Abstract
Tropical streams host diverse benthic macroinvertebrates, essential for ecological processes and bioindicators of ecosystem health. However, land use changes, such as sugarcane cultivation, negatively impact stream structure and function. This study examined these effects by comparing streams in sugarcane-dominated areas with those in native vegetation. Streams with native vegetation showed 2226 individuals across 107 taxa, 39 families, and 52 genera, with Chironomidae (Chironominae, Tanypodinae), Leptoceridae, Leptophlebiidae, and Calamoceratidae being the most abundant. In contrast, sugarcane streams showed 692 individuals from 47 taxa, 24 families, and 19 genera, with Chironomidae (Chironominae, Tanypodinae), Dryopidae, and Simuliidae dominating. The first hypothesis suggested that sugarcane plantations reduce taxonomic and functional diversity. The results partially confirmed this, showing lower abundance and richness in sugarcane streams, though functionality remained unchanged. The second hypothesis proposed greater seasonal taxonomic variation in sugarcane streams due to hydrological differences. Results supported this, revealing stronger seasonal shifts, particularly during the rainy season. These findings highlight the significant impact of sugarcane cultivation on aquatic ecosystems. Continuous monitoring of macroinvertebrates in agricultural landscapes is crucial for assessing environmental impacts and guiding conservation strategies.
1. Introduction
Deforestation and agricultural expansion can significantly alter the quantity and quality of water in aquatic ecosystems, with widespread effects on both terrestrial and aquatic environments [,]. Such land-use changes disrupt natural landscapes, creating inhospitable conditions for many species and leading to a decline in biodiversity [,,,]. Understanding the scale of these impacts is complex, as the consequences of anthropogenic activities manifest at multiple spatial levels [,,]. These impacts are influenced not only by land use at the watershed level but also by conditions along the riparian corridor and at the stream reach scale, all of which interact to shape aquatic biota []. In agricultural landscapes, the proximity and intensity of land-use practices near watercourses can result in increased sedimentation, nutrient loading, and degradation of water quality, which are reflected in changes in biological indicators such as macroinvertebrate assemblages [,].
In Brazil, sugarcane is a major crop that supports ethanol and sugar production, helping to reduce reliance on fossil fuels. However, this crop’s cultivation is also a primary contributor to stream degradation, posing a significant environmental challenge [,]. Freshwater ecosystems globally bear the burden of agricultural growth, which uses about 85% of global freshwater, often leading to water quality deterioration through chemical and physical alterations []. In sugarcane-dominated landscapes, such as those found in southeastern Brazil, stream degradation is closely linked to changes in land use that include the removal of riparian vegetation, increased soil erosion, and the input of agrochemicals and sediments into water bodies [,]. These pressures affect not only abiotic parameters, such as conductivity and turbidity, but also biological communities [,]. Furthermore, studies have highlighted that the proximity of intensive agricultural areas to stream margins intensifies these effects, reinforcing the importance of maintaining and restoring riparian buffers as a key strategy for mitigating environmental impacts [,,].
Headwater streams are highly interlinked with their surrounding landscapes, creating meta-ecosystem complexes where biotic and abiotic processes closely interact []. The ecological condition of these terrestrial surroundings strongly influences stream structure and functioning [,,,]. This sensitivity is particularly evident in small streams, which are highly reactive to human activities due to their extensive connectivity with adjacent areas []. This interdependence underscores the importance of integrating landscape-level assessments when evaluating freshwater ecosystems, as land use and habitat structure at both local and broader scales directly influence stream biodiversity and ecological integrity [,,]. In particular, the degradation of riparian zones and watershed modifications have been shown to disrupt key ecosystem functions, such as organic matter decomposition, nutrient cycling, and biological regulation [,,]. Moreover, the spatial configuration and intensity of land use practices—especially agriculture—can trigger threshold responses in biotic communities, leading to abrupt losses in species richness and functional diversity once critical environmental limits are crossed [,]. Such effects are especially pronounced in agricultural or fragmented landscapes, where reduced vegetation cover and increased sediment and pollutant loads compromise the ecological services provided by riparian systems [,,,].
Riparian vegetation plays a crucial role in maintaining stream health by serving as a physical buffer that reduces sediment, fertilizer, and pesticide runoff from agricultural lands [,]. The narrowing of riparian zones directly impacts stream biota, leading to sedimentation that disrupts benthic habitat structure, reduces resources such as shelter and food, and ultimately diminishes species diversity over time []. In addition to acting as a filter for pollutants, riparian vegetation regulates stream temperature through shading, stabilizes stream banks against erosion, and contributes organic material essential for aquatic food webs [,,]. The loss or degradation of this vegetation—often due to the expansion of intensive agriculture—compromises these functions, creating unstable habitats and promoting homogenization of aquatic communities. This is particularly concerning in tropical agricultural landscapes, where high rainfall can exacerbate surface runoff and soil loss in the absence of adequate vegetative buffers [,,].
To assess these environmental changes, biological assessments are essential, as aquatic organisms reflect both direct and indirect responses to habitat quality []. Biological assessments use bioindicator species, including bacteria, fungi, fish, protozoa, algae, macrophytes, and benthic macroinvertebrates, to evaluate ecosystem health and detect environmental disturbances [,]. Among these indicators, benthic macroinvertebrates are particularly valuable due to their widespread distribution, taxonomic diversity, varying tolerance levels to pollution, limited mobility, and relatively long life cycles, all of which make them responsive indicators of ecological conditions [,].
The use of benthic communities as bioindicators is widespread due to their effectiveness in assessing ecosystem structure, providing insights for watershed restoration, and measuring resilience against environmental pressures such as extreme spates [,]. Functional feeding groups (FGs), a classification that has been in use for over 30 years, is a powerful approach to examining these communities. FGs help clarify trophic relationships and community dynamics, illustrating species interactions and roles within ecosystems [,,].
Given the anticipated expansion of sugarcane cultivation in Brazil, it is critical to examine how this crop affects headwater stream ecosystems, particularly the benthic macroinvertebrate community. This study aims to compare the taxonomic and functional traits of benthic macroinvertebrates in streams within sugarcane-dominated landscapes against those in streams bordered by native vegetation (Brazilian Woodland Savannas—Cerrado biome). The study hypothesizes the following: (i) benthic macroinvertebrate communities will show reduced taxonomic and functional diversity in streams draining sugarcane plantations, and (ii) different periods of sampling would influence the macroinvertebrate community.
2. Materials and Methods
This study was conducted in the municipalities of São Carlos and Itirapina in São Paulo State, Brazil. The local climate is subtropical, characterized by hot summers with temperatures exceeding 22 °C and more than 30 mm of rainfall in the driest month. This climate is classified as Cfa under the Köppen system and falls within the Cerrado biome []. The research focused on five catchments draining first-order streams, with two catchments primarily occupied by sugarcane plantations and three covered by native vegetation (Figure 1). These catchments were selected based on their dominant land cover, determined using Quantum GIS and Google Earth.
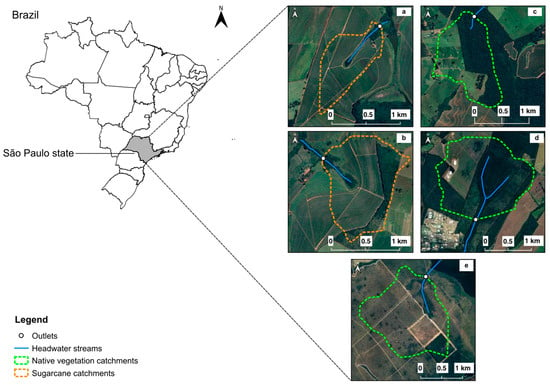
Figure 1.
Catchments selected for the study, where 3 are covered by sugarcane plantations ((a,b)—orange polygons) and 2 are occupied by native vegetation ((c–e)—green polygons). The streams are in the countryside of the state of São Paulo. Coordinates of each sampling catchment are labeled: (a) 21°56′50″ S 47°51′53″ W; (b) 21°56′28″ S 47°51′30″ W; (c) 21°58′46″ S 47°52′23″ W; (d) 21°58′00″ S 47°50′33″ W; (e) 22°11′36″ S 47°53′52″ W.
Sampling was conducted during two campaigns, one in December 2018 (wet season) and another in October 2019 (dry season). A 100 m stretch of each stream was sampled, with five replicates taken per stream using a D-net with a 250 mm mesh. Sampling involved stirring the substrate for 3 min near the net and exploring different bank conditions and bottom substrate types []. At each stream, water parameters—including pH, temperature, electrical conductivity, dissolved oxygen, and redox potential—were measured on-site with a YSI Professional Series multiparameter probe (YSI, Yellow Springs, OH, USA).
Following sampling, benthic macroinvertebrates were stored in plastic bags with water from each sampling site, frozen, and then sorted. Sorting involved transferring samples to white polyethylene trays with backlighting, where substrates (leaves, sticks, logs, rocks, and sediment) were washed with running water over a 250 µm mesh to remove any attached macroinvertebrates. The collected macroinvertebrates were preserved in 70% ethanol and identified to the family level, or the lowest possible taxonomic level, using a DI 224 stereoscope and identification manuals [,]. Morphological and behavioral characteristics were also considered for classification []. Benthic macroinvertebrates were assigned to seven functional feeding groups: collector-gatherer, collector-filterer, herbivorous-sucker, predator, scraper, scraper-grazer, and shredder [,,].
Univariate statistical analyses were performed to compare the water’s physical and chemical parameters, with visualizations provided via boxplots. Median differences in these parameters were assessed using the Mann-Whitney test. To examine the effects of land use on benthic macroinvertebrate communities, we calculated the relative abundance, taxon richness and functional feeding groups across different seasons and land uses. We conducted a Redundancy analysis (RDA) followed by ANOVA to assess community structure across land uses. All analyses were performed in R, using the “factoextra”, “ggplot2”, “ggpubr”, and “vegan” packages.
3. Results
3.1. Physical and Chemical Characteristics of Stream Water Draining Sugarcane Cultivation and Native Vegetation
The physical and chemical water parameters in the streams exhibited significant differences between land uses associated with sugarcane cultivation and native vegetation. Streams draining sugarcane plantations showed consistently higher values and concentrations across all assessed parameters (Figure 2). Regarding the sampling periods, higher water temperatures were observed in December, while higher conductivity values were recorded in September (Figure 2).
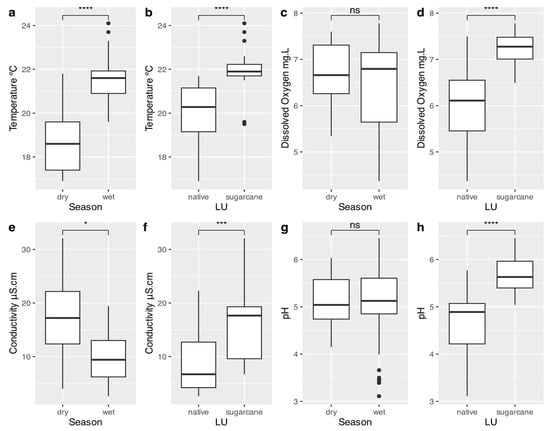
Figure 2.
Boxplots of the physicochemical characteristics of the water representing the medians and quartiles of the different parameters evaluated, comparing the different land uses (LU) and the different seasonal periods (Season); Dots represents outliers. * p ≤ 0.1, *** p ≤ 0.01, **** p ≤ 0.001. (a) Water temperature between seasons; (b) water temperature between land uses; (c) dissolved oxygen concentrations between seasons; (d) dissolved oxygen concentrations between land uses; (e) water conductivity between seasons; (f) water conductivity between land uses; (g) water pH between seasons, and (h) water pH between land uses.
3.2. Taxonomic and Functional Characteristics of the Benthic Macroinvertebrate Community in Streams Draining Sugarcane and Native Vegetation
A total of 2918 individuals were sampled from both land uses (Supplementary Material S1). A total of 2226 individuals were collected in streams draining native vegetation; these individuals were distributed among 107 taxa, 39 families, and 52 genera. The most abundant families were Chironomidae (subfamily Chironominae and Tanypodinae) (n = 1182), Leptoceridae (n = 118), Leptophlebiidae (n = 283), and Calamoceratidae (n = 68). The streams draining sugarcane had 692 individuals divided into 47 taxa, 24 families, and 19 genera, with the families Chironomidae (Chironominae and Tanypodiinae) (n = 405), Dryopidae (n = 60), and Simuliidae (n = 73) being the most abundant.
There was a distinct difference in the benthic macroinvertebrate community composition between streams draining native vegetation and those draining sugarcane plantations. Streams in sugarcane areas showed higher relative abundances of Diptera and Trichoptera, with Diptera predominating in the second sampling period and Trichoptera predominating in the first (Figure 3a,c). In contrast, streams draining native vegetation exhibited higher relative abundances of Diptera, Ephemeroptera, and Trichoptera, with Diptera dominant in the second period and Ephemeroptera and Trichoptera dominant in the first period (Figure 3a,c). Regarding functional groups, streams draining sugarcane plantations had greater relative abundances of collector-gatherers, collector-filterers, and predators compared to those in native vegetation (Figure 3b). Conversely, streams in native vegetation showed higher abundances of shredders and scraper-grazers (Figure 3b). Seasonal differences also influenced functional group composition, with higher abundances of collector-filterers and shredders in the second sampling period and more predators and scrapers in the first period (Figure 3d).
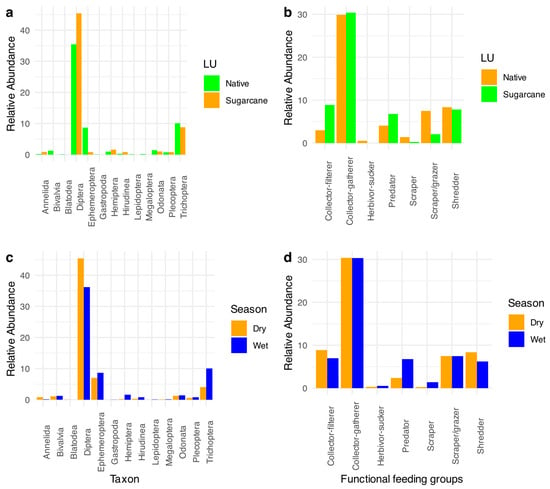
Figure 3.
Taxonomical and functional relative abundances of benthic macroinvertebrates in different land uses and seasons in the sampled headwater streams. (a) Relative abundances of benthic invertebrates between different land uses; (b) relative abundances of the functional feeding groups of benthic invertebrates between different land uses; (c) relative abundances of benthic invertebrates between different seasons; (d) relative abundances of the functional feeding groups of benthic invertebrates between different seasons.
Redundancy Analysis (RDA) revealed that the benthic macroinvertebrate community at the order level was significantly influenced by both land use and season (LU:Df = 1, F = 8.65, p = 0.008; Season: Df = 1, F = 4.75, p = 0.04) (Figure 4). The taxa most strongly associated with streams draining native forests were Trichoptera and Ephemeroptera during the wet season, while Diptera was more closely related to streams draining sugarcane in both seasons. The variance explained by RDA axis 1 was 91%, while axis 2 explained 8%.
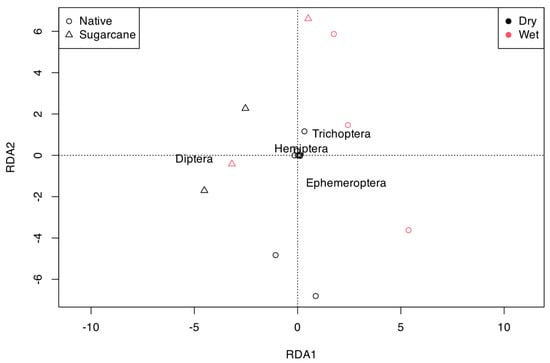
Figure 4.
Redundancy analysis using the benthic macroinvertebrate community at order level for ordinating the orders, land uses, and seasons.
4. Discussion
The physicochemical characteristics of the streams in this study showed significant differences. Streams draining sugarcane plantations exhibited higher water temperature, pH, conductivity, and dissolved oxygen levels. Additionally, differences in stream depth and width were observed. These results can be attributed to the conversion of native vegetation into sugarcane crops. The removal of native vegetation often leads to increased water temperature due to reduced shading, and higher concentrations of dissolved ions, which directly contribute to increased conductivity []. The higher dissolved oxygen levels may be linked to enhanced primary production, driven by increased solar radiation after vegetation removal, as well as higher ion concentrations, which can stimulate photosynthetic activity in benthic algae []. Furthermore, land-use changes like this can also lead to greater sediment and nutrient inputs, which can further alter water quality. Seasonal differences were also evident, with higher water temperatures during the rainy season, likely due to the coincidence of the rainy season with the summer months in tropical regions, and increased conductivity during the dry season, driven by reduced rainfall and the lack of dilution.
The first hypothesis of this study was that the benthic macroinvertebrate community is taxonomically and functionally impoverished in streams occupied by sugarcane plantations. Our results showed that the streams draining sugarcane plantations had lower abundance and taxonomic richness, but in terms of the functional feeding groups, the streams showed no significant differences, partially corroborating the proposed hypothesis. The streams with native vegetation had a greater abundance of macroinvertebrates (n = 2226), unlike the streams occupied by sugarcane (n = 692), possibly due to the adjacent structural characteristics and the diversity of microhabitats provided by the native vegetation []. Studies analyzing the link between conserved riparian vegetation and benthic macroinvertebrate structure have shown a decrease in the diversity and richness of sensitive organisms in streams following partial or total vegetation degradation [,,,,,]. Streams with conserved native vegetation tend to have specialized fauna that are more vulnerable to habitat alterations, while modified environments tend to show high rates of abundance of organisms, although for groups that tolerate impacts []. Therefore, a significant benefit to stream biodiversity would be to protect an even larger riparian zone around small watercourses (up to 10 m or more) []. Studies conducted in Brazil that evaluated different land uses (urbanization, agriculture, and pasture) revealed that after modifying riparian vegetation, low richness and changes in the composition of benthic macroinvertebrates were observed [,,,]. In a study conducted in the same streams, it was observed that the structure of the streams was also different in relation to the different land uses, showing more degraded conditions in the streams draining sugarcane crops, while differences in habitat structure were observed as well. Considering that habitat structure is directly related to the dynamics and structure of the benthic community, these results show that this factor may be more important than changes in anthropized landscapes [].
The family of benthic macroinvertebrates with the highest abundance in both land uses was Chironomidae, which combined the subfamilies Chironominae and Tanypodinae (reed beds n = 405 and native vegetation n = 1182), and these organisms were categorized as collectors/gatherers. This family is relevant for biomonitoring and has considerable use as a bioindicator in several countries. They are very abundant organisms in freshwater aquatic ecosystems and are ecologically important in the bioturbation process (sediment—water) [,]. The results of this study also revealed a greater abundance of the EPT group in streams draining native vegetation catchments. Organisms of the EPT orders (Ephemeroptera, Plecoptera, Trichoptera) are inherent to sites with good water quality [,]. These groups of macroinvertebrates are more sensitive and show a decline in abundance in streams adjacent to monocultures, indicating the impact generated by this activity since they are organisms that are sensitive to environmental changes and serve as bioindicators of environmental quality []. In the study by [], which compared macroinvertebrates between pastures and native forest streams in New Zealand, a greater abundance of macroinvertebrates was observed in the pasture areas than in the native forest areas, but with a high quantity of Chironomidae and mollusks, while the forested areas had a high abundance of the EPT group. Studies show that the degradation, suppression, or change of native vegetation by monocultures promotes homogeneity in environments [,,]. Therefore, our results highlight the importance of maintaining riparian forests in agricultural streams to preserve aquatic biodiversity and ecosystem functions.
The second hypothesis of this study proposed that differences between sampling periods influence the taxonomic composition of the macroinvertebrate community. Our results support this hypothesis, as seasonality had a significant effect on the redundancy analysis. During the dry season, Diptera dominated the community, whereas Trichoptera and Ephemeroptera were more abundant in the wet season. This seasonal shift is likely driven by increased streamflow during the wet season, which transports insects downstream, enhances oxygen saturation, and contributes to sediment and organic matter input. Seasonal hydrological changes also play a key role in shaping macroinvertebrate assemblages. During the rainy season, an influx of allochthonous organic matter from terrestrial sources affects electrical conductivity, water depth, substrate deposition, and habitat composition for invertebrates [,]. Increased streamflow changes the benthic zone and increases dissolved oxygen concentrations and water temperature [,]. As a result, these environmental changes during the wet season often support higher macroinvertebrate abundance [].
Ephemeroptera, Plecoptera, and Trichoptera (EPT) are common in low- and mid-order streams and serve as effective bioindicators of water quality due to their sensitivity to anthropogenic disturbances [,]. These taxa thrive in clean streams with well-preserved riparian vegetation, stable temperatures, high dissolved oxygen levels, and diverse mesohabitats [,,]. Seasonal variations influence EPT distribution as observed in a study which found that Ephemeroptera and Plecoptera were more prevalent in summer and autumn, showing some tolerance to climatic variation, whereas Trichoptera was more sensitive to seasonal shifts and dissolved oxygen fluctuations []. Similarly, ref. [] found that reduced flow and lower oxygen levels led to declines in rheophilic taxa-like EPT, which prefer well-aerated water []. Our findings suggest that higher streamflow and increased dissolved oxygen concentrations in forested streams support greater abundances of sensitive taxa, particularly Trichoptera and Ephemeroptera.
Our findings strongly emphasize the critical role of riparian forests in mitigating the adverse effects of sugarcane cultivation on stream ecosystems. These ecosystems are intricately linked to the surrounding landscape, and the presence of native riparian vegetation acts as a crucial buffer that modulates physical, chemical, and biological processes within stream environments. The conversion of native vegetation into sugarcane monocultures leads to pronounced alterations in stream conditions—such as increased water temperature, conductivity, and ion concentrations, along with decreased habitat heterogeneity—directly impacting benthic macroinvertebrate communities. This transformation results in reduced taxonomic richness and abundance, particularly among sensitive groups like Ephemeroptera, Plecoptera, and Trichoptera (EPT), which are recognized globally as reliable bioindicators of aquatic ecosystem health []. Given this context, maintaining and restoring riparian buffers is not merely a conservation ideal but a practical necessity for sustainable land and water management [,]. Riparian forests contribute to the regulation of stream temperatures by providing shade, which limits solar radiation and reduces thermal stress on aquatic organisms [,]. They also stabilize streambanks, reduce erosion, and filter sediments and agrochemical runoff before these pollutants reach aquatic systems []. The root systems of riparian vegetation help trap and cycle nutrients, which can otherwise lead to eutrophication when present in excess, particularly in agricultural catchments []. Furthermore, riparian zones contribute to habitat complexity by supplying allochthonous organic matter (e.g., leaf litter and woody debris), which serves as both food and habitat for macroinvertebrates, especially shredders and collectors [].
To effectively safeguard aquatic biodiversity and ecosystem functions in regions dominated by sugarcane cultivation, a multifaceted management approach is necessary. Practical and evidence-based recommendations include the following: (i) Riparian Buffer Width Regulations: Current legislation in many regions, including parts of Brazil, mandates minimum riparian buffer widths based on watercourse size. However, our findings and other studies suggest that wider buffers—extending beyond 10 m, and ideally 30 m or more—can significantly improve stream conditions. These wider zones provide better protection against runoff, create more diverse microhabitats, and sustain higher biodiversity levels. Policy adjustments and enforcement mechanisms should ensure that riparian buffer widths are science-based and context-specific []. (ii) Riparian Reforestation and Passive Restoration: In areas where riparian vegetation has been degraded or removed, reforestation using native plant species can gradually restore ecological functions. Passive restoration allowing natural regeneration by fencing off riparian areas from grazing and cultivation—can also be effective, especially when combined with strategic planting in more degraded sections. Restoration programs should prioritize connectivity between forest patches to facilitate species dispersal and resilience [,]. (iii) Best Management Practices (BMPs) in Agriculture: Contour Farming: Planting along the natural contours of the land helps reduce surface runoff, thereby minimizing the transport of sediments and agrochemicals into watercourses []. Vegetated Buffer Strips: Establishing herbaceous or shrubby vegetation strips between cropland and streams can capture and absorb runoff, further filtering pollutants before they reach aquatic ecosystems []. Reduced agrochemical use near water bodies: Precision agriculture techniques, such as controlled fertilizer application and integrated pest management (IPM), can reduce our reliance on chemical inputs and prevent their accumulation in nearby aquatic environments. Establishing no-spray buffer zones adjacent to streams is a low-cost yet effective strategy []. Sediment Traps and Constructed Wetlands: Installing sediment traps or small constructed wetlands downstream of agricultural fields can help capture suspended solids and nutrients before they reach the stream []. (iv) Environmental Monitoring and Adaptive Management: Long-term ecological monitoring programs are essential to assess the effectiveness of restoration and management interventions. Biological indicators such as EPT richness and composition should be integrated into regular water quality monitoring protocols. By comparing baseline and post-intervention data, land managers and policymakers can adjust strategies based on observed ecological responses, ensuring adaptive and evidence-based decision-making []. (v) Environmental Education and Community Engagement: Involving local communities, particularly landowners and farmers, in conservation efforts is fundamental. Outreach programs should focus on raising awareness about the ecological importance of riparian zones, the ecosystem services they provide (e.g., water purification, erosion control, biodiversity conservation), and the long-term benefits of sustainable land use. Providing incentives, such as payments for ecosystem services (PES), tax breaks, or technical support, can encourage the voluntary adoption of BMPs and riparian conservation measures []. (vi) Intersectoral Coordination and Policy Integration: Effective riparian management requires coordination among multiple sectors, including agriculture, environment, water resources, and rural development. Policies that integrate conservation objectives with agricultural production goals can create synergies rather than conflicts between land use and environmental protection. In Brazil, the Watershed Committees should discuss these problems. Landscape-scale planning, where agricultural expansion is aligned with ecological conservation priorities, can help prevent fragmentation of riparian corridors and maintain ecological connectivity. (vii) Research and Innovation: Future studies should explore the long-term ecological effects of riparian restoration on biodiversity and ecosystem services in sugarcane-dominated landscapes. Research could focus on optimal buffer widths for different environmental settings, the effectiveness of various native plant species in improving water quality, and the role of functional traits in macroinvertebrate recovery following restoration. In addition, integrating socio-economic research can help us understand the barriers and opportunities for implementing conservation practices at the farm level.
Considering the clear relationship between land-use changes and declines in sensitive aquatic taxa, it becomes evident that the health of freshwater ecosystems cannot be disconnected from terrestrial management. The protection and rehabilitation of riparian zones must be considered as a core component of watershed management and agricultural sustainability strategies. Moreover, incorporating ecological principles into agricultural policies is crucial to harmonize food production with biodiversity conservation. The development of agroecological models that maintain or enhance ecosystem functions while ensuring economic viability for farmers can serve as a guiding framework. Ultimately, the resilience of stream ecosystems in agricultural landscapes depends on the ability to maintain or restore habitat heterogeneity, water quality, and ecological connectivity. Riparian forests play a pivotal role in achieving this, serving as keystone elements of aquatic–terrestrial interface ecosystems. Their preservation is not only an ecological imperative but a foundational strategy for ensuring the long-term sustainability of both biodiversity and agricultural productivity. By taking decisive actions rooted in science and guided by practical, scalable strategies, it is possible to mitigate the negative consequences of sugarcane cultivation on freshwater systems. Implementing these recommendations requires collaboration across disciplines, sectors, and stakeholders, but the benefits—healthier ecosystems, more stable water supplies, and enhanced biodiversity—are well worth the effort.
5. Conclusions
Although agricultural expansion in the state of São Paulo—particularly sugarcane monoculture—continues to play a significant role in Brazil’s economy, our findings highlight the vulnerability of first-order streams to such land use changes. These headwater systems, which are critical for maintaining regional biodiversity and ecological integrity, demonstrated clear alterations in physicochemical conditions and benthic macroinvertebrate communities associated with sugarcane cultivation. The streams draining sugarcane-dominated catchments supported a taxonomically and numerically impoverished macroinvertebrate fauna, particularly with declines in sensitive groups such as Ephemeroptera, Plecoptera, and Trichoptera (EPT). These changes were not only influenced by land use but also by seasonal variation, underscoring the importance of temporal dynamics in shaping aquatic communities. This study reinforces the value of using benthic macroinvertebrates as bioindicators of stream health, as their community structure provides a sensitive and integrative measure of both land-use impacts and seasonal shifts. Biological assessments should be integrated into long-term water quality monitoring programs and environmental impact assessments, particularly in regions undergoing intensive agricultural development. Importantly, our findings advocate for the urgent protection, restoration, and expansion of riparian buffers as a key management strategy to mitigate the impacts of sugarcane cultivation on freshwater ecosystems. By maintaining riparian vegetation and adopting sustainable agricultural practices, it is possible to reduce sediment and nutrient runoff, stabilize streambanks, and preserve the microhabitats necessary to support diverse aquatic life. In conclusion, balancing economic development with ecological sustainability requires science-based land-use planning and effective conservation strategies. Protecting small streams and their surrounding riparian zones should be considered a fundamental component of watershed management. The resilience of freshwater ecosystems in agricultural landscapes depends on our collective ability to preserve habitat complexity, ensure water quality, and maintain ecological connectivity—goals that are essential not only for biodiversity conservation but also for the long-term productivity and sustainability of agricultural systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/limnolrev25020013/s1, Supplementary Material S1: Impact of Sugarcane Cultivation on Benthic Macroinvertebrate Communities in Tropical Streams.
Author Contributions
Writing, M.V.F. and R.H.T.; conceptualization, M.V.F. and R.H.T.; design methodology, M.V.F. and R.H.T.; data acquisition and analysis, M.V.F. and R.H.T.; supervision, R.H.T.; writing—reviewing and editing, M.V.F., R.H.T., E.M.S., W.S.S. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the “Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq”, Process 407502/2018-1, the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil” (CAPES—Código de financiamento 001), and the São Paulo Research Foundation FAPESP (grant #17/02455-6).
Institutional Review Board Statement
This study did not require institutional approval, as it did not involve humans or animal experimentation (CEUA UFABC - Decreto Federal nº 6029/07).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article supplementary material.
Acknowledgments
We are grateful to the “Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq”, the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil”, the São Paulo Research Foundation FAPESP, and the Environmental Science and Technology Post-Graduate Program of the Federal University of ABC.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Allan, J.D. Landscapes and Riverscapes: The Influence of Land Use on Stream Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Woodward, G.; Gessner, M.O.; Giller, P.S.; Gulis, V.; Hladyz, S.; Lecerf, A.; Malmqvist, B.; Mckie, B.G.; Tiegs, S.D.; Cariss, H.; et al. Continental-Scale Effects of Nutrient Pollution on Stream Ecosystem Functioning. Science 2012, 336, 1438–1440. [Google Scholar] [CrossRef] [PubMed]
- Lammert, M.; Allan, J.D. Environmental Auditing: Assessing Biotic Integrity of Streams: Effects of Scale in Measuring the Influence of Land Use/Cover and Habitat Structure on Fish and Macroinvertebrates. Environ. Manag. 1999, 23, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Grashof-Bokdam, C.J.; Van Langevelde, F. Green Veining: Landscape Determinants of Biodiversity in European Agricultural Landscapes. Landsc. Ecol. 2005, 20, 417–439. [Google Scholar] [CrossRef]
- Bruno, D.; Belmar, O.; Sánchez-Fernández, D.; Guareschi, S.; Millán, A.; Velasco, J. Responses of Mediterranean aquatic and riparian communities to human pressures at different spatial scales. Ecol. Indic. 2014, 45, 456–464. [Google Scholar] [CrossRef]
- Fierro, P.; Bertrán, C.; Tapia, J.; Hauenstein, E.; Peña-Cortés, F.; Vergara, C.; Cerna, C.; Vargas-Chacoff, L. Effects of local land-use on riparian vegetation, water quality, and the functional organization of macroinvertebrate assemblages. Sci. Total Environ. 2017, 609, 724–734. [Google Scholar] [CrossRef]
- Stewart, J.S.; Wang, L.; Lyons, J.; Horwatich, J.A.; Bannerman, R. Influences of watershed, riparian-corridor, and reach-scale characteristics on aquatic biota in agricultural watersheds. J. Am. Water Resour. Assoc. 2001, 37, 1475–1487. [Google Scholar] [CrossRef]
- Tanaka, M.O.; Souza, A.L.T.D.; Moschini, L.E.; Oliveira, A.K.D. Influence of watershed land use and riparian characteristics on biological indicators of stream water quality in southeastern Brazil. Agric. Ecosyst. Environ. 2016, 216, 333–339. [Google Scholar] [CrossRef]
- Tran, C.P.; Bode, R.W.; Smith, A.J.; Kleppel, G.S. Land-use proximity as a basis for assessing stream water quality in New York State (USA). Ecol. Indic. 2010, 10, 727–733. [Google Scholar] [CrossRef]
- Filoso, S.; Carmo, J.B.D.; Mardegan, S.F.; Lins, S.R.M.; Gomes, T.F.; Martinelli, L.A. Reassessing the environmental impacts of sugarcane ethanol production in Brazil to help meet sustainability goals. Renew. Sustain. Energy Rev. 2015, 52, 1847–1856. [Google Scholar] [CrossRef]
- Taniwaki, R.H.; Cassiano, C.C.; Filoso, S.; Ferraz, S.F.D.B.; Camargo, P.B.D.; Martinelli, L.A. Impacts of converting low-intensity pastureland to high-intensity bioenergy cropland on the water quality of tropical streams in Brazil. Sci. Total Environ. 2017, 584, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Callisto, M.; Solar, R.; Silveira, F.A.O.; Saito, V.S.; Hughes, R.M.; Fernandes, G.W.; Gonçalves-Júnior, J.F.; Leitão, R.P.; Massara, R.L.; Macedo, D.R.; et al. A Humboldtian approach to mountain conservation and freshwater ecosystem services. Front. Environ. Sci. 2019, 7, 195. [Google Scholar] [CrossRef]
- Chen, K.; Olden, J.D. Threshold responses of riverine fish communities to land use conversion across regions of the world. Glob. Change Biol. 2020, 26, 4952–4965. [Google Scholar] [CrossRef] [PubMed]
- Dala-Corte, R.B.; Melo, A.S.; Siqueira, T.; Bini, L.M.; Martins, R.T.; Cunico, A.M.; Pes, A.M.; Magalhães, A.L.B.; Godoy, B.S.; Leal, C.G.; et al. Thresholds of freshwater biodiversity in response to riparian vegetation loss in the Neotropical region. J. Appl. Ecol. 2020, 57, 1391–1402. [Google Scholar] [CrossRef]
- Mello, K.D.; Taniwaki, R.H.; Paula, F.R.D.; Valente, R.A.; Randhir, T.O.; Macedo, D.R.; Leal, C.G.; Rodrigues, C.B.; Hughes, R.M. Multiscale land use impacts on water quality: Assessment, planning, and future perspectives in Brazil. J. Environ. Manag. 2020, 270, 110879. [Google Scholar] [CrossRef]
- Riis, T.; Kelly-Quinn, M.; Aguiar, F.C.; Manolaki, P.; Bruno, D.; Bejarano, M.D.; Clerici, N.; Fernandes, M.R.; Franco, J.C.; Pettit, N.; et al. Global overview of ecosystem services provided by riparian vegetation. BioScience 2020, 70, 501–514. [Google Scholar] [CrossRef]
- Dudgeon, D. Riparian wetlands of tropical streams. In Tropical Stream Ecology, 1st ed.; Wantzen, K.M., Yule, C.M., Tockner, K., Junk, W.J., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2008; pp. 199–217. [Google Scholar]
- Pusey, B.J.; Arthington, A.H. Importance of the riparian zone to the conservation and management of freshwater fish: A review. Mar. Freshw. Res. 2003, 54, 1–16. [Google Scholar] [CrossRef]
- Dosskey, M.G.; Vidon, P.; Gurwick, N.P.; Allan, C.J.; Duval, T.P.; Lowrance, R. The role of riparian vegetation in protecting and improving chemical water quality in streams. J. Am. Water Resour. Assoc. 2010, 46, 261–277. [Google Scholar] [CrossRef]
- Rabeni, C.F.; Smale, M.A. Effects of siltation on stream fishes and the potential mitigating role of the buffering riparian zone. Hydrobiologia 1995, 303, 211–219. [Google Scholar] [CrossRef]
- Buss, D.F.; Baptista, D.F.; Nessimian, J.L.; Egler, M. Substrate specificity, environmental degradation and disturbance structuring macroinvertebrate assemblages in neotropical streams. Hydrobiologia 2004, 518, 179–188. [Google Scholar] [CrossRef]
- Sagova-Mareckova, M.; Boenigk, J.; Bouchez, A.; Cermakova, K.; Chonova, T.; Cordier, T.; Eisendle, U.; Elersek, T.; Fazi, S.; Fleituch, T.; et al. Expanding ecological assessment by integrating microorganisms into routine freshwater biomonitoring. Water Res. 2021, 191, 116767. [Google Scholar] [CrossRef]
- Van Rees, C.B.; Waylen, K.A.; Schmidt-Kloiber, A.; Thackeray, S.J.; Kalinkat, G.; Martens, K.; Domisch, S.; Lillebø, A.I.; Hermoso, V.; Grossart, H.-P.; et al. Safeguarding freshwater life beyond 2020: Recommendations for the new global biodiversity framework from the European experience. Conserv. Lett. 2021, 14, 12771. [Google Scholar] [CrossRef]
- Resh, V.H. Which group is best? Attributes of different biological assemblages used in freshwater biomonitoring programs. Environ. Monit. Assess. 2008, 138, 131–138. [Google Scholar] [PubMed]
- Veríssimo, H.; Neto, J.M.; Teixeira, H.; Franco, J.N.; Fath, B.D.; Marques, J.C.; Patrício, J. Ability of benthic indicators to assess ecological quality in estuaries following management. Ecol. Indic. 2012, 19, 130–143. [Google Scholar] [CrossRef]
- Fellows, C.S.; Clapcott, J.E.; Udy, J.W.; Bunn, S.E.; Harch, B.D.; Smith, M.J.; Davies, P.M. Benthic metabolism as an indicator of stream ecosystem health. Hydrobioloia 2006, 572, 71–87. [Google Scholar] [CrossRef]
- Orozco-González, C.E.; Ocasio-Torres, M.E. Aquatic macroinvertebrates as bioindicators of water quality: A study of an ecosystem regulation service in a tropical river. Ecologies 2023, 4, 209–228. [Google Scholar] [CrossRef]
- Cummins, K.W.; Merritt, R.W.; Andrade, P.C. The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil. Stud. Neotrop. Fauna Environ. 2005, 40, 69–89. [Google Scholar] [CrossRef]
- Fu, L.; Jiang, Y.; Ding, J.; Liu, Q.; Peng, Q.Z.; Kang, M.Y. Impacts of land use and environmental factors on macroinvertebrate functional feeding groups in the Dongjiang River basin, southeast China. J. Freshw. Ecol. 2016, 31, 21–35.31. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, N.; Vanhooren, G. Method for biological quality assessment of watercourses in Belgium. Hydrobiologia 1983, 100, 153–168. [Google Scholar] [CrossRef]
- Hamada, N.; Nessimian, J.L.; Querino, R.B. Insetos Aquáticos na Amazônia Brasileira: Taxonomia, Biologia e Ecologia; INPA: Manaus, Brasil, 2014; pp. 173–723. [Google Scholar]
- Hamada, N.; Thorp, J.H.; Rogers, C.D. Thorp and Covich’s Freshwater Invertebrates: Volume 3: Keys to Neotropical Hexapoda, 4th ed.; Academic Press: Cambridge, MA, USA; Elsevier: Cambridge, MA, USA, 2018; pp. 61–793. [Google Scholar]
- Hauer, F.R.; Resh, V.H. Methods in Stream Ecology, Volume 1: Ecosystem Structure, 3rd ed.; Academic Press: Cambridge, MA, USA; Elsevier: Cambridge, MA, USA, 2017; pp. 297–319. [Google Scholar]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B. An Introduction to the Aquatic Insects of North America, 4th ed.; Kendall Hunt Publishing: Dubuque, IA, USA, 2008; p. 1214. [Google Scholar]
- Correa-González, J.C.; Chávez-Parga, M.a.D.C.; Cortés, J.A.; Pérez-Munguía, R.M. Photosynthesis, respiration and reaeration in a stream with complex dissolved oxygen pattern and temperature dependence. Ecol. Model. 2014, 273, 220–227. [Google Scholar] [CrossRef]
- Villéger, S.; Miranda, J.R.; Hernández, D.F.; Mouillot, D. Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol. Appl. 2010, 20, 1512–1522. [Google Scholar] [CrossRef]
- Corbi, J.J.; Trivinho-Strixino, S. Relationship between sugar cane cultivation and stream macroinvertebrate communities. Braz. Arch. Biol. Technol. 2008, 51, 569–579. [Google Scholar] [CrossRef]
- Hepp, L.U.; Santos, S. Benthic communities of streams related to different land uses in a hydrographic basin in southern Brazil. Environ. Monit. Assess. 2009, 157, 305–318. [Google Scholar] [CrossRef]
- Iñiguez-Armijos, C.; Hampel, H.; Breuer, L. Land-use effects on structural and functional composition of benthic and leaf-associated macroinvertebrates in four Andean streams. Aquat. Ecol. 2018, 52, 77–92. [Google Scholar] [CrossRef]
- Nessimian, J.L.; Venticinque, E.M.; Zuanon, J.; De Marco, P.; Gordo, M.; Fidelis, L.; Batista, J.D.; Juen, L. Land use, habitat integrity, and aquatic insect assemblages in Central Amazonian streams. Hydrobiologia 2008, 614, 117–131. [Google Scholar] [CrossRef]
- Roque, F.O.; Trivinho-Strixino, S.; Strixino, G.; Agostinho, R.C.; Fogo, J.C. Benthic Macroinvertebrates in Streams of the Jaragua State Park (Southeast of Brazil) Considering Multiple Spatial Scales. J. Insect Conserv. 2003, 7, 63–72. [Google Scholar] [CrossRef]
- Moraes, A.B.; Wilhelm, A.E.; Boelter, T.; Stenert, C.; Schulz, U.H.; Maltchik, L. Reduced riparian zone width compromises aquatic macroinvertebrate communities in streams of southern Brazil. Environ. Monit. Assess. 2014, 186, 7063–7074. [Google Scholar] [CrossRef]
- França, M.V.; Shimabukuro, E.M.; Fushita, Â.T.; Smith, W.S.; Benassi, R.F.; Cunha, D.G.F.; Taniwaki, R.H. Structural characteristics of tropical headwater streams draining native vegetation and sugarcane cultivation. Limnologica 2023, 101, 126099. [Google Scholar] [CrossRef]
- Corbi, J.J.; Trivinho-Strixino, S. Chironomid species are sensitive to sugarcane cultivation. Hydrobiologia 2017, 785, 91–99. [Google Scholar] [CrossRef]
- Entrekin, S.A.; Wallace, J.B.; Eggert, S.L. The response of Chironomidae (Diptera) to a long-term exclusion of terrestrial organic matter. Hydrobiologia 2007, 575, 401–413. [Google Scholar] [CrossRef]
- Corbi, J.J.; Kleine, P.; Trivinho-Strixino, S. Are aquatic insect species sensitive to banana plant cultivation? Ecol. Indic. 2013, 25, 156–161. [Google Scholar] [CrossRef]
- Quinn, J.M.; Cooper, A.B.; Davies-Colley, R.J.; Rutherford, J.C.; Williamson, R.B. Land use effects on habitat, water quality, periphyton, and benthic invertebrates in Waikato, New Zealand, hill-country streams. N. Z. J. Mar. Fresh. 1997, 31, 579–597. [Google Scholar] [CrossRef]
- Cunha, E.J.; Juen, L. Impacts of oil palm plantations on changes in environmental heterogeneity and Heteroptera (Gerromorpha and Nepomorpha) diversity. J. Insect. Conserv. 2017, 21, 111–119. [Google Scholar] [CrossRef]
- Juen, L.; Cunha, E.J.; Carvalho, F.G.; Ferreira, M.C.; Begot, T.O.; Andrade, A.L.; Shimano, Y.; Leão, H.; Pompeu, O.S.; Montag, L.F.A. Effects of Oil Palm Plantations on the Habitat Structure and Biota of Streams in Eastern Amazon: Effect of Oil Palm on the Structure of Stream. River Res. Appl. 2016, 32, 2081–2094. [Google Scholar] [CrossRef]
- Phillips, H.R.P.; Newbold, T.; Purvis, A. Land-use effects on local biodiversity in tropical forests vary between continents. Biodivers. Conserv. 2017, 26, 2251–2270. [Google Scholar] [CrossRef]
- Lisboa, L.K.; Lemes-Silva, A.L.; Siegloch, A.E.; Gonçalvez-Junior, J.F.; Petrucio, M.M. Temporal dynamics of allochthonous coarse particulate organic matter in a subtropical Atlantic rainforest Brazilian stream. Mar. Freshw. Res. 2015, 66, 674–680. [Google Scholar] [CrossRef]
- Piggott, J.J.; Lange, K.; Townsend, C.R.; Matthaei, C.D. Multiple Stressors in Agricultural Streams: A Mesocosm Study of Interactions among Raised Water Temperature, Sediment Addition and Nutrient Enrichment. Solan M, organizador. PLoS ONE. 2012, 7, 49873. [Google Scholar] [CrossRef]
- Schmitt, R.; Siegloch, A.E.; Lemes Da Silva, A.L.; Lisboa, L.K.; Petrucio, M.M. Temporal variation in the Ephemeroptera, Plecoptera and Trichoptera community in response to environmental drivers in a subtropical stream. J. Insect Biodivers. 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Akamagwuna, F.C.; Mensah, P.K.; Nnadozie, C.F.; Odume, O.N. Evaluating the responses of taxa in the orders Ephemeroptera, Plecoptera and Trichoptera (EPT) to sediment stress in the Tsitsa River and its tributaries, Eastern Cape, South Africa. Environ. Monit. Assess. 2019, 191, 664. [Google Scholar] [CrossRef] [PubMed]
- Luiza-Andrade, A.; Brasil, L.S.; Benone, N.L.; Shimano, Y.; Farias, A.P.J.; Montag, L.F.; Dolédec, S.; Juen, L. Influence of oil palm monoculture on the taxonomic and functional composition of aquatic insect communities in eastern Brazilian Amazonia. Ecol. Indic. 2017, 82, 478–483. [Google Scholar] [CrossRef]
- Monteiro Do Amaral, P.H.; De Almeida Gonçalves, E.; Da Silveira, L.S.; Da Gama Alves, R. Richness and distribution of Ephemeroptera, Plecoptera and Trichoptera in Atlantic forest streams. Acta Oecol. 2019, 99, 103441. [Google Scholar] [CrossRef]
- Bispo, P.C.; Oliveira, L.G. Diversity and structure of Ephemeroptera, Plecoptera and Trichoptera (Insecta) assemblages from riffles in mountain streams of Central Brazil. Rev. Bras. Zool. 2007, 24, 283–293. [Google Scholar] [CrossRef]
- Bueno, A.A.P.; Bond-Buckup, G.; Ferreira, B.D.P. Estrutura da comunidade de invertebrados bentônicos em dois cursos d’água do Rio Grande do Sul, Brasil. Rev. Bras. Zool. 2003, 20, 115–125. [Google Scholar] [CrossRef]
- Júnior, A.P.; Conceição, C.S.; Lobo, R.R.; Santos, C.O.R.; Sardinha, A.S. Association between ephemeroter, plecoptera and trichoptera and the limnimetric parameters of the water quality index. Braz. Appl. Sci. Rev. 2019, 3, 839–863. [Google Scholar]
- Pardo, I.; García, L. Water abstraction in small lowland streams: Unforeseen hypoxia and anoxia effects. Sci. Total Environ. 2016, 568, 226–235. [Google Scholar] [CrossRef]
- Calapez, A.R.; Branco, P.; Santos, J.M.; Ferreira, T.; Hein, T.; Brito, A.G.; Feio, M.J. Macroinvertebrate short-term responses to flow variation and oxygen depletion: A mesocosm approach. Sci. Total Environ. 2017, 599, 1202–1212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).