Assessment of the Impact of Nanowarming on Microstructure of Cryopreserved Fibroblast-Containing 3D Tissue Models Using Mueller Polarimetry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples’ Preparation and Characterization
2.1.1. Chemicals for Cell Culture
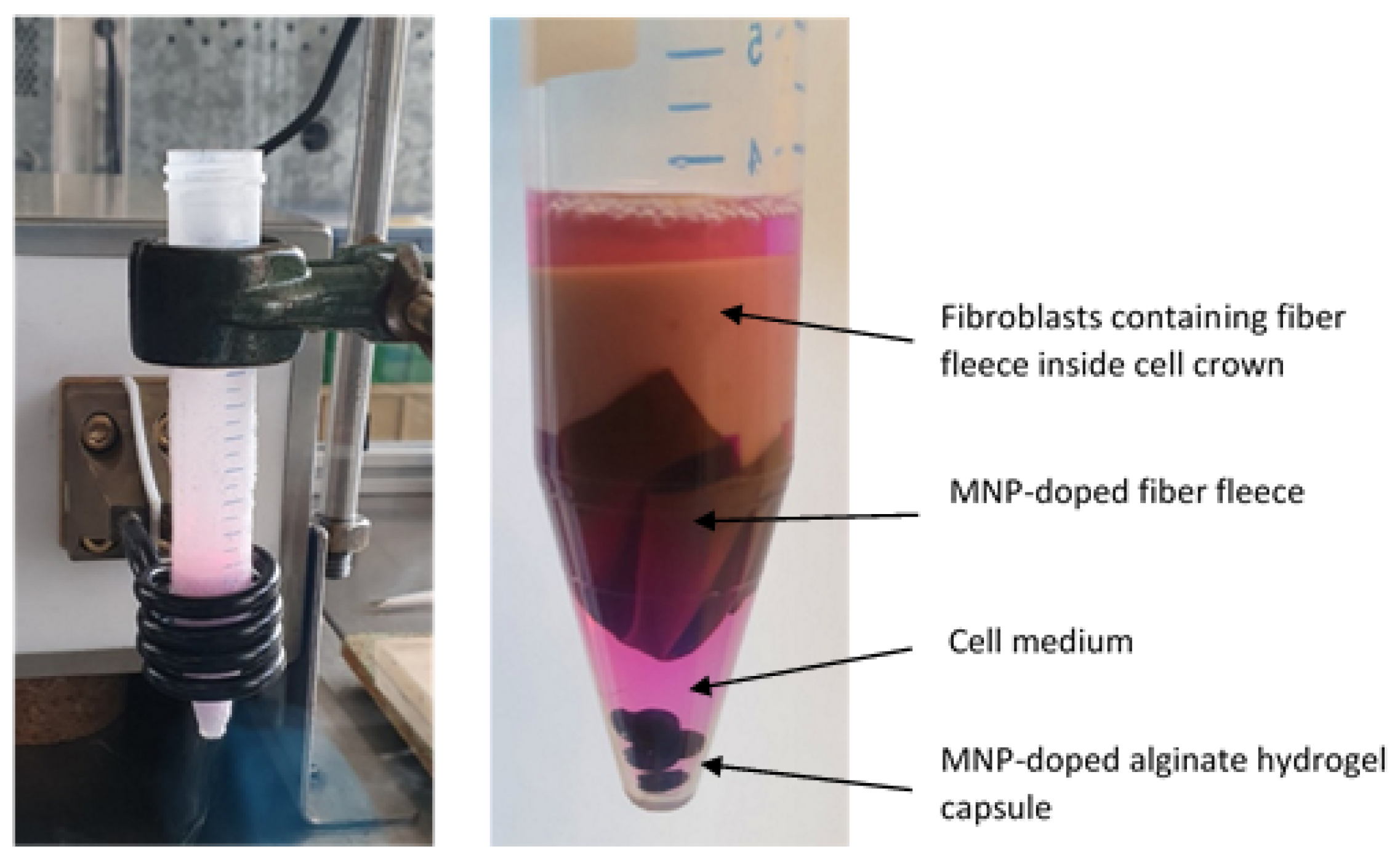
2.1.2. Preparation of MNP-Doped Fibers and Capsules
2.1.3. Cultivation of Fibroblasts on MNP-Doped Fleeces
2.1.4. Preparation and Characterization of Tissue Sections
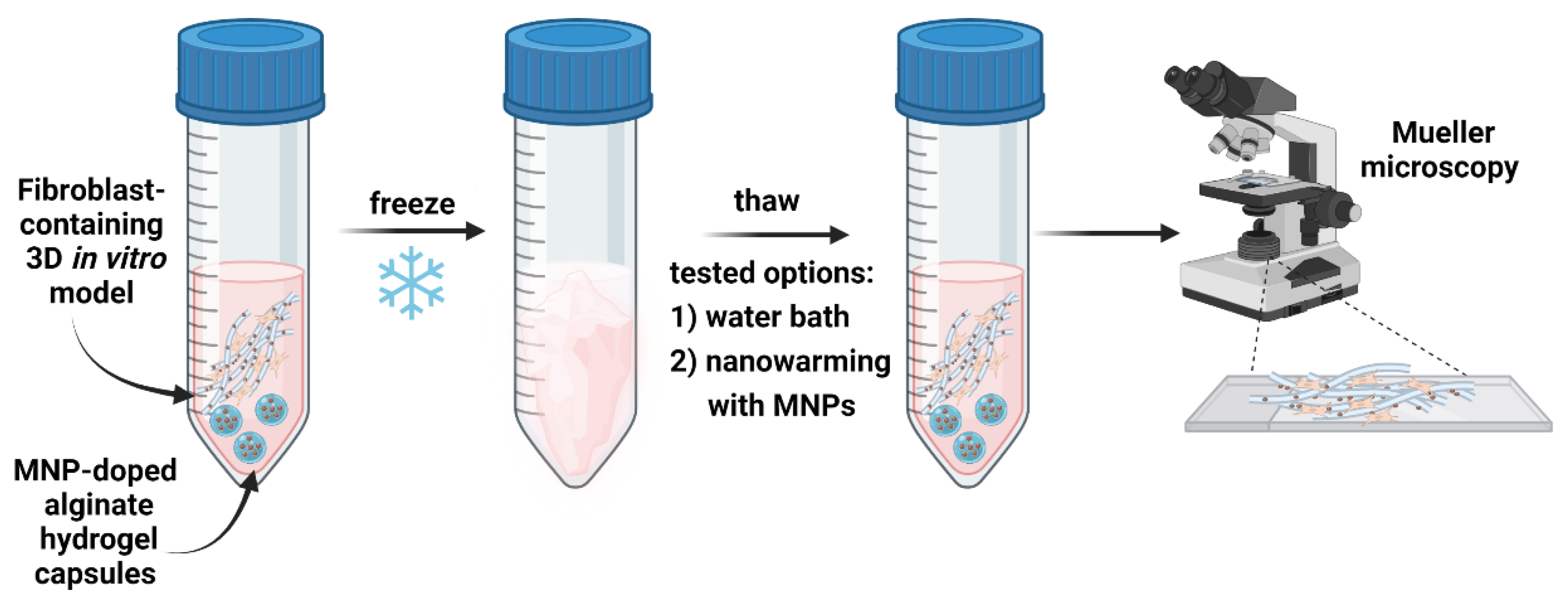
2.1.5. Induction Thawing Method
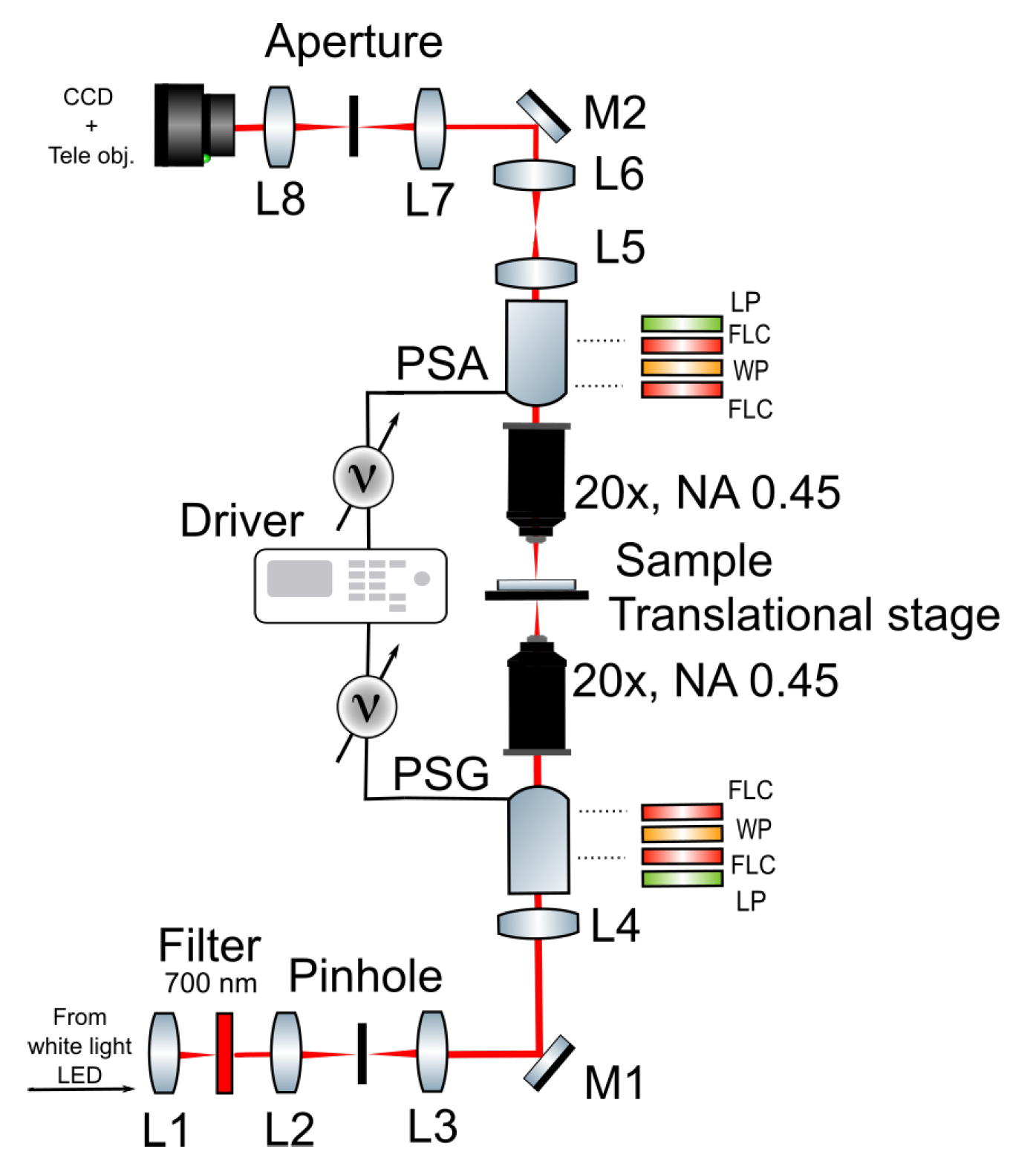
2.2. Polarimetric Experimental Set-Up
2.3. Post-Processing of Polarimetric Data with Differential Decomposition
3. Results and Discussion
3.1. Preparation and Characterization of Fibroblasts-Containing Models
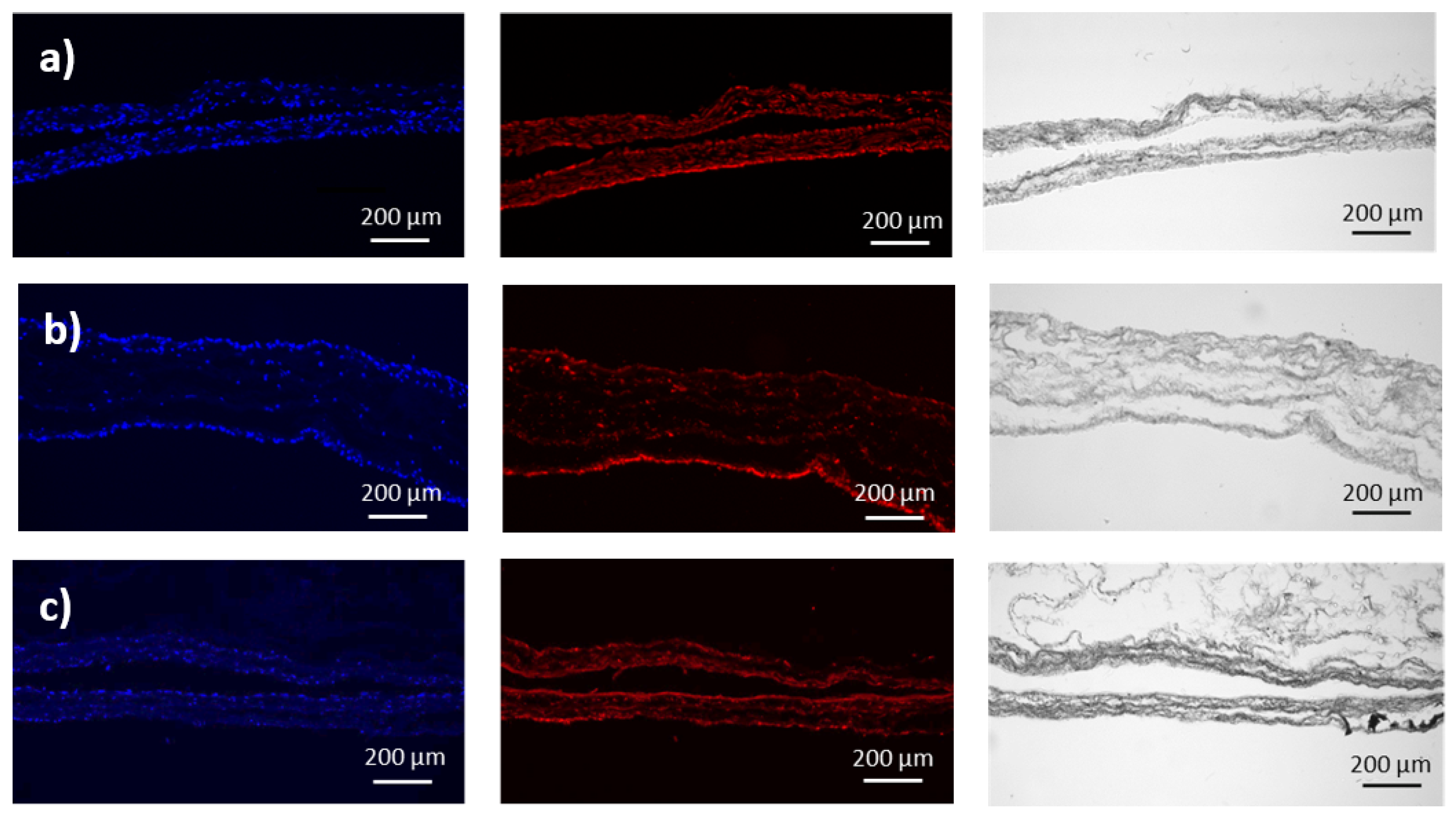
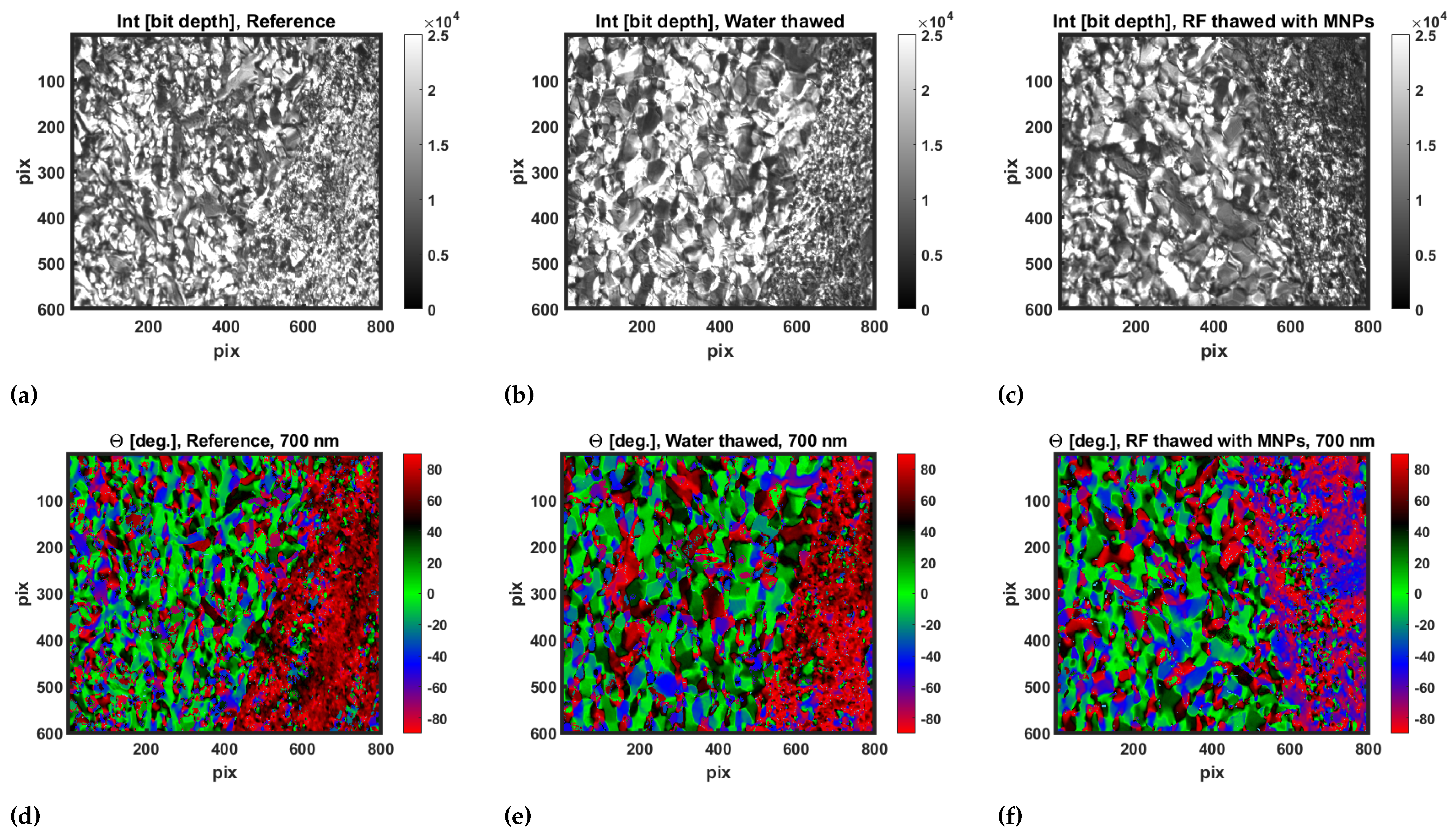
3.2. Transmission Mueller Microscopy
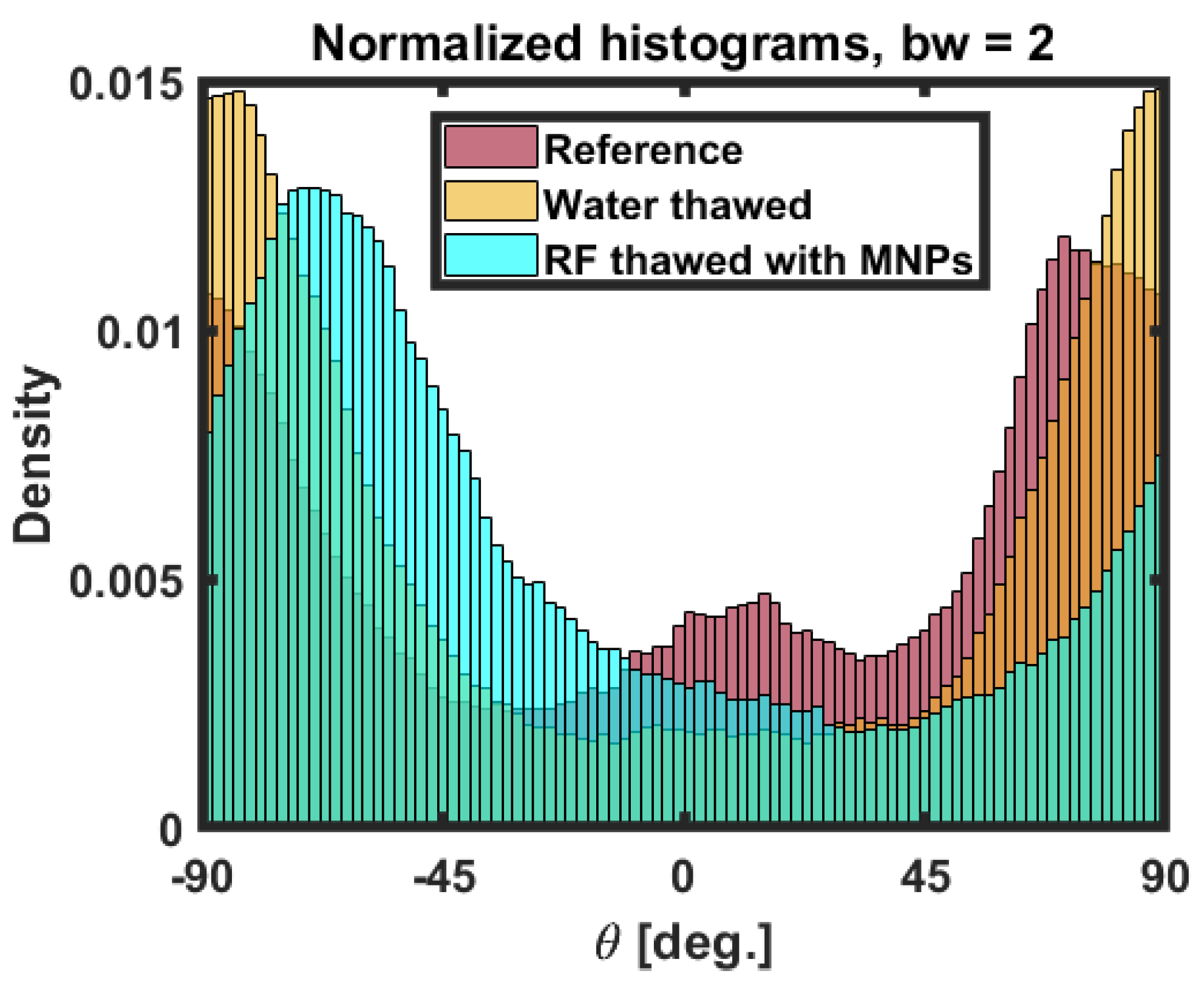
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPA | Cryoprotectants |
| MNP | Magnetic nanoparticle |
| RF | Radio frequency |
| 3D | Three dimensional |
| LED | Light-emitting diode |
| FWHM | Full width at half maximum |
| PSG | Polarization state generator |
| PSA | Polarization state analyzer |
| LP | Linear polarizer |
| WP | Wave plate |
| FLC | Feroelectric liquid crystal |
| NA | Numerical apperture |
| CCD | Charge-coupled devices |
| ROI | Region of interest |
| ECM | Extracellular matrix |
References
- Mazur, P. Freezing of living cells: Mechanisms and implications. Am. J. Physiol. 1984, 247, C125. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.O.; Toner, M. Long-term storage of tissues by cryopreservation: Critical issues. Biomaterials 1996, 17, 243. [Google Scholar] [CrossRef] [PubMed]
- Ladanyi, C.; Mor, A.; Christianson, M.S.; Dhillon, N.; Segars, J.H. Recent advances in the field of ovarian tissue cryopreservation and opportunities for research. J. Assist. Reprod. Genet. 2017, 34, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Xie, Y.; Wang, Y.; Li, S. Vitrification versus slow freezing for human ovarian tissue cryopreservation: A systematic review and meta-analysis. Sci. Rep. 2018, 7, 8538. [Google Scholar] [CrossRef] [PubMed]
- Stanzel, B.V.; Espanac, E.M.; Grueterich, M.; Kawakita, T.; Parel, J.M.; Tseng, S.C.; Binder, S. Amniotic membrane maintains the phenotype of rabbit retinal epithelial cells in culture. Exp. Eye Res. 2005, 80, 103. [Google Scholar] [CrossRef] [PubMed]
- Scudellari, M. Core Concept: Cryopreservation aims to engineer novel ways to freeze, store, and thaw organs. Proc. Natl. Acad. Sci. USA 2017, 114, 13060. [Google Scholar] [CrossRef]
- Chang, T.; Zhao, G. Ice Inhibition for Cryopreservation: Materials, Strategies and Challenges. Adv. Sci. 2021, 8, 2002425. [Google Scholar] [CrossRef]
- Ito, A.; Yoshioka, K.; Masumoto, S.; Sato, K.; Hatae, Y.; Nakai, T.; Yamazaki, T.; Takahashi, M.; Tanoue, S.; Horie, M. Magnetic heating of nanoparticles as a scalable cryopreservation technology for human induced pluripotent stem cells. Sci. Rep. 2020, 10, 13605. [Google Scholar] [CrossRef]
- Zhao, G.; Ring, H.L.; Sharma, A.; Namsrai, B.; Tran, N.; Finger, E.; Garwood, M.; Haynes, C.; Bischof, J. Preparation of Scalable Silica-Coated Iron Oxide Nanoparticles for Nanowarming. Adv. Sci. 2020, 7, 1901624. [Google Scholar]
- Manuchehrabadi, N.; Gao, Z.; Zhang, J.; Ring, H.; Shao, Q.; Liu, F.; McDermott, M.; Fok, A.; Rabin, Y.; Brockbank, K.; et al. Improved tissue cryopreservation using inductive heating of magnetic nanoparticles. Sci. Transl. Med. 2017, 9, eaah4586. [Google Scholar] [CrossRef]
- Chiu-Lam, A.; Staples, E.; Pepine, C.; Rinaldi, C. Perfusion, cryopreservation, and nanowarming of whole hearts using colloidally stable magnetic cryopreservation agent solutions. Sci. Adv. 2021, 7, 1901624. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J. Immunofluorescence Staining. Curr. Protoc. Cell Biol. 2001. [Google Scholar] [CrossRef]
- Yousaf, M.; Khurshid, A.; Mahmood, R.; Ikram, M. Polarimetric comparison of fresh and frozen skeletal muscle tissues of goat. Photodiagn. Photodyn. 2020, 32, 102071. [Google Scholar] [CrossRef]
- Wu, P.; Walsh, J. Stokes polarimetry imaging of rat tail tissue in a turbid medium: Degree of linear polarization image maps using incident linearly polarized light. J. Biomed. Opt. 2006, 11, 014031. [Google Scholar] [CrossRef]
- Pierangelo, A.; Manhas, S.; Benali, A.; Antonelli, M.-R.; Novikova, T.; Validire, P.; Gayet, B.; De Martino, A. Use of Mueller polarimetric imaging for the staging of human colon cancer. Proc. SPIE Opt. Biopsy IX 2011, 7895, 78950E. [Google Scholar]
- Robinson, D.; Kleijn, W.B.; Hoong, K.; Doronin, A.; Rehbinder, J.; Vizet, J.; Pierangelo, A.; Novikova, T. Polarimetric imaging for cervical pre-cancer screening aided by machine learning: Ex vivo studies. J. Biomed. Opt. 2023, 28, 102904. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nunez, O.; Novikova, T. Polarimetric techniques for the structural studies and diagnosis of brain tissue. Adv. Opt. Technol. 2022, 11, 157. [Google Scholar] [CrossRef]
- Lee, H.R.; Li, P.; Yoo, T.; Lotz, C.; Groeber-Becker, K.; Dembski, S.; Garcia-Caurel, E.; Ossikovski, R.; Ma, H.; Novikova, T. Digital histology with Mueller microscopy: How to mitigate an impact of tissue cut thickness fluctuations. J Biomed. Opt. 2019, 24, 076004. [Google Scholar] [CrossRef]
- Li, P.; Lee, H.R.; Chandel, S.; Lotz, C.; Groeber-Becker, K.; Dembski, S.; Osikovski, R.; Ma, H.; Novikova, T. Analysis of tissue microsctructure with Mueller microscopy: Logarithmic decomposition and Monte Carlo modeling. J. Biomed. Opt. 2020, 25, 015002. [Google Scholar] [CrossRef]
- Lee, H.R.; Lotz, C.; Groeber-Becker, K.; Dembski, S.; Novikova, T. Digital histology with Mueller polarimetry and FastDBSCAN. Appl. Opt. 2022, 61, 9616–9624. [Google Scholar] [CrossRef]
- Kim, M.; Lee, H.R.; Ossikovski, R.; Malfait-Jobart, A.; Lamarque, D.; Novikova, T. Optical diagnosis of gastric tissue biopsies with Mueller microscopy and statistical analysis. J. Eur. Opt. Soc. Rapid. Publ. 2022, 18, 1–10. [Google Scholar] [CrossRef]
- Kobayashi, A.; Golash, N.H.; Kirschvink, J.L. A first test of the hypothesis of biogenic magnetite-based heterogeneous ice-crystal nucleation in cryopreservation. Cryobiology 2016, 72, 216e224. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Horikawa, M.; Kirschvink, J.L.; Golash, H.N. Magnetic control of heterogeneous ice nucleation with nanophase magnetite: Biophysical and agricultural implications. Proc. Natl. Acad. Sci. USA 2018, 115, 5383–5388. [Google Scholar] [CrossRef] [PubMed]
- Granath, T.; Loebmann, P.; Mandel, K. Oxidative Precipitation as a Versatile Method to Obtain Ferromagnetic Fe3O4 Nano- and Mesocrystals Adjustable in Morphology and Magnetic Properties. Part. Part. Syst. Charact. 2021, 38, 2000307. [Google Scholar] [CrossRef]
- Stauch, C.; Spaeth, S.; Ballweg, T.; Luxenhofer, R.; Mandel, K. Nanostructured micro-raspberries from superparamagnetic iron oxide nanoparticles: Studying agglomeration degree and redispersibility of nanoparticulate powders via magnetisation measurements. J. Colloid Interf. Sci. 2017, 505, 605–614. [Google Scholar] [CrossRef]
- Weigel, T.; Malkmus, C.; Weigel, V.; Wussmann, M.; Berger, C.; Brennecke, J.; Groeber-Becker, F.; Hansmann, J. Fully Synthetic 3D Fibrous Scaffolds for Stromal Tissues—Replacement of Animal-Derived Scaffold Materials Demonstrated by Multilayered Skin. Adv. Mater. 2022, 34, 2106780. [Google Scholar] [CrossRef]
- Zimmermann, H.; Shirley, S.; Zimmermann, U. Alginate-based encapsulation of cells: Past, present, and future. Curr. Diabetes Rep. 2007, 7, 314–320. [Google Scholar] [CrossRef]
- Agarwal, N.; Yoon, J.; Garcia-Caurel, E.; Novikova, T.; Vanel, J.-C.; Pierangelo, A.; Bykov, A.; Popov, A.; Meglinski, I.; Ossikovski, R. Spatial evolution of depolarization in homogeneous turbid media within the differential Mueller matrix formalism. Opt. Lett. 2015, 40, 5634. [Google Scholar] [CrossRef]
- Lee, H.R.; Saytashev, I.; Du Le, V.N.; Mahendroo, M.; Ramella-Roman, J.C.; Novikova, T. Mueller matrix imaging for collagen scoring in mice model of pregnancy. Sci. Rep. 2021, 11, 15621. [Google Scholar] [CrossRef]
- Tyo, J.S. Signal-To-Noise Ratio and Minimization of Systematic Error. Appl. Opt. 2002, 41, 619–630. [Google Scholar] [CrossRef]
- Compain, E.; Poirier, S.; Drevillon, B. General and self-consistent method for the calibration of polarization modulators, polarimeters and Mueller-matrix ellipsometers. Appl. Opt. 1999, 38, 3490–3502. [Google Scholar] [CrossRef] [PubMed]
- Azzam, R.M.A. Propagation of partially polarized light through anisotropic media with or without depolarization: A differential 4 × 4 matrix calculus. J. Opt. Soc. Am. 1978, 68, 1756–1767. [Google Scholar] [CrossRef]
- Ossikovski, R. Differential matrix formalism for depolarizing anisotropic media. Opt. Let. 2011, 36, 2330–2332. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mondrinos, M.; Gandhi, M.; Ko, F.; Weiss, A.; Lelkes, P. Electrospun protein fibers as matrices for tissue engineering. Biomaterials 2005, 26, 5999–6008. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M. Handbook of Immunohistochemistry and In Situ Hybridization of Human Carcinomas, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Mould, R. Introductory Medical Statistics, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, D.; Hoeppel, A.; Weigel, T.; Ossikovski, R.; Dembski, S.; Novikova, T. Assessment of the Impact of Nanowarming on Microstructure of Cryopreserved Fibroblast-Containing 3D Tissue Models Using Mueller Polarimetry. Photonics 2023, 10, 1129. https://doi.org/10.3390/photonics10101129
Ivanov D, Hoeppel A, Weigel T, Ossikovski R, Dembski S, Novikova T. Assessment of the Impact of Nanowarming on Microstructure of Cryopreserved Fibroblast-Containing 3D Tissue Models Using Mueller Polarimetry. Photonics. 2023; 10(10):1129. https://doi.org/10.3390/photonics10101129
Chicago/Turabian StyleIvanov, Deyan, Anika Hoeppel, Tobias Weigel, Razvigor Ossikovski, Sofia Dembski, and Tatiana Novikova. 2023. "Assessment of the Impact of Nanowarming on Microstructure of Cryopreserved Fibroblast-Containing 3D Tissue Models Using Mueller Polarimetry" Photonics 10, no. 10: 1129. https://doi.org/10.3390/photonics10101129
APA StyleIvanov, D., Hoeppel, A., Weigel, T., Ossikovski, R., Dembski, S., & Novikova, T. (2023). Assessment of the Impact of Nanowarming on Microstructure of Cryopreserved Fibroblast-Containing 3D Tissue Models Using Mueller Polarimetry. Photonics, 10(10), 1129. https://doi.org/10.3390/photonics10101129






