Instrumentation in Diffuse Optical Imaging
Abstract
:1. Introduction
2. System Architecture
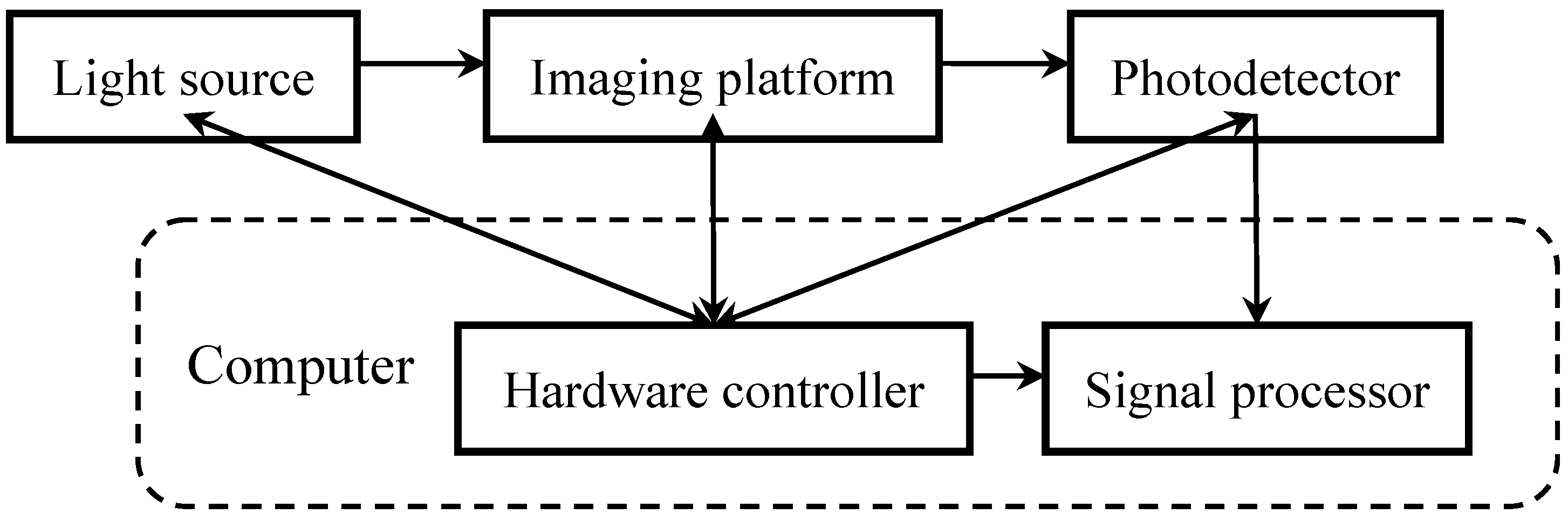
2.1. Light Coupling
2.2. Subject Positioning
3. Light Source
3.1. Lamp
3.2. Laser
3.3. Light Emitting Diode
| Specifications | LED | LD |
|---|---|---|
| Technology | Semiconductor | Semiconductor |
| Photo-emission mechanism | Electroluminescence | Stimulated emission |
| Optical power density | Low | High |
| Power consumption | Low | High |
| Power efficiency | High | Low |
| Optical output linearity | Linear | Linear above threshold |
| Temperature stability | High | Low |
| Spectral width | Broad (~50–100 nm) | Narrow (~1–10 nm) |
| Wavelength choices | Less | More |
| Wavelength tunability | No | Yes (limited) |
| Directionality | None | High |
| Coherence | No | Yes |
| Polarization | No | Yes |
| Speckling effect | No | Yes |
| Mode of operation | Multimode | Single- or multi-mode |
| Modulation bandwidth | Low | High |
| Lifespan | Longer | Long |
| Cost | Low | High |
| Operation | Easy/simple | Difficult/complex |
4. Photo-Detection
4.1. Discrete Photo-Sensitive Element
4.2. Integrated Photo-Sensing Array
5. Spectral Separation
6. Signal Modulation
6.1. Amplitude Modulation
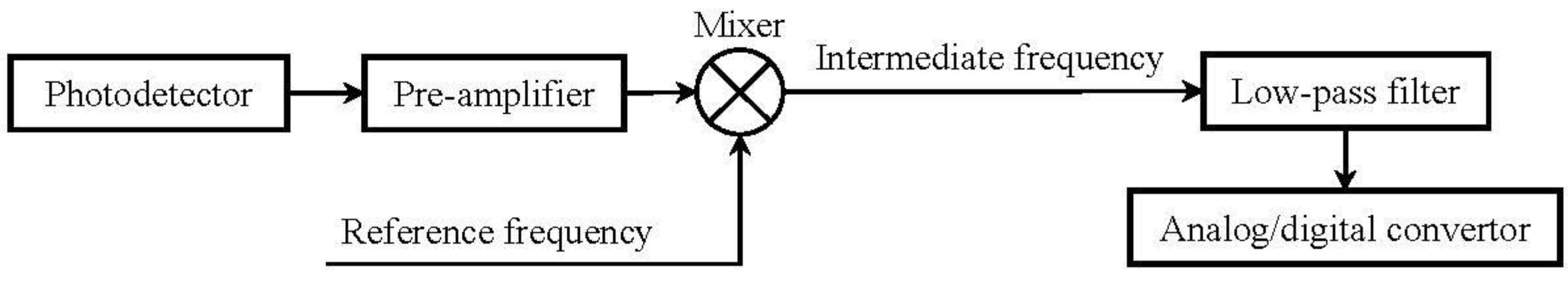
6.2. Temporal Modulation
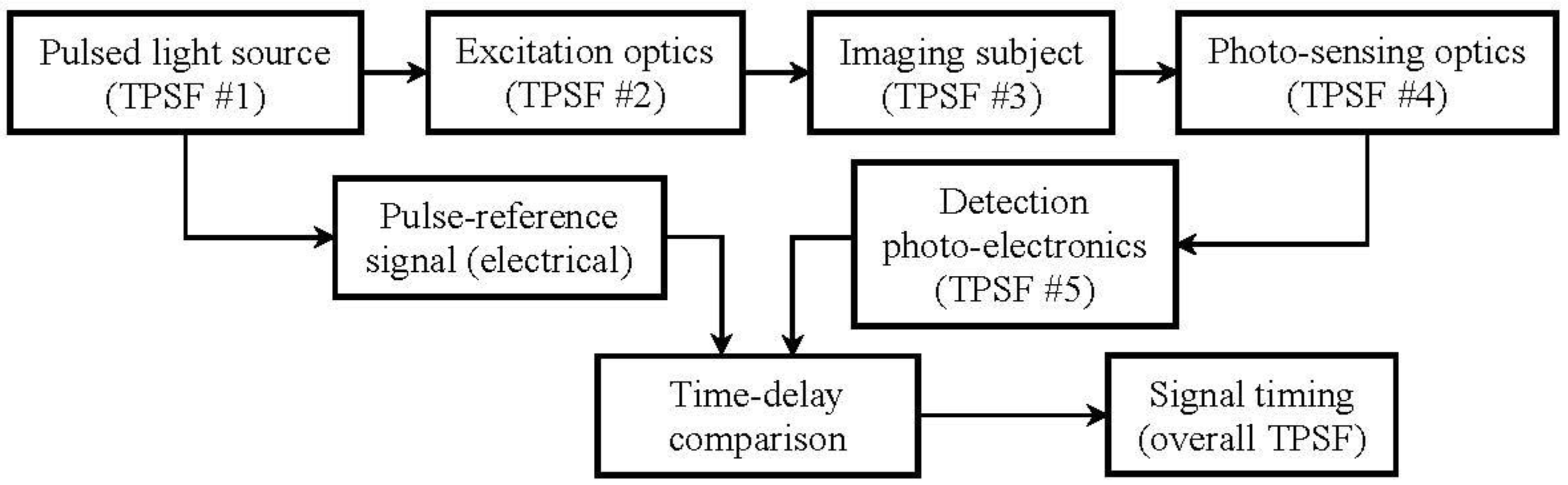
6.3. Spatial Modulation
6.4. Fourier Analysis
7. Imaging Contrast
7.1. Tissue Absorption and Scattering
7.2. Fluorescence
7.3. Bioluminescence
7.4. High-Energy Radiation
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Boas, D.A.; Dale, A.M.; Franceschini, M.A. Diffuse optical imaging of brain activation: Approaches to optimizing image sensitivity, resolution, and accuracy. Neuroimage 2004, 23, S275–S288. [Google Scholar] [CrossRef]
- Intes, X.; Chance, B. Non-PET functional imaging techniques: Optical. Radiol. Clin. North Am. 2005, 43, 221–234. [Google Scholar] [CrossRef]
- Hielscher, A.H. Optical tomographic imaging of small animals. Curr. Opin. Biotechnol. 2005, 16, 79–88. [Google Scholar] [CrossRef]
- Gibson, A.P.; Hebden, J.C.; Arridge, S.R. Recent advances in diffuse optical imaging. Phys. Med. Biol. 2005, 50, R1–R43. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, F.; Tanikawa, Y.; Yamada, Y. Time-resolved diffuse optical tomography and its application to in vitro and in vivo imaging. J. Biomed. Opt. 2007, 12, 062107. [Google Scholar] [CrossRef]
- Dehghani, H.; Srinivasan, S.; Pogue, B.W.; Gibson, A. Numerical modelling and image reconstruction in diffuse optical tomography. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 3073–3093. [Google Scholar] [CrossRef]
- Leblond, F.; Davis, S.C.; Valdes, P.A.; Pogue, B.W. Pre-clinical whole-body fluorescence imaging: Review of instruments, methods and applications. J. Photochem. Photobiol. B 2010, 98, 77–94. [Google Scholar] [CrossRef]
- Brown, J.Q.; Bydlon, T.M.; Richards, L.M.; Yu, B.; Kennedy, S.A.; Geradts, J.; Wilke, L.G.; Junker, M.; Gallagher, J.; Barry, W.; et al. Optical assessment of tumor resection margins in the breast. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 530–544. [Google Scholar] [CrossRef]
- Arridge, S.R. Methods in diffuse optical imaging. Philos. Trans. Ser. A 2011, 369, 4558–4576. [Google Scholar] [CrossRef]
- O’Sullivan, T.D.; Cerussi, A.E.; Cuccia, D.J.; Tromberg, B.J. Diffuse optical imaging using spatially and temporally modulated light. J. Biomed. Opt. 2012, 17, 071311. [Google Scholar]
- Busch, D.R.; Choe, R.; Durduran, T.; Yodh, A.G. Towards non-invasive characterization of breast cancer and cancer metabolism with diffuse optics. PET Clin. 2013, 8, 345–356. [Google Scholar] [CrossRef]
- Boas, D.A.; Gaudette, T.; Strangman, G.; Cheng, X.; Marota, J.J.; Mandeville, J.B. The accuracy of near infrared spectroscopy and imaging during focal changes in cerebral hemodynamics. Neuroimage 2001, 13, 76–90. [Google Scholar]
- Zhang, X.; Toronov, V.; Webb, A. Simultaneous integrated diffuse optical tomography and functional magnetic resonance imaging of the human brain. Opt. Express 2005, 13, 5513–5521. [Google Scholar] [CrossRef]
- Gratton, E.; Toronov, V.; Wolf, U.; Wolf, M.; Webb, A. Measurement of brain activity by near-infrared light. J. Biomed. Opt. 2005, 10, 11008. [Google Scholar] [CrossRef]
- Intes, X.; Ripoll, J.; Chen, Y.; Nioka, S.; Yodh, A.G.; Chance, B. In vivo continuous-wave optical breast imaging enhanced with Indocyanine Green. Med. Phys. 2003, 30, 1039–1047. [Google Scholar] [CrossRef]
- Tromberg, B.J.; Pogue, B.W.; Paulsen, K.D.; Yodh, A.G.; Boas, D.A.; Cerussi, A.E. Assessing the future of diffuse optical imaging technologies for breast cancer management. Med. Phys. 2008, 35, 2443–2451. [Google Scholar] [CrossRef]
- Brown, J.Q.; Wilke, L.G.; Geradts, J.; Kennedy, S.A.; Palmer, G.M.; Ramanujam, N. Quantitative optical spectroscopy: A robust tool for direct measurement of breast cancer vascular oxygenation and total hemoglobin content in vivo. Cancer Res. 2009, 69, 2919–2926. [Google Scholar] [CrossRef]
- Hillman, E.M.; Moore, A. All-optical anatomical co-registration for molecular imaging of small animals using dynamic contrast. Nat. Photonics 2007, 1, 526–530. [Google Scholar] [CrossRef]
- Zhang, X.; Badea, C.; Hood, G.; Wetzel, A.; Qi, Y.; Stiles, J.; Johnson, G.A. High-resolution reconstruction of fluorescent inclusions in mouse thorax using anatomically guided sampling and parallel Monte Carlo computing. Biomed. Opt. Express 2011, 2, 2449–2460. [Google Scholar] [CrossRef]
- Vasquez, K.O.; Casavant, C.; Peterson, J.D. Quantitative whole body biodistribution of fluorescent-labeled agents by non-invasive tomographic imaging. PLoS One 2011, 6, e20594. [Google Scholar]
- Ale, A.; Ermolayev, V.; Herzog, E.; Cohrs, C.; de Angelis, M.H.; Ntziachristos, V. FMT-XCT: In vivo animal studies with hybrid fluorescence molecular tomography-X-ray computed tomography. Nat. Methods 2012, 9, 615–620. [Google Scholar] [CrossRef]
- Boas, D.A.; Campbell, L.E.; Yodh, A.G. Scattering and imaging with diffusing temporal field correlations. Phys. Rev. Lett. 1995, 75, 1855–1858. [Google Scholar] [CrossRef]
- He, L.; Lin, Y.; Shang, Y.; Shelton, B.J.; Yu, G.Q. Using optical fibers with different modes to improve the signal-to-noise ratio of diffuse correlation spectroscopy flow-oximeter measurements. J. Biomed. Opt. 2013, 18, 037001. [Google Scholar] [CrossRef]
- McGinily, S.J.; Abram, R.H.; Riis, E.; Ferguson, A.I. Efficient coupling of several broad area laser diodes into an optical fiber. Rev. Sci. Instrum. 2006, 77, 116101. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, Y.; Iftimia, N. Experimental three-dimensional optical image reconstruction of heterogeneous turbid media from continuous-wave data. Opt. Express 2000, 7, 204–209. [Google Scholar] [CrossRef]
- Franceschini, M.A.; Toronov, V.; Filiaci, M.; Gratton, E.; Fantini, S. On-line optical imaging of the human brain with 160-ms temporal resolution. Opt. Express 2000, 6, 49–57. [Google Scholar] [CrossRef]
- McBride, T.O.; Pogue, B.W.; Jiang, S.; Osterberg, U.L.; Paulsen, K.D. A parallel-detection frequency-domain near-infrared tomography system for hemoglobin imaging of the breast in vivo. Rev. Sci. Instrum. 2001, 72, 1817–1824. [Google Scholar] [CrossRef]
- Toronov, V.Y.; Zhang, X.; Webb, A.G. A spatial and temporal comparison of hemodynamic signals measured using optical and functional magnetic resonance imaging during activation in the human primary visual cortex. Neuroimage 2007, 34, 1136–1148. [Google Scholar] [CrossRef]
- Fu, H.L.; Yu, B.; Lo, J.Y.; Palmer, G.M.; Kuech, T.F.; Ramanujam, N. A low-cost, portable, and quantitative spectral imaging system for application to biological tissues. Opt. Express 2010, 18, 12630–12645. [Google Scholar] [CrossRef]
- Deliolanis, N.; Lasser, T.; Hyde, D.; Soubret, A.; Ripoll, J.; Ntziachristos, V. Free-space fluorescence molecular tomography utilizing 360 degrees geometry projections. Opt. Lett. 2007, 32, 382–384. [Google Scholar] [CrossRef]
- Zhang, X.; Badea, C.T.; Johnson, G.A. Three-dimensional reconstruction in free-space whole-body fluorescence tomography of mice using optically reconstructed surface and atlas anatomy. J. Biomed. Opt. 2009, 14, 064010. [Google Scholar] [CrossRef]
- Koenig, A.; Herve, L.; Josserand, V.; Berger, M.; Boutet, J.; da Silva, A.; Dinten, J.M.; Peltie, P.; Coll, J.L.; Rizo, P. In vivo mice lung tumor follow-up with fluorescence diffuse optical tomography. J. Biomed. Opt. 2008, 13, 011008. [Google Scholar]
- Pogue, B.W.; Gibbs, S.L.; Chen, B.; Savellano, M. Fluorescence imaging in vivo: Raster scanned point-source imaging provides more accurate quantification than broad beam geometries. Technol. Cancer Res. Treat. 2004, 3, 15–21. [Google Scholar]
- Ale, A.; Schulz, R.B.; Sarantopoulos, A.; Ntziachristos, V. Imaging performance of a hybrid x-ray computed tomography-fluorescence molecular tomography system using priors. Med. Phys. 2010, 37, 1976–1986. [Google Scholar] [CrossRef]
- Yan, H.; Lin, Y.; Barber, W.C.; Unlu, M.B.; Gulsen, G. A gantry-based tri-modality system for bioluminescence tomography. Rev. Sci. Instrum. 2012, 83, 043708. [Google Scholar] [CrossRef]
- Li, C.; Mitchell, G.S.; Dutta, J.; Ahn, S.; Leahy, R.M.; Cherry, S.R. A three-dimensional multispectral fluorescence optical tomography imaging system for small animals based on a conical mirror design. Opt. Express 2009, 17, 7571–7585. [Google Scholar] [CrossRef]
- Graves, E.E.; Ripoll, J.; Weissleder, R.; Ntziachristos, V. A submillimeter resolution fluorescence molecular imaging system for small animal imaging. Med. Phys. 2003, 30, 901–911. [Google Scholar] [CrossRef]
- Corlu, A.; Choe, R.; Durduran, T.; Rosen, M.A.; Schweiger, M.; Arridge, S.R.; Schnall, M.D.; Yodh, A.G. Three-dimensional in vivo fluorescence diffuse optical tomography of breast cancer in humans. Opt. Express 2007, 15, 6696–6716. [Google Scholar] [CrossRef]
- Zhang, X.; Badea, C.; Jacob, M.; Johnson, G.A. Development of a noncontact 3-D fluorescence tomography system for small animal in vivo imaging. Proc. Soc. Photo Opt. Instrum. Eng. 2009, 7191. [Google Scholar] [CrossRef]
- Muller, A.; Marshall, S.; Jensen, O.B.; Fricke, J.; Wenzel, H.; Sumpf, B.; Andersen, P.E. Diode laser based light sources for biomedical applications. Laser Photonics Rev. 2013, 7, 605–627. [Google Scholar] [CrossRef]
- Chance, B.; Zhao, Z.; Wen, S.; Chen, Y. Simple ac circuit for breast cancer detection and object detection. Rev. Sci. Instrum. 2006, 77. [Google Scholar] [CrossRef]
- Rodat-Boutonnet, A.; Naccache, P.; Morin, A.; Fabre, J.; Feurer, B.; Couderc, F. A comparative study of LED-induced fluorescence and laser-induced fluorescence in SDS-CGE: Application to the analysis of antibodies. Electrophoresis 2012, 33, 1709–1714. [Google Scholar] [CrossRef]
- Bargigia, I.; Tosi, A.; Bahgat Shehata, A.; Della Frera, A.; Farina, A.; Bassi, A.; Taroni, P.; Dalla Mora, A.; Zappa, F.; Cubeddu, R.; et al. Time-resolved diffuse optical spectroscopy up to 1700 nm by means of a time-gated InGaAs/InP single-photon avalanche diode. Appl. Spectrosc. 2012, 66, 944–950. [Google Scholar] [CrossRef]
- Becker, W.; Bergmann, A.; Hink, M.A.; Konig, K.; Benndorf, K.; Biskup, C. Fluorescence lifetime imaging by time-correlated single-photon counting. Microsc. Res. Tech. 2004, 63, 58–66. [Google Scholar] [CrossRef]
- Bloch, S.; Lesage, F.; McIntosh, L.; Gandjbakhche, A.; Liang, K.; Achilefu, S. Whole-body fluorescence lifetime imaging of a tumor-targeted near-infrared molecular probe in mice. J. Biomed. Opt. 2005, 10, 054003. [Google Scholar] [CrossRef]
- Brambilla, M.; Spinelli, L.; Pifferi, A.; Torricelli, A.; Cubeddu, R. Time-resolved scanning system for double reflectance and transmittance fluorescence imaging of diffusive media. Rev. Sci. Instrum. 2008, 79, 013103. [Google Scholar] [CrossRef]
- Lapointe, E.; Pichette, J.; Berube-Lauziere, Y. A multi-view time-domain non-contact diffuse optical tomography scanner with dual wavelength detection for intrinsic and fluorescence small animal imaging. Rev. Sci. Instrum. 2012, 83, 063703. [Google Scholar] [CrossRef]
- Godavarty, A.; Eppstein, M.J.; Zhang, C.; Theru, S.; Thompson, A.B.; Gurfinkel, M.; Sevick-Muraca, E.M. Fluorescence-enhanced optical imaging in large tissue volumes using a gain-modulated ICCD camera. Phys. Med. Biol. 2003, 48, 1701–1720. [Google Scholar] [CrossRef]
- Patwardhan, S.; Bloch, S.; Achilefu, S.; Culver, J. Time-dependent whole-body fluorescence tomography of probe bio-distributions in mice. Opt. Express 2005, 13, 2564–2577. [Google Scholar] [CrossRef]
- Hernandez-Palacios, J.; Randeberg, L.L. Intercomparison of EMCCD- and sCMOS-based imaging spectrometers for biomedical applications in low-light conditions. Proc. Soc. Photo Opt. Instrum. Eng. 2012, 8215. [Google Scholar] [CrossRef]
- Valdes, P.A.; Jacobs, V.L.; Wilson, B.C.; Leblond, F.; Roberts, D.W.; Paulsen, K.D. System and methods for wide-field quantitative fluorescence imaging during neurosurgery. Opt. Lett. 2013, 38, 2786–2788. [Google Scholar]
- Baillard, X.; Gauguet, A.; Bize, S.; Lemonde, P.; Laurent, P.; Clairon, A.; Rosenbusch, P. Interference-filter-stabilized external-cavity diode lasers. Opt. Commu. 2006, 266, 609–613. [Google Scholar] [CrossRef]
- Gebhart, S.C.; Thompson, R.C.; Mahadevan-Jansen, A. Liquid-crystal tunable filter spectral imaging for brain tumor demarcation. Appl. Opt. 2007, 46, 1896–1910. [Google Scholar] [CrossRef]
- Hueber, D.M.; Stevenson, C.L.; Vo-Dinh, T. Fast scanning synchronous luminescence spectrometer based on acousto-optic tunable filters. Appl. Spectrosc. 1995, 49, 1624–1631. [Google Scholar] [CrossRef]
- Xu, H.; Rice, B.W. In-vivo fluorescence imaging with a multivariate curve resolution spectral unmixing technique. J. Biomed. Opt. 2009, 14, 064011. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, K.; Martin, M.; Wintenberg, A.; Lenarduzzi, R.; Panjehpour, M.; Overholt, B.F.; Vo-Dinh, T. Development of a synchronous fluorescence imaging system and data analysis methods. Opt. Express 2007, 15, 12583–12594. [Google Scholar] [CrossRef]
- Toronov, V.; D’Amico, E.; Hueber, D.; Gratton, E.; Barbieri, B.; Webb, A. Optimization of the signal-to-noise ratio of frequency-domain instrumentation for near-infrared spectro-imaging of the human brain. Opt. Express 2003, 11, 2717–2729. [Google Scholar] [CrossRef]
- Fantini, S.; Franceschinifantini, M.A.; Maier, J.S.; Walker, S.A.; Barbieri, B.; Gratton, E. Frequency-domain multichannel optical-detector for noninvasive tissue spectroscopy and oximetry. Opt. Eng. 1995, 34, 32–42. [Google Scholar]
- Siegel, A.; Marota, J.J.; Boas, D. Design and evaluation of a continuous-wave diffuse optical tomography system. Opt. Express 1999, 4, 287–298. [Google Scholar] [CrossRef]
- Yu, G.; Durduran, T.; Furuya, D.; Greenberg, J.H.; Yodh, A.G. Frequency-domain multiplexing system for in vivo diffuse light measurements of rapid cerebral hemodynamics. Appl. Opt. 2003, 42, 2931–2939. [Google Scholar] [CrossRef]
- Joseph, D.K.; Huppert, T.J.; Franceschini, M.A.; Boas, D.A. Diffuse optical tomography system to image brain activation with improved spatial resolution and validation with functional magnetic resonance imaging. Appl. Opt. 2006, 45, 8142–8151. [Google Scholar] [CrossRef]
- Arridge, S.R.; Cope, M.; Delpy, D.T. The theoretical basis for the determination of optical pathlengths in tissue: Temporal and frequency analysis. Phys. Med. Biol. 1992, 37, 1531–1560. [Google Scholar] [CrossRef]
- Chance, B.; Cope, M.; Gratton, E.; Ramanujam, N.; Tromberg, B. Phase measurement of light absorption and scatter in human tissue. Rev. Sci. Instrum. 1998, 69, 3457–3481. [Google Scholar] [CrossRef]
- Alford, K.; Wickramasinghe, Y. Phase-amplitude crosstalk in intensity modulated near infrared spectroscopy. Rev. Sci. Instrum. 2000, 71, 2191–2195. [Google Scholar] [CrossRef]
- Arridge, S.R.; Hebden, J.C. Optical imaging in medicine: II. Modeling and reconstruction. Phys. Med. Biol. 1997, 42, 841–853. [Google Scholar] [CrossRef]
- Hall, D.; Ma, G.; Lesage, F.; Wang, Y. Simple time-domain optical method for estimating the depth and concentration of a fluorescent inclusion in a turbid medium. Opt. Lett. 2004, 29, 2258–2260. [Google Scholar] [CrossRef]
- Selb, J.; Dale, A.M.; Boas, D.A. Linear 3D reconstruction of time-domain diffuse optical imaging differential data: Improved depth localization and lateral resolution. Opt. Express 2007, 15, 16400–16412. [Google Scholar] [CrossRef]
- Leblond, F.; Dehghani, H.; Kepshire, D.; Pogue, B.W. Early-photon fluorescence tomography: Spatial resolution improvements and noise stability considerations. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2009, 26, 1444–1457. [Google Scholar] [CrossRef]
- Chen, J.; Venugopal, V.; Intes, X. Monte Carlo based method for fluorescence tomographic imaging with lifetime multiplexing using time gates. Biomed. Opt. Express 2011, 2, 871–886. [Google Scholar] [CrossRef]
- Pulkkinen, A.; Tarvainen, T. Truncated Fourier-series approximation of the time-domain radiative transfer equation using finite elements. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2013, 30, 470–478. [Google Scholar] [CrossRef]
- Yoo, K.M.; Das, B.B.; Alfano, R.R. Imaging of a translucent object hidden in a highly scattering medium from the early portion of the diffuse component of a transmitted ultrafast laser pulse. Opt. Lett. 1992, 17, 958–960. [Google Scholar] [CrossRef]
- Niedre, M.J.; de Kleine, R.H.; Aikawa, E.; Kirsch, D.G.; Weissleder, R.; Ntziachristos, V. Early photon tomography allows fluorescence detection of lung carcinomas and disease progression in mice in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 19126–19131. [Google Scholar]
- Idlahcen, S.; Mees, L.; Roze, C.; Girasole, T.; Blaisot, J.B. Time gate, optical layout, and wavelength effects on ballistic imaging. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2009, 26, 1995–2004. [Google Scholar] [CrossRef]
- Madsen, S.J.; Wilson, B.C.; Patterson, M.S.; Park, Y.D.; Jacques, S.L.; Hefetz, Y. Experimental tests of a simple diffusion model for the estimation of scattering and absorption coefficients of turbid media from time-resolved diffuse reflectance measurements. Appl. Opt. 1992, 31, 3509–3517. [Google Scholar] [CrossRef]
- Kumar, A.T.; Raymond, S.B.; Bacskai, B.J.; Boas, D.A. Comparison of frequency-domain and time-domain fluorescence lifetime tomography. Opt. Lett. 2008, 33, 470–472. [Google Scholar] [CrossRef]
- Keren, S.; Gheysens, O.; Levin, C.S.; Gambhir, S.S. A comparison between a time domain and continuous wave small animal optical imaging system. IEEE Trans. Med. Imaging 2008, 27, 58–63. [Google Scholar] [CrossRef]
- Li, Z.; Niedre, M. Hybrid use of early and quasi-continuous wave photons in time-domain tomographic imaging for improved resolution and quantitative accuracy. Biomed. Opt. Express 2011, 2, 665–679. [Google Scholar] [CrossRef]
- Arridge, S.R.; Schotland, J.C. Optical tomography: Forward and inverse problems. Inverse Probl. 2009, 25, 123010. [Google Scholar] [CrossRef]
- Turner, G.M.; Zacharakis, G.; Soubret, A.; Ripoll, J.; Ntziachristos, V. Complete-angle projection diffuse optical tomography by use of early photons. Opt. Lett. 2005, 30, 409–411. [Google Scholar] [CrossRef]
- Cuccia, D.J.; Bevilacqua, F.; Durkin, A.J.; Tromberg, B.J. Modulated imaging: Quantitative analysis and tomography of turbid media in the spatial-frequency domain. Opt. Lett. 2005, 30, 1354–1356. [Google Scholar] [CrossRef]
- D’Andrea, C.; Ducros, N.; Bassi, A.; Arridge, S.; Valentini, G. Fast 3D optical reconstruction in turbid media using spatially modulated light. Biomed. Opt. Express 2010, 1, 471–481. [Google Scholar] [CrossRef]
- Lousberg, G.P.; Lundeberg, L.D.; Boiko, D.L.; Kapon, E. Space-domain lock-in amplifier based on a liquid-crystal spatial light modulator. Opt. Lett. 2006, 31, 990–992. [Google Scholar] [CrossRef]
- Dognitz, N.; Wagnieres, G. Determination of tissue optical properties by steady-state spatial frequency-domain reflectometry. Laser Med. Sci. 1998, 13, 55–65. [Google Scholar] [CrossRef]
- Smith, P.J.; Taylor, C.M.; Shaw, A.J.; McCabe, E.M. Programmable array microscopy with a ferroelectric liquid-crystal spatial light modulator. Appl. Opt. 2000, 39, 2664–2669. [Google Scholar] [CrossRef]
- Grosse, M.; Buehl, J.; Babovsky, H.; Kiessling, A.; Kowarschik, R. 3D shape measurement of macroscopic objects in digital off-axis holography using structured illumination. Opt. Lett. 2010, 35, 1233–1235. [Google Scholar] [CrossRef]
- Gregory, D.A.; Juday, R.D.; Sampsell, J.; Gale, R.; Cohn, R.W.; Monroe, S.E., Jr. Optical characteristics of a deformable-mirror spatial light modulator. Opt. Lett. 1988, 13, 10–12. [Google Scholar] [CrossRef]
- Warde, C.; Schiller, C.M.; Bounds, J.; Horsky, T.N.; Melnik, G.; Dillon, R. Charge-transfer-plate spatial light modulators. Appl. Opt. 1992, 31, 3971–3979. [Google Scholar] [CrossRef]
- Bansal, V.; Saggau, P. Digital micromirror devices: Principles and applications in imaging. Cold Spring Harb. Protoc. 2013, 2013, 404–411. [Google Scholar]
- Hillman, E.M.; Boas, D.A.; Dale, A.M.; Dunn, A.K. Laminar optical tomography: Demonstration of millimeter-scale depth-resolved imaging in turbid media. Opt. Lett. 2004, 29, 1650–1652. [Google Scholar] [CrossRef]
- Hillman, E.M.; Devor, A.; Bouchard, M.B.; Dunn, A.K.; Krauss, G.W.; Skoch, J.; Bacskai, B.J.; Dale, A.M.; Boas, D.A. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. Neuroimage 2007, 35, 89–104. [Google Scholar] [CrossRef]
- Mazhar, A.; Cuccia, D.J.; Gioux, S.; Durkin, A.J.; Frangioni, J.V.; Tromberg, B.J. Structured illumination enhances resolution and contrast in thick tissue fluorescence imaging. J. Biomed. Opt. 2010, 15, 010506. [Google Scholar] [CrossRef]
- Laughney, A.M.; Krishnaswamy, V.; Rice, T.B.; Cuccia, D.J.; Barth, R.J.; Tromberg, B.J.; Paulsen, K.D.; Pogue, B.W.; Wells, W.A. System analysis of spatial frequency domain imaging for quantitative mapping of surgically resected breast tissues. J. Biomed. Opt. 2013, 18, 036012. [Google Scholar] [CrossRef]
- Ziegler, R.; Nielsen, T.; Koehler, T.; Grosenick, D.; Steinkellner, O.; Hagen, A.; Macdonald, R.; Rinneberg, H. Nonlinear reconstruction of absorption and fluorescence contrast from measured diffuse transmittance and reflectance of a compressed-breast-simulating phantom. Appl. Opt. 2009, 48, 4651–4662. [Google Scholar] [CrossRef]
- Ripoll, J. Hybrid Fourier-real space method for diffuse optical tomography. Opt. Lett. 2010, 35, 688–690. [Google Scholar] [CrossRef]
- Yoo, K.M.; Xing, Q.; Alfano, R.R. Imaging objects hidden in highly scattering media using femtosecond second-harmonic-generation cross-correlation time gating. Opt. Lett. 1991, 16, 1019–1021. [Google Scholar] [CrossRef]
- Sato, H.; Kiguchi, M.; Kawaguchi, F.; Maki, A. Practicality of wavelength selection to improve signal-to-noise ratio in near-infrared spectroscopy. Neuroimage 2004, 21, 1554–1562. [Google Scholar] [CrossRef]
- Chance, B.; Luo, Q.; Nioka, S.; Alsop, D.C.; Detre, J.A. Optical investigations of physiology: A study of intrinsic and extrinsic biomedical contrast. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1997, 352, 707–716. [Google Scholar] [CrossRef]
- Spichtig, S.; Hornung, R.; Brown, D.W.; Haensse, D.; Wolf, M. Multifrequency frequency-domain spectrometer for tissue analysis. Rev. Sci. Instrum. 2009, 80, 024301. [Google Scholar] [CrossRef]
- Nioka, S.; Chance, B. NIR spectroscopic detection of breast cancer. Technol. Cancer Res. Treat. 2005, 4, 497–512. [Google Scholar]
- Kepshire, D.S.; Gibbs-Strauss, S.L.; O’Hara, J.A.; Hutchins, M.; Mincu, N.; Leblond, F.; Khayat, M.; Dehghani, H.; Srinivasan, S.; Pogue, B.W. Imaging of glioma tumor with endogenous fluorescence tomography. J. Biomed. Opt. 2009, 14, 030501. [Google Scholar] [CrossRef]
- Gulsen, G.; Birgul, O.; Unlu, M.B.; Shafiiha, R.; Nalcioglu, O. Combined diffuse optical tomography (DOT) and MRI system for cancer imaging in small animals. Technol. Cancer Res. Treat. 2006, 5, 351–363. [Google Scholar]
- Nothdurft, R.E.; Patwardhan, S.V.; Akers, W.; Ye, Y.; Achilefu, S.; Culver, J.P. In vivo fluorescence lifetime tomography. J. Biomed. Opt. 2009, 14, 024004. [Google Scholar] [CrossRef]
- Mitsiades, C.S.; Mitsiades, N.S.; Bronson, R.T.; Chauhan, D.; Munshi, N.; Treon, S.P.; Maxwell, C.A.; Pilarski, L.; Hideshima, T.; Hoffman, R.M.; et al. Fluorescence imaging of multiple myeloma cells in a clinically relevant SCID/NOD in vivo model: Biologic and clinical implications. Cancer Res. 2003, 63, 6689–6696. [Google Scholar]
- Chen, J.; Tung, C.H.; Mahmood, U.; Ntziachristos, V.; Gyurko, R.; Fishman, M.C.; Huang, P.L.; Weissleder, R. In vivo imaging of proteolytic activity in atherosclerosis. Circulation 2002, 105, 2766–2771. [Google Scholar] [CrossRef]
- Chen, W.T.; Mahmood, U.; Weissleder, R.; Tung, C.H. Arthritis imaging using a near-infrared fluorescence folate-targeted probe. Arthritis Res. Ther. 2005, 7, R310–R317. [Google Scholar] [CrossRef]
- Shepherd, J.; Hilderbrand, S.A.; Waterman, P.; Heinecke, J.W.; Weissleder, R.; Libby, P. A fluorescent probe for the detection of myeloperoxidase activity in atherosclerosis-associated macrophages. Chem. Biol. 2007, 14, 1221–1231. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Sosnovik, D.E.; Waterman, P.; Swirski, F.K.; Pande, A.N.; Aikawa, E.; Figueiredo, J.L.; Pittet, M.J.; Weissleder, R. Dual channel optical tomographic imaging of leukocyte recruitment and protease activity in the healing myocardial infarct. Circ. Res. 2007, 100, 1218–1225. [Google Scholar] [CrossRef]
- Aikawa, E.; Nahrendorf, M.; Figueiredo, J.L.; Swirski, F.K.; Shtatland, T.; Kohler, R.H.; Jaffer, F.A.; Aikawa, M.; Weissleder, R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 2007, 116, 2841–2850. [Google Scholar] [CrossRef]
- Haller, J.; Hyde, D.; Deliolanis, N.; de Kleine, R.; Niedre, M.; Ntziachristos, V. Visualization of pulmonary inflammation using noninvasive fluorescence molecular imaging. J. Appl. Physiol. 2008, 104, 795–802. [Google Scholar]
- Cortez-Retamozo, V.; Swirski, F.K.; Waterman, P.; Yuan, H.; Figueiredo, J.L.; Newton, A.P.; Upadhyay, R.; Vinegoni, C.; Kohler, R.; Blois, J.; et al. Real-time assessment of inflammation and treatment response in a mouse model of allergic airway inflammation. J. Clin. Investig. 2008, 118, 4058–4066. [Google Scholar] [CrossRef]
- Vinegoni, C.; Botnaru, I.; Aikawa, E.; Calfon, M.A.; Iwamoto, Y.; Folco, E.J.; Ntziachristos, V.; Weissleder, R.; Libby, P.; Jaffer, F.A. Indocyanine green enables near-infrared fluorescence imaging of lipid-rich, inflamed atherosclerotic plaques. Sci. Transl. Med. 2011, 3. [Google Scholar] [CrossRef]
- Stepnoski, R.A.; LaPorta, A.; Raccuia-Behling, F.; Blonder, G.E.; Slusher, R.E.; Kleinfeld, D. Noninvasive detection of changes in membrane potential in cultured neurons by light scattering. Proc. Natl. Acad. Sci. USA 1991, 88, 9382–9386. [Google Scholar]
- Rector, D.M.; Poe, G.R.; Kristensen, M.P.; Harper, R.M. Light scattering changes follow evoked potentials from hippocampal Schaeffer collateral stimulation. J. Neurophys. 1997, 78, 1707–1713. [Google Scholar]
- Rector, D.M.; Carter, K.M.; Volegov, P.L.; George, J.S. Spatio-temporal mapping of rat whisker barrels with fast scattered light signals. Neuroimage 2005, 26, 619–627. [Google Scholar] [CrossRef]
- Gratton, G.; Fabiani, M. The event-related optical signal (EROS) in visual cortex: Replicability, consistency, localization, and resolution. Psychophysiology 2003, 40, 561–571. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Di Vacri, A.; Romani, G.L.; Merla, A. Fast optical signal in visual cortex: Improving detection by General Linear Convolution Model. Neuroimage 2012, 66C, 194–202. [Google Scholar]
- Jiang, H. Frequency-domain fluorescent diffusion tomography: A finite-element-based algorithm and simulations. Appl. Opt. 1998, 37, 5337–5343. [Google Scholar] [CrossRef]
- Kumar, A.T.; Raymond, S.B.; Dunn, A.K.; Bacskai, B.J.; Boas, D.A. A time domain fluorescence tomography system for small animal imaging. IEEE Trans. Med. Imaging 2008, 27, 1152–1163. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef]
- Jiang, P.C.; Grundfest, W.S.; Stafsudd, O.M. Quasi-real-time fluorescence imaging with lifetime dependent contrast. J. Biomed. Opt. 2011, 16, 086001. [Google Scholar] [CrossRef]
- McGinty, J.; Stuckey, D.W.; Soloviev, V.Y.; Laine, R.; Wylezinska-Arridge, M.; Wells, D.J.; Arridge, S.R.; French, P.M.W.; Hajnal, J.V.; Sardini, A. In vivo fluorescence lifetime tomography of a FRET probe expressed in mouse. Biomed. Opt. Express 2011, 2, 1907–1917. [Google Scholar] [CrossRef]
- Marcu, L. Fluorescence lifetime techniques in medical applications. Ann. Biomed. Eng. 2012, 40, 304–331. [Google Scholar] [CrossRef]
- Liu, X.; Yang, P.S.; Yang, W.; Yue, D.T. Enzyme-inhibitor-like tuning of Ca(2+) channel connectivity with calmodulin. Nature 2010, 463, 968–972. [Google Scholar]
- Shen, Z.; Lu, Z.; Chhatbar, P.Y.; O’Herron, P.; Kara, P. An artery-specific fluorescent dye for studying neurovascular coupling. Nat. Methods 2012, 9, 273–276. [Google Scholar]
- Dmitriev, R.I.; Zhdanov, A.V.; Nolan, Y.M.; Papkovsky, D.B. Imaging of neurosphere oxygenation with phosphorescent probes. Biomaterials 2013, 34, 9307–9317. [Google Scholar] [CrossRef]
- Hille, C.; Berg, M.; Bressel, L.; Munzke, D.; Primus, P.; Lohmannsroben, H.G.; Dosche, C. Time-domain fluorescence lifetime imaging for intracellular pH sensing in living tissues. Anal. Bioanal. Chem. 2008, 391, 1871–1879. [Google Scholar] [CrossRef]
- Caldwell, B.J.; Wellner, M.; Mitrea, B.G.; Pertsov, A.M.; Zemlin, C.W. Probing field-induced tissue polarization using transillumination fluorescent imaging. Biophys. J. 2010, 99, 2058–2066. [Google Scholar] [CrossRef]
- Ho, Y.P.; Chen, H.H.; Leong, K.W.; Wang, T.H. Evaluating the intracellular stability and unpacking of DNA nanocomplexes by quantum dots-FRET. J. Control Release 2006, 116, 83–89. [Google Scholar] [CrossRef]
- Becker, W. Fluorescence lifetime imaging--techniques and applications. J. Microsc. 2012, 247, 119–136. [Google Scholar] [CrossRef]
- Vinegoni, C.; Razansky, D.; Hilderbrand, S.A.; Shao, F.; Ntziachristos, V.; Weissleder, R. Transillumination fluorescence imaging in mice using biocompatible upconverting nanoparticles. Opt. Lett. 2009, 34, 2566–2568. [Google Scholar] [CrossRef]
- Wang, M.; Abbineni, G.; Clevenger, A.; Mao, C.; Xu, S. Upconversion nanoparticles: Synthesis, surface modification and biological applications. Nanomedicine 2011, 7, 710–729. [Google Scholar] [CrossRef]
- Cong, W.; Wang, G.; Kumar, D.; Liu, Y.; Jiang, M.; Wang, L.; Hoffman, E.; McLennan, G.; McCray, P.; Zabner, J.; et al. Practical reconstruction method for bioluminescence tomography. Opt. Express 2005, 13, 6756–6771. [Google Scholar] [CrossRef]
- Alexandrakis, G.; Rannou, F.R.; Chatziioannou, A.F. Tomographic bioluminescence imaging by use of a combined optical-PET (OPET) system: A computer simulation feasibility study. Phys. Med. Biol. 2005, 50, 4225–4241. [Google Scholar] [CrossRef]
- Chaudhari, A.J.; Darvas, F.; Bading, J.R.; Moats, R.A.; Conti, P.S.; Smith, D.J.; Cherry, S.R.; Leahy, R.M. Hyperspectral and multispectral bioluminescence optical tomography for small animal imaging. Phys. Med. Biol. 2005, 50, 5421–5441. [Google Scholar] [CrossRef]
- Pratx, G.; Carpenter, C.M.; Sun, C.; Xing, L. X-ray luminescence computed tomography via selective excitation: A feasibility study. IEEE Trans. Med. Imaging 2010, 29, 1992–1999. [Google Scholar] [CrossRef]
- Cong, W.; Shen, H.; Wang, G. Spectrally resolving and scattering-compensated X-ray luminescence/fluorescence computed tomography. J. Biomed. Opt. 2011, 16, 066014. [Google Scholar] [CrossRef]
- Li, C.; Di, K.; Bec, J.; Cherry, S.R. X-ray luminescence optical tomography imaging: Experimental studies. Opt. Lett. 2013, 38, 2339–2341. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, S.; Yi, H.; Zhang, X.; Chen, D.; Liang, J.; Tian, J. Cone beam x-ray luminescence computed tomography: A feasibility study. Med. Phys. 2013, 40, 031111. [Google Scholar] [CrossRef]
- Cherenkov, P.A. Radiation from high-speed particles. Science 1960, 131, 136–142. [Google Scholar]
- Ross, H.H. Measurement of beta-emitting nuclides using cerenkov radiation. Anal. Chem. 1969, 41, 1260–1265. [Google Scholar] [CrossRef]
- Cheng, Z.; Levi, J.; Xiong, Z.; Gheysens, O.; Keren, S.; Chen, X.; Gambhir, S.S. Near-infrared fluorescent deoxyglucose analogue for tumor optical imaging in cell culture and living mice. Bioconjug. Chem. 2006, 17, 662–669. [Google Scholar] [CrossRef]
- Dothager, R.S.; Goiffon, R.J.; Jackson, E.; Harpstrite, S.; Piwnica-Worms, D. Cerenkov radiation energy transfer (CRET) imaging: A novel method for optical imaging of PET isotopes in biological systems. PLoS One 2010, 5, e13300. [Google Scholar]
- Mitchell, G.S.; Gill, R.K.; Boucher, D.L.; Li, C.; Cherry, S.R. In vivo Cerenkov luminescence imaging: A new tool for molecular imaging. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 4605–4619. [Google Scholar] [CrossRef]
- Zhang, X.; Kuo, C.; Moore, A.; Ran, C. In vivo optical imaging of interscapular brown adipose tissue with (18)F-FDG via Cerenkov luminescence imaging. PLoS one 2013, 8, e62007. [Google Scholar] [CrossRef]
- Spinelli, A.E.; Ferdeghini, M.; Cavedon, C.; Zivelonghi, E.; Calandrino, R.; Fenzi, A.; Sbarbati, A.; Boschi, F. First human Cerenkography. J. Biomed. Opt. 2013, 18, 20502. [Google Scholar] [CrossRef]
- Newman, F.; Asadi-Zeydabadi, M.; Durairaj, V.D.; Ding, M.; Stuhr, K.; Kavanagh, B. Visual sensations during megavoltage radiotherapy to the orbit attributable to Cherenkov radiation. Med. Phys. 2008, 35, 77–80. [Google Scholar] [CrossRef]
- Axelsson, J.; Davis, S.C.; Gladstone, D.J.; Pogue, B.W. Cerenkov emission induced by external beam radiation stimulates molecular fluorescence. Med. Phys. 2011, 38, 4127–4132. [Google Scholar] [CrossRef]
- Demers, J.L.; Davis, S.C.; Zhang, R.; Gladstone, D.J.; Pogue, B.W. Cerenkov excited fluorescence tomography using external beam radiation. Opt. Lett. 2013, 38, 1364–1366. [Google Scholar] [CrossRef]
- Glaser, A.K.; Zhang, R.; Davis, S.C.; Gladstone, D.J.; Pogue, B.W. Time-gated Cherenkov emission spectroscopy from linear accelerator irradiation of tissue phantoms. Opt. Lett. 2012, 37, 1193–1195. [Google Scholar] [CrossRef]
- Glaser, A.K.; Davis, S.C.; McClatchy, D.M.; Zhang, R.; Pogue, B.W.; Gladstone, D.J. Projection imaging of photon beams by the Cerenkov effect. Med. Phys. 2013, 40, 012101. [Google Scholar] [CrossRef]
- Zhang, R.; Fox, C.J.; Glaser, A.K.; Gladstone, D.J.; Pogue, B.W. Superficial dosimetry imaging of Cerenkov emission in electron beam radiotherapy of phantoms. Phys. Med. Biol. 2013, 58, 5477–5493. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, X. Instrumentation in Diffuse Optical Imaging. Photonics 2014, 1, 9-32. https://doi.org/10.3390/photonics1010009
Zhang X. Instrumentation in Diffuse Optical Imaging. Photonics. 2014; 1(1):9-32. https://doi.org/10.3390/photonics1010009
Chicago/Turabian StyleZhang, Xiaofeng. 2014. "Instrumentation in Diffuse Optical Imaging" Photonics 1, no. 1: 9-32. https://doi.org/10.3390/photonics1010009
APA StyleZhang, X. (2014). Instrumentation in Diffuse Optical Imaging. Photonics, 1(1), 9-32. https://doi.org/10.3390/photonics1010009




