Photobiomodulation and Growth Factors in Dentistry: A Systematic Review
Abstract
:1. Introduction
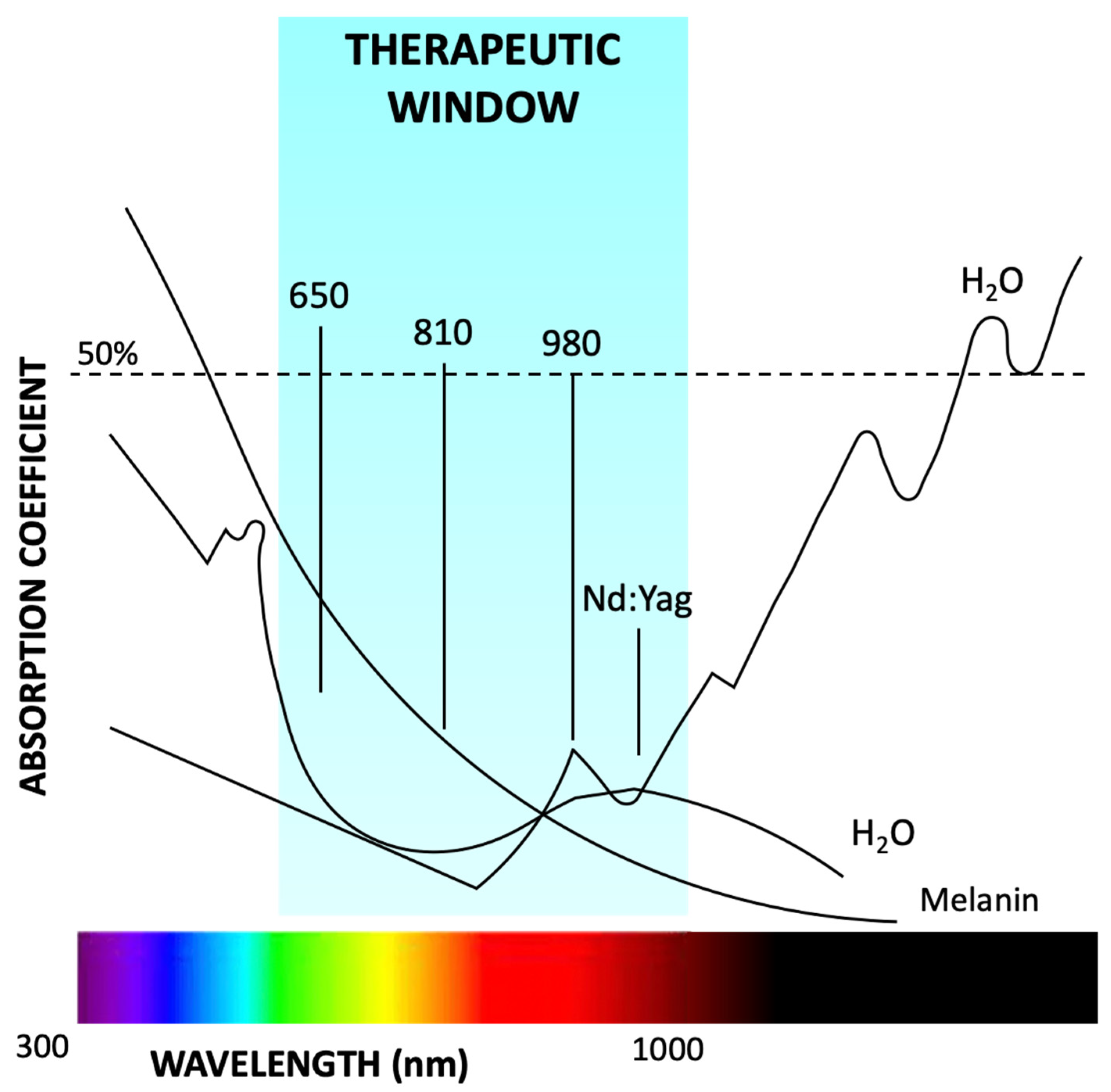
1.1. Photobiomodulation
1.2. PBM Mechanisms of Action
- -
- Endodontics
- -
- Maxillofacial
- -
- Oral pathology
- -
- Oral surgery
- -
- Orthodontics
- -
- Pediatric dentistry
- -
- Periodontics
- -
- Prosthodontics
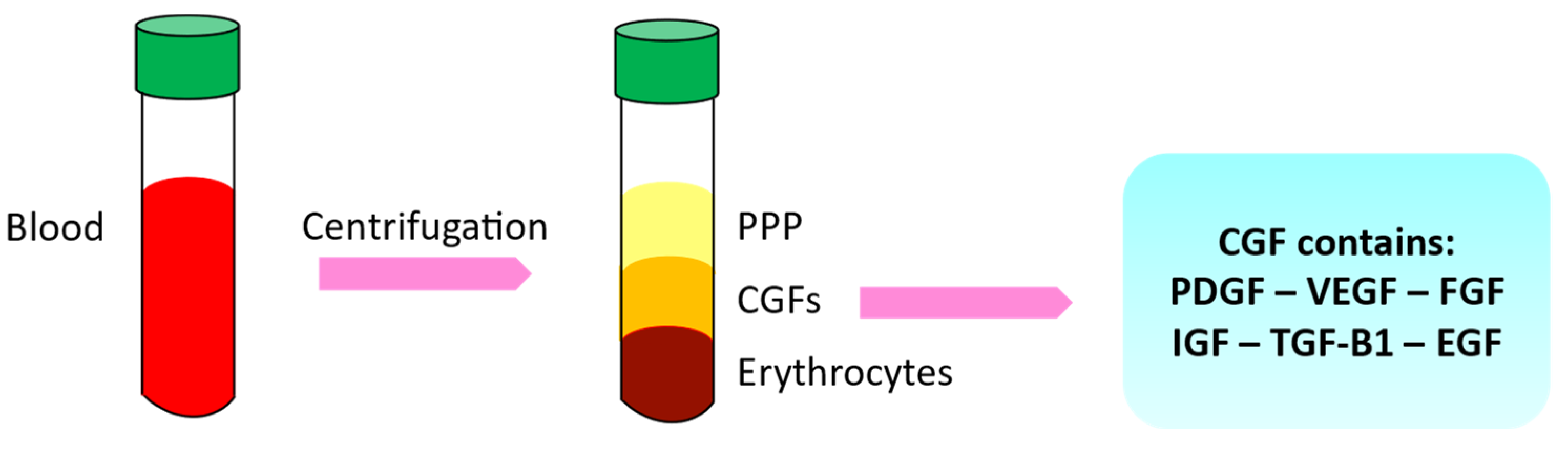
1.3. Growth Factors and Autologous Platelet Concentrates
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Processing
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Data Processing
2.6. Quality Assessment
3. Results
Quality Assessment and Risk of Bias
4. Discussion
4.1. LLLT, GF, and Oral Cancer
4.2. LLLT, GF, and Surgery
4.3. LLLT, GF, and Orthodontics
4.4. LLLT, GF and Periodontology
4.5. Energy Dose and Photobiomodulation
5. Limitations
- Limited sample size: Some of the studies mentioned in the text had a small sample size, which can limit the generalizability of the findings. Small sample sizes may not accurately represent the larger population and can increase the risk of bias.
- Lack of control groups: In some studies, there was a lack of control groups or inadequate comparison groups. Without proper control groups, it becomes challenging to determine the specific effects of LLLT and growth factors independently.
- Varied study designs: The studies mentioned in the text had different designs, including clinical trials, randomized trials, and observational studies. While each study design has its merits, the lack of consistency in study designs makes it difficult to draw definitive conclusions and compare the results across studies.
- Limited understanding of mechanisms: Although the studies suggest potential interactions between LLLT, growth factors, and oral conditions, the exact underlying mechanisms of action are not well understood. Further research is needed to elucidate the precise mechanisms involved and to determine the optimal parameters for LLLT application.
- Need for more rigorous research: While the studies discussed provide preliminary evidence, additional well-designed and rigorous research is necessary to establish the efficacy, safety, and long-term effects of LLLT in conjunction with growth factors. Further investigations should include larger sample sizes, standardized protocols, and control groups to strengthen the scientific validity of the findings.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APCs | Autologous platelet concentrates |
| ATP | Adenosine triphosphate |
| bFGF | basic fibroblast growth factor |
| BMP2 | Bone Morphogenetic Protein 2 |
| BTA | black triangle area |
| BTH | black triangle height |
| CBCT | Cone-beam computed tomography |
| CGF | Concentrated growth factor |
| EGF | Epidermal growth factor |
| FGF | Fibroblast Growth Factor |
| GFs | Growth factors |
| HSCT | hematopoietic stem cell transplantation |
| IGF-1 | Insulin-like Growth Factor |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| L-PRF | Leukocyte and platelet-rich fibrin |
| LEDs | light emitting semiconductors |
| LLLT | Low-level laser therapy |
| LPCGF | Liquid Phase Concentrated Growth Factors |
| MMP | Matrix Metalloproteinase |
| MS | miniscrew |
| NCI | National Cancer Institute |
| Nd:YAG | Neodymium-doped yttrium aluminum garnet |
| OHRQoL | Oral health-related quality of life |
| OM | Oral Mucositis |
| OSMF | Oral submucous fibrosis |
| PAI-1 | plasminogen activator inhibitor 1 |
| PBM | photobiomodulation |
| PBMT | Photobiomodulation therapy |
| PDGF | Platelet-Derived Growth Factor |
| PICF | peri-implant crevicular fluid |
| PMCF | peri-miniscrew crevicular fluid |
| PRF | Platelet-Rich Fibrin |
| PRP | Platelet-rich plasma |
| RBD | Relative bone density |
| SRP | scaling and root planning |
| TGF | transforming growth factor |
| tPA | tissue plasminogen activator |
| VEGF | Vascular Endothelial Growth Factor |
| WHO | Word Health Organization |
References
- Almasri, M.; Mandal, N.; Kulkarni, P.; Raj, A.; Zeya, A.; Mann, N.; Tiwari, R.C. Low-Level Laser Therapy Role in Surgical Extractions: An Original Research. J. Pharm. Bioall. Sci. 2022, 14, 245. [Google Scholar] [CrossRef]
- John, S.S.; Mohanty, S.; Chaudhary, Z.; Sharma, P.; Kumari, S.; Verma, A. Comparative Evaluation of Low Level Laser Therapy and Cryotherapy in Pain Control and Wound Healing Following Orthodontic Tooth Extraction: A Double Blind Study. J. Cranio-Maxillofac. Surg. 2020, 48, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.P.; Da Silva, M.A.; Almeida, A.P.F.; Junior, I.L.; Matos, A.P. Laser Therapy in the Tissue Repair Process: A Literature Review. Photomed. Laser Surg. 2010, 28, 17–21. [Google Scholar] [CrossRef]
- Vermesan, D.; Inchingolo, F.; Patrascu, J.M.; Trocan, I.; Prejbeanu, R.; Florescu, S.; Damian, G.; Benagiano, V.; Abbinante, A.; Caprio, M.; et al. Anterior Cruciate Ligament Reconstruction and Determination of Tunnel Size and Graft Obliquity. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 357–364. [Google Scholar] [PubMed]
- Mester, E.; Mester, A.F.; Mester, A. The Biomedical Effects of Laser Application. Lasers Surg. Med. 1985, 5, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.M.; Malcangi, G.; Ferrara, I.; Viapiano, F.; Netti, A.; Buongiorno, S.; Latini, G.; Azzollini, D.; De Leonardis, N.; De Ruvo, E.; et al. Laser Surgical Approach of Upper Labial Frenulum: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 1302. [Google Scholar] [CrossRef] [PubMed]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Topi, S.; Gnoni, A.; Dipalma, G.; Mancini, A.; Di Domenico, M.; Tartaglia, G.M.; Scarano, A.; et al. The Gaseous Ozone Therapy as a Promising Antiseptic Adjuvant of Periodontal Treatment: A Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 985. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Inchingolo, A.M.; Dipalma, G. Comparison between Traditional Surgery, CO2 and Nd:Yag Laser Treatment for Generalized Gingival Hyperplasia in Sturge-Weber Syndrome: A Retrospective Study. J. Investig. Clin. Dent. 2010, 1, 85–89. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Liebert, A. Photobiomodulation Therapy Mechanisms Beyond Cytochrome c Oxidase. Photobiomodul. Photomed. Laser Surg. 2022, 40, 75–77. [Google Scholar] [CrossRef]
- Sun, G.; Tunér, J. Low-Level Laser Therapy in Dentistry. Dent. Clin. N. Am. 2004, 48, 1061–1076. [Google Scholar] [CrossRef]
- Arany, P.R. Photobiomodulation-Activated Latent Transforming Growth Factor-Β1: A Critical Clinical Therapeutic Pathway and an Endogenous Optogenetic Tool for Discovery. Photobiomodul. Photomed. Laser Surg. 2022, 40, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Arany, P.R.; Cho, A.; Hunt, T.D.; Sidhu, G.; Shin, K.; Hahm, E.; Huang, G.X.; Weaver, J.; Chen, A.C.-H.; Padwa, B.L.; et al. Photoactivation of Endogenous Latent Transforming Growth Factor–Β1 Directs Dental Stem Cell Differentiation for Regeneration. Sci. Transl. Med. 2014, 6, 238ra69. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Sharma, S.K.; Carroll, J.; Hamblin, M.R. Biphasic Dose Response in Low Level Light Therapy—An Update. Dose Response 2011, 9, 602–618. [Google Scholar] [CrossRef] [PubMed]
- De Benedittis, M.; Petruzzi, M.; Pastore, L.; Inchingolo, F.; Serpico, R. Nd:YAG Laser for Gingivectomy in Sturge-Weber Syndrome. J. Oral Maxillofac. Surg. 2007, 65, 314–316. [Google Scholar] [CrossRef]
- Cronshaw, M.; Parker, S.; Anagnostaki, E.; Mylona, V.; Lynch, E.; Grootveld, M. Photobiomodulation and Oral Mucositis: A Systematic Review. Dent. J. 2020, 8, 87. [Google Scholar] [CrossRef]
- Serrage, H.; Heiskanen, V.; Palin, W.M.; Cooper, P.R.; Milward, M.R.; Hadis, M.; Hamblin, M.R. Under the Spotlight: Mechanisms of Photobiomodulation Concentrating on Blue and Green Light. Photochem. Photobiol. Sci. 2019, 18, 1877–1909. [Google Scholar] [CrossRef]
- Karu, T. Primary and Secondary Mechanisms of Action of Visible to Near-IR Radiation on Cells. J. Photochem. Photobiol. B Biol. 1999, 49, 1–17. [Google Scholar] [CrossRef]
- Navratil, L.; Kymplova, J. Contraindications in Noninvasive Laser Therapy: Truth and Fiction. J. Clin. Laser Med. Surg. 2002, 20, 341–343. [Google Scholar] [CrossRef]
- Qamar, Z.; Alghamdi, A.M.S.; Haydarah, N.K.B.; Balateef, A.A.; Alamoudi, A.A.; Abumismar, M.A.; Shivakumar, S.; Cicciù, M.; Minervini, G. Impact of Temporomandibular Disorders on Oral Health-related Quality of Life: A Systematic Review and Meta-analysis. J. Oral Rehabil. 2023. [Google Scholar] [CrossRef]
- Grassi, F.R.; Ciccolella, F.; D’Apolito, G.; Papa, F.; Iuso, A.; Salzo, A.E.; Trentadue, R.; Nardi, G.M.; Scivetti, M.; De Matteo, M.; et al. Effect of Low-Level Laser Irradiation on Osteoblast Proliferation and Bone Formation. J. Biol. Regul. Homeost. Agents 2011, 25, 603–614. [Google Scholar]
- Tarullo, A.; Laino, L.; Tarullo, A.; Inchingolo, F.; Flace, P.; Inchingolo, A.M.; Inchingolo, A.D.; Dipalma, G.; Podo Brunetti, S.; Cagiano, R. Use of a Diode Laser in an Excisional Biopsy of Two Spoonlike Neoformations on the Tongue Tip. Acta Biomed. 2011, 82, 63–68. [Google Scholar] [PubMed]
- Carroll, J.D.; Milward, M.R.; Cooper, P.R.; Hadis, M.; Palin, W.M. Developments in Low Level Light Therapy (LLLT) for Dentistry. Dent. Mater. 2014, 30, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Stone, W.L.; Leavitt, L.; Varacallo, M. Physiology, Growth Factor; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Ronsivalle, V.; Shapira, I.; Cicciù, M. Prevalence of Temporomandibular Disorders in Subjects Affected by Parkinson Disease: A Systematic Review and Metanalysis. J. Oral Rehabil. 2023. [Google Scholar] [CrossRef] [PubMed]
- Intini, G. The Use of Platelet-Rich Plasma in Bone Reconstruction Therapy. Biomaterials 2009, 30, 4956–4966. [Google Scholar] [CrossRef]
- Mijiritsky, E.; Assaf, H.D.; Peleg, O.; Shacham, M.; Cerroni, L.; Mangani, L. Use of PRP, PRF and CGF in Periodontal Regeneration and Facial Rejuvenation—A Narrative Review. Biology 2021, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Ballini, A.; Cagiano, R.; Inchingolo, A.D.; Serafini, M.; De Benedittis, M.; Cortelazzi, R.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; et al. Immediately Loaded Dental Implants Bioactivated with Platelet-Rich Plasma (PRP) Placed in Maxillary and Mandibular Region. Clin. Ter. 2015, 166, e146–e152. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Ceci, S.; Patano, A.; Inchingolo, A.M.; Montenegro, V.; Di Pede, C.; Malcangi, G.; Marinelli, G.; Coloccia, G.; Garibaldi, M.; et al. Elastodontic Therapy of Hyperdivergent Class II Patients Using AMCOP® Devices: A Retrospective Study. Appl. Sci. 2022, 12, 3259. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Dohan, D.M. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part IV: Clinical Effects on Tissue Healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e56–e60. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Inchingolo, F.; Sammartino, G.; Charrier, J.-B. Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Human Cell Cultures: Growth Factor Release and Contradictory Results. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, 418–421. [Google Scholar] [CrossRef]
- Islam, M.S.; Greco, S.; Janjusevic, M.; Ciavattini, A.; Giannubilo, S.R.; D’Adderio, A.; Biagini, A.; Fiorini, R.; Castellucci, M.; Ciarmela, P. Growth Factors and Pathogenesis. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 34, 25–36. [Google Scholar] [CrossRef]
- Mummolo, S.; Mancini, L.; Quinzi, V.; D’Aquino, R.; Marzo, G.; Marchetti, E. Rigenera® Autologous Micrografts in Oral Regeneration: Clinical, Histological, and Radiographical Evaluations. Appl. Sci. 2020, 10, 5084. [Google Scholar] [CrossRef]
- Rochira, A.; Siculella, L.; Damiano, F.; Palermo, A.; Ferrante, F.; Carluccio, M.A.; Calabriso, N.; Giannotti, L.; Stanca, E. Concentrated Growth Factors (CGF) Induce Osteogenic Differentiation in Human Bone Marrow Stem Cells. Biology 2020, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Shirbhate, U.; Bajaj, P. Third-Generation Platelet Concentrates in Periodontal Regeneration: Gaining Ground in the Field of Regeneration. Cureus 2022, 14, e28072. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.J.; Seroyer, S.T.; Filardo, G.; Bajaj, S.; Fortier, L.A. Platelet-Rich Plasma: Where Are We Now and Where Are We Going? Sports Health 2010, 2, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; Di Stasio, D.; Dipalma, G.; Cantore, S.; Ambrosio, P.; Coppola, M.; Quagliuolo, L.; Scarano, A.; Malcangi, G.; Borsani, E.; et al. Steroids and Growth Factors in Oral Squamous Cell Carcinoma: Useful Source of Dental-Derived Stem Cells to Develop a Steroidogenic Model in New Clinical Strategies. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8730–8740. [Google Scholar] [CrossRef]
- Everts, P.A.M.; Knape, J.T.A.; Weibrich, G.; Schönberger, J.P.A.M.; Hoffmann, J.; Overdevest, E.P.; Box, H.A.M.; van Zundert, A. Platelet-Rich Plasma and Platelet Gel: A Review. J. Extra Corpor. Technol. 2006, 38, 174–187. [Google Scholar] [CrossRef]
- Borsani, E.; Bonazza, V.; Buffoli, B.; Nocini, P.F.; Albanese, M.; Zotti, F.; Inchingolo, F.; Rezzani, R.; Rodella, L.F. Beneficial Effects of Concentrated Growth Factors and Resveratrol on Human Osteoblasts In Vitro Treated with Bisphosphonates. Biomed. Res. Int. 2018, 2018, 4597321. [Google Scholar] [CrossRef]
- Naik, B.; Karunakar, P.; Jayadev, M.; Marshal, V.R. Role of Platelet Rich Fibrin in Wound Healing: A Critical Review. J. Conserv. Dent. 2013, 16, 284–293. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, S.-H.; Sándor, G.K.; Kim, Y.-D. Comparison of Platelet-Rich Plasma (PRP), Platelet-Rich Fibrin (PRF), and Concentrated Growth Factor (CGF) in Rabbit-Skull Defect Healing. Arch. Oral Biol. 2014, 59, 550–558. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.; Yu, J.; Wang, J.; Zhai, P.; Chen, S.; Liu, M.; Zhou, Y. Platelet-Rich Fibrin as a Bone Graft Material in Oral and Maxillofacial Bone Regeneration: Classification and Summary for Better Application. BioMed Res. Int. 2019, 2019, 3295756. [Google Scholar] [CrossRef]
- Bonazza, V.; Borsani, E.; Buffoli, B.; Parolini, S.; Inchingolo, F.; Rezzani, R.; Rodella, L.F. In Vitro Treatment with Concentrated Growth Factors (CGF) and Sodium Orthosilicate Positively Affects Cell Renewal in Three Different Human Cell Lines. Cell Biol. Int. 2018, 42, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; Ferrante, F.; Stanca, E.; Damiano, F.; Gnoni, A.; Batani, T.; Carluccio, M.A.; Demitri, C.; Siculella, L. Release of VEGF from Dental Implant Surface (IML® Implant) Coated with Concentrated Growth Factors (CGF) and the Liquid Phase of CGF (LPCGF): In Vitro Results and Future Expectations. Appl. Sci. 2019, 9, 2114. [Google Scholar] [CrossRef]
- Wang, L.; Wan, M.; Li, Z.; Zhong, N.; Liang, D.; Ge, L. A Comparative Study of the Effects of Concentrated Growth Factors in Two Different Forms on Osteogenesis In vitro. Mol. Med. Rep. 2019, 20, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Bielecki, T.; Mishra, A.; Borzini, P.; Inchingolo, F.; Sammartino, G.; Rasmusson, L.; Everts, P.A. In Search of a Consensus Terminology in the Field of Platelet Concentrates for Surgical Use: Platelet-Rich Plasma (PRP), Platelet-Rich Fibrin (PRF), Fibrin Gel Polymerization and Leukocytes. Curr. Pharm. Biotechnol. 2012, 13, 1131–1137. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Bielecki, T.; Jimbo, R.; Barbé, G.; Del Corso, M.; Inchingolo, F.; Sammartino, G. Do the Fibrin Architecture and Leukocyte Content Influence the Growth Factor Release of Platelet Concentrates? An Evidence-Based Answer Comparing a Pure Platelet-Rich Plasma (P-PRP) Gel and a Leukocyte- and Platelet-Rich Fibrin (L-PRF). Curr. Pharm. Biotechnol. 2012, 13, 1145–1152. [Google Scholar] [CrossRef]
- Stanca, E.; Calabriso, N.; Giannotti, L.; Nitti, P.; Damiano, F.; Stanca, B.D.C.; Carluccio, M.A.; De Benedetto, G.E.; Demitri, C.; Palermo, A.; et al. Analysis of CGF Biomolecules, Structure and Cell Population: Characterization of the Stemness Features of CGF Cells and Osteogenic Potential. Int. J. Mol. Sci. 2021, 22, 8867. [Google Scholar] [CrossRef]
- Nicosia, R.F.; Nicosia, S.V.; Smith, M. Vascular Endothelial Growth Factor, Platelet-Derived Growth Factor, and Insulin-like Growth Factor-1 Promote Rat Aortic Angiogenesis in Vitro. Am. J. Pathol. 1994, 145, 1023–1029. [Google Scholar]
- Dohan Ehrenfest, D.M.; Bielecki, T.; Del Corso, M.; Inchingolo, F.; Sammartino, G. Shedding Light in the Controversial Terminology for Platelet-Rich Products: Platelet-Rich Plasma (PRP), Platelet-Rich Fibrin (PRF), Platelet-Leukocyte Gel (PLG), Preparation Rich in Growth Factors (PRGF), Classification and Commercialism. J. Biomed. Mater. Res. A 2010, 95, 1280–1282. [Google Scholar] [CrossRef]
- Throckmorton, D.C.; Brogden, A.P.; Min, B.; Rasmussen, H.; Kashgarian, M. PDGF and TGF-β Mediate Collagen Production by Mesangial Cells Exposed to Advanced Glycosylation End Products. Kidney Int. 1995, 48, 111–117. [Google Scholar] [CrossRef]
- Oton-Leite, A.F.; Silva, G.B.L.; Morais, M.O.; Silva, T.A.; Leles, C.R.; Valadares, M.C.; Pinezi, J.C.D.; Batista, A.C.; Mendonça, E.F. Effect of Low-Level Laser Therapy on Chemoradiotherapy-Induced Oral Mucositis and Salivary Inflammatory Mediators in Head and Neck Cancer Patients: Effect of Low-Level Laser Therapy in Cancer Patients. Lasers Surg. Med. 2015, 47, 296–305. [Google Scholar] [CrossRef]
- Silva, G.B.L.; Sacono, N.T.; Othon-Leite, A.F.; Mendonça, E.F.; Arantes, A.M.; Bariani, C.; Duarte, L.G.L.; Abreu, M.H.N.; Queiroz-Júnior, C.M.; Silva, T.A.; et al. Effect of Low-Level Laser Therapy on Inflammatory Mediator Release during Chemotherapy-Induced Oral Mucositis: A Randomized Preliminary Study. Lasers Med. Sci. 2015, 30, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.; Porto, F.A.; Miraglia, P.; Brunetto, A.L. Low-Level Infrared Laser Therapy in Chemotherapy-Induced Oral Mucositis: A Randomized Placebo-Controlled Trial in Children. J. Pediatr. Hematol. Oncol. 2009, 31, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Sukanya, D.; Upasana, L.; Deepak, T.; Abhinethra, M.; Choudary, S. Determination of Effectiveness of Photobiomodulation in the Treatment of Oral Submucous Fibrosis. J. Pharm. Bioall. Sci. 2022, 14, 475. [Google Scholar] [CrossRef] [PubMed]
- Ozturan, S.; Sirali, A.; Sur, H. Effects of Nd:YAG Laser Irradiation for Minimizing Edema and Pain After Sinus Lift Surgery: Randomized Controlled Clinical Trial. Photomed. Laser Surg. 2015, 33, 193–199. [Google Scholar] [CrossRef]
- Arakeeb, M.A.A.; Zaky, A.A.; Harhash, T.A.-H.; Salem, W.S.; El-Mofty, M. Effect of Combined Application of Growth Factors and Diode Laser Bio-Stimulation on the Osseo Integration of Dental Implants. Open Access Maced. J. Med. Sci. 2019, 7, 2520–2527. [Google Scholar] [CrossRef]
- Gokmenoglu, C.; Ozmeric, N.; Erguder, I.; Elgun, S. The Effect of Light-Emitting Diode Photobiomodulation on Implant Stability and Biochemical Markers in Peri-Implant Crevicular Fluid. Photomed. Laser Surg. 2014, 32, 138–145. [Google Scholar] [CrossRef]
- Kamal, A.; Salman, B.; Razak, N.H.A.; Samsudin, A.R. A Comparative Clinical Study between Concentrated Growth Factor and Low-Level Laser Therapy in the Management of Dry Socket. Eur. J. Dent. 2020, 14, 613–620. [Google Scholar] [CrossRef]
- Üretürk, S.E.; Saraç, M.; Fıratlı, S.; Can, Ş.B.; Güven, Y.; Fıratlı, E. The Effect of Low-Level Laser Therapy on Tooth Movement during Canine Distalization. Lasers Med. Sci. 2017, 32, 757–764. [Google Scholar] [CrossRef]
- Yassaei, S.; Kordi, S.; Aghili, H.; Zavar Reza, J.; Ebrahiminik, Z. Diode Laser Irradiation Effects on Miniscrew Stability and IL-1β and TGF-Β1 Levels: A Split-Mouth Randomized Controlled Clinical Trial. J. Lasers Med. Sci. 2023, 14, e3. [Google Scholar] [CrossRef]
- Marrelli, M.; Tatullo, M.; Dipalma, G.; Inchingolo, F. Oral Infection by Staphylococcus Aureus in Patients Affected by White Sponge Nevus: A Description of Two Cases Occurred in the Same Family. Int. J. Med. Sci. 2012, 9, 47–50. [Google Scholar] [CrossRef]
- Felmeden, D. Angiogenesis: Basic Pathophysiology and Implications for Disease. Eur. Heart J. 2003, 24, 586–603. [Google Scholar] [CrossRef] [PubMed]
- Adina, S.; Dipalma, G.; Bordea, I.R.; Lucaciu, O.; Feurdean, C.; Inchingolo, A.D.; Septimiu, R.; Malcangi, G.; Cantore, S.; Martin, D.; et al. Orthopedic Joint Stability Influences Growth and Maxillary Development: Clinical Aspects. J. Biol. Regul. Homeost. Agents 2020, 34, 747–756. [Google Scholar]
- Folkman, J.; Klagsbrun, M. Angiogenic Factors. Science 1987, 235, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.-P.; Kirsner, R.S. Angiogenesis in Wound Repair: Angiogenic Growth Factors and the Extracellular Matrix. Microsc. Res. Tech. 2003, 60, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Liekens, S.; De Clercq, E.; Neyts, J. Angiogenesis: Regulators and Clinical Applications. Biochem. Pharmacol. 2001, 61, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Miao, D.; Zhang, L.; Zhong, L.; Liu, N.; Chen, Y. Efficacy of Concentrated Growth Factor with Low-Level Laser for the Regeneration of Interdental Papilla Defects. Odontology 2022, 110, 795–804. [Google Scholar] [CrossRef]
- Xue, G.; Wang, S.; Liu, Q.; Zhang, K.; Xin, P. Analysis of the Effects of Concentrated Growth Factor and Low-Level Laser Therapy on the Bone Healing. Heliyon 2023, 9, e12800. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciù, M. Economic Inequalities and Temporomandibular Disorders: A Systematic Review with Meta-analysis. J. Oral Rehabil. 2023, 50, 715–723. [Google Scholar] [CrossRef]
- Gkogkos, A.S.; Karoussis, I.K.; Prevezanos, I.D.; Marcopoulou, K.E.; Kyriakidou, K.; Vrotsos, I.A. Effect of Nd:YAG Low Level Laser Therapy on Human Gingival Fibroblasts. Int. J. Dent. 2015, 2015, 258941. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Crimi, S.; Badnjević, A.; Cervino, G.; Bianchi, A.; Cicciù, M. Correlation between Temporomandibular Disorders (TMD) and Posture Evaluated Trough the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD): A Systematic Review with Meta-Analysis. J. Clin. Med. 2023, 12, 2652. [Google Scholar] [CrossRef]
- Pamuk, F.; Lütfioğlu, M.; Aydoğdu, A.; Koyuncuoglu, C.Z.; Cifcibasi, E.; Badur, O.S. The Effect of Low-Level Laser Therapy as an Adjunct to Non-Surgical Periodontal Treatment on Gingival Crevicular Fluid Levels of Transforming Growth Factor-Beta 1, Tissue Plasminogen Activator and Plasminogen Activator Inhibitor 1 in Smoking and Non-Smoki. J. Periodont. Res. 2017, 52, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Gündoğar, H.; Şenyurt, S.Z.; Erciyas, K.; Yalım, M.; Üstün, K. The Effect of Low-Level Laser Therapy on Non-Surgical Periodontal Treatment: A Randomized Controlled, Single-Blind, Split-Mouth Clinical Trial. Lasers Med. Sci. 2016, 31, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciù, M. Prevalence of Temporomandibular Disorders (TMD) in Pregnancy: A Systematic Review with Meta-analysis. J. Oral Rehabil. 2023, 50, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Arany, P.R. Craniofacial Wound Healing with Photobiomodulation Therapy: New Insights and Current Challenges. J. Dent. Res. 2016, 95, 977–984. [Google Scholar] [CrossRef] [PubMed]


| Authors | Study Type | Number of Patients | Average Age | Material and Methods | Results |
|---|---|---|---|---|---|
| Oton-Leite AF et al. (2015) [51] | Double-blinded, placebo-controlled RCT | 30 | 18 years | To evaluate the effect of LLLT on the severity of oral mucositis (OM) and the release of salivary molecules during chemoradiation treatment for head and neck cancer. The study aimed to investigate the potential benefits of LLLT in reducing the severity of OM, a common side effect of chemotherapy and radiotherapy, and to understand its impact on the release of inflammatory mediators and growth factors involved in the pathogenesis of OM. | The severity of OM, as assessed by both the National Cancer Institute (NCI) and Word Health Organization (WHO) grading scales, was significantly lower in the laser group compared to the control group at the 7th, 21st, and 35th sessions of radiotherapy. Salivary concentrations of interleukin-6 (IL-6), FGF, EGF, and VEGF were lower in the laser group compared to the control group, although not all differences reached statistical significance. Matrix metalloproteinase (MMP) levels showed a slight decrease in the laser group compared to the control group, but the difference was not statistically significant. The study also mentions a reduction in IL-6 concentrations at the end of radiotherapy after 21 sessions of laser therapy. |
| Silva et al. (2015) [52] | Randomized, controlled, and single-blinded clinical trial | 25 | N.D. | Patients were randomly assigned to either the laser group or the control group. Salivary and blood samples were collected at multiple time points. Laser therapy was administered using a diode laser with specific parameters. The severity of oral mucositis was assessed using the WHO mucositis scale. Saliva and plasma samples were analyzed for cytokine, growth factor, and enzyme concentrations. | The study demonstrated a significant reduction in the severity of oral mucositis in the laser group compared to the control group. The laser group had a higher proportion of patients without ulcers and a lower proportion of patients with severe mucositis. The levels of certain cytokines, growth factors, and enzymes showed differences between the laser and control groups, indicating potential modulatory effects of low-level laser therapy. |
| Ali Arakeeb et al. (2019) [53] | RCCT | 40 | 40 Males (average age: 35.2 years) | The patients were randomly allocated into four groups, each consisting of 10 patients: Group A: Control Group-Implant procedure without growth factors or LLLT. Group B: LLLT Group-Implant procedure with LLLT (Diode laser 808 nm). Group C: L-PRF Group-Implant procedure with the addition of L-PRF. Group D: Combined Group-Implant procedure with both L-PRF and LLLT. | Cone-beam computed tomography (CBCT) assessed implant relative bone density (RBD) at 1, 6, and 12 weeks. Results showed the best outcomes at 12 weeks. Group A had decreased RBD at 6 weeks, while other treatment groups demonstrated increases. L-PRF exhibited the highest effect. |
| Kamal et al. (2020) [54] | RCCT | 60 | 38 Males (average age: 34.5 years) 22 Females (average age: 27.1 years) | 60 patients with individual dry sockets at University Dental Hospital Sharjah were assigned to three treatment groups based on their preferences. Group I (n = 30) received conventional treatment involving gentle socket curettage and saline irrigation. Group II (n = 15) received CGF treatment, while Group III (n = 15) underwent LLLT. Patients were assessed for pain score, perisocket inflammation, perisocket tenderness, and granulation tissue formation at day 0 and followed up at 4, 7, 14, and 21 days. | CGF and LLLT speed up gum tissue formation and pain reduction. CGF outperforms LLLT by promoting faster gum tissue growth and eliminating pain within a week. |
| Üretürk et al. (2017) [55] | RCT | 15 | 8 Males-7 Females | Split-mouth study: distalization of the canine after extraction of the maxillary first premolar; LLLT irradiation (gallium-aluminum-arsenide diode laser with a power of 20 mW on days 0, 3, 7, 14, 21, 30, 33, 37, 60, 63 and 67); crevicular fluid collection and cytokine measurement IL-1β and TGF-β1; 3D scan to measure the extent of displacement | Increased TGF-β1 concentration and accelerated movement by 40% in the study group |
| Yassaei et al. (2023) [56] | RCT | 18 | N.D. | Split-mouth study: distalization of the canine after extraction of the maxillary first premolar and use of miniscrew (MS) (diode laser in continuous wave mode, the wavelength is 980 nm, and the output power is 100 mW at four-time points); crevicular fluid collection and cytokine measurement IL-1β and TGF-β1 | TGF-β1 lower in the laser group but the difference was not statistically significant |
| Chen et al., 2022 [57] | RCT | 87 sites from 12 participants | Female participants | Liquid Phase Concentrated Growth Factors (LPCGF) was injected at time 0 and 2, 4, 8, 16, and 24 weeks after the initial injection into the connective tissue layer measuring. Then, Nd:YAG or Semiconductor laser was irradiated to the surface of the gingival papilla at the labial and lingual surface. CBCT was used to measure the BTH (black triangle height) and BTA (black triangle area) | Low-level laser treatment has been widely employed, but concentrated growth factor (CGF) was formerly thought to be the sole material capable of soft tissue regeneration. |
| Pamuk et al., 2017 [58] | 60 | N.D. | On the day that SRP was applied, as well as on days 2 and 7, LLLT was also used. Clinical indicators were noted before and after day 30. On days 7, 14, and 30, samples of gingival crevicular fluid were taken before and during follow-up visits. ELISpot test was used to assess the levels of TGF-1, tissue plasminogen activator (tPA), and Plasminogen Activator Inhibitor (PAI)-1. | Particularly in smokers with chronic periodontitis, LLLT may be thought to have a role in the regulation of periodontal tissue tPA and PAI-1 gingival crevicular fluid levels. | |
| Gündoğar et al., 2016 [59] | RCT | 25 adults with chronic periodontitis | 9 Males, 16 Females | Gingival index (GI), plaque index (PI), and clinical attachment level (CAL) are measured to determine the periodontal status. Gingival crevicular fluid samples are taken at baseline, 1 week, and 1 month after treatment. Strips of paper (Periopaper®) are installed inside the crack until slight resistance has occurred. Levels of cytokines, chemokines, and growth factors are determined using a MAGPIX system. | They showed no statistical significance between-group changes in these biochemical parameters at any time point |
| Gokmenoglu et al., 2014 [60] | RCT | 15 patients | Unspecified | In the trial, 15 patients (8 control, 7 LED) took part. Three times each week beginning on the day of the procedure, an LED device was placed for 20 min over the surgical region for three weeks. In postoperative weeks 4 and 12, peri-implant crevicular fluid (PICF) samples were taken, and the levels of IL-1b, TGF-b, PGE2, and NO were measured. | Changes in biochemical parameters were found to be similar between groups over time. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dipalma, G.; Inchingolo, A.M.; Patano, A.; Palumbo, I.; Guglielmo, M.; Trilli, I.; Netti, A.; Ferrara, I.; Viapiano, F.; Inchingolo, A.D.; et al. Photobiomodulation and Growth Factors in Dentistry: A Systematic Review. Photonics 2023, 10, 1095. https://doi.org/10.3390/photonics10101095
Dipalma G, Inchingolo AM, Patano A, Palumbo I, Guglielmo M, Trilli I, Netti A, Ferrara I, Viapiano F, Inchingolo AD, et al. Photobiomodulation and Growth Factors in Dentistry: A Systematic Review. Photonics. 2023; 10(10):1095. https://doi.org/10.3390/photonics10101095
Chicago/Turabian StyleDipalma, Gianna, Angelo Michele Inchingolo, Assunta Patano, Irene Palumbo, Mariafrancesca Guglielmo, Irma Trilli, Anna Netti, Irene Ferrara, Fabio Viapiano, Alessio Danilo Inchingolo, and et al. 2023. "Photobiomodulation and Growth Factors in Dentistry: A Systematic Review" Photonics 10, no. 10: 1095. https://doi.org/10.3390/photonics10101095
APA StyleDipalma, G., Inchingolo, A. M., Patano, A., Palumbo, I., Guglielmo, M., Trilli, I., Netti, A., Ferrara, I., Viapiano, F., Inchingolo, A. D., Favia, G., Dongiovanni, L., Palermo, A., Inchingolo, F., & Limongelli, L. (2023). Photobiomodulation and Growth Factors in Dentistry: A Systematic Review. Photonics, 10(10), 1095. https://doi.org/10.3390/photonics10101095












