Biological Photonic Devices Designed for the Purpose of Bio-Imaging with Bio-Diagnosis
Abstract
:1. Introduction
2. Limitations as Well as Possible Improvements
2.1. Cell-Constructed Biological Photonic Waveguides
2.2. Biologically Inspired Light Guidance
2.3. Cell-Constructed Biologically Photonic Waveguides
2.4. Biologically Medical Uses
2.5. Further Limitations and Potential Improvements
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, M.J.; Hughes, C.S.; Hollywood, K.A. Biophotonics: Vibrational Spectroscopic Diagnostics; Morgan & Claypool Publishers: San Rafael, CA, USA, 2016. [Google Scholar]
- Xin, H.; Namgung, B.; Lee, L. Nanoplasmonic optical antennas for life sciences and medicine. Nat. Rev. Mater. 2018, 3, 228–243. [Google Scholar]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar]
- Bai, W.; Yang, H.; Ma, Y.; Chen, H.; Shin, J.; Liu, Y.; Yang, Q.; Kandela, I.; Liu, Z.; Kang, S.-K.; et al. Flexible Transient Optical Waveguides and Surface-Wave Biosensors Constructed from Monocrystalline Silicon. Adv. Mater. 2018, 30, 1801584. [Google Scholar] [CrossRef]
- Andreu, N.; Zelmer, A.; Wiles, S. Noninvasive biophotonic imaging for studies of infectious disease. FEMS Microbiol. Rev. 2011, 35, 360–394. [Google Scholar]
- Wu, L.; Sedgwick, A.C.; Sun, X.; Bull, S.D.; He, X.-P.; James, T.D. Reaction-Based Fluorescent Probes for the Detection and Imaging of Reactive Oxygen, Nitrogen, and Sulfur Species. Acc. Chem. Res. 2019, 52, 2582–2597. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Yang, L. Optical whispering-gallery mode barcodes for high-precision and wide-range temperature measurements. Light Sci. Appl. 2021, 10, 32. [Google Scholar]
- Yang, S.-T.; Cao, L.; Luo, P.G.; Lu, F.; Wang, X.; Wang, H.; Meziani, M.J.; Liu, Y.; Qi, G.; Sun, Y.-P. Carbon Dots for Optical Imaging in Vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Isikman, S.O.; Mudanyali, O.; Greenbaum, A.; Ozcan, A. Optical imaging techniques for point-of-care diagnostics. Lab. Chip 2013, 13, 51–67. [Google Scholar]
- Zhang, K.Y.; Yu, Q.; Wei, H.; Liu, S.; Zhao, Q.; Huang, W. Long-lived emissive probes for time-resolved photoluminescence bioimaging and biosensing. Chem. Rev. 2018, 118, 1770–1839. [Google Scholar] [PubMed]
- Stender, A.S.; Marchuk, K.; Liu, C.; Sander, S.; Meyer, M.W.; Smith, E.A.; Neupane, B.; Wang, G.; Li, J.; Cheng, J.-X. Single cell optical imaging and spectroscopy. Chem. Rev. 2013, 113, 2469–2527. [Google Scholar] [PubMed]
- Lu, H.; Li, W.; Dong, H.; Wei, M. Graphene quantum dots for optical bioimaging. Small 2019, 15, 1902136. [Google Scholar] [CrossRef]
- Doong, R.-A.; Lee, P.-S.; Anitha, K. Simultaneous determination of biomarkers for Alzheimer’s disease using sol–gel-derived optical array biosensor. Biosens. Bioelectron. 2010, 25, 2464–2469. [Google Scholar] [CrossRef]
- Law, W.-C.; Yong, K.-T.; Baev, A.; Prasad, P.N. Sensitivity improved surface plasmon resonance biosensor for cancer biomarker detection based on plasmonic enhancement. ACS Nano 2011, 5, 4858–4864. [Google Scholar] [CrossRef] [PubMed]
- Budz, H.; Ali, M.; Li, Y.; LaPierre, R. Photoluminescence model for a hybrid aptamer-GaAs optical biosensor. J. Appl. Phys. 2010, 107, 104702. [Google Scholar] [CrossRef]
- Yao, J.; Yang, M.; Duan, Y. Chemistry, biology, and medicine of fluorescent nanomaterials and related systems: New insights into biosensing, bioimaging, genomics, diagnostics, and therapy. Chem. Rev. 2014, 114, 6130–6178. [Google Scholar] [PubMed]
- Cennamo, N.; Varriale, A.; Pennacchio, A.; Staiano, M.; Massarotti, D.; Zeni, L.; D’Auria, S. An innovative plastic optical fiber-based biosensor for new bio/applications. The case of celiac disease. Sens. Actuators B Chem. 2013, 176, 1008–1014. [Google Scholar] [CrossRef]
- Feng, K.; Qiu, L.-P.; Yang, Y.; Wu, Z.-S.; Shen, G.-L.; Yu, R.-Q. Label-free optical bifunctional oligonucleotide probe for homogeneous amplification detection of disease markers. Biosens. Bioelectron. 2011, 29, 66–75. [Google Scholar] [CrossRef]
- Pan, T.; Lu, D.; Xin, H.; Li, B. Biophotonic probes for bio-detection and imaging. Light Sci. Appl. 2021, 10, 124. [Google Scholar] [CrossRef]
- Xiong, R.; Luan, J.; Kang, S.; Ye, C.; Singamaneni, S.; Tsukruk, V.V. Biopolymeric photonic structures: Design, fabrication, and emerging applications. Chem. Soc. Rev. 2020, 49, 983–1031. [Google Scholar] [CrossRef]
- Ballato, J.; Ebendorff-Heidepriem, H.; Zhao, J.; Petit, L.; Troles, J. Glass and process development for the next generation of optical fibers: A review. Fibers 2017, 5, 11. [Google Scholar] [CrossRef]
- Qiu, J.; Miura, K.; Hirao, K. Femtosecond laser-induced microfeatures in glasses and their applications. J. Non-Cryst. Solids 2008, 354, 1100–1111. [Google Scholar] [CrossRef]
- Bradley, D.; Hugtenburg, R.; Nisbet, A.; Rahman, A.T.A.; Issa, F.; Noor, N.M.; Alalawi, A. Review of doped silica glass optical fibre: Their TL properties and potential applications in radiation therapy dosimetry. Appl. Radiat. Isot. 2012, 71, 2–11. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, Q.-T.; Wang, Z.; Liu, Y.-X.; Qiu, C.-W.; Yang, L.; Gong, Q.; Xiao, Y.-F. Symmetry-breaking-induced nonlinear optics at a microcavity surface. Nat. Photonics 2019, 13, 21–24. [Google Scholar] [CrossRef]
- Xing, X.; Zhu, H.; Wang, Y.; Li, B. Ultracompact photonic coupling splitters twisted by PTT nanowires. Nano Lett. 2008, 8, 2839–2843. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.J.; Liu, Z.; Qian, Y.J.; Shi, K.; Sun, Y.; Wu, C.; Gong, Q.; Xiao, Y.F. A tunable optofluidic microlaser in a photostable conjugated polymer. Adv. Mater. 2018, 30, 1804556. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Gattass, R.R.; Ashcom, J.B.; He, S.; Lou, J.; Shen, M.; Maxwell, I.; Mazur, E. Subwavelength-diameter silica wires for low-loss optical wave guiding. Nature 2003, 426, 816–819. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Tadepalli, S.; Slocik, J.M.; Gupta, M.K.; Naik, R.R.; Singamaneni, S. Bio-Optics and Bio-Inspired Optical Materials. Chem. Rev. 2017, 117, 12705–12763. [Google Scholar] [CrossRef]
- Humar, M.; Kwok, S.J.J.; Choi, M.; Yetisen, A.K.; Cho, S.; Yun, S.-H. Toward biomaterial-based implantable photonic devices. Nanophotonics 2017, 6, 414–434. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Fan, X. Biological Lasers for Biomedical Applications. Adv. Opt. Mater. 2019, 7, 1900377. [Google Scholar] [CrossRef]
- Apter, B.; Lapshina, N.; Handelman, A.; Rosenman, G. Light waveguiding in bioinspired peptide nanostructures. J. Pept. Sci. 2019, 25, e3164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gong, X.; Yuan, Z.; Wang, W.; Chen, Y.-C. DNA Self-Switchable Microlaser. ACS Nano 2020, 14, 16122–16130. [Google Scholar] [CrossRef]
- Mysliwiec, J.; Cyprych, K.; Sznitko, L.; Miniewicz, A. Biomaterials in light amplification. J. Opt. 2017, 19, 033003. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Liu, Y.-C.; Zhang, Y.; Xiao, Y.-F.; Li, B. Optical Forces: From Fundamental to Biological Applications. Adv. Mater. 2020, 32, 2001994. [Google Scholar] [CrossRef]
- Puchkova, A.; Vietz, C.; Pibiri, E.; Wünsch, B.; Sanz Paz, M.; Acuna, G.P.; Tinnefeld, P. DNA Origami Nanoantennas with over 5000-fold Fluorescence Enhancement and Single-Molecule Detection at 25 μM. Nano Lett. 2015, 15, 8354–8359. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Liu, X.; Li, B. Escherichia coli-Based Biophotonic Waveguides. Nano Lett. 2013, 13, 3408–3413. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-H.; Moon, H.; Kim, H.; Lee, G.H.; Kwon, W.; Yoo, S.; Myung, D.; Yun, S.H.; Bao, Z.; Hahn, S.K. Multifunctional materials for implantable and wearable photonic healthcare devices. Nat. Rev. Mater. 2020, 5, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Kwok, S.J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 2017, 1, 0008. [Google Scholar] [CrossRef] [PubMed]
- Turco, A.; Corvaglia, S.; Pompa, P.P.; Malitesta, C. An innovative and simple all electrochemical approach to functionalize electrodes with a carbon nanotubes/polypyrrole molecularly imprinted nanocomposite and its application for sulfamethoxazole analysis. J. Colloid Interface Sci. 2021, 599, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhu, X.; Wu, X.J.; Zhang, B.; Liu, H.L. Selectively Manipulating Upconversion Emission Channels with Tunable Biological Photonic Crystals. J. Phys. Chem. C 2021, 125, 732–739. [Google Scholar] [CrossRef]
- Fan, X.; Yun, S.-H. The potential of optofluidic biolasers. Nat. Methods 2014, 11, 141–147. [Google Scholar] [CrossRef]
- Hales, J.E.; Matmon, G.; Dalby, P.A.; Ward, J.M.; Aeppli, G. Virus lasers for biological detection. Nat. Commun. 2019, 10, 3594. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Humar, M.; Martino, N.; Yun, S.H. Laser particle stimulated emission microscopy. Phys. Rev. Lett. 2016, 117, 193902. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, H.; Lee, W.; Sun, Y.; Zhu, D.; Pei, H.; Fan, C.; Fan, X. Self-assembled DNA tetrahedral optofluidic lasers with precise and tunable gain control. Lab A Chip 2013, 13, 3351–3354. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Volckaert, K.; Karl, M.; Morton, A.; Liehm, P.; Miles, G.B.; Powis, S.J.; Gather, M.C. Lasing in live mitotic and non-phagocytic cells by efficient delivery of microresonators. Sci. Rep. 2017, 7, 40877. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, Y.-C.; Zhang, Z.; Wu, B.; Coleman, R.; Fan, X. An integrated microwell array platform for cell lasing analysis. Lab A Chip 2017, 17, 2814–2820. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, Q.; Fan, X. Lasing in blood. Optica 2016, 3, 809–815. [Google Scholar] [CrossRef]
- Gongora, J.S.T.; Fratalocchi, A. Optical force on diseased blood cells: Towards the optical sorting of biological matter. Opt. Lasers Eng. 2016, 76, 40–44. [Google Scholar] [CrossRef]
- Song, Q.; Xiao, S.; Xu, Z.; Liu, J.; Sun, X.; Drachev, V.; Shalaev, V.M.; Akkus, O.; Kim, Y.L. Random lasing in bone tissue. Opt. Lett. 2010, 35, 1425–1427. [Google Scholar] [CrossRef]
- Chudakov, D.M.; Matz, M.V.; Lukyanov, S.; Lukyanov, K.A. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol. Rev. 2010, 90, 1103–1163. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, A.J.; Gather, M.C. Organic lasers: Recent developments on materials, device geometries, and fabrication techniques. Chem. Rev. 2016, 116, 12823–12864. [Google Scholar] [CrossRef] [PubMed]
- Gather, M.C.; Yun, S.H. Bio-optimized energy transfer in densely packed fluorescent protein enables near-maximal luminescence and solid-state lasers. Nat. Commun. 2014, 5, 5722. [Google Scholar] [CrossRef]
- Oh, H.J.; Gather, M.C.; Song, J.-J.; Yun, S.H. Lasing from fluorescent protein crystals. Opt. Express 2014, 22, 31411–31416. [Google Scholar] [CrossRef] [PubMed]
- Jonáš, A.; Aas, M.; Karadag, Y.; Manioğlu, S.; Anand, S.; McGloin, D.; Bayraktar, H.; Kiraz, A. In vitro and in vivo biolasing of fluorescent proteins suspended in liquid microdroplet cavities. Lab A Chip 2014, 14, 3093–3100. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Q.; Sun, Y.; Fan, X. Bio-inspired optofluidic lasers with luciferin. Appl. Phys. Lett. 2013, 102, 203706. [Google Scholar] [CrossRef]
- Nizamoglu, S.; Gather, M.C.; Yun, S.H. All-Biomaterial Laser Using Vitamin and Biopolymers. Adv. Mater. 2013, 25, 5943–5947. [Google Scholar] [CrossRef]
- Feng, J.; Yang, X.; Wang, T.; Du, T.; Zhuo, J.; Zhang, P.; Zhang, L.; Sun, H.; Han, Y.; Xia, Y.; et al. Reassembled One-Dimensional VB2 Submicrorods with Enhanced Photosensitivity and H2O2 Supply for Efficient Antibacterial Therapy. ACS Sustain. Chem. Eng. 2023, 11, 13081–13095. [Google Scholar] [CrossRef]
- Siegman, A.E. Lasers; University Science Books: Herndon, VA, USA, 1986. [Google Scholar]
- Toropov, N.; Cabello, G.; Serrano, M.P.; Gutha, R.R.; Rafti, M.; Vollmer, F. Review of biosensing with whispering-gallery mode lasers. Light Sci. Appl. 2021, 10, 42. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Tan, X.; Sun, Q.; Chen, Q.; Wang, W.; Fan, X. Laser-emission imaging of nuclear biomarkers for high-contrast cancer screening and immunodiagnosis. Nat. Biomed. Eng. 2017, 1, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Glyn MacDonald, R.; Yakovlev, A.; Pacheco-Peña, V. Time derivatives via interconnected waveguides. Sci. Rep. 2023, 13, 13126. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Steude, A.; Liehm, P.; Kronenberg, N.M.; Karl, M.; Campbell, E.C.; Powis, S.J.; Gather, M.C. Lasing within Live Cells Containing Intracellular Optical Microresonators for Barcode-Type Cell Tagging and Tracking. Nano Lett. 2015, 15, 5647–5652. [Google Scholar] [CrossRef] [PubMed]
- Humar, M.; Gather, M.C.; Yun, S.-H. Cellular dye lasers: Lasing thresholds and sensing in a planar resonator. Opt. Express 2015, 23, 27865–27879. [Google Scholar] [CrossRef] [PubMed]
- Gather, M.C.; Yun, S.H. Single-cell biological lasers. Nat. Photonics 2011, 5, 406–410. [Google Scholar] [CrossRef]
- Gather, M.C.; Yun, S.H. Lasing from Escherichia coli bacteria genetically programmed to express green fluorescent protein. Opt. Lett. 2011, 36, 3299–3301. [Google Scholar] [CrossRef]
- Abbadessa, A.; Blokzijl, M.M.; Mouser, V.H.; Marica, P.; Malda, J.; Hennink, W.E.; Vermonden, T. A thermo-responsive and photo-polymerizable chondroitin sulfate-based hydrogel for 3D printing applications. Carbohydr. Polym. 2016, 149, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Karl, M.; Dietrich, C.; Schubert, M.; Samuel, I.; Turnbull, G.; Gather, M. Single cell induced optical confinement in biological lasers. J. Phys. D Appl. Phys. 2017, 50, 084005. [Google Scholar] [CrossRef]
- Nizamoglu, S.; Lee, K.B.; Gather, M.C.; Kim, K.S.; Jeon, M.; Kim, S.; Humar, M.; Yun, S.H. A simple approach to biological single-cell lasers via intracellular dyes. Adv. Opt. Mater. 2015, 3, 1197–1200. [Google Scholar] [CrossRef]
- Fikouras, A.H.; Schubert, M.; Karl, M.; Kumar, J.D.; Powis, S.J.; Di Falco, A.; Gather, M.C. Non-obstructive intracellular nanolasers. Nat. Commun. 2018, 9, 4817. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, X. Distinguishing DNA by Analog-to-Digital-like Conversion by Using Optofluidic Lasers. Angew. Chem. Int. Ed. 2012, 51, 1236–1239. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, Q.; Zhang, T.; Wang, W.; Fan, X. Versatile tissue lasers based on high-Q Fabry–Pérot microcavities. Lab A Chip 2017, 17, 538–548. [Google Scholar] [CrossRef]
- Septiadi, D.; Barna, V.; Saxena, D.; Sapienza, R.; Genovese, D.; De Cola, L. Biolasing from Individual cells in a low-Q resonator enables spectral fingerprinting. Adv. Opt. Mater. 2020, 8, 1901573. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Su, X.; Gao, D.; Yu, H. CdTe quantum dot-based self-supporting films with enhanced stability for flexible light-emitting devices. Soft Matter. 2022, 18, 7324–7332. [Google Scholar] [CrossRef] [PubMed]
- Matsko, A.B.; Ilchenko, V.S. Optical resonators with whispering-gallery modes-part I: Basics. IEEE J. Sel. Top. Quantum Electron. 2006, 12, 3–14. [Google Scholar] [CrossRef]
- Jiang, X.; Qavi, A.J.; Huang, S.H.; Yang, L. Whispering-gallery sensors. Matter 2020, 3, 371–392. [Google Scholar] [CrossRef]
- Zhi, Y.; Yu, X.C.; Gong, Q.; Yang, L.; Xiao, Y.F. Single nanoparticle detection using optical microcavities. Adv. Mater. 2017, 29, 1604920. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Özdemir, Ş.K.; Yang, L. Whispering gallery microcavity lasers. Laser Photonics Rev. 2013, 7, 60–82. [Google Scholar] [CrossRef]
- Guo, C.; Hu, J.; Kao, L.; Pan, D.; Luo, K.; Li, N.; Gu, Z. Pepetide Dendron-Functionalized Mesoporous Silica Nanoparticle-Based Nanohybrid: Biocompatibility and Its Potential as Imaging Probe. ACS Biomater. Sci. Eng. 2016, 2, 860–870. [Google Scholar] [CrossRef]
- Kamoshita, A.; Kohno, J.-Y. Cavity-Enhanced Fluorescence in Colliding Droplets of Rhodamine 6G Aqueous Solutions. J. Phys. Chem. A 2023, 127, 7605–7611. [Google Scholar] [CrossRef]
- Ma, Y.; Nolte, R.J.; Cornelissen, J.J. Virus-based nanocarriers for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 811–825. [Google Scholar] [CrossRef]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef]
- Kutter, E.; De Vos, D.; Gvasalia, G.; Alavidze, Z.; Gogokhia, L.; Kuhl, S.; Abedon, S.T. Phage therapy in clinical practice: Treatment of human infections. Curr. Pharm. Biotechnol. 2010, 11, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Morales, N.M.; McClean, M.N. Engineered bacteria self-organize to sense pressure. Nat. Biotechnol. 2017, 35, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Peivandi, A.; Tian, L.; Mahabir, R.; Hosseinidoust, Z. Hierarchically structured, self-healing, fluorescent, bioactive hydrogels with self-organizing bundles of phage nanofilaments. Chem. Mater. 2019, 31, 5442–5449. [Google Scholar] [CrossRef]
- Lee, J.H.; Warner, C.M.; Jin, H.-E.; Barnes, E.; Poda, A.R.; Perkins, E.J.; Lee, S.-W. Production of tunable nanomaterials using hierarchically assembled bacteriophages. Nat. Protoc. 2017, 12, 1999–2013. [Google Scholar] [CrossRef] [PubMed]
- Arter, J.A.; Taggart, D.K.; McIntire, T.M.; Penner, R.M.; Weiss, G.A. Virus-PEDOT nanowires for biosensing. Nano Lett. 2010, 10, 4858–4862. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, H.; Song, D.; Xu, K.; Zheng, Y.; Xiao, H.; Liu, Y.; Li, J.; Song, X. Simultaneous detection of three zoonotic pathogens based on phage display peptide and multicolor quantum dots. Anal. Biochem. 2020, 608, 113854. [Google Scholar] [CrossRef]
- Yuan, Z.; Cheng, X.; Zhou, Y.; Tan, X.; Gong, X.; Rivy, H.; Gong, C.; Fan, X.; Wang, W.-J.; Chen, Y.-C. Distinguishing Small Molecules in Microcavity with Molecular Laser Polarization. ACS Photonics 2020, 7, 1908–1914. [Google Scholar] [CrossRef]
- Ignesti, E.; Tommasi, F.; Fini, L.; Martelli, F.; Azzali, N.; Cavalieri, S. A new class of optical sensors: A random laser based device. Sci. Rep. 2016, 6, 35225. [Google Scholar] [CrossRef]
- Polson, R.C.; Vardeny, Z.V. Random lasing in human tissues. Appl. Phys. Lett. 2004, 85, 1289–1291. [Google Scholar] [CrossRef]
- Briones-Herrera, J.; Cuando-Espitia, N.; Sánchez-Arévalo, F.; Hernández-Cordero, J. Evaluation of mechanical behavior of soft tissue by means of random laser emission. Rev. Sci. Instrum. 2013, 84, 104301. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; He, N.; Jacques, S.L.; Thomsen, S.L. Biological laser action. Appl. Opt. 1996, 35, 1775–1779. [Google Scholar] [CrossRef] [PubMed]
- Lahoz, F.; Acebes, A.; González-Hernández, T.; de Armas-Rillo, S.; Soler-Carracedo, K.; Cuesto, G.; Mesa-Infante, V. Random lasing in brain tissues. Org. Electron. 2019, 75, 105389. [Google Scholar] [CrossRef]
- Lahoz, F.; Martín, I.R.; Urgellés, M.; Marrero-Alonso, J.; Marín, R.; Saavedra, C.J.; Boto, A.; Díaz, M. Random laser in biological tissues impregnated with a fluorescent anticancer drug. Laser Phys. Lett. 2015, 12, 045805. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, Z.; Qiu, Z.; Zhang, P.; Wu, J.; Zhang, D.; Xiang, T. Random lasing in human tissues embedded with organic dyes for cancer diagnosis. Sci. Rep. 2017, 7, 8385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Y.; Tang, J.; Mu, H. Random laser marked PLCD1 gene therapy effect on human breast cancer. J. Appl. Phys. 2019, 125, 203102. [Google Scholar] [CrossRef]
- Hohmann, M.; Dörner, D.; Mehari, F.; Chen, C.; Späth, M.; Müller, S.; Albrecht, H.; Klämpfl, F.; Schmidt, M. Investigation of random lasing as a feedback mechanism for tissue differentiation during laser surgery. Biomed. Opt. Express 2019, 10, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Humar, M.; Dobravec, A.; Zhao, X.; Yun, S.H. Biomaterial microlasers implantable in the cornea, skin, and blood. Optica 2017, 4, 1080–1085. [Google Scholar] [CrossRef]
- Martino, N.; Kwok, S.J.J.; Liapis, A.C.; Forward, S.; Jang, H.; Kim, H.-M.; Wu, S.J.; Wu, J.; Dannenberg, P.H.; Jang, S.-J.; et al. Wavelength-encoded laser particles for massively multiplexed cell tagging. Nat. Photonics 2019, 13, 720–727. [Google Scholar] [CrossRef]
- Jiang, X.F.; Zou, C.L.; Wang, L.; Gong, Q.; Xiao, Y.F. Whispering-gallery microcavities with unidirectional laser emission. Laser Photonics Rev. 2016, 10, 40–61. [Google Scholar] [CrossRef]
- Tang, S.-J.; Dannenberg, P.H.; Liapis, A.C.; Martino, N.; Zhuo, Y.; Xiao, Y.-F.; Yun, S.-H. Laser particles with omnidirectional emission for cell tracking. Light Sci. Appl. 2021, 10, 23. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Wang, W.Y.; Wu, X.; Li, Y.; Tan, X.; Matera, D.L.; Baker, B.M.; Paulus, Y.M.; Fan, X. Optical coherence tomography and fluorescence microscopy dual-modality imaging for in vivo single-cell tracking with nanowire lasers. Biomed. Opt. Express 2020, 11, 3659–3672. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chen, Q.; Wu, X.; Tan, X.; Wang, J.; Fan, X. A robust tissue laser platform for analysis of formalin-fixed paraffin-embedded biopsies. Lab A Chip 2018, 18, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chen, Q.; Tan, X.; Chen, G.; Bergin, I.; Aslam, M.N.; Fan, X. Chromatin laser imaging reveals abnormal nuclear changes for early cancer detection. Biomed. Opt. Express 2019, 10, 838–854. [Google Scholar] [CrossRef]
- Tan, X.; Chen, Q.; Zhu, H.; Zhu, S.; Gong, Y.; Wu, X.; Chen, Y.-C.; Li, X.; Li, M.W.-H.; Liu, W. Fast and reproducible ELISA laser platform for ultrasensitive protein quantification. ACS Sens. 2019, 5, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Li, X.; Zhu, H.; Weng, W.-H.; Tan, X.; Chen, Q.; Sun, Y.; Wang, X.; Fan, X. Monitoring neuron activities and interactions with laser emissions. ACS Photonics 2020, 7, 2182–2189. [Google Scholar] [CrossRef]
- Schubert, M.; Woolfson, L.; Barnard, I.R.; Dorward, A.M.; Casement, B.; Morton, A.; Robertson, G.B.; Appleton, P.L.; Miles, G.B.; Tucker, C.S. Monitoring contractility in cardiac tissue with cellular resolution using biointegrated microlasers. Nat. Photonics 2020, 14, 452–458. [Google Scholar] [CrossRef]
- Lv, Y.; Xiong, X.; Liu, Y.; Yao, J.; Li, Y.J.; Zhao, Y.S. Controlled Outcoupling of Whispering-Gallery-Mode Lasers Based on Self-Assembled Organic Single-Crystalline Microrings. Nano Lett. 2019, 19, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, A.; Wittmann, T. Fluorescence live cell imaging. Methods Cell Biol. 2014, 123, 77–94. [Google Scholar]
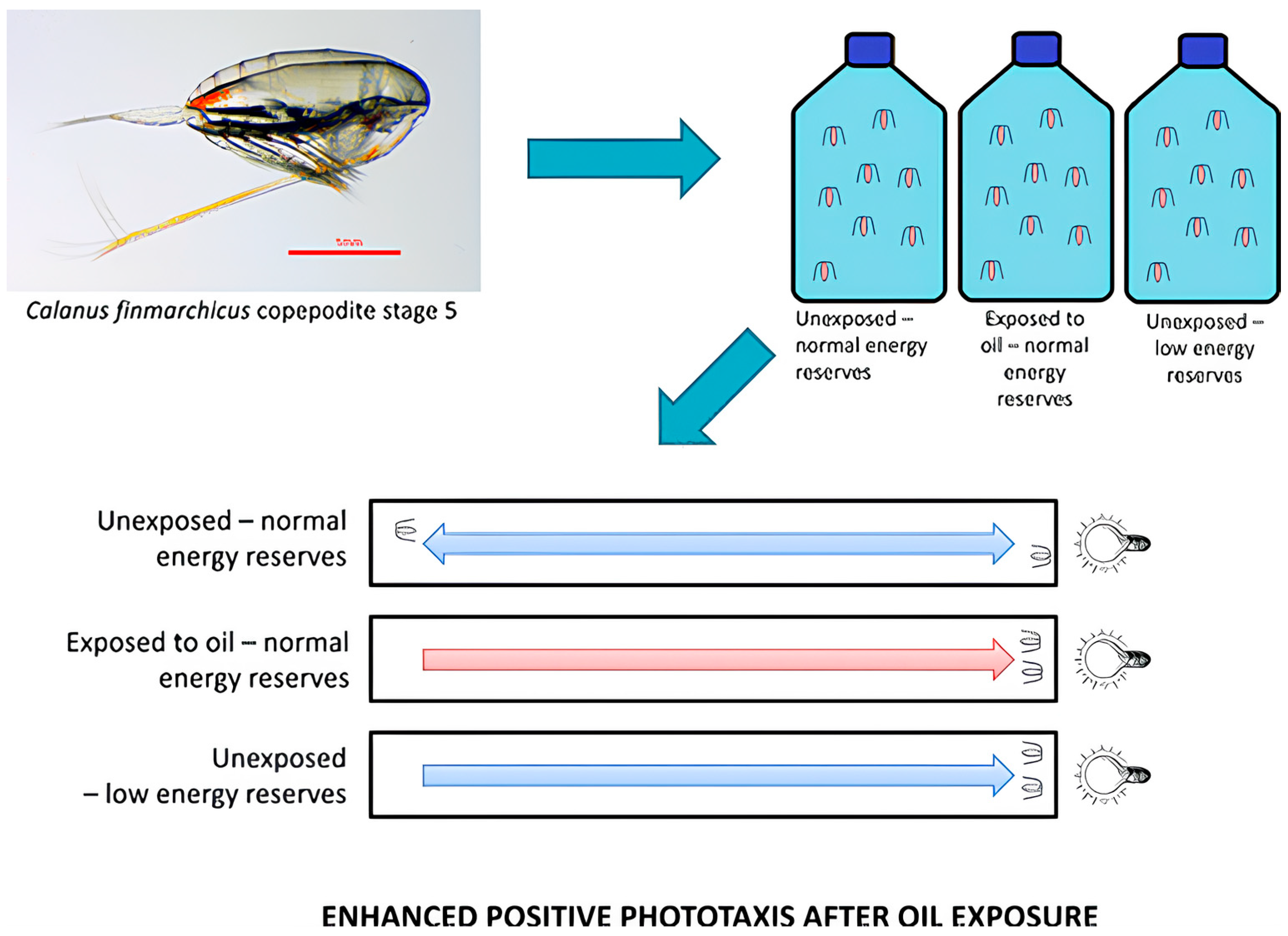
- Miljeteig, C.; Olsen, A.J.; Nordtug, T.; Altin, D.; Jenssen, B.M. Sublethal Exposure to Crude Oil Enhances Positive Phototaxis in the Calanoid Copepod Calanus finmarchicus. Environ. Sci. Technol. 2013, 47, 14426–14433. [Google Scholar] [CrossRef]
- Wang, C.; Tao, H.; Cheng, L.; Liu, Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials 2011, 32, 6145–6154. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yao, Y.; Zhao, X.; Tan, N. Programmable site-specific delivery of an alkaline phosphatase-activatable prodrug and a mitochondria-targeted cyclopeptide for combination therapy in colon cancer. Biomater. Sci. 2023. [Google Scholar] [CrossRef] [PubMed]
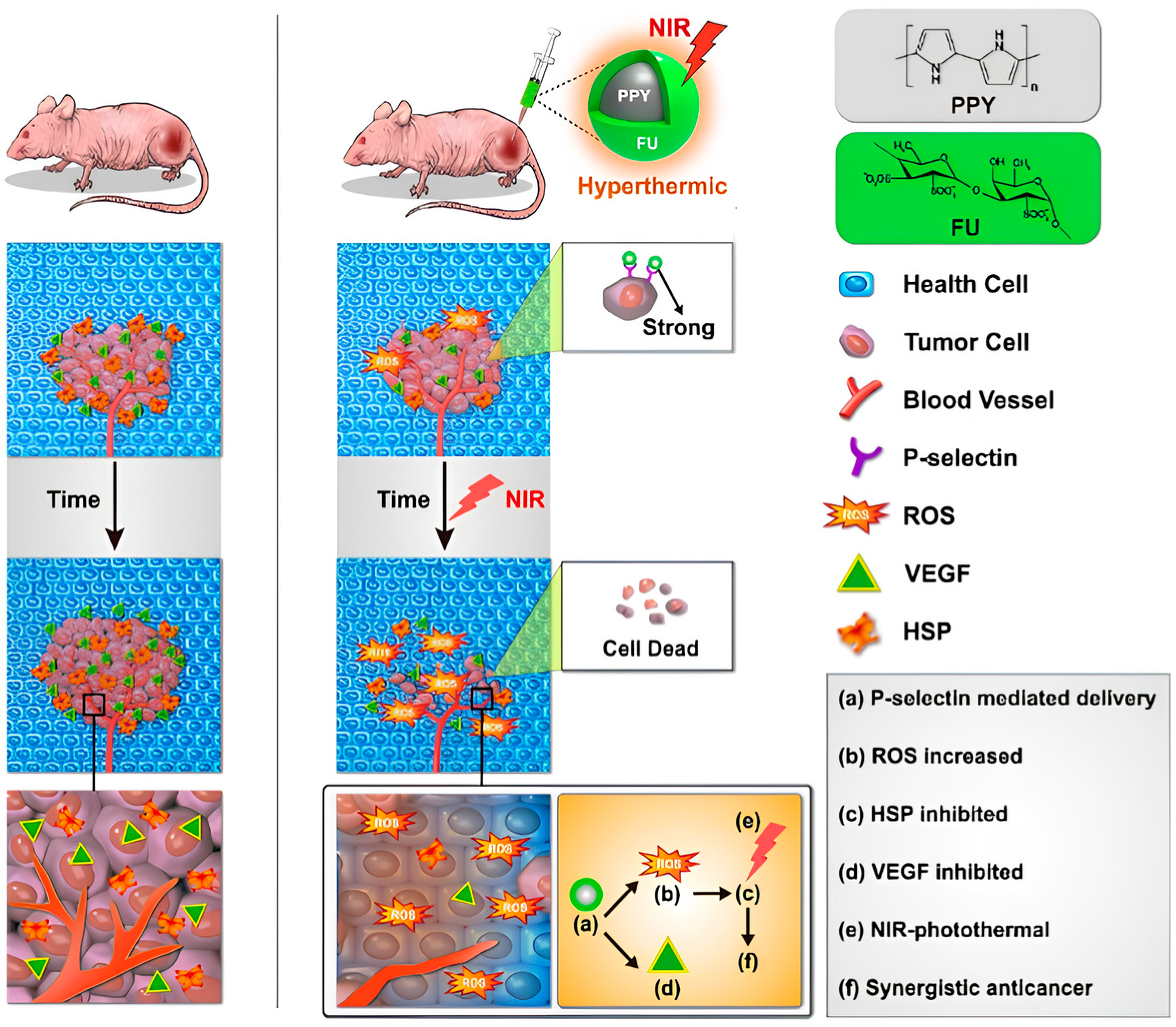
- Lu, K.-Y.; Jheng, P.-R.; Lu, L.-S.; Rethi, L.; Mi, F.-L.; Chuang, E.-Y. Enhanced anticancer effect of ROS-boosted photothermal therapy by using fucoidan-coated polypyrrole nanoparticles. Int. J. Biol. Macromol. 2021, 166, 98–107. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Jheng, P.-R.; Nguyen, H.T.; Chen, Y.-H.; Manga, Y.B.; Lu, L.-S.; Rethi, L.; Chen, C.-H.; Huang, T.-W.; Lin, J.-D.; et al. Photothermal-Irradiated Polyethyleneimine–Polypyrrole Nanopigment Film-Coated Polyethylene Fabrics for Infrared-Inspired with Pathogenic Evaluation. ACS Appl. Mater. Interfaces 2021, 13, 2483–2495. [Google Scholar] [CrossRef] [PubMed]
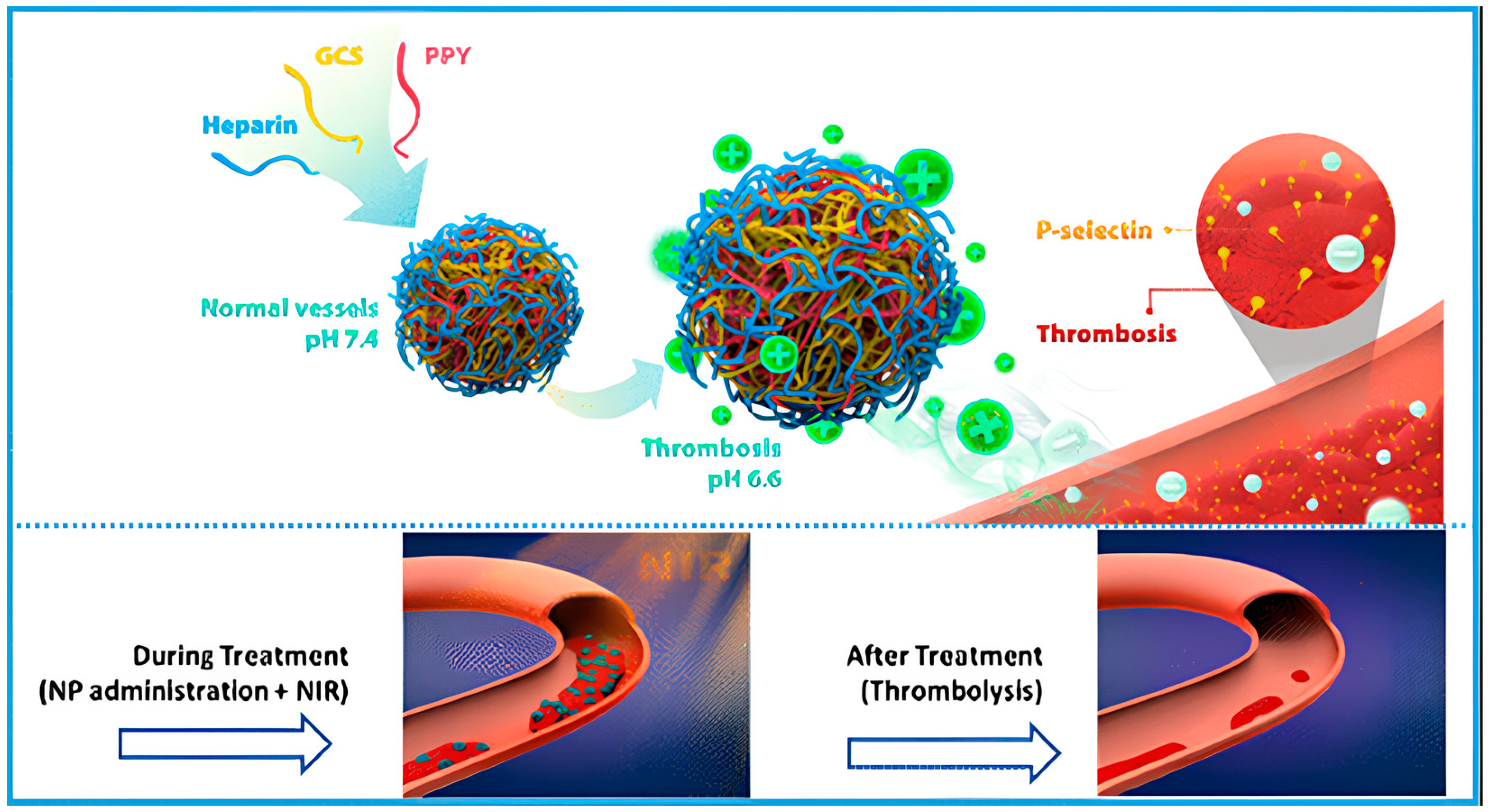
- Lu, T.-Y.; Chiang, C.-Y.; Fan, Y.-J.; Jheng, P.-R.; Quiñones, E.D.; Liu, K.-T.; Kuo, S.-H.; Hsieh, H.Y.; Tseng, C.-L.; Yu, J.; et al. Dual-Targeting Glycol Chitosan/Heparin-Decorated Polypyrrole Nanoparticle for Augmented Photothermal Thrombolytic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 10287–10300. [Google Scholar] [CrossRef]
- Dianov, E.M. Bismuth-doped optical fibers: A challenging active medium for near-IR lasers and optical amplifiers. Light Sci. Appl. 2012, 1, e12. [Google Scholar] [CrossRef]
- Haddock, S.H.; Moline, M.A.; Case, J.F. Bioluminescence in the sea. Annu. Rev. Mar. Sci. 2010, 2, 443–493. [Google Scholar] [CrossRef] [PubMed]
- Oertner, T.G. The Bright Side of Life; Nature Publishing Group: London, UK, 2006. [Google Scholar]
- Lee, H.-J.; Ha, J.-H.; Kim, S.-G.; Choi, H.-K.; Kim, Z.H.; Han, Y.-J.; Kim, J.-I.; Oh, Y.; Fragoso, V.; Shin, K. Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots. Sci. Signal. 2016, 9, ra106. [Google Scholar] [CrossRef] [PubMed]
- Sundar, V.C.; Yablon, A.D.; Grazul, J.L.; Ilan, M.; Aizenberg, J. Fibre-optical features of a glass sponge. Nature 2003, 424, 899–900. [Google Scholar] [CrossRef]
- Franze, K.; Grosche, J.; Skatchkov, S.N.; Schinkinger, S.; Foja, C.; Schild, D.; Uckermann, O.; Travis, K.; Reichenbach, A.; Guck, J. Müller cells are living optical fibers in the vertebrate retina. Proc. Natl. Acad. Sci. USA 2007, 104, 8287–8292. [Google Scholar] [CrossRef]
- Agte, S.; Junek, S.; Matthias, S.; Ulbricht, E.; Erdmann, I.; Wurm, A.; Schild, D.; Käs, J.A.; Reichenbach, A. Müller glial cell-provided cellular light guidance through the vital guinea-pig retina. Biophys. J. 2011, 101, 2611–2619. [Google Scholar] [CrossRef] [PubMed]
- Pena-Francesch, A.; Florez, S.; Jung, H.; Sebastian, A.; Albert, I.; Curtis, W.; Demirel, M.C. Materials fabrication from native and recombinant thermoplastic squid proteins. Adv. Funct. Mater. 2014, 24, 7401–7409. [Google Scholar] [CrossRef]
- Shan, D.; Gerhard, E.; Zhang, C.; Tierney, J.W.; Xie, D.; Liu, Z.; Yang, J. Polymeric biomaterials for biophotonic applications. Bioact. Mater. 2018, 3, 434–445. [Google Scholar] [CrossRef]
- Jeevarathinam, A.S.; Pai, N.; Huang, K.; Hariri, A.; Wang, J.; Bai, Y.; Wang, L.; Hancock, T.; Keys, S.; Penny, W. A cellulose-based photoacoustic sensor to measure heparin concentration and activity in human blood samples. Biosens. Bioelectron. 2019, 126, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Nazempour, R.; Zhang, Q.; Fu, R.; Sheng, X. Biocompatible and implantable optical fibers and waveguides for biomedicine. Materials 2018, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Monks, J.N.; Yan, B.; Hawkins, N.; Vollrath, F.; Wang, Z. Spider silk: Mother nature’s bio-superlens. Nano Lett. 2016, 16, 5842–5845. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.T.; Domachuk, P.; Amsden, J.; Bressner, J.; Lewis, J.A.; Kaplan, D.L.; Omenetto, F.G. Biocompatible silk printed optical waveguides. Adv. Mater. 2009, 21, 2411–2415. [Google Scholar] [CrossRef]
- Li, D.; Wang, L. Cellulose acetate polymer film modified microstructured polymer optical fiber towards a nitrite optical probe. Opt. Commun. 2010, 283, 2841–2844. [Google Scholar] [CrossRef]
- Roxby, D.N.; Yuan, Z.; Krishnamoorthy, S.; Wu, P.; Tu, W.C.; Chang, G.E.; Lau, R.; Chen, Y.C. Enhanced biophotocurrent generation in living photosynthetic optical resonator. Adv. Sci. 2020, 7, 1903707. [Google Scholar] [CrossRef]
- Huby, N.; Vié, V.; Renault, A.; Beaufils, S.; Lefevre, T.; Paquet-Mercier, F.; Pézolet, M.; Bêche, B. Native spider silk as a biological optical fiber. Appl. Phys. Lett. 2013, 102, 123702. [Google Scholar] [CrossRef]
- Sun, Y.-L.; Sun, S.-M.; Wang, P.; Dong, W.-F.; Zhang, L.; Xu, B.-B.; Chen, Q.-D.; Tong, L.-M.; Sun, H.-B. Customization of Protein Single Nanowires for Optical Biosensing. Small 2015, 11, 2869–2876. [Google Scholar] [CrossRef]
- Shabahang, S.; Kim, S.; Yun, S.H. Light-guiding biomaterials for biomedical applications. Adv. Funct. Mater. 2018, 28, 1706635. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Li, B. Bacteria-based branched structures for bionanophotonics. Laser Photonics Rev. 2015, 9, 554–563. [Google Scholar] [CrossRef]
- Fardad, S.; Salandrino, A.; Heinrich, M.; Zhang, P.; Chen, Z.; Christodoulides, D.N. Plasmonic resonant solitons in metallic nanosuspensions. Nano Lett. 2014, 14, 2498–2504. [Google Scholar] [CrossRef]
- Kelly, T.S.; Ren, Y.-X.; Samadi, A.; Bezryadina, A.; Christodoulides, D.; Chen, Z. Guiding and nonlinear coupling of light in plasmonic nanosuspensions. Opt. Lett. 2016, 41, 3817–3820. [Google Scholar] [CrossRef]
- Yashin, V.; Chizhov, S.; Sabirov, R.; Starchikova, T.; Vysotina, N.; Rozanov, N.; Semenov, V.; Smirnov, V.; Fedorov, S. Formation of soliton-like light beams in an aqueous suspension of polystyrene particles. Opt. Spectrosc. 2005, 98, 466–469. [Google Scholar] [CrossRef]
- Bezryadina, A.; Hansson, T.; Gautam, R.; Wetzel, B.; Siggins, G.; Kalmbach, A.; Lamstein, J.; Gallardo, D.; Carpenter, E.J.; Ichimura, A. Nonlinear self-action of light through biological suspensions. Phys. Rev. Lett. 2017, 119, 058101. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Xiang, Y.; Lamstein, J.; Liang, Y.; Bezryadina, A.; Liang, G.; Hansson, T.; Wetzel, B.; Preece, D.; White, A. Optical force-induced nonlinearity and self-guiding of light in human red blood cell suspensions. Light Sci. Appl. 2019, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Perez, N.; Chambers, J.; Chen, Z.; Bezryadina, A. Nonlinear self-trapping and guiding of light at different wavelengths with sheep blood. Opt. Lett. 2021, 46, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Chen, C.H.; Tan, S.J.; Tseng, C.L.; Lu, K.Y.; Chang, L.H.; Nyambat, B.; Huang, S.C.; Jheng, P.R.; Aditya, R.N.; et al. A bioinspired hyperthermic macrophage-based polypyrrole-polyethylenimine (Ppy-PEI) nanocomplex carrier to prevent and disrupt thrombotic fibrin clots. Acta Biomater. 2019, 96, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Li, S.; Cao, W.; Mo, C.; Zhang, Z.; Huang, K.; Li, X. Bacteria-Templated and Dual Enzyme-Powered Microcapsule Motors To Promote Thrombus Penetration and Thrombolytic Efficacy. ACS Appl. Mater. Interfaces 2022, 14, 37553–37565. [Google Scholar] [CrossRef]
- Chang, L.H.; Chuang, E.Y.; Cheng, T.M.; Lin, C.; Shih, C.M.; Wu, A.T.; Jheng, P.R.; Lu, H.Y.; Shih, C.C.; Mi, F.L. Thrombus-specific theranostic nanocomposite for codelivery of thrombolytic drug, algae-derived anticoagulant and NIR fluorescent contrast agent. Acta Biomater. 2021, 134, 686–701. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Liu, C.-H.; Pan, W.-Y.; Jheng, P.-R.; Hsieh, Y.S.; Burnouf, T.; Fan, Y.-J.; Chiang, C.-C.; Chen, T.-Y.; Chuang, E.-Y. Biomimetic Platelet Nanomotors for Site-Specific Thrombolysis and Ischemic Injury Alleviation. ACS Appl. Mater. Interfaces 2023, 15, 32967–32983. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.T.; Quiñones, E.D.; Liu, M.H.; Lin, C.W.; Chen, Y.T.; Chiang, C.C.; Wu, K.C.W.; Fan, Y.J.; Chuang, E.Y.; Yu, J. A Biomimicking and Multiarm Self-Indicating Nanoassembly for Site-Specific Photothermal-Potentiated Thrombolysis Assessed in Microfluidic and In Vivo Models. Adv. Healthc. Mater. 2023, 12, 2300682. [Google Scholar] [CrossRef]
- Liu, C.H.; Liu, M.C.; Jheng, P.R.; Yu, J.; Fan, Y.J.; Liang, J.W.; Hsiao, Y.C.; Chiang, C.W.; Bolouki, N.; Lee, J.W. Plasma-Derived Nanoclusters for Site-Specific Multimodality Photo/Magnetic Thrombus Theranostics. Adv. Healthc. Mater. 2023, 2301504. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Jheng, P.-R.; Rethi, L.; Godugu, C.; Lee, C.Y.; Chen, Y.-T.; Nguyen, H.T.; Chuang, E.-Y. P-Selectin mediates targeting of a self-assembling phototherapeutic nanovehicle enclosing dipyridamole for managing thromboses. J. Nanobiotechnol. 2023, 21, 260. [Google Scholar] [CrossRef]
- Liang, J.-W.; Liu, C.-H.; Wu, K.C.-W.; Jheng, P.-R.; Chuang, E.-Y. Plasma-treated nano-enabled multimodal coatings made of phototherapeutic molybdenum disulfide and fucoidan prevent catheter-associated urinary tract issues. Chem. Eng. J. 2023, 468, 143749. [Google Scholar] [CrossRef]
- Burnouf, T.; Jheng, P.-R.; Chen, Y.-H.; Rethi, L.; Rethi, L.; Lu, L.-S.; Ho, Y.-C.; Chuang, E.-Y. Near-infrared-driven photoablation of lung cancer tumors utilizing biomimetic platelet-polyethyleneimine-polypyrrole drug-free nanoparticles. Mater. Des. 2022, 215, 110481. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, H.; Ye, C.; Chen, S.; Ning, J.; Halim, M.A.; Donaev, S.B.; Wang, S. Fabrication of Iron Pyrite Thin Films and Photovoltaic Devices by Sulfurization in Electrodeposition Method. Nanomaterials 2021, 11, 2844. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-W.; Chuang, E.-Y. Biofunctional core-shell polypyrrole–polyethylenimine nanocomplex for a locally sustained photothermal with reactive oxygen species enhanced therapeutic effect against lung cancer. Int. J. Nanomed. 2019, 14, 1575–1585. [Google Scholar] [CrossRef]
- Mi, F.L.; Burnouf, T.; Lu, S.Y.; Lu, Y.J.; Lu, K.Y.; Ho, Y.C.; Kuo, C.Y.; Chuang, E.Y. Self-Targeting, Immune Transparent Plasma Protein Coated Nanocomplex for Noninvasive Photothermal Anticancer Therapy. Adv. Healthc. Mater. 2017, 6, 1700181. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Nyambat, B.; Chiang, C.-W.; Chen, C.-H.; Wong, P.-C.; Ho, P.-H.; Jheng, P.-R.; Burnouf, T.; Tseng, C.-L.; Chuang, E.-Y. A gelatin hydrogel-containing nano-organic PEI–Ppy with a photothermal responsive effect for tissue engineering applications. Molecules 2018, 23, 1256. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-W.; Hsiao, Y.-C.; Jheng, P.-R.; Chen, C.-H.; Manga, Y.B.; Lekha, R.; Chao, K.-M.; Ho, Y.-C.; Chuang, E.-Y. Strontium ranelate-laden near-infrared photothermal-inspired methylcellulose hydrogel for arthritis treatment. Mater. Sci. Eng. C 2021, 123, 111980. [Google Scholar] [CrossRef] [PubMed]
- Bolouki, N.; Hsu, Y.-N.; Hsiao, Y.-C.; Jheng, P.-R.; Hsieh, J.-H.; Chen, H.-L.; Mansel, B.W.; Yeh, Y.-Y.; Chen, Y.-H.; Lu, C.-X. Cold atmospheric plasma physically reinforced substances of platelets-laden photothermal-responsive methylcellulose complex restores burn wounds. Int. J. Biol. Macromol. 2021, 192, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Chuang, E.-Y.; Huang, W.-H.; Ho, T.-L.; Wang, P.-C.; Hsiao, Y.-C. IR-inspired visual display/response device fabricated using photothermal liquid crystals for medical and display applications. Chem. Eng. J. 2022, 429, 132213. [Google Scholar] [CrossRef]
- Li, Y.; Xin, H.; Zhang, Y.; Lei, H.; Zhang, T.; Ye, H.; Saenz, J.J.; Qiu, C.-W.; Li, B. Living Nanospear for Near-Field Optical Probing. ACS Nano 2018, 12, 10703–10711. [Google Scholar] [CrossRef]
- Wu, T.; Chen, X.; Gong, Z.; Li, Y.; Zhang, Y. Waveguiding and focusing in a bio-medium with an optofluidic cell chain. Acta Biomater. 2020, 103, 165–171. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Xu, X.; Xin, H.; Zhang, Y.; Li, B. Red-Blood-Cell Waveguide as a Living Biosensor and Micromotor. Adv. Funct. Mater. 2019, 29, 1905568. [Google Scholar] [CrossRef]
- Arano-Martinez, J.A.; Martínez-González, C.L.; Salazar, M.I.; Torres-Torres, C. A Framework for Biosensors Assisted by Multiphoton Effects and Machine Learning. Biosensors 2022, 12, 710. [Google Scholar] [CrossRef]


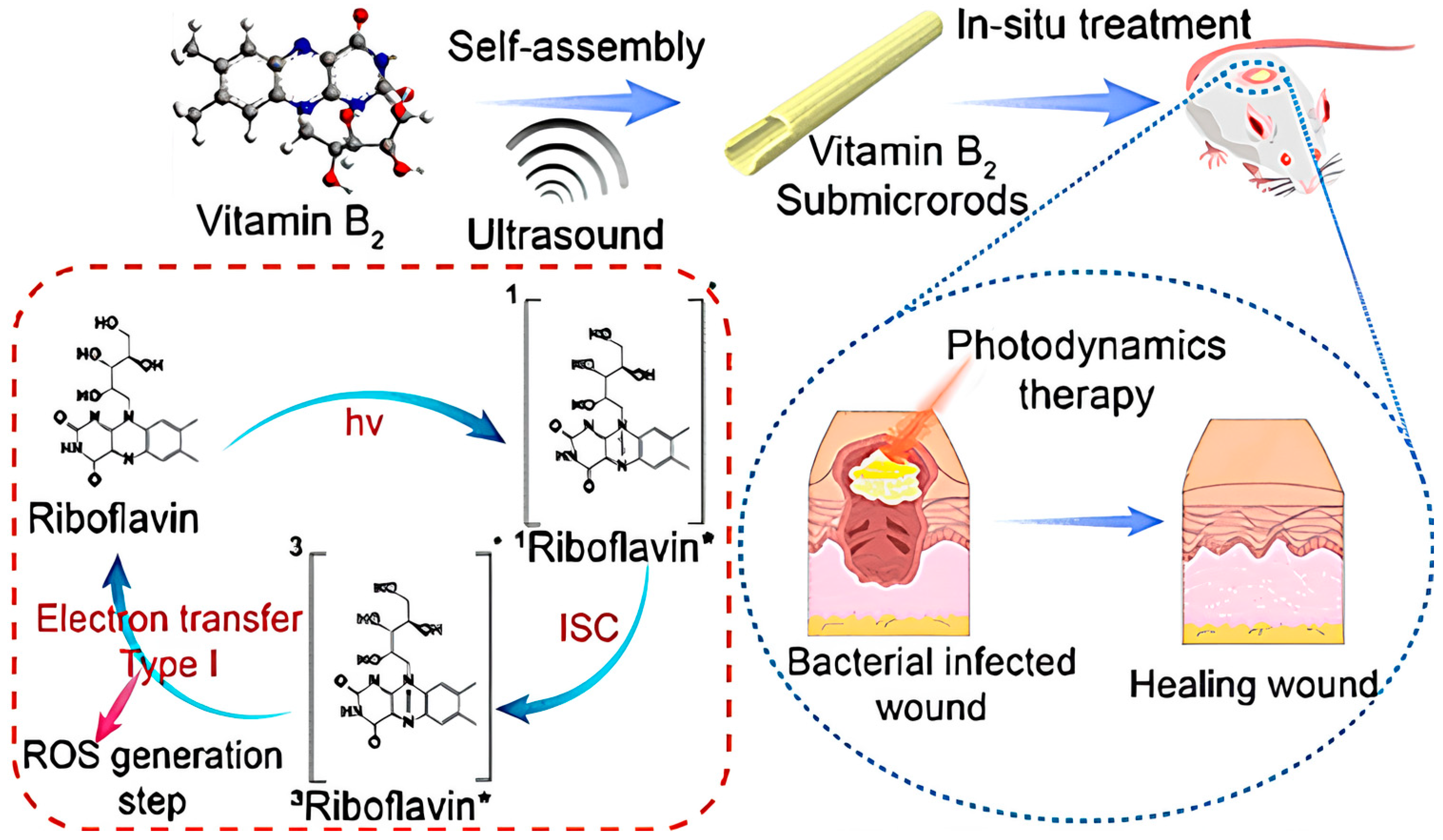
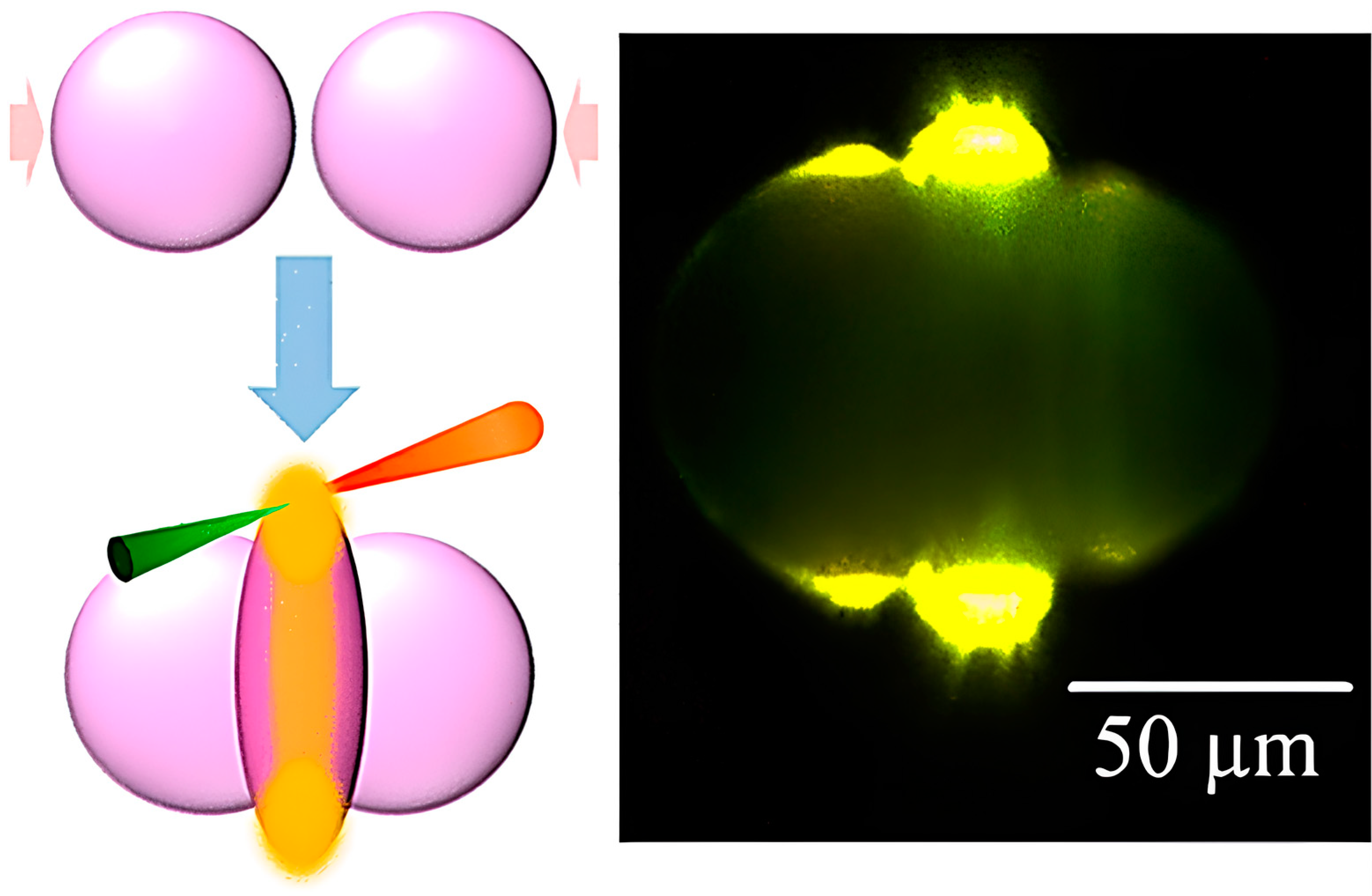
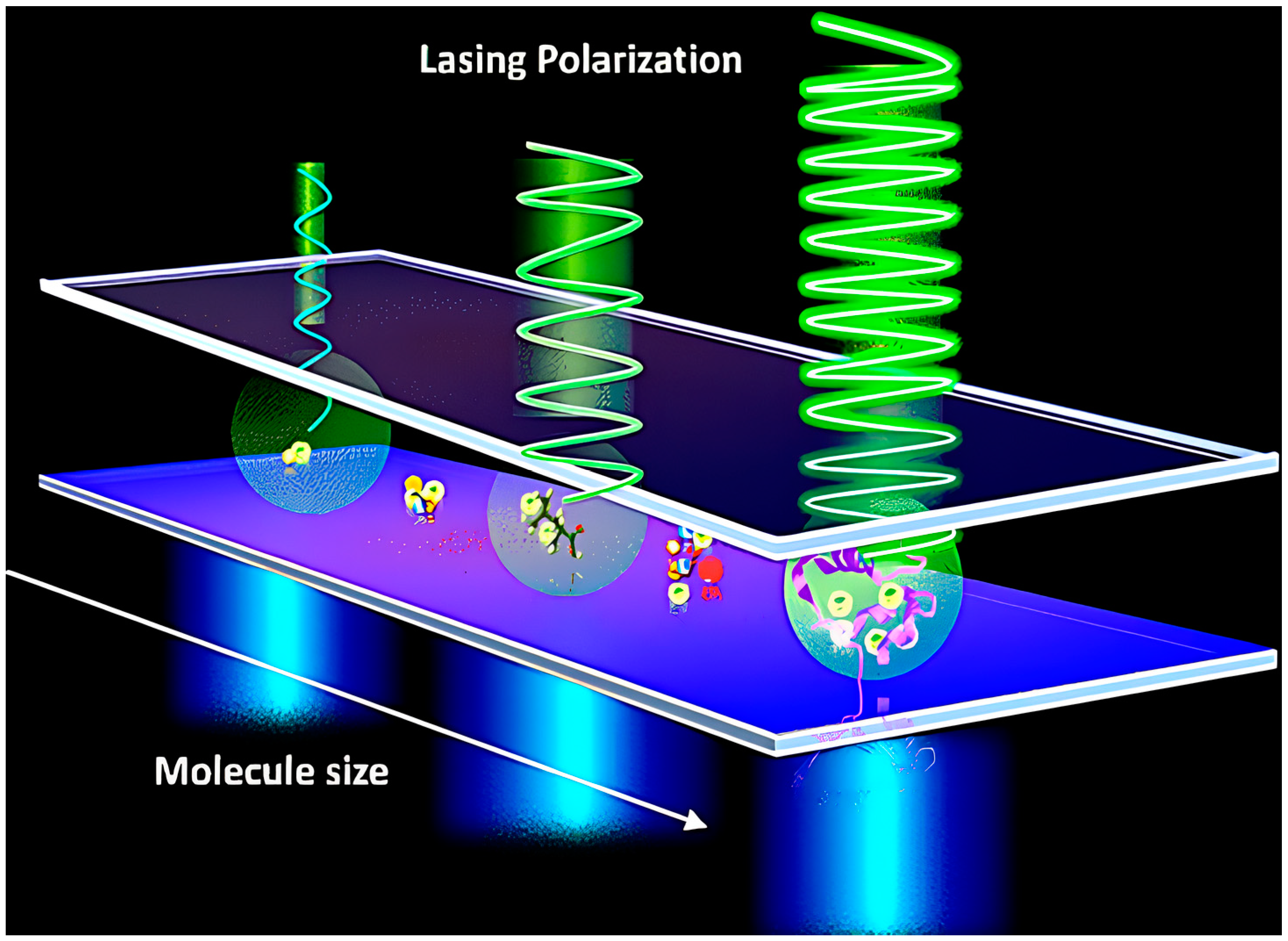
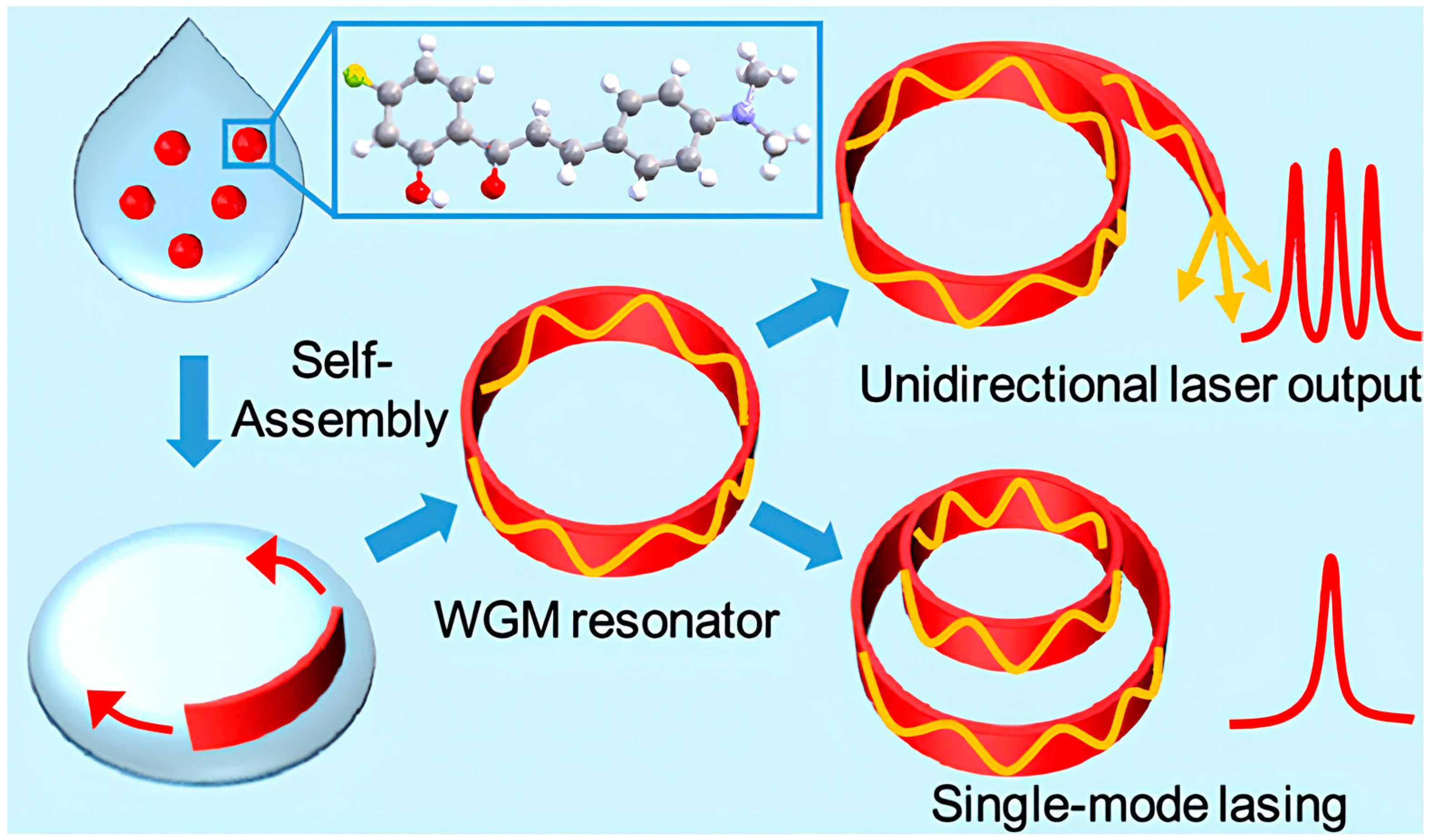

| Issue | Limitations | Possible Improvements |
|---|---|---|
| Fabrication challenges | Generating long-length cellular-based biological photonic waveguides/devices remains a challenge because of the geometric properties of cells. Moreover, maintaining the biological functionality as well as the viability of living cells in fabrication is crucial, limiting the ways to those that can create these waveguides without harming living cells. | Further research is vital for exploring novel ways of producing long-living cellular-based biological photonic waveguides/devices under biologically compatible situations. |
| Limited penetration depth of light | The depth to which light can be delivered along formed biological cellular-based waveguides/devices remains constrained, posing a hurdle for applications in vivo. | Developing methods to overcome scattering losses and enhance light transmission efficiency is essential to achieve deeper tissue penetration in the body. |
| Cellular morphology dependence | The optical properties of cell-based bio-micro lenses are greatly influenced by various factors, including cell morphology and the surrounding medium. | Precisely controlling these factors for consistent and reliable performance is needed and it is required for further investigation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, S.-C.; Yu, S.-A.; Hung, P.-C.; Lu, H.-T.; Nguyen, H.-T.; Chuang, E.-Y. Biological Photonic Devices Designed for the Purpose of Bio-Imaging with Bio-Diagnosis. Photonics 2023, 10, 1124. https://doi.org/10.3390/photonics10101124
Chuang S-C, Yu S-A, Hung P-C, Lu H-T, Nguyen H-T, Chuang E-Y. Biological Photonic Devices Designed for the Purpose of Bio-Imaging with Bio-Diagnosis. Photonics. 2023; 10(10):1124. https://doi.org/10.3390/photonics10101124
Chicago/Turabian StyleChuang, Sih-Chi, Shih-An Yu, Pei-Chia Hung, Hsien-Tsung Lu, Hieu-Trung Nguyen, and Er-Yuan Chuang. 2023. "Biological Photonic Devices Designed for the Purpose of Bio-Imaging with Bio-Diagnosis" Photonics 10, no. 10: 1124. https://doi.org/10.3390/photonics10101124
APA StyleChuang, S.-C., Yu, S.-A., Hung, P.-C., Lu, H.-T., Nguyen, H.-T., & Chuang, E.-Y. (2023). Biological Photonic Devices Designed for the Purpose of Bio-Imaging with Bio-Diagnosis. Photonics, 10(10), 1124. https://doi.org/10.3390/photonics10101124






