Abstract
The question of the structure of aqueous solutions is one of the most fundamental and complex, while it is relevant to all natural science disciplines. An important parameter of the dynamically equilibrium structure of an aqueous solution is the number of free water molecules. To date, there are no reliable and fully justified methods for determining this parameter. Recently, the terahertz time-domain spectroscopy (THz-TDS) method has been developing. It makes it possible to record the spectra of the complex permittivity in the THz region, where an orientation relaxation band of free water molecules is detected for aqueous solutions. The purpose of this work is to establish the relationship of the parameters of THz dielectric permittivity with the number of free water molecules. For this purpose, the process of polarization of water in the THz region was theoretically considered using the formalism of electrodynamics of continuous media. The Onsager theory is taken as a basis and its development is carried out for the case of high-frequency fields. As a result, an analytical ratio was obtained for calculating the proportion of free water molecules in solutions. A comparison with other well-known, more simplified and poorly substantiated approaches is presented. Calculations of the fraction of free molecules for a number of aqueous solutions have been carried out. It can be argued that the first theoretically justified approach to calculating the population of free water molecules in a solution, which does not contain internal contradictions, is presented.
1. Introduction
The structure of water is one of the fundamental objects of research, which, despite the efforts of several generations of scientists, is still largely unexplored. In this context, first of all, it is worth noting the classical works of D.J. Bernal and R.H. Fowler [1], J.I. Frenkel [2], O.Ya. Samoilov [3], and I.Z. Fisher [4]. An important achievement was the understanding that the concept of “structure” of water contains not only a spatial aspect (the mutual arrangement of molecules), but also a temporal aspect. In [4] it is shown that the result of the analysis of the structure of water depends on the time of its registration. It can be instantaneous (<<10−13 s), vibrational-averaged (10−13–10−11 s) and diffusion–averaged (>>10−11 s) structure. The characteristic times may vary greatly for different structural experimental methods; therefore, the result will be different.
A statistical description of the structure of water in solution is often used in the form of decomposition of water molecules into fractions based on binding energy, mobility, and the number of hydrogen bonds. An important parameter in this context is the number of free water molecules. These are molecules that are not bound by any hydrogen bonds and are not in the ion field, which significantly affects their mobility. The proportion of free water molecules can be considered as an integral characteristic of the connectedness (or structuredness) of water in solution [5]. This parameter was attempted to be determined in different ways, and the values obtained vary greatly. Thus, according to various data, free molecules in pure water at room temperature are less than 1% [6], about 2–3% [5], or even more than 10% [7]. Obviously, the discrepancies are related to the different specifics of the methods used and their sensitivity to the binding of water molecules.
The appearance of the terahertz time-domain spectroscopy (THz-TDS) method has opened up new possibilities in studying the structure of aqueous solutions. The energy and times corresponding to the terahertz range are characteristic of the intermolecular structure and dynamics of water. In addition, the THz-TDS method allows us to obtain not only absorption spectra, but also much more informative spectra of complex dielectric permittivity. A weakly pronounced relaxation band of water was found in the THz region [8,9,10], which differs from the well-known Debye relaxation band from the gigahertz region [11,12]. To date, it is understood that this high-frequency band refers to the orientation relaxation of free water molecules [5,13]. Obviously, the parameters of this band should be directly related to the number of free water molecules. Despite the technical ability to obtain high-quality spectra in the THz range, there are still no reliable approaches for calculating the number of free water molecules in solutions. This requires the development of an appropriate theory.
Due to the absence of such a theory, when calculating the proportion of free water molecules in solutions based on THz spectra, the ratio of the amplitudes of the relaxation bands of free and bound molecules is usually used [13,14,15]. However, this is a crude approach that does not take into account a number of important factors. For example, relaxation processes cannot be considered completely independent of other processes of molecular dynamics in an external electric field. This issue was considered in [16], which describes an approach for calculating the proportion of free water molecules, taking into account the influence of electric field shielding on their orientation polarization from faster polarization processes. However, this approach is criticized because it is based on the use of the Langevin–Debye theory, which is considered untenable for liquids with high dielectric permittivity [17], which is water.
In this paper, a theory is developed that, based on the analysis of water polarization processes in the THz frequency range, allows us to obtain the relationship of the parameters of the complex permittivity of an aqueous solution with the number of free water molecules.
2. Theoretical Background
2.1. Models of Water Polarization
A water molecule has an electric dipole moment. In an external electric field, dipoles tend to orient themselves along the field, while thermal motion disorders them. Based on such a simple consideration, it is possible to express the orientational polarizability of water molecules through a linear term of the Langevin function and associate it with the permittivity [18]:
where N is the number of molecules per unit volume, d is the electric dipole moment of a water molecule, is the vacuum permittivity, k is the Boltzmann constant, T is the absolute temperature, is the static permittivity. The parameter is called the high-frequency permittivity, which describes in the sum all faster polarization processes than the orientation polarization of molecules (electronic, deformation, etc.).
The polarization of a substance is an integral characteristic associated with both the dipole moment of individual molecules and their intermolecular interaction. Since Equation (1) does not take into account the interaction of molecules in any way, it can be used only for rarefied gases, but not for an aqueous solution.
A deeper consideration of the polarization of dipole molecules in a substance requires taking into account the local field. The local field differs from the external field by the presence of an additional polarization field. Usually, this field is called the Lorentz local field, accounting for which leads to the well-known Langevin–Debye equation [18]:
Using the well-known Clausius–Mossotti equation for high-frequency polarizability:
the Langevin–Debye equation can be rewritten in the following form:
As it was later shown, Equation (4) contains a fundamental defect called the Mossotti catastrophe [17], since it leads to ferroelectric properties of water at room temperature, which is not observed in reality. This approach is considered applicable for substances with a small dielectric constant. Nevertheless, it is still often taken as the basis for describing the polarization of water.
Further development of the theory of water polarization was made by Onsager [19], showing that the inconsistency of the Langevin–Debye approach lies in the inaccuracy of the expression for the Lorentz local field. In Onsager’s theory, a water molecule is considered as a spherical object with a permittivity , having a dipole moment . The molecule is surrounded by a continuous medium with a dielectric constant . The theory takes into account not only the influence of the polarization of the environment on the dipole, but also the fact that the polarization of the environment itself changes under the influence of the dipole field. This change in the environment leads to the appearance of an additional member of the local field, which also affects the polarization of the dipole molecule. This additional field was called the Onsager reaction field. The Onsager formula can be written as follows:
The most developed theory of polarization of polar liquids is the Kirkwood theory [20], which takes into account the correlations of the mutual orientation of the dipole moments of molecules:
The Kirkwood Equation (6) differs from the Onsager Equation (5) only by the coefficient g, which is determined based on models of the near-order structure of water or experimentally. Its value for water at room temperature is approximately 2.5 [21]. This theory is considered to be the most justified in describing the polarization of water and allows us to obtain an almost perfect correspondence between the theoretical and experimental values of .
2.2. Polarization in an Alternating Electric Field
So far, the polarization of water in an electrostatic field has been considered, and the equations for have been given. In the electrodynamics of continuous media, polarization in alternating fields is often considered. In this case, the static permittivity is replaced by the complex permittivity , which depends on the frequency ω. The function makes it possible to describe the polarization of matter at different frequencies, including in the dispersion regions where the absorption of the electromagnetic field is observed. The form of all equations obtained for the electrostatic field remains unchanged for the case of alternating fields under certain conditions [22,23]. For example, the Langevin–Debye Equation (2) for an electrostatic field passes into the Clausius–Mossotti Equation (3) or the Lorentz–Lorentz equation [24], describing polarization in the infrared and optical frequency ranges. The form of the equations remains the same, only the contribution of the orientation polarization of the molecules disappears due to the fact that at such high frequencies the molecules do not have time to reorient themselves.
2.3. Complex Permittivity of Water in the THz Range
The dielectric characteristics of water in the THz range are determined by several reliably established molecular dynamics processes. Firstly [25], it is the orientational relaxation of bound water molecules (Debye relaxation) with characteristic times of ~10 ps and the orientational relaxation of free water molecules (sometimes called “fast relaxation”) at ~0.3 ps. Intermolecular oscillations also appear on the high-frequency side of the THz range [26] and some others. With this in mind, the permittivity of water in the THz range can be described in a standard way for dielectric spectroscopy [9,14]:
where is the relaxation time of bound and free water molecules; is the contribution of the corresponding relaxation processes to the overall dielectric response; is the high–frequency dielectric constant; ω is the cyclic frequency; i is an imaginary unit, and the term describes the dielectric losses associated with ionic conductivity ( is dc-conductivity, is the vacuum permittivity). If necessary, additional terms describing high-frequency processes of molecular dynamics [26,27], which in this case are included in , can be explicitly included in Equation (7).
The calculation of the parameters of the model complex permittivity (7) of aqueous solutions can be performed on the basis of fitting experimental spectra. The spectra of the complex permittivity can be measured using the terahertz time-domain spectroscopy (THz-TDS) method, the principles of which are described, for example, in [28]. This method makes it possible to simultaneously determine the transmission spectra Tr() and the refractive index n(). From these two spectra, the spectra of the real and imaginary parts of the complex permittivity can be calculated using the following equations:
where and are the real and imaginary parts of complex permittivity, is sample thickness, is wavenumber ().
2.4. Methods for Calculating the Proportion of Free Water Molecules in Aqueous Solutions Based on Permittivity Parameters in the THz Range
When calculating the proportion of free water molecules using the permittivity parameters of a solution in the THz range, as a rule, an approach based on the ratio of the amplitudes of the relaxation bands and is used, or a similar approach with minor variations [13,14,15]:
The number of free water molecules is considered to be proportional , and the total number is proportional . Equation (9) implies that the process of orientation polarization of free water molecules does not depend on other polarization processes, which is not quite true. In [16], the orientational polarization of free water molecules was considered on the basis of the combined Langevin–Debye and Clausius–Mossotti theory, and the need to take into account the shielding of the field by faster polarization processes was shown. The relationship of the proportion of free water molecules with the parameters and , as well as with the parameters of high-frequency processes (bending, stretching intermolecular modes, etc.) which, however, can be combined into one parameter , is established:
where the parameters’ definitions are the same as in Equations (1) and (7). This approach, however, is imperfect, since the underlying Langevin–Debye theory, as applied to the polarization of water, leads to the above-mentioned Mossotti catastrophe. As a result, despite the existence of reasonable aspects of this theory, it is unclear what the error of calculations according to Equation (10) is.
3. Calculation of the Proportion of Free Water Molecules in Aqueous Solutions Based on the Permittivity Parameters in the THz Range Using the Onsager Theory
As shown in Section 2.3, several types of molecular dynamics can be distinguished in water, described by Equation (7): orientation relaxation of bound molecules, orientation relaxation of free molecules and other high-frequency processes. Each of these types of molecular dynamics is associated with a corresponding type of polarization of water: orientation polarization of bound molecules, orientation polarization of free molecules and other, faster, polarization processes. Each type of polarization is realized for its characteristic times and gives a certain contribution to the overall dielectric response. Therefore, as is known, static permittivity is determined by the sum of the contributions of all polarization processes:
Despite the additivity of the polarization processes for the electrostatic case, considering each polarization process separately at its characteristic times requires, as mentioned above, taking into account the shielding from faster polarization processes. For the orientational polarization of free water molecules, all faster processes are described by the value . At the same time, the orientation polarization of bound molecules is much slower and does not have time to shield the external electric field during the orientation polarization of free molecules. Indeed, at the peak of the orientation relaxation band of free molecules (at the frequency ), the contribution of the polarization of bound molecules is with the total permittivity of water at this frequency ( ps [27], ps and [29]).
Consider the process of polarization of water at a frequency of ( ≈ 0.6 THz), that is, on a spectral scale between two relaxation bands of water (Figure 1). At this frequency, the orientation polarization of free water molecules is almost completely realized, since , as well as the polarization of all faster processes described by . At the same time, the polarization of bound water molecules practically does not have time to be realized, since .
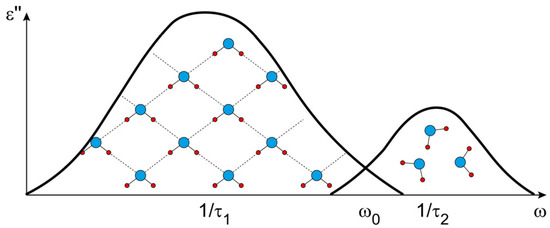
Figure 1.
Schematic description of the location of orientation relaxation bands of bound and free water molecules. is the selected frequency at which the process of orientational polarization of free water molecules is considered.
By analogy with the static permittivity (11), at a frequency of , the permittivity of an aqueous solution can be represented as the sum of . To describe the orientation polarization of free water molecules at the frequency , we will then use the Onsager Equation (5), where instead of the static permeability , we need to substitute . In addition, taking into account the selected frequency domain, where the orientation polarization of only free molecules is realized, it is necessary, instead of the total number of molecules N, to substitute the number of free water molecules (in unit volume):
As a rule, it is not the absolute number of free water molecules that is of interest, but their fraction n of the total number:
Based on Equations (12) and (13), we obtain an expression for calculating the proportion of free water molecules:
where the parameters’ definitions are the same as in Equations (1) and (7).
4. Discussion
Equation (14) for calculating the proportion of free water molecules in solution was obtained on the basis of theoretical consideration of the process of orientation polarization of free molecules with the involvement of the Onsager theory. Despite the fact that the Onsager theory has been substantially modified by us for application in the case of high-frequency fields, its main aspects have remained unchanged. Firstly, water molecules are considered not to interact with each other in any way other than Coulomb, that is, there are no correlations of the relative location of neighboring molecules. Secondly, a water molecule is considered to be a sphere filled with a dielectric medium with a permittivity of .
As is known, not taking into account the correlations of the relative position of water molecules leads to an underestimation of the magnitude of the static permittivity of water, which was corrected in Kirkwood’s theory [20] by the introduction of the correlation g-factor (6). However, when considering the orientation polarization of the fraction of free water molecules at their characteristic times, the Onsager approach turns out to be the most appropriate. Taking into account the Kirkwood g-factor in this case would be erroneous, since it refers to bound water molecules whose orientation polarization is not realized at the considered times.
The spherical symmetry of the water molecule in the Onsager approach may also seem like a rough approximation. However, when considering the processes of polarization, it is not the physical sphericity that is important, but the spherical symmetry of the polarizability of the water molecule. In this case, this is quite justified, since it has been experimentally shown that the polarizability of a water molecule at THz frequencies is close to isotropic [30].
Thus, the Onsager theory, which is unacceptable for describing the polarization of water in the electrostatic case, after appropriate modification turned out to be much more suitable for describing the orientation polarization of free molecules in water at terahertz frequencies than other well-known Langevin–Debye or Kirkwood models.
Table 1 presents a comparison of the results of calculating the number of free water molecules in various solutions using the three approaches described above: according to Equations (9) and (10) and according to Equation (14) obtained in this work.

Table 1.
Calculation of the proportion of free water molecules (in % of the total number) using three approaches: based on the ratio of the amplitudes of relaxation processes (9), based on the Langevin–Debye theory (10), based on the theory developed in this paper (14).
It follows from the data in Table 1 that simplified approaches (9) and (10), as a rule, underestimate the number of free water molecules. Interestingly, the seemingly crude approach (10), based on the Langevin–Debye theory, underestimates the number of free water molecules by only 15–20% relative to the values obtained in this work.
The results of calculating the proportion of free water molecules based on the theory developed in this paper are consistent with modern concepts of hydration in aqueous solutions. In the Hoffmeister series for Ca2+, K+, Cs+ cations, an increase in the number of free water molecules is observed. This is explained by a decrease in the charge density of the ion on the surface, which leads to a weakening of the binding of water in the solution. In fact, the increase in the number of free molecules in this series is even greater if we take into account the effect of water displacement by ions, since the total volume of ions in the solution increases in this series. The presence of protein, sugar and DNA in the solution leads to a slight increase in the proportion of free molecules. This phenomenon is observed for the vast majority of biomolecules during their hydration [29,31,36]. The explanation of this fact was first proposed in [31]. Energetically strong binding of water molecules to hydrophilic sites of biomolecules in primary hydrate shells leads to distortion of the structure of water and does not allow the formation of undisturbed (“normally bound”) structure of water outside the primary hydrate layer for steric reasons. At the same time, beyond the primary hydrate layer, there is no longer an energetically strong binding of water molecules. That is, the hydrate shell of the biomolecule, roughly speaking, consists of two layers: a strongly bound primary and a partially destroyed secondary, outside of which the structure of undisturbed water is formed. It should be particularly noted that the calculation of the proportion of free water molecules in the solutions of biomolecules indicated in Table 1 was carried out on the basis of the dielectric permittivity of the aqueous phase of these solutions. The dielectric contribution of the biomolecules themselves was excluded from the experimental permittivity of solutions using effective medium models [22,37] and did not affect the result.
5. Conclusions
A consistent theory of orientation polarization of the fraction of free water molecules in an aqueous solution is presented. This theory is based on the theory of Onsager polarization, modified for use in the case of high-frequency fields. Based on the presented theory and experimental spectra of the complex permittivity in the THz range, it is possible to calculate the proportion of free water molecules in any aqueous solution. The calculations show that the previously known similar approaches are greatly simplified and underestimate the number of free water molecules.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Part of this work was performed on the instrument base of the Optical Microscopy and Spectrophotometry Core Facility, ICB RAS, Federal Research Center “Pushchino Scientific Center for Biological Research of the Russian Academy of Sciences” (https://www.pbcras.ru/services/tskp/).
Conflicts of Interest
The author declares no conflict of interest.
References
- Bernal, J.D.; Fowler, R. A Theory of Water and Ionic Solution, with Particular Reference to Hydrogen and Hydroxyl Ions. J. Chem. Phys. 1933, 1, 515–548. [Google Scholar] [CrossRef]
- Frenkel, J. Kinetic Theory of Liquids; Oxford University Press: Oxford, UK, 1946. [Google Scholar]
- Samoilov, O.Y. Structure of Aqueous Electrolyte Solutions and the Hydration of Ions; Consultants Bureau: New York, NY, USA, 1965. [Google Scholar]
- Fisher, I.Z. Statistical Theory of Liquids; University of Chicago Press: Chicago, IL, USA, 1964. [Google Scholar]
- Penkov, N.; Shvirst, N.; Yashin, V.; Fesenko, E., Jr.; Fesenko, E. Terahertz Spectroscopy Applied for Investigation of Water Structure. J. Phys. Chem. B 2015, 119, 12664–12670. [Google Scholar] [CrossRef] [PubMed]
- Marti, J.; Padro, J.A.; Guardia, E. Molecular Dynamics Simulation of Liquid Water Along the Coexistence Curve: Hydrogen Bonds and Vibrational Spectra. J. Chem. Phys. 1996, 105, 639–649. [Google Scholar] [CrossRef]
- Walrafen, G.E.; Fisher, M.R.; Hokmabadi, M.S.; Yang, W.-H. Temperature Dependence of the Low- and High-Frequency Raman Scattering from Liquid Water. J. Chem. Phys. 1986, 85, 6970–6982. [Google Scholar] [CrossRef]
- Barthel, J.; Bachhuber, K.; Buchner, R.; Hetzenauer, H. Dielectric Spectra of Some Common Solvents in the Microwave Region. Water and Lower Alcohols. Chem. Phys. Lett. 1990, 165, 369–373. [Google Scholar] [CrossRef]
- Buchner, R.; Barthel, J.; Stauber, J. The Dielectric Relaxation of Water Between 0° C and 35° C. Chem. Phys. Lett. 1999, 306, 57–63. [Google Scholar] [CrossRef]
- Møller, U.; Cooke, D.G.; Tanaka, K.; Jepsen, P.U. Terahertz Reflection Spectroscopy of Debye Relaxation in Polar Liquids. J. Opt. Soc. Am. B 2009, 26, A113–A125. [Google Scholar] [CrossRef]
- Von Hippel, A.R. The Dielectric Relaxation Spectra of Water, Ice, and Aqueous Solutions, and Their Interpretation. 2. Tentative Interpretation of the Relaxation Spectrum of Water in the Time and Frequency Domain. IEEE Trans. Electr. Insul. 1988, 23, 817–823. [Google Scholar] [CrossRef]
- Laage, D.; Hynes, J.T. A Molecular Jump Mechanism of Water Reorientation. Science 2006, 311, 832–835. [Google Scholar] [CrossRef]
- Yada, H.; Nagai, M.; Tanaka, K. Origin of the Fast Relaxation Component of Water and Heavy Water Revealed by Terahertz Time-Domain Attenuated Total Reflection Spectroscopy. Chem. Phys. Lett. 2008, 464, 166–170. [Google Scholar] [CrossRef]
- Shiraga, K.; Suzuki, T.; Kondo, N.; De Baerdemaeker, J.; Ogawa, Y. Quantitative Characterization of Hydration State and Destructuring Effect of Monosaccharides and Disaccharides on Water Hydrogen Bond Network. Carbohydr. Res. 2015, 406, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Shiraga, K.; Adachi, A.; Nakamura, M.; Tajima, T.; Ajito, K.; Ogawa, Y. Characterization of the Hydrogen-Bond Network of Water Around Sucrose and Trehalose: Microwave and Terahertz Spectroscopic Study. J. Chem. Phys. 2017, 146, 105102. [Google Scholar] [CrossRef] [PubMed]
- Penkov, N.V.; Yashin, V.A.; Fesenko, E.E., Jr.; Fesenko, E.E. Calculation of the Amount of Free Water Molecules in Aqueous Solutions by Means of Spectral Parameters from the Terahertz Frequency Domain Taking into Account Processes of Screening. Biophysics 2014, 59, 347–350. [Google Scholar] [CrossRef]
- Fröhlich, H. Theory of Dielectrics, 2nd ed.; Clarendon Pres: Oxford, UK, 1958; 192p. [Google Scholar] [CrossRef]
- Debye, P.J.W. Polar Molecules; Dover Publications: New York, NY, USA, 1929; 172p. [Google Scholar]
- Onsager, L. Electric Moments of Molecules in Liquids. J. Am. Chem. Soc. 1936, 58, 1486–1493. [Google Scholar] [CrossRef]
- Kirkwood, J.G. The Dielectric Polarization of Polar Liquids. J. Chem. Phys. 1939, 7, 911–919. [Google Scholar] [CrossRef]
- Robinson, R.A.; Stokes, R.H. Electrolyte Solutions: The Measurement and Interpretation of Conductance, Chemical Potential and Diffusion, 2nd ed.; Academic Press: New York, NY, USA, 1955; 512p. [Google Scholar]
- Choy, T.C. Effective Medium Theory: Principle and Applications, 1st ed.; Oxford University Press: Oxford, UK, 1999; 200p. [Google Scholar]
- Sihvola, A. Mixing Rules with Complex Dielectric Coefficients. Subsurf. Sens. Technol. Appl. 2000, 1, 393–415. [Google Scholar] [CrossRef]
- Lorentz, H.A. The Theory of Electrons, 2nd ed.; Dover Publications: New York, NY, USA, 1952; 343p. [Google Scholar]
- Nazarov, M.M.; Cherkasova, O.P.; Shkurinov, A.P. Study of the Dielectric Function of Aqueous Solutions of Glucose and Albumin by THz Time-Domain Spectroscopy. Quantum Electron. 2016, 46, 488–495. [Google Scholar] [CrossRef]
- Penkov, N.V.; Shvirst, N.E.; Yashin, V.A.; Fesenko, E.E. On Singularities of Molecular Relaxation in Water Solutions. Biophysics 2013, 58, 731–738. [Google Scholar] [CrossRef]
- Ellison, W.J. Permittivity of Pure Water, at Standard Atmospheric Pressure, Over the Frequency Range 0−25 THz and the Temperature Range 0−100 °C. J. Phys. Chem. Ref. Data 2007, 36, 1–18. [Google Scholar] [CrossRef]
- Lee, Y.-S. Principles of Terahertz Science and Technology; Springer: New York, NY, USA, 2009. [Google Scholar]
- Penkov, N.V.; Penkova, N. Key Differences of the Hydrate Shell Structures of ATP and Mg·ATP Revealed by Terahertz Time-Domain Spectroscopy and Dynamic Light Scattering. J. Phys. Chem. B 2021, 125, 4375–4382. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.F. The Rayleigh Depolarization Ratio and Rotational Raman Spectrum of Water Vapor and the Polarizability Components for the Water Molecule. J. Chem. Phys. 1977, 67, 5877–5882. [Google Scholar] [CrossRef]
- Penkov, N.; Yashin, V.; Fesenko, E., Jr.; Manokhin, A.; Fesenko, E. A Study of the Effect of a Protein on the Structure of Water in Solution Using Terahertz Time-Domain Spectroscopy. Appl. Spectrosc. 2018, 72, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Penkov, N.V.; Yashin, V.A.; Belosludtsev, K.N. Hydration Shells of DPPC Liposomes from the Point of View of Terahertz Time-Domain Spectroscopy. Appl. Spectrosc. 2021, 75, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Penkova, N.A.; Sharapov, M.G.; Penkov, N.V. Hydration Shells of DNA From the Point of View of Terahertz Time-Domain Spectroscopy. Int. J. Mol. Sci. 2021, 22, 11089. [Google Scholar] [CrossRef]
- Penkov, N.V. Relationships Between Molecular Structure of Carbohydrates and Their Dynamic Hydration Shells Revealed by Terahertz Time-Domain Spectroscopy. Int. J. Mol. Sci. 2021, 22, 11969. [Google Scholar] [CrossRef] [PubMed]
- The International Association for the Properties of Water and Steam. Gaithersburg, MD, USA, 9–14 September 2001. Available online: http://www.iapws.org/relguide/fundam.pdf (accessed on 28 December 2022).
- Penkov, N.V.; Penkova, N.A.; Lobyshev, V.I. Special Role of Mg2+ in the Formation of the Hydration Shell of Adenosine Triphosphate. Phys. Wave Phenom. 2022, 30, 344–350. [Google Scholar] [CrossRef]
- Penkov, N.V.; Penkova, N.A. Effective Medium Model Applied to Biopolymer Solutions. Appl. Spectrosc. 2021, 75, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).