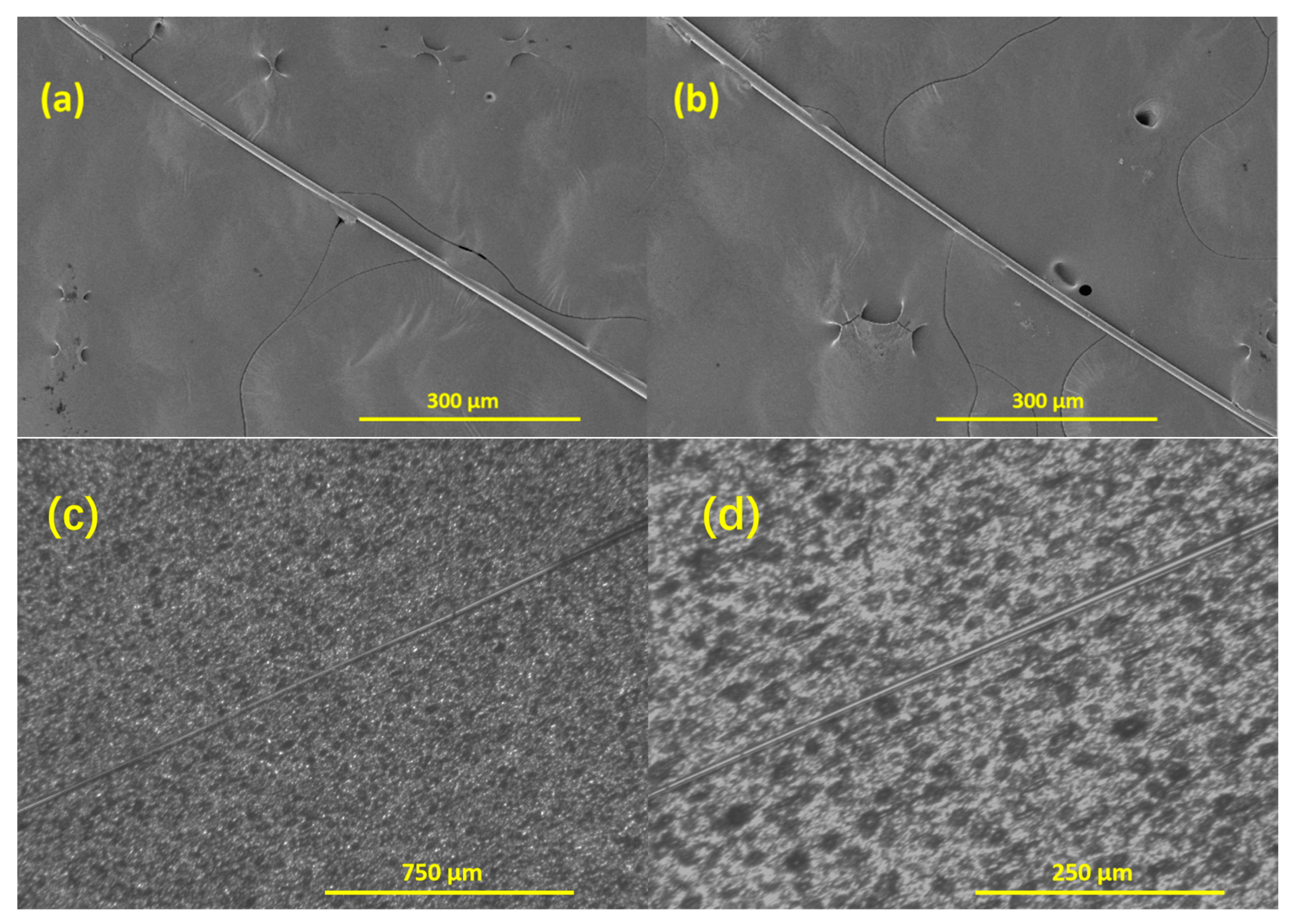
2.4. Fabrication of Taper/Taper-in-Taper Fiber Structure
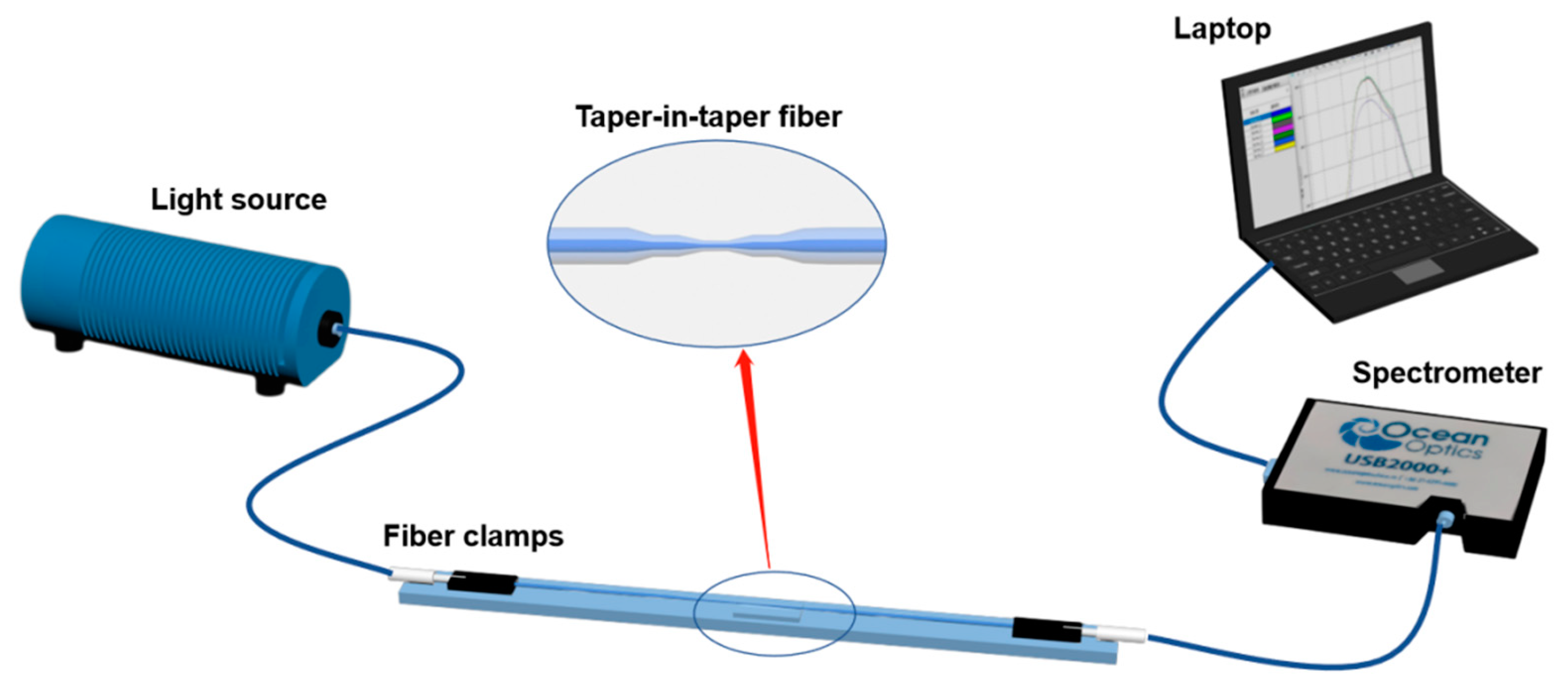
2.5. Experimental Setup for Spectrum Measurement
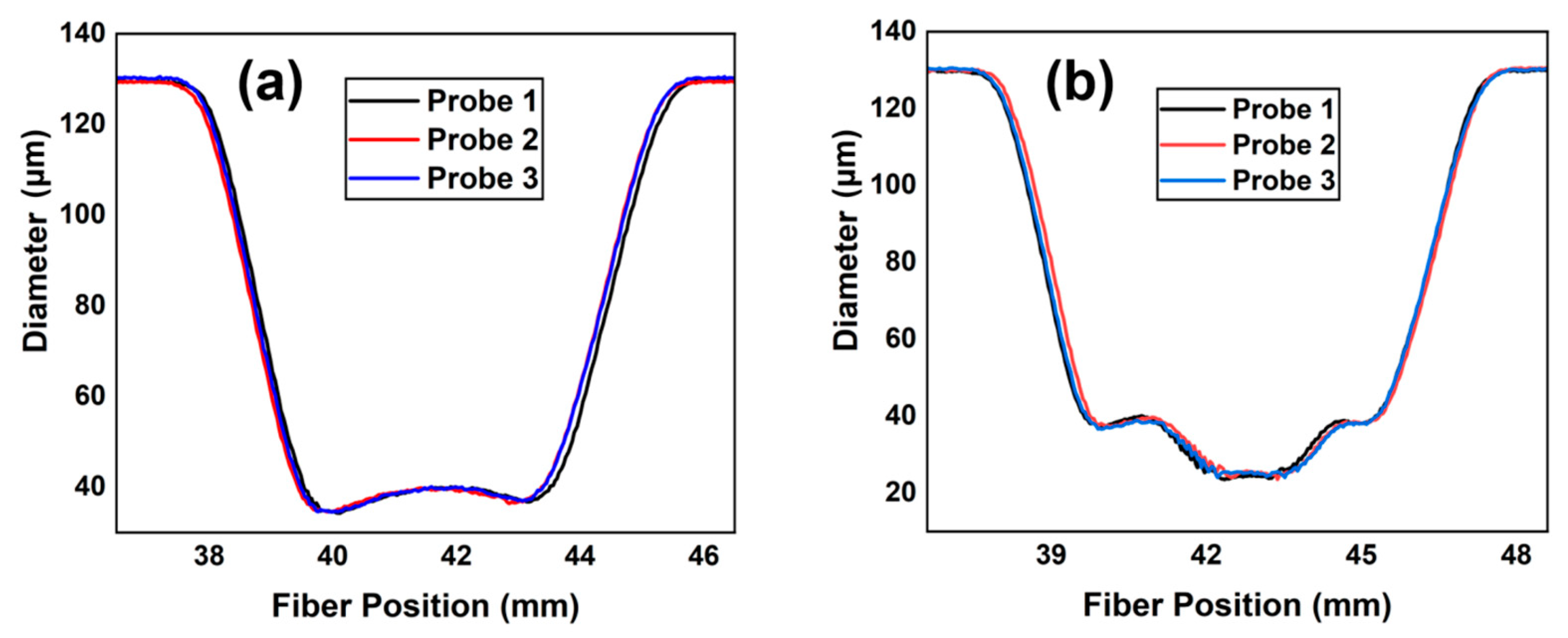
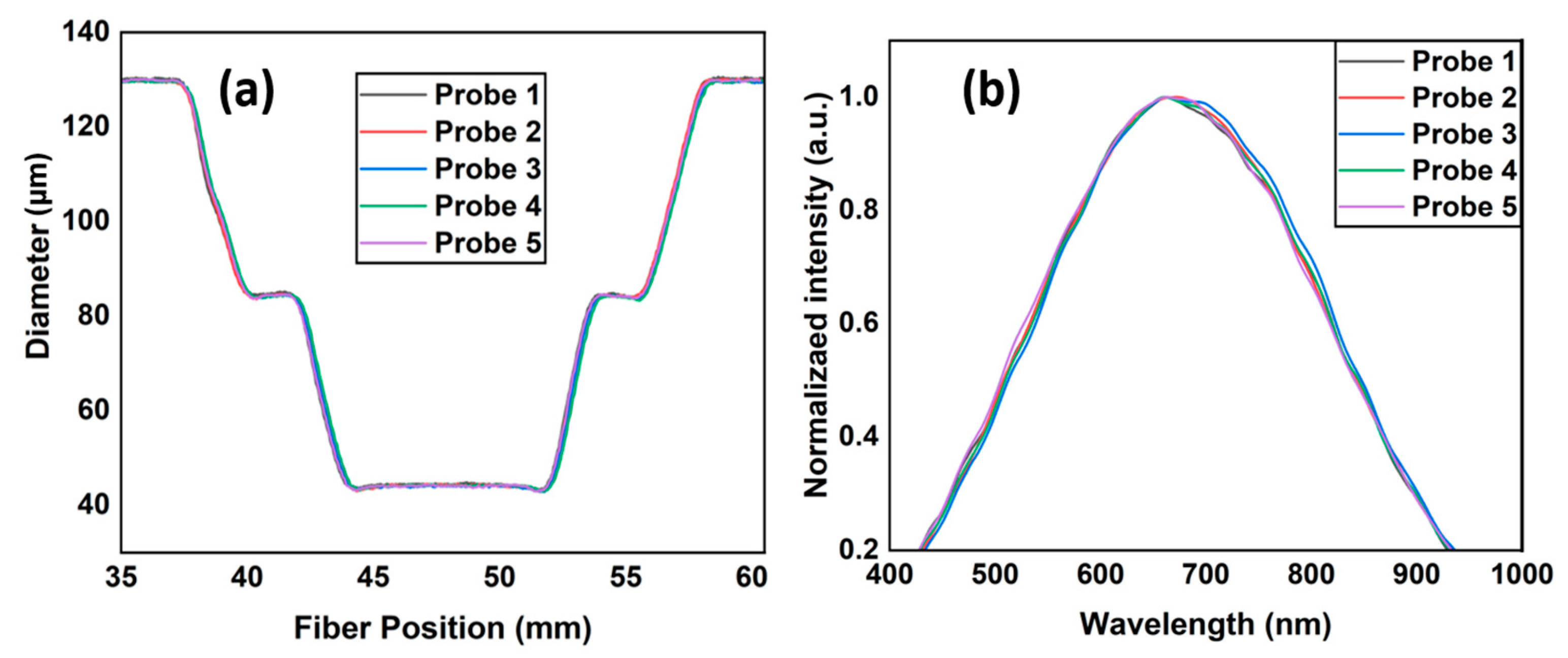
3. Structural Analysis and Optimization
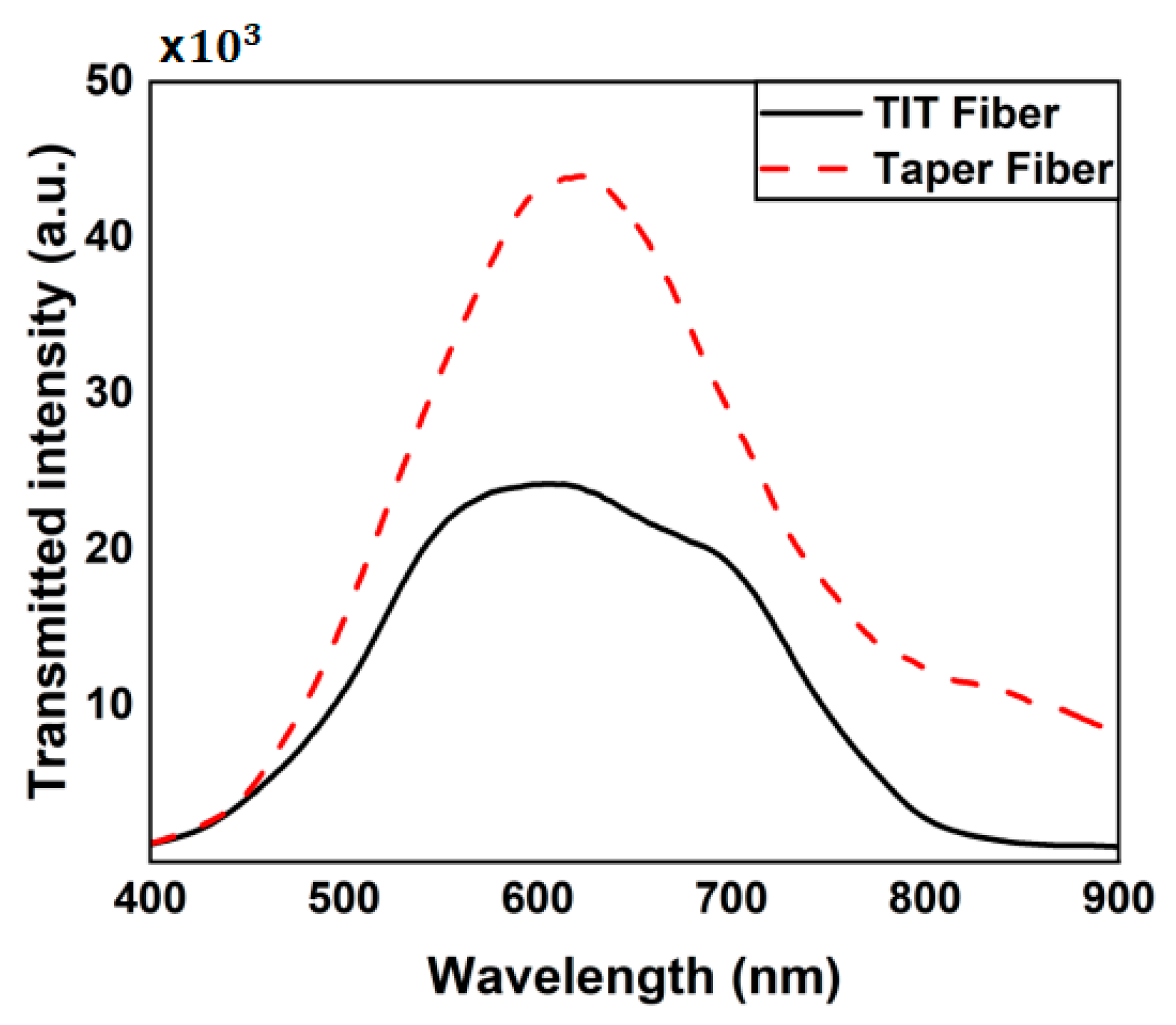
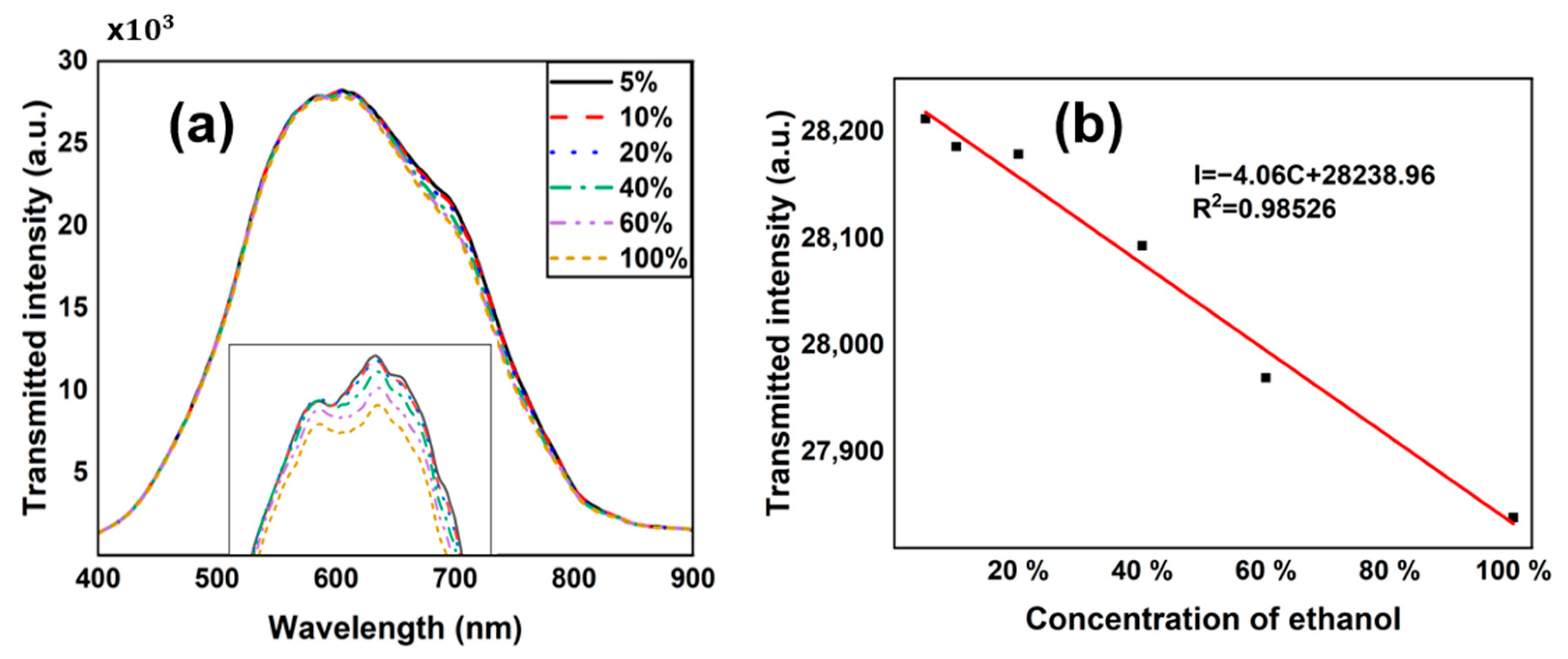
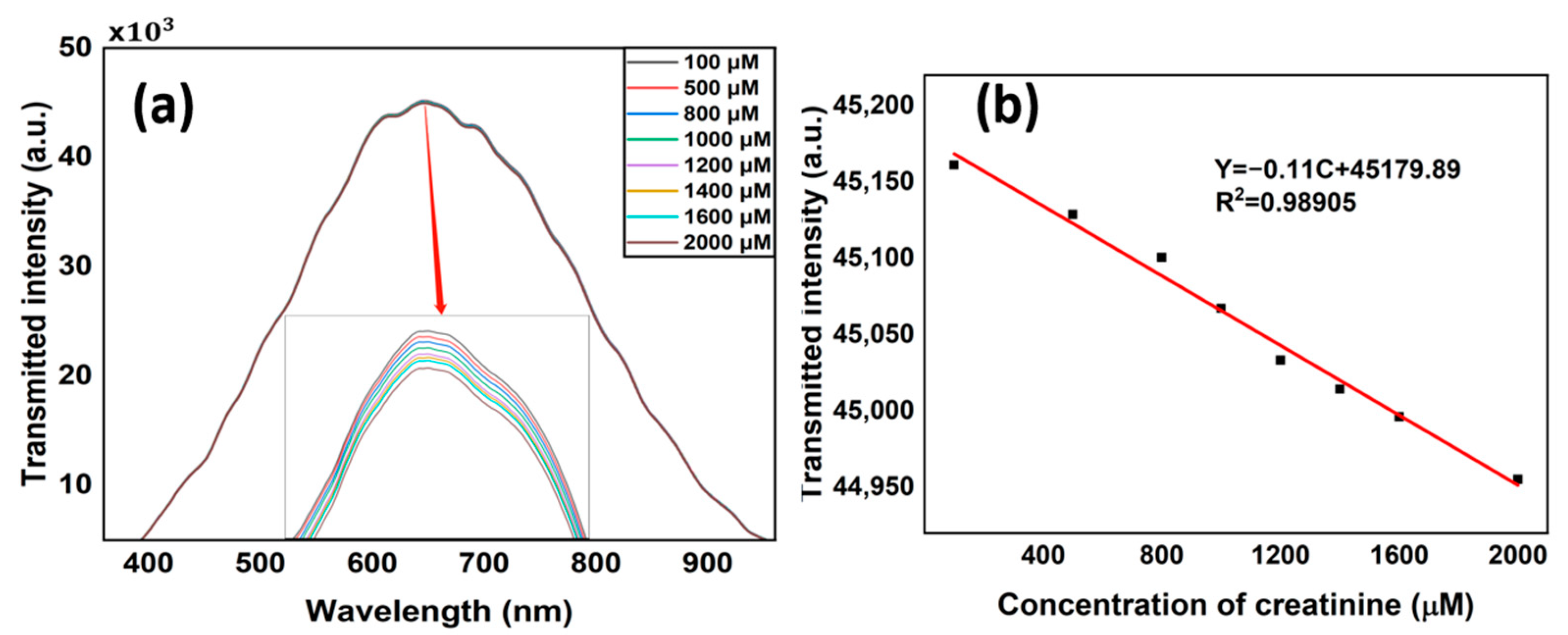
4. Results and Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, X.; Zhang, Y.; Jin, W.; Hu, Y.; Cui, Y. Carbon Nanotube Field-Effect Transistor-Based Chemical and Biological Sensors. Sensors 2021, 21, 995. [Google Scholar] [CrossRef] [PubMed]
- Perri, C.; Arcadio, F.; D’Agostino, G.; Cennamo, N.; Porto, G.; Zeni, L. Chemical and Biological Applications Based on Plasmonic Optical Fiber Sensors. IEEE Instrum. Meas. Mag. 2021, 24, 50–55. [Google Scholar] [CrossRef]
- Zain, H.A.; Batumalay, M.; Rahim, H.R.A.; Yasin, M.; Harun, S.W. Single-walled carbon nanotubes coated D-shaped fiber for aqueous ethanol detection. Optoelectron. Lett. 2022, 18, 430–433. [Google Scholar] [CrossRef]
- Sampath, U.; Kim, D.-G.; Song, M. Hydrous Ethanol Detection Using a Graphene Oxide-Coated Fresnel Reflection Sensor. IEEE Sens. J. 2019, 19, 4069–4073. [Google Scholar] [CrossRef]
- Aziz, A.; Lim, H.N.; Girei, S.H.; Yaacob, M.H.; Mahdi, M.A.; Huang, N.M.; Pandikumar, A. Silver/graphene nanocomposite-modified optical fiber sensor platform for ethanol detection in water medium. Sens. Actuators B 2015, 206, 119–125. [Google Scholar]
- Alula, M.T.; Karamchand, L.; Hendricks, N.R.; Blackburn, J.M. Citrate-capped silver nanoparticles as a probe for sensitive and selective colorimetric and spectrophotometric sensing of creatinine in human urine. Anal. Chim. Acta 2018, 1007, 40–49. [Google Scholar] [CrossRef]
- Krishanu, S. A Review on the Development of Spectroscopic Sensors for the Detection of Creatinine in Human Blood Serum. Int. J. Life Sci. Pharma Res. 2022, 11, 91–101. [Google Scholar] [CrossRef]
- Pundir, C.S.; Kumar, P.; Jaiwal, R. Biosensing methods for determination of creatinine: A review. Biosens. Bioelectron. 2019, 126, 707–724. [Google Scholar] [CrossRef]
- Coresh, J.; Astor, B.C.; Greene, T.; Eknoyan, G.; Levey, A.S. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. 2003, 41, 1–12. [Google Scholar] [CrossRef]
- Li, M.; Singh, R.; Soares, M.S.; Marques, C.; Zhang, B.; Kumar, S. Convex fiber-tapered seven core fiber-convex fiber (CTC) structure-based biosensor for creatinine detection in aquaculture. Opt. Express 2022, 30, 13898–13914. [Google Scholar] [CrossRef]
- Zhu, J.; Pan, J.; Li, Y.; Yang, J.; Ye, B. Enzyme-nanozyme cascade colorimetric sensor platform: A sensitive method for detecting human serum creatinine. Anal. Bioanal. Chem. 2022, 414, 6271–6280. [Google Scholar] [CrossRef] [PubMed]
- Kerr, E.; West, C.; Hartwell, S.K. Quantitative TLC-Image Analysis of Urinary Creatinine Using Iodine Staining and RGB Values. J. Chromatogr. Sci. 2016, 54, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Shrivastav, A.M.; Gupta, B.D. Lossy Mode Resonance Based Fiber Optic Creatinine Sensor Fabricated Using Molecular Imprinting Over Nanocomposite of MoS2/SnO2. IEEE Sens. J. 2020, 20, 4251–4259. [Google Scholar] [CrossRef]
- Kumar, V.; Hebbar, S.; Kalam, R.; Panwar, S.; Prasad, S.; Srikanta, S.S.; Krishnaswamy, P.R.; Bhat, N. Creatinine-Iron Complex and Its Use in Electrochemical Measurement of Urine Creatinine. IEEE Sens. J. 2018, 18, 830–836. [Google Scholar] [CrossRef]
- Stasyuk, N.Y.; Gayda, G.Z.; Zakalskiy, A.E.; Fayura, L.R.; Zakalska, O.M.; Nisnevitch, M.; Gonchar, M.V. Amperometric biosensors for L-arginine and creatinine assay based on recombinant deiminases and ammonium-sensitive Cu/Zn(Hg) S nanoparticles. Talanta 2022, 238, 122996. [Google Scholar] [CrossRef] [PubMed]
- Jayasekhar Babu, P.; Tirkey, A.; Rao, T.J.M.; Chanu, N.B.; Lalchhandama, K.; Singh, Y.D. Conventional and nanotechnology based sensors for creatinine (A kidney biomarker) detection: A consolidated review. Anal. Biochem. 2022, 645, 114622. [Google Scholar] [CrossRef]
- Li, M.-J.; Nolan, D.A. Optical Transmission Fiber Design Evolution. J. Light. Technol. 2008, 26, 1079–1092. [Google Scholar] [CrossRef]
- Qiang, Z.; Junyang, L.; Yanling, Y.; Libo, G.; Chenyang, X. Micro double tapered optical fiber sensors based on the evanescent field-effect and surface modification. Optik 2014, 125, 4614–4617. [Google Scholar] [CrossRef]
- Zi, X.; Chen, J.; Zhang, S.; Li, X.; Wang, S.; Wei, J.; Lu, H.; Sheng, K.; Niu, Y.; Gong, S. Ultrasensitive and stable pressure sensor based on tapered optical fibres. Opt. Commun. 2022, 518, 128337. [Google Scholar] [CrossRef]
- Li, Y.C.; Xin, H.B.; Zhang, Y.; Li, B.J. Optical Fiber Technologies for Nanomanipulation and Biodetection: A Review. J. Light. Technol. 2021, 39, 251–262. [Google Scholar] [CrossRef]
- Zhang, X.; Broere, W. Design of a distributed optical fiber sensor system for measuring immersed tunnel joint deformations. Tunn. Undergr. Space Technol. 2023, 131, 104770. [Google Scholar] [CrossRef]
- Ge, J.; Liu, J.; Gui, K.; Ye, L. Atmospheric icing measurement and online ice type recognition for aircraft utilizing optical fiber sensor and machine learning algorithms. Measurement 2022, 205, 112215. [Google Scholar] [CrossRef]
- Martan, T.; Mares, D.; Prajzler, V. Local detection of gaseous carbon dioxide using optical fibers and fiber tapers of single-cell dimensions. Sens. Actuators B 2023, 375, 132887. [Google Scholar] [CrossRef]
- Rachana, M.; Charles, I.; Swarnakar, S.; Krishna, S.V.; Kumar, S. Recent advances in photonic crystal fiber-based sensors for biomedical applications. Opt. Fiber Technol. 2022, 74, 103085. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, R.Y.M.; Faisal, M. Efficient diaphragm-based Fiber Bragg grating vacuum sensor. Vacuum 2023, 207, 111566. [Google Scholar] [CrossRef]
- Du, C.; Wang, Q.; Zhao, S.; Deng, X. Biological sensors based on long period fiber grating. Opt. Laser Technol. 2023, 158, 108936. [Google Scholar] [CrossRef]
- Karimi-Alavijeh, H.; Taslimi, A.; Maghsoudian, M.H.; Poorghadiri, M.H.; Kazemzadeh, M. Fabrication of low-loss adiabatic optical microfibers using an attainable arc-discharge fiber tapering setup. Opt. Commun. 2022, 522, 128669. [Google Scholar] [CrossRef]
- Gong, Z.; Lei, Y.; Wang, Z.; Zhang, J.; Sun, Z.; Li, Y.; Huang, J.; Chan, C.; Ouyang, X. A Taper-in-Taper Structured Interferometric Optical Fiber Sensor for Cu2+ ion Detection. Sensors 2022, 22, 2709. [Google Scholar] [CrossRef]
- Korposh, S.; James, S.W.; Lee, S.W.; Tatam, R.P. Tapered Optical Fibre Sensors: Current Trends and Future Perspectives. Sensors 2019, 19, 2294. [Google Scholar] [CrossRef]
- Bojarczuk, J.; Araszkiewicz, P.; Markowski, K. Numerical analysis of the F-P TCFBG strain sensor spectral response and its multiplexation capabilities achieved with DSP and FFT analysis. Appl. Opt. 2022, 61, 1139–1149. [Google Scholar] [CrossRef]
- Markowski, K.; Araszkiewicz, P.; Bojarczuk, J.; Perlicki, K. High-sensitivity chirped tapered fiber-Bragg-grating-based Fabry-Perot cavity for strain measurements. Opt. Lett. 2020, 45, 2838–2841. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Singh, R.; Marques, C.; Jha, R.; Zhang, B.; Kumar, S. Taper-in-taper fiber structure-based LSPR sensor for alanine aminotransferase detection. Opt. Express 2021, 29, 43793. [Google Scholar] [CrossRef]
- Idris, A.O.; Akanji, S.P.; Orimolade, B.O.; Olorundare, F.O.G.; Azizi, S.; Mamba, B.; Maaza, M. Using Nanomaterials as Excellent Immobilisation Layer for Biosensor Design. Biosensors 2023, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lin, X.; Peng, Z.; Xu, S.; Jin, L.; Zheng, X.; Luo, H. Materials and Methods of Biosensor Interfaces with Stability. Front. Mater. 2021, 7, 583739. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, A.; Su, M. Nanomaterials for Biosensing Applications. Nanomaterials 2016, 6, 58. [Google Scholar] [CrossRef]
- Chou, H.-T.; Huang, W.-H.; Wu, T.-M.; Yu, Y.-K.; Hsu, H.-C. LSPR effects of Au nanoparticles/ZnO nano-composite films. Sens. Bio-Sens. Res. 2017, 14, 17–20. [Google Scholar] [CrossRef]
- Thangavel, K.; Balamurugan, A.; Venkatachalam, T.; Kumar, E.R. Structural, morphological and optical properties of ZnO nano-fibers. Superlattices Microstruct. 2016, 90, 45–52. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gupta, J.; Sharma, I. Fiber optic evanescent wave absorption-based sensors: A detailed review of advancements in the last decade (2007–18). Optik 2019, 183, 1008–1025. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, W.H.; Wu, N.; Zou, X.T.; Wang, X.W. Tapered Optical Fiber Sensor for Label-Free Detection of Biomolecules. Sensors 2011, 11, 3780–3790. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, G.; Singh, L.; Wang, Y.; Singh, R.; Zhang, B.; Zhang, X.; Kumar, S. Highly sensitive and selective sensor probe using glucose oxidase/gold nanoparticles/graphene oxide functionalized tapered optical fiber structure for detection of glucose. Optik 2020, 208, 164536. [Google Scholar] [CrossRef]
- Kyunghwan, O.; Choi, S.; Yongmin, J.; Lee, J.W. Novel hollow optical fibers and their applications in photonic devices for optical communications. J. Light. Technol. 2005, 23, 524–532. [Google Scholar] [CrossRef]
- Choochalerm, P.; Martin, W.E.; Jones, H.R.A.; Errmann, R.; Yerolatsitis, S.; Wright, T.A.; Buisset, C. Transmission properties of tapered optical fibres: Simulations and experimental measurements. Opt. Fiber Technol. 2021, 66, 102632. [Google Scholar] [CrossRef]
- Shabaneh, A.A.; Girei, S.H.; Arasu, P.T.; Rahman, W.B.W.A.; Bakar, A.A.A.; Sadek, A.Z.; Lim, H.N.; Huang, N.M.; Yaacob, M.H. Reflectance response of tapered optical fiber coated with graphene oxide nanostructured thin film for aqueous ethanol sensing. Opt. Commun. 2014, 331, 320–324. [Google Scholar] [CrossRef]
- Yadav, S.; Devi, R.; Kumar, A.; Pundir, C.S. Tr-enzyme functionalized ZnO-NPs/CHIT/c-MWCNT/PANI composite film for amperometric determination of creatinine. Biosens. Bioelectron. 2011, 28, 64–70. [Google Scholar] [CrossRef]
- Dudala, S.; Dubey, S.K.; Javed, A.; Ganguly, A.; Kapur, S.; Goel, S. Portable Chemiluminescence Detection Platform and Its Application in Creatinine Detection. IEEE Sens. J. 2022, 22, 7177–7184. [Google Scholar] [CrossRef]
- Boobphahom, S.; Ruecha, N.; Rodthongkum, N.; Chailapakul, O.; Remcho, V.T. A copper oxide-ionic liquid/reduced graphene oxide composite sensor enabled by digital dispensing: Non-enzymatic paper-based microfluidic determination of creatinine in human blood serum. Anal. Chim. Acta 2019, 1083, 110–118. [Google Scholar] [CrossRef]



| Materials Used | Linear Range | Sensitivity | Ref. |
|---|---|---|---|
| Ag-NPs | 0–4.2 μM | 4.2 μM | [6] |
| ZnO-NPs/CHIT/c-MWCNT/PANI | 10–650 μM | 0.03 μAμM−1cm−2 | [44] |
| Cobalt (II) ions | 40–160 μM | n.r. a | [45] |
| CuO/IL/ERGO/SPCE | 0.01–2.0 mM | n.r. a | [46] |
| ZnO-NPs | 0–2000 μM | 0.11 a.u./μM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Zhang, W.; Lang, X.; Liu, X.; Singh, R.; Li, G.; Xie, Y.; Zhang, B.; Kumar, S. Development of Taper-in-Taper-Based Optical Fiber Sensors for Chemical and Biological Sensing. Photonics 2023, 10, 567. https://doi.org/10.3390/photonics10050567
Liu F, Zhang W, Lang X, Liu X, Singh R, Li G, Xie Y, Zhang B, Kumar S. Development of Taper-in-Taper-Based Optical Fiber Sensors for Chemical and Biological Sensing. Photonics. 2023; 10(5):567. https://doi.org/10.3390/photonics10050567
Chicago/Turabian StyleLiu, Fei, Wen Zhang, Xianzheng Lang, Xuecheng Liu, Ragini Singh, Guoru Li, Yiyan Xie, Bingyuan Zhang, and Santosh Kumar. 2023. "Development of Taper-in-Taper-Based Optical Fiber Sensors for Chemical and Biological Sensing" Photonics 10, no. 5: 567. https://doi.org/10.3390/photonics10050567
APA StyleLiu, F., Zhang, W., Lang, X., Liu, X., Singh, R., Li, G., Xie, Y., Zhang, B., & Kumar, S. (2023). Development of Taper-in-Taper-Based Optical Fiber Sensors for Chemical and Biological Sensing. Photonics, 10(5), 567. https://doi.org/10.3390/photonics10050567







