Influence of Experimental Eutrophication on Macrozoobenthos in Tufa-Depositing System of Plitvice Lakes National Park, Croatia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Nutrient-Diffusing Substrate Design and Placement
2.3. Measurement of Physicochemical Water Parameters and Chlorophyll a Analysis
2.4. Macrozoobenthos and Tufa Deposition Analysis
2.5. Data Analysis
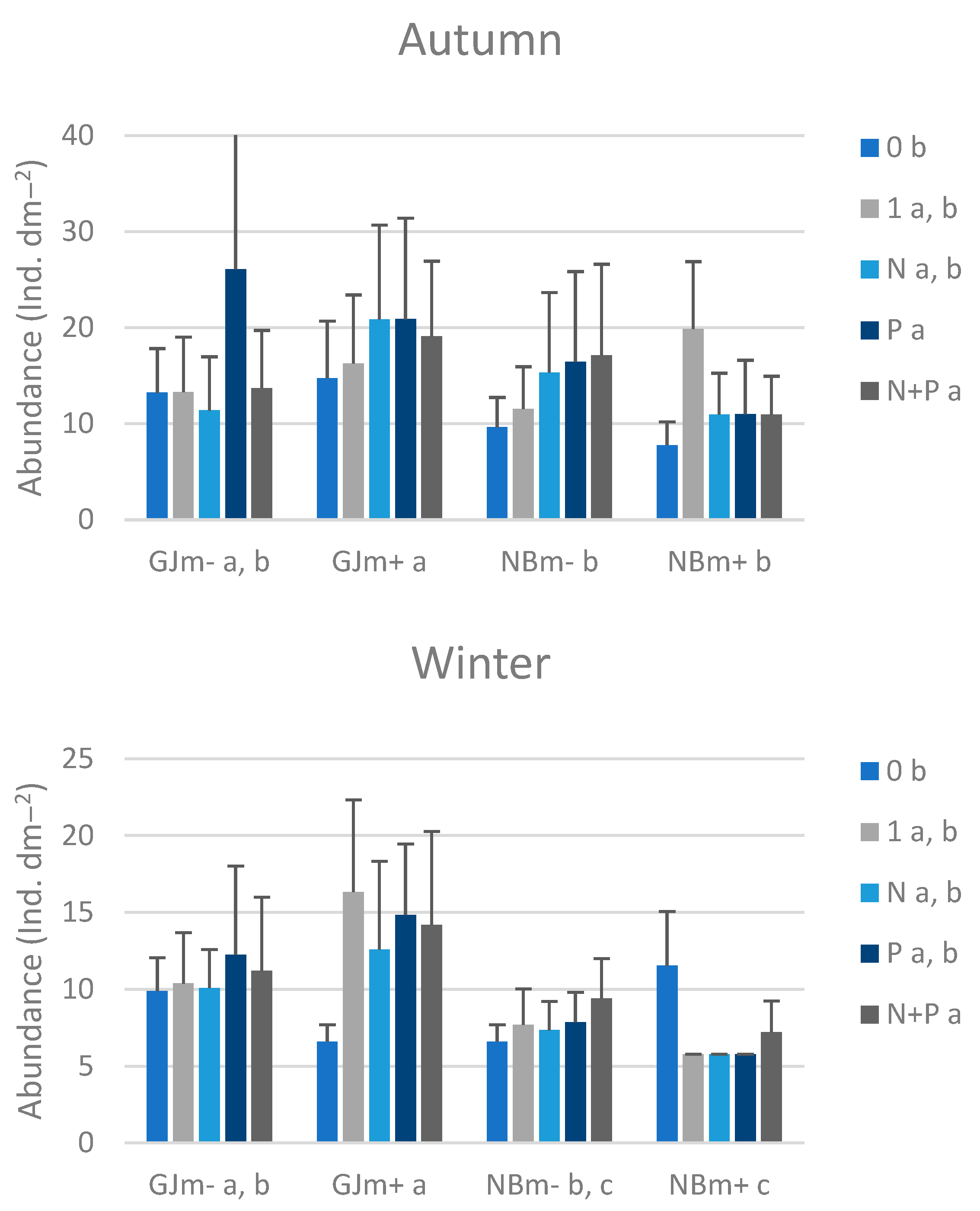
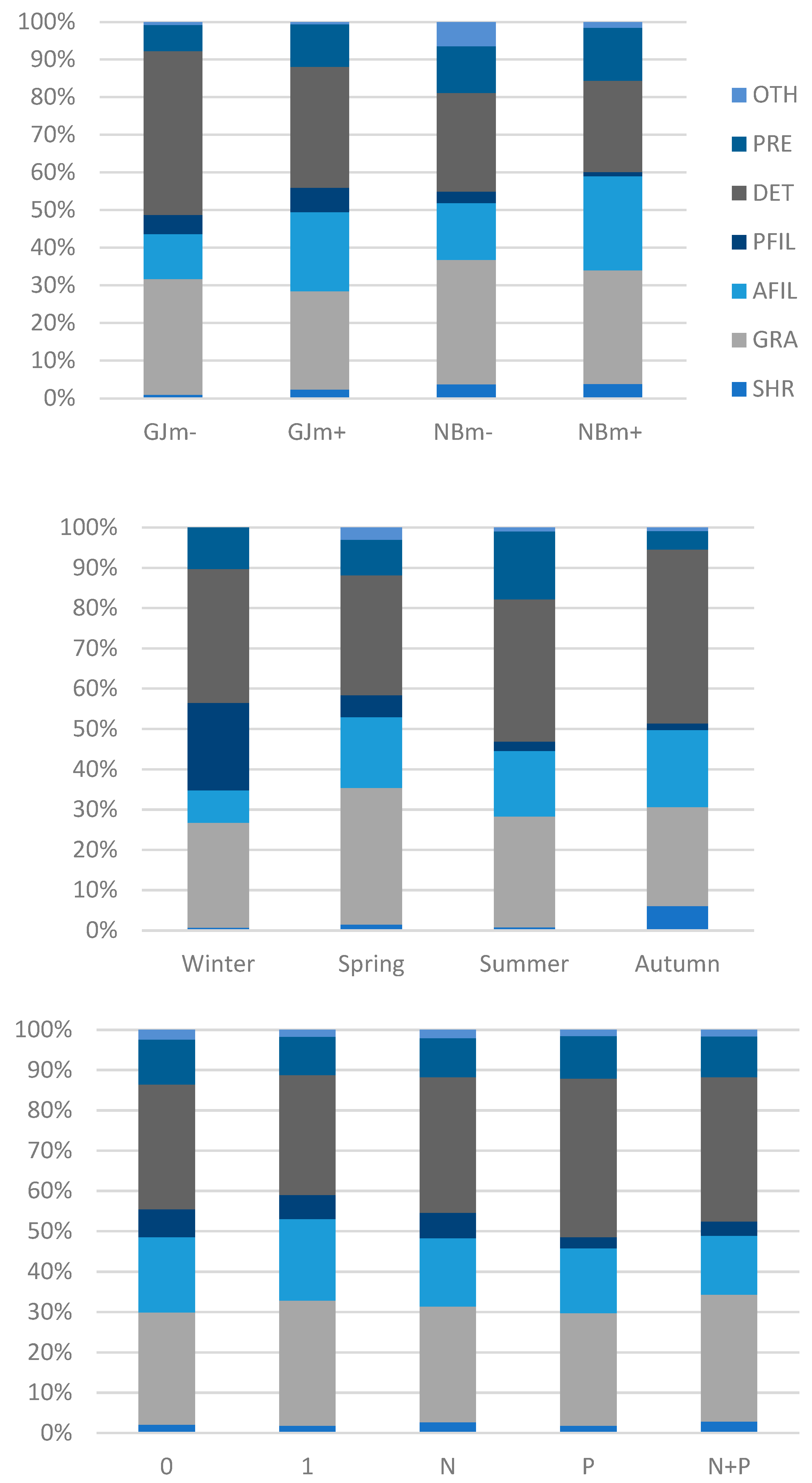
3. Results
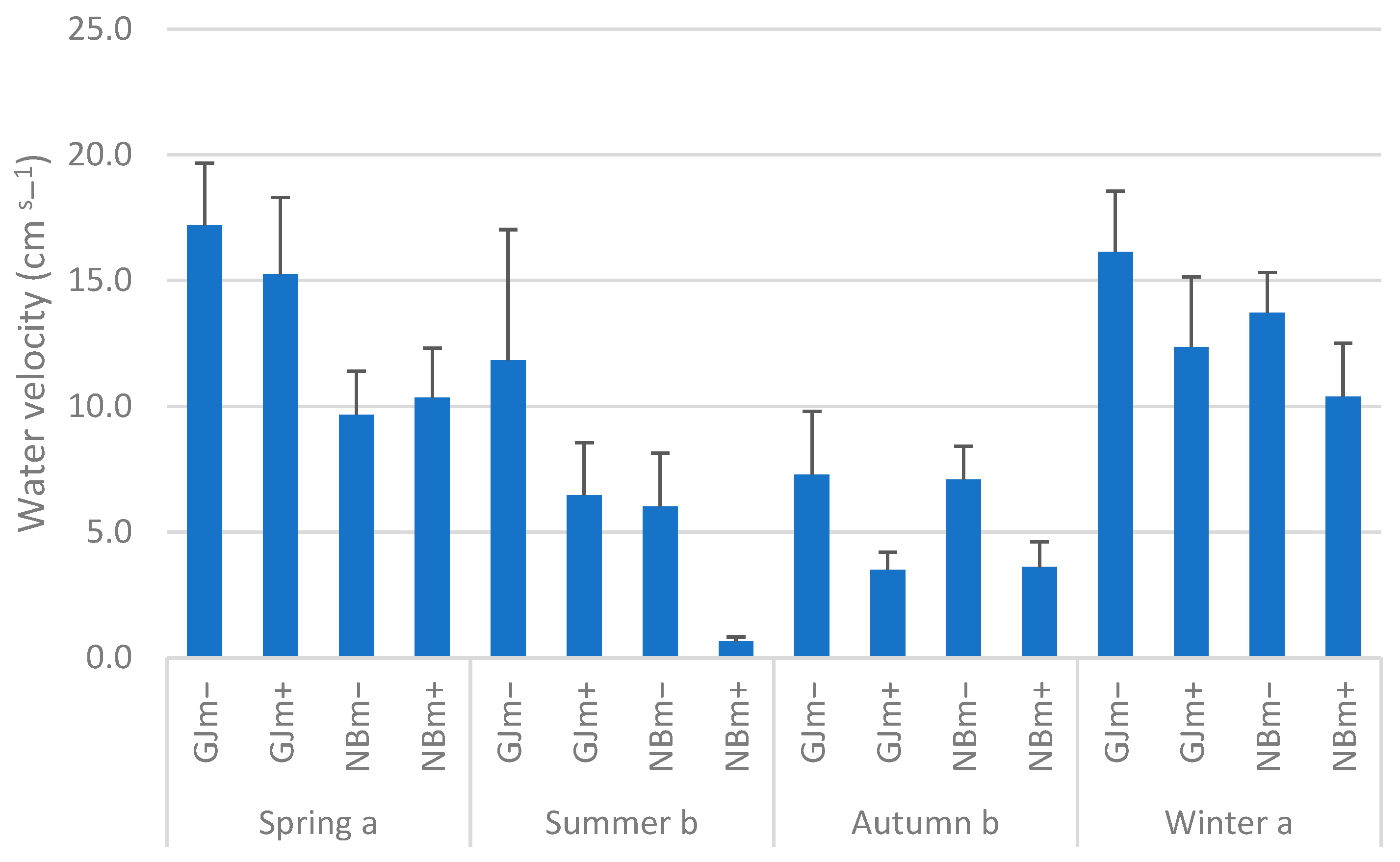
3.1. Water Velocity and Physicochemical Parameters
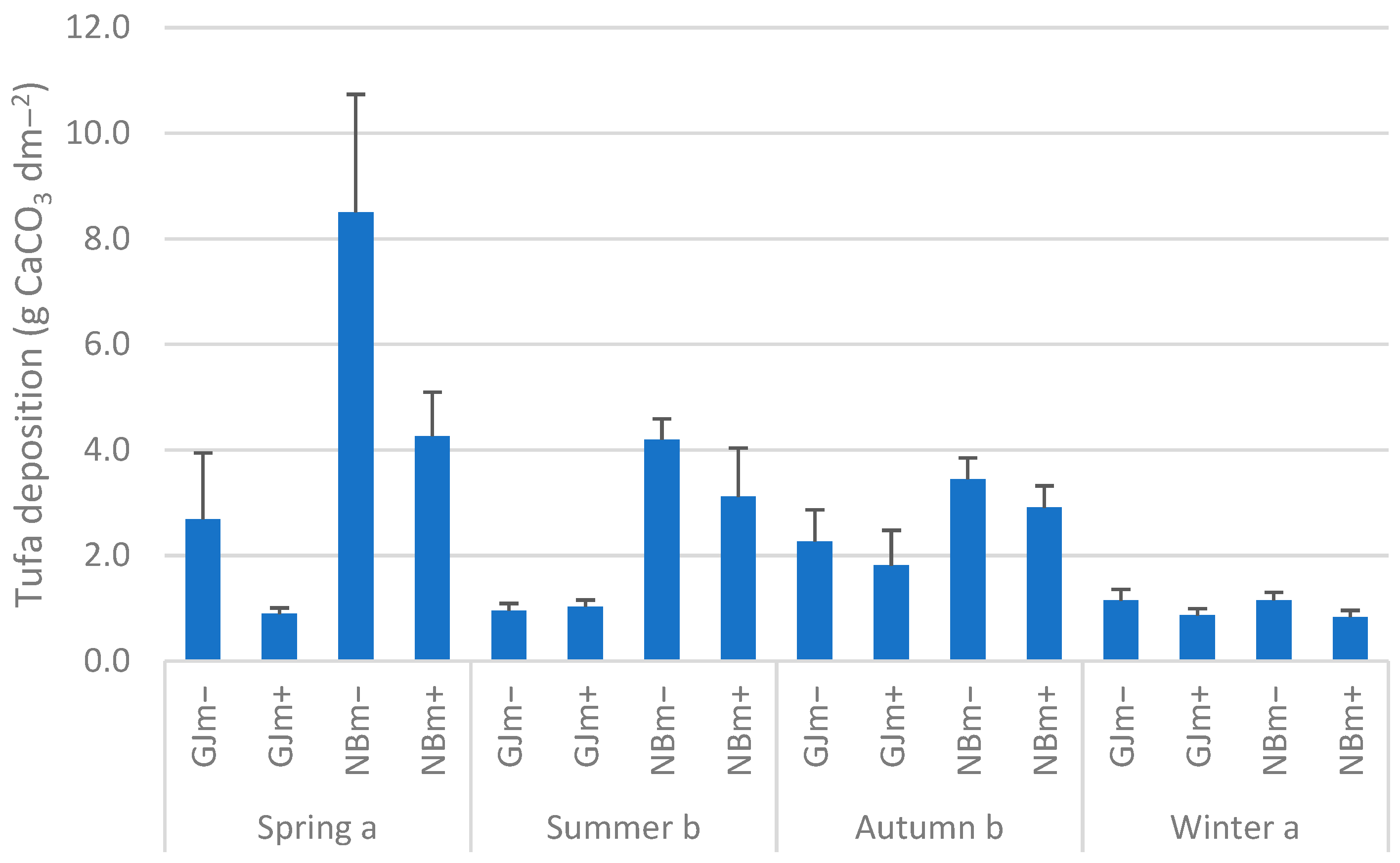
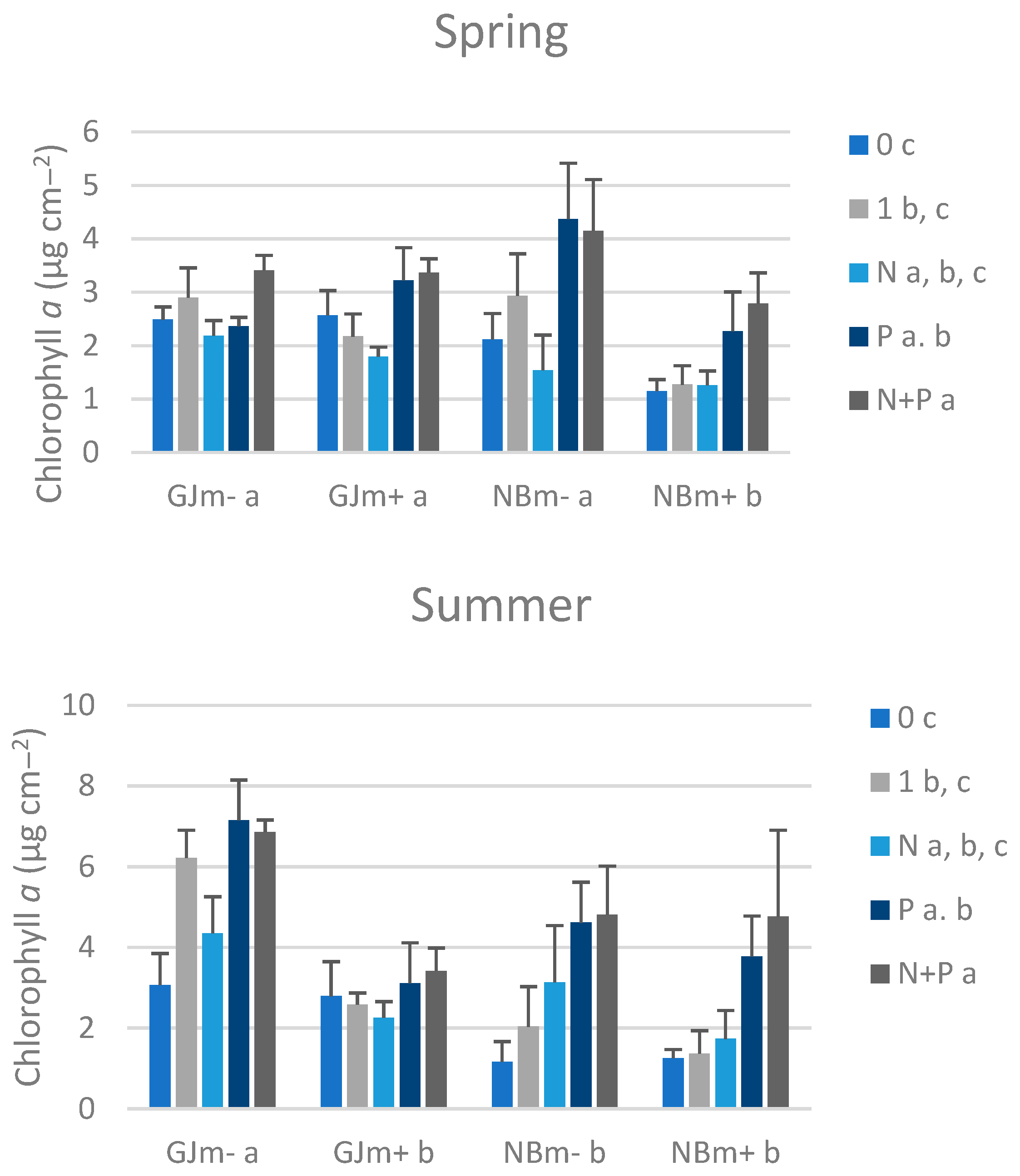
3.2. Tufa Deposition and Chlorophyll a on Nutrient-Diffusing Substrata
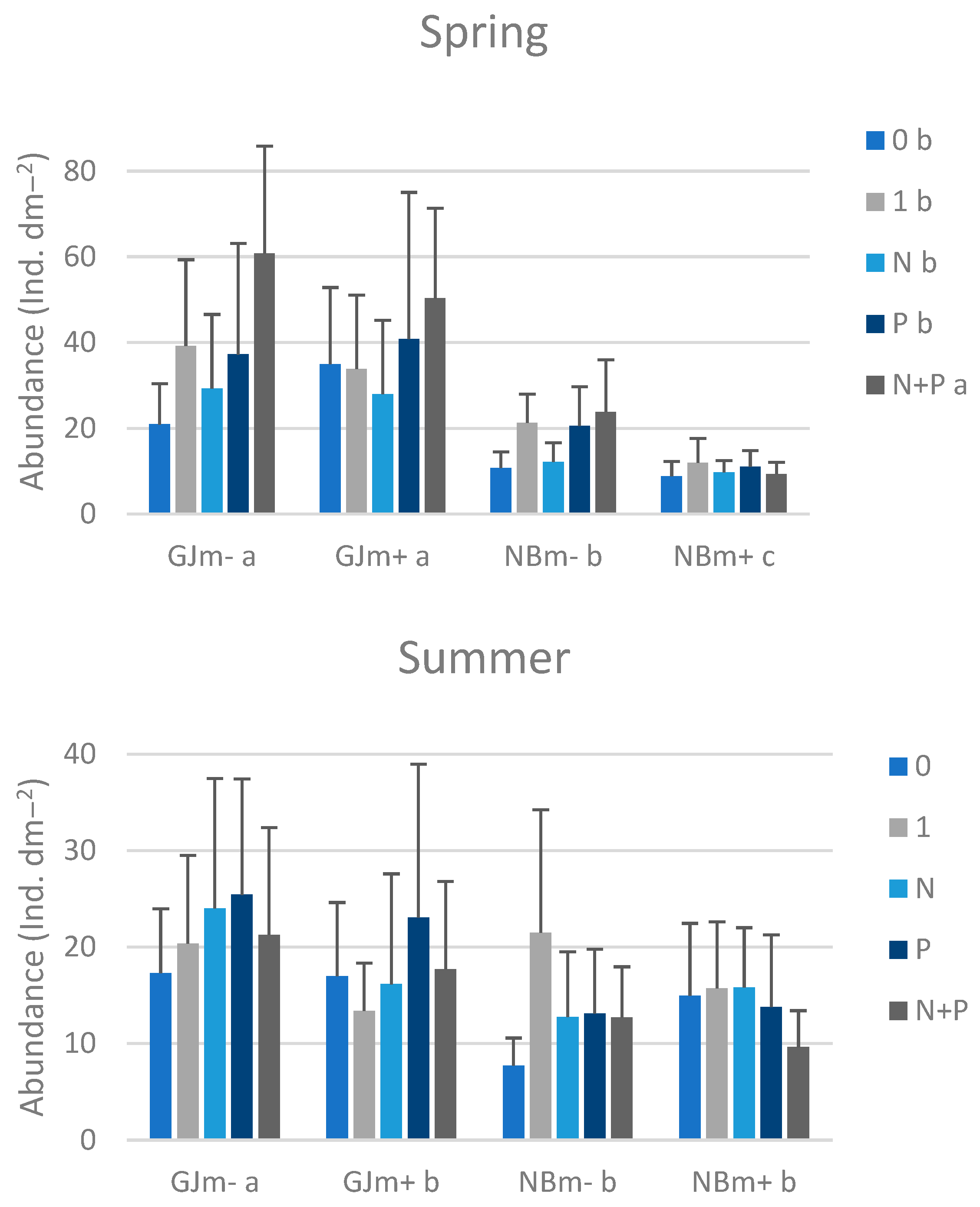
3.3. Macrozoobenthos on Nutrient-Diffusing Substrata
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NDS | Nutrient-diffusing substrates |
| GJm− | Gradinsko jezero tufa barriers without macrophytes |
| GJm+ | Gradinsko jezero tufa barriers with macrophytes |
| NBm− | Novakovića brod tufa barriers without macrophytes |
| NBm+ | Novakovića brod tufa barriers with macrophytes |
| TN | Total nitrogen |
| TP | Total phosphorus |
| Chl a | Chlorophyll a |
| MDS | Multidimensional scaling |
| FFGs | Functional feeding groups |
| SHRs | Shredders |
| GRAs | Grazers |
| AFILs | Active filter feeders |
| PFILs | Passive filter feeders |
| DETs | Detrivore collectors |
| PREs | Predators |
| OTHs | Others |
Appendix A
Appendix A.1
| Location | Microhabitats | Replicates | Coordinates |
|---|---|---|---|
| Novakovića brod lake outlet (NB) | NBm− | A | 15°36′35.633″ E 44°54′6.442″ N |
| B | 15°36′35.452″ E 44°54′6.649″ N | ||
| C | 15°36′35.521″ E 44°54′6.919″ N | ||
| NBm+ | D | 15°36′38.055″ E 44°54′7.96″ N | |
| E | 15°36′37.339″ E 44°54′7.989″ N | ||
| F | 15°36′38.445″ E 44°54′8.019″ N | ||
| Gradinsko jezero lake outlet (GJ) | GJm− | G | 15°36′47.803″ E 44°52′46.472″ N |
| H | 15°36′48.164″ E 44°52′46.469″ N | ||
| I | 15°36′48.496″ E 44°52′46.458″ N | ||
| GJm+ | J | 15°36′48.69″ E 44°52′46.057″ N | |
| K | 15°36′48.973″ E 44°52′46.11″ N | ||
| L | 15°36′49.208″ E 44°52′46.262″ N |
Appendix A.2
| Spring | Summer | Autumn | Winter | ANOVA Results | |
|---|---|---|---|---|---|
| Temperature (°C) | 13.22 ± 3.11 | 20.24 ± 1.96 | 11.74 ± 2.40 | 4.34–1.18 | F3.42 = 99.4 p < 0.001 |
| Dissolved oxygen (mg O2 L−1) | 10.69 ± 0.81 | 9.53 ± 0.45 | 10.49 ± 0.57 | 12.27 ± 0.54 | F3.42 = 42.3 p < 0.001 |
| Electrical conductivity (µS cm−1) | 352.42 ± 5.70 | 325.42 ± 7.42 | 348.42 ± 9.64 | 367.08 ± 5.12 | F3.42 = 85.6 p < 0.001 |
| pH | 8.35 ± 0.06 | 8.17 ± 0.05 | 8.25 ± 0.07 | 8.35 ± 0.12 | F3.42 = 23.5 p < 0.001 |
| Total hardness (mg CaCO3 L−1) | 228.10 ± 5.18 | 211.90 ± 5.56 | 227.91 ± 11.02. | 236.02 ± 1.56 | F3.26 = 17.5 p < 0.001 |
| Nitrates (mg N L−1) | 0.65 ± 0.07 | 0.47 ± 0.07 | 0.55 ± 0.08 | 0.73 ± 0.02 | F3.26 = 23.9 p < 0.001 |
| Orthophosphates (mg P L−1) | 0.02 ± 0.02 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.00 ± 0.00 | F3.26 = 5.1 p < 0.01 |
| Total nitrogen (mg TN L−1) | 1.35 ± 0.99 | 0.77 ± 0.37 | 0.70 ± 0.14 | 0.89 ± 0.46 | N.S. |
| Total phosphorus (mg TP L−1) | 0.15 ± 0.06 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.14 ± 0.06 | F3.26 = 26.1 p < 0.001 |
Appendix A.3
| Taxa | NBm− | NBm+ | GJm− | GJm+ |
|---|---|---|---|---|
| Turbellaria | ||||
| Turbellaria non det. | 4 | |||
| Gastropoda | ||||
| Bithynia tentaculata (Linnaeus, 1758) | 3 | |||
| Holandriana holandrii (C. Pfeiffer, 1828) | 3 | |||
| Oligochaeta | ||||
| Oligochaeta non det. | 45 | 63 | 524 | 304 |
| Hydrachnidia | ||||
| Hydrachnidia non det. | 10 | 4 | 4 | 5 |
| Ostracoda | ||||
| Ostracoda non det. | 1 | |||
| Cladocera | ||||
| Alona sp. | 50 | 83 | 22 | 45 |
| Copepoda | ||||
| Copepoda non det. | 6 | 28 | 1 | 5 |
| Ephemeroptera | ||||
| Baetidae juv. | 7 | 39 | 5 | |
| Baetis alpinus (Pictet, 1843) | 1 | 2 | 2 | |
| Baetis sp. | 7 | 1 | 4 | 1 |
| Centroptilum luteolum (Muller, 1776) | 8 | 41 | 24 | |
| Centroptilum sp. | 3 | 3 | 5 | |
| Ephemera danica (Muller, 1764) | 1 | 1 | 1 | |
| Ephemeridae juv. | 1 | 2 | ||
| Ephemerella sp. | 1 | 1 | 19 | 5 |
| Ephemeroptera non det. | 25 | 15 | 12 | 12 |
| Habroleptoides sp. | 4 | |||
| Leptophlebiidae juv. | 1 | |||
| Paraleptophlebia sp. | 3 | 3 | 28 | 10 |
| Plecoptera | ||||
| Amphinemura sp. | 1 | |||
| Isoperla sp. | 12 | 1 | 9 | 12 |
| Leuctra sp. | 4 | 1 | ||
| Nemouridae juv. | 3 | |||
| Nemurella sp. | 80 | 16 | 4 | 53 |
| Perlodes sp. | 2 | 1 | ||
| Perlodidae juv. | 12 | 7 | 6 | 12 |
| Perloidea juv. | 1 | 5 | 3 | |
| Plecoptera non det. | 67 | 21 | 48 | 116 |
| Protonemura sp. | 1 | 11 | ||
| Odonata | ||||
| Corduliidae ili Libellulidae | 2 | 2 | 2 | |
| Coleoptera | ||||
| Coleoptera non det. | 1 | |||
| Coleoptera pupae | 1 | |||
| Dryopidae | 1 | |||
| Dryopidae imago | 1 | 1 | 1 | |
| Elmidae | 6 | 16 | 67 | 3 |
| Elmidae imago | 1 | |||
| Elodes sp. | 13 | 2 | ||
| Trichoptera | ||||
| Agraylea multipunctata (Curtis, 1834) | 1 | |||
| Apatania sp. | 1 | |||
| Athripsodes sp. | 1 | 2 | ||
| Beraeidae | 9 | |||
| Ecnomidae juv. | 4 | |||
| Hydropsyche angustipennis (Curtis, 1834) | 6 | 1 | 4 | |
| Hydropsyche incognita (T. Pitsch, 1993) | 1 | |||
| Hydropsyche instabilis (Curtis, 1834) | 9 | |||
| Hydropsyche sp. | 1 | 11 | ||
| Hydropsychidae juv. | 1 | |||
| Hydroptila occulta (Eaton, 1873) | 1 | 2 | ||
| Hydroptila sp. | 69 | |||
| Hydroptilidae juv. | 2 | 1 | 1 | 2 |
| Hydroptilidae pupae | 2 | |||
| Limnephilidae non det. | 1 | |||
| Limnephilus sp. | 1 | |||
| Orthotrichia sp. | 8 | 3 | ||
| Oxyethira flavicornis (F.J. Pictet, 1834) | 5 | 3 | ||
| Philopotamidae juv. | 3 | 1 | 3 | 1 |
| Philopotamus sp. | 2 | |||
| Philopotamus variegatus (Scopoli, 1763) | 1 | 1 | ||
| Plectrocnemia conspersa (Curtis, 1834) | 1 | |||
| Polycentropodidae juv. | 1 | 2 | 1 | |
| Polycentropus flavomaculatus (Pictet, 1834) | 1 | |||
| Polycentropus sp. | 1 | 3 | ||
| Rhyacophila sp. | 1 | 6 | ||
| Sericostoma sp. | 1 | 18 | 1 | |
| Stactobia sp. | 102 | 25 | 59 | |
| Trichoptera non det. | 1 | |||
| Trichoptera pupae | 5 | |||
| Wormaldia occipitalis (Pictet, 1834) | 1 | 1 | 4 | |
| Wormaldia sp. | 5 | 3 | 1 | |
| Diptera | ||||
| Athericidae | 5 | |||
| Ceratopogonidae | 7 | 1 | ||
| Chironomidae | 196 | 82 | 652 | 207 |
| Chironomidae pupae | 1 | 4 | 3 | |
| Chironominae | 1 | 3 | 5 | 8 |
| Chironomini | 43 | 21 | 94 | 195 |
| Diptera pupae | 3 | 4 | 26 | 57 |
| Empididae | 16 | 13 | 15 | 83 |
| Limoniidae | 1 | |||
| Simulium sp. | 127 | 4 | 125 | |
| Tanypodinae | 108 | 99 | 179 | 156 |
| Tanytarsini | 186 | 155 | 451 | 596 |
| Total | 1253 | 712 | 2366 | 2147 |
References
- Ford, T.D.; Pedley, H.M. A review of tufa and travertine deposits of the world. Earth-Sci. Rev. 1996, 41, 117–175. [Google Scholar] [CrossRef]
- Pedley, H.M.; Andrews, J.; Ordonez, S.; Del Cura, M.A.G.; Martin, J.-A.G.; Taylor, D. Does climate control the morphological fabric of freshwater carbonates? A comparative study of Holocene barrage tufas from Spain and Britain. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1996, 121, 236–257. [Google Scholar] [CrossRef]
- Lin, Y.P.; Singer, P.C. Inhibition of calcite precipitation by orthophosphate: Speciation and thermodynamic considerations. Geochim. Cosmochim. Acta 2006, 70, 2530–2539. [Google Scholar] [CrossRef]
- Dokulil, M.T.; Teubner, K. Eutrophication and Climate Change: Present Situation and Future Scenarios. In Eutrophication: Causes, Consequences and Control; Ansari, A.A., Gill, S.S., Lanza, G.R., Rast, W., Eds.; Springer Science+Business Media B.V.: Dordrecht, The Netherlands, 2011; pp. 1–16. [Google Scholar]
- Illyovà, M.; Pastuhovà, Z. The zooplankton communities of small water reservoirs with different trophic conditions in two catchments in western Slovakia. Limnologica 2012, 42, 271–281. [Google Scholar] [CrossRef]
- Jònasson, P.M. Benthic Invertebrates. In The Lakes Handbook—Limnology and Limnetic Ecology; O’Sullivan, P.E., Reynolds, C.S., Eds.; Blackwell Publishing: Oxford, UK, 2004; Volume 1, pp. 341–416. [Google Scholar]
- Ansari, A.A.; Gill, S.S.; Khan, F.A. Eutrophication: Threat to Aquatic Ecosystems. In Eutrophication: Causes, Consequences and Control; Ansari, A.A., Gill, S.S., Lanza, G.R., Rast, W., Eds.; Springer Science+Business Media B.V.: Dordrecht, The Netherlands, 2011; pp. 143–170. [Google Scholar]
- Wiik, E.; Bennion, H.; Sayer, C.D.; Davidson, T.A.; Clarke, S.J.; McGowan, S.; Prentice, S.; Simpson, G.L.; Stone, L. The coming and going of a marl lake: Multi-indicator paleolimnology reveals abrupt ecological change and alternative views of reference conditions. Front. Ecol. Evol. 2015, 3, 82. [Google Scholar] [CrossRef]
- Thakur, R.K.; Jindal, R.; Singh, U.B.; Ahluwalia, A.S. Plankton diversity and water quality assessment of three freshwater lakes of Mandi (Himachal Pradesh, India) with special reference to planktonic indicators. Environ. Monit. Assess. 2013, 185, 8355–8373. [Google Scholar] [CrossRef]
- Winsborough, B.M. Diatoms and benthic microbial carbonates. In Microbial Sediments; Riding, R.E., Awramik, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 76–83. [Google Scholar]
- Riding, R. Microbial carbonates: The geological record of calcified bacterial-algal mats and biofilms. Sedimentology 2000, 47 (Suppl. 1), 179–214. [Google Scholar] [CrossRef]
- Čanjevac, I.; Martinić, I.; Radišić, M.; Rubinić, J.; Meaški, H. Hydrology, hydrogeology and Hydromorphology of the Plitvice Lakes Area. In Plitvice Lakes; Miliša, M., Ivković, M., Eds.; Springer Water, Springer Nature Switzerland AG: Cham, Switzerland, 2023; pp. 17–64. [Google Scholar]
- Plitvice Lakes National Park Management Plan 2019–2028. In Management Plan; Kovačević, T., Ed.; Plitvice Lakes National Park Public Institution: Plitvice Lakes, Croatia, 2019. [Google Scholar]
- Brozinčević, A.; Vurnek, M.; Frketić, T. Water Chemistry. In Plitvice Lakes; Miliša, M., Ivković, M., Eds.; Springer Water, Springer Nature Switzerland AG: Cham, Switzerland, 2023; pp. 65–95. [Google Scholar]
- Habdija, I.; Stilinović, B.; Erben, R.; Maloseja, Ž.; Primc, B.; Plenković, A.; Krga, M.; Futač, N. Ekološka Istraživanja na Trajnim Plohama u Akvatičkom Dijelu Ekosistema Nacionalnog Parka Plitvička Jezera. Expert study, Faculty of Science, University of Zagreb, Zagreb, Croatia, 1986. [Google Scholar]
- Maloseja, Ž. Vertikalni raspored fitoplanktona u jezeru Kozjak (Nacionalni park Plitvice). Ekologija 1985, 20, 64–74. [Google Scholar]
- Wiik, E.; Bennion, H.; Sayer, C.D.; Davidson, T.A.; McGowan, S.; Patmore, I.R.; Clarke, S.J. Ecological sensitivity of marl lakes to nutrient enrichment: Evidence from Hawes Water, UK. Freshwater Biol. 2015, 60, 2226–2247. [Google Scholar] [CrossRef]
- Miliša, M.; Matoničkin Kepčija, R.; Radanović, I.; Ostojić, A.; Habdija, I. The impact of aquatic macrophyte (Salix sp. and Cladium mariscus (L.) Pohl.) removal on habitat conditions and macroinvertebrates of tufa barriers (Plitvice Lakes, Croatia). Hydrobiologia 2006, 573, 183–197. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.; Sun, H.; Liao, C.; Li, H.; Wang, J.; Wu, K. Diurnal Variations of Hydrochemistry in a Travertine-depositing Stream at Baishuitai, Yunnan, SW China. Aquat. Geochem. 2006, 12, 103–121. [Google Scholar] [CrossRef]
- Huntsman, A.G. Fertility and Fertilization of Streams. J. Fish. Res. Board Can. 1948, 7b, 248–253. [Google Scholar] [CrossRef]
- Tank, J.L.; Bernot, M.J.; Rosi-Marshall, E.J. Nitrogen limitation and uptake. In Methods in Stream Ecology, 2nd ed.; Hauer, F.R., Lamberti., G.A., Eds.; Academic Press: San Diego, CA, USA, 2006; pp. 213-238. [Google Scholar]
- Matoničkin Kepčija, R.; Miliša, M.; Sertić Perić, M.; Matijić Cvjetović, M.; Primc-Habdija, B. Response of periphyton to nutrient addition in a tufa depositing environment. Aquat. Microb. Ecol. 2011, 65, 183–195. [Google Scholar] [CrossRef]
- Hart, D.D.; Robinson, C.T. Resource Limitation in a Stream Community: Phosphorus Enrichment Effects on Periphyton and Grazers. Ecology 1990, 71, 1494–1502. [Google Scholar] [CrossRef]
- Kiffney, P.M.; Richardson, J.S. Interactions among Nutrients, Periphyton, and Invertebrate and Vertebrate (Ascaphus truei) Grazers in Experimental Channels. Copeia 2001, 2, 422–429. [Google Scholar] [CrossRef]
- Biggs, B.J.F.; Kilroy, C. Stream Periphyton Monitoring Manual; The New Zealand Ministry for the Environment, NIWA: Christchurch, New Zealand, 2000. [Google Scholar]
- Allan, J.D.; Castillo, M.M. Stream Ecology: Structure and Function of Running Waters, 2nd ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 13–32. [Google Scholar]
- Directive 2000/60/EC of the European Parliament and of the council of 23 October 2000 establishing a framework for community action in the field of water policy. Off. J. Eur. Communities 2000, L327, 1–72.
- Habdija, I.; Primc-Habdija, B.; Matonickin, R.; Kucinic, M.; Radanovic, I.; Milisa, M.; Mihaljevic, Z. Current velocity and food supply as factors affecting the composition of macroinvertebrates in bryophyte habitats in karst running water. Biol. Brat. 2004, 59, 577–594. [Google Scholar]
- Powell, K.E.; Oliver, T.H.; Johns, T.; González-Suárez, M.; England, J.; Roy, D.B. Abundance trends for river macroinvertebrates vary across taxa, trophic group and river typology. Glob. Change Biol. 2023, 29, 1282–1295. [Google Scholar] [CrossRef]
- Davis, S.J.; Mellander, P.E.; Matthaei, C.D.; Piggott, J.J.; Kelly-Quinn, M. Chronic nutrient inputs affect stream macroinvertebrate communities more than acute inputs: An experiment manipulating phosphorus, nitrogen and sediment. Sci. Total Environ. 2019, 683, 9–20. [Google Scholar] [CrossRef]
- Babinka, S. Multi-Tracer Study of Karst Waters and Lake Sediments in Croatia and Bosnia Herzegovina: Plitvice Lakes National Park and Bihać Area. Ph.D. thesis, Universität Bonn, Bonn, Germany, 2007. [Google Scholar]
- Vurnek, M.; Brozinčević, A.; Matoničkin Kepčija, R.; Frketić, T. Analyses of long-term trend sin water quality dana of Plitvice Lakes National Park. Fundam. Appl. Limnol. 2021, 194, 155–169. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E. (Eds.) Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Nusch, E.A. Comparison of different methods for chlorophyll and phaeopigment determination. Arch. Hydrobiol. 1980, 14, 14–36. [Google Scholar]
- Sundermann, A.; Lohse, S.; Beck, L.A.; Haase, P. Key to larval stages of aquatic true flies (Diptera), based on the operational taxa list for running water in Germany. Ann. Limnol. 2007, 43, 61–74. [Google Scholar] [CrossRef]
- Bauernfeind, E.; Humpesch, U.H. Die Eintagsfliegen Zentraleuropas (Insecta: Ephemeroptera): Bestimmung und Ökologie; Verlag des Naturhistorischen Museums: Wien, Austria, 2001. [Google Scholar]
- Waringer, J.; Graf, W. Atlas der Österreichischen Köcherfliegenlarven; Facultas Universitätsverlag: Wien, Austria, 1997. [Google Scholar]
- Birmingham, M.; Heimdal, D.; Hubbard, T.; Krier, K.; Leopold, R.; Luzier, J.; Neely, J.; Soenen, B.; Wilton, T. Benthic Macroinvertebrate Key. IOWATER Volunteer Water Quality Monitoring. 2005. Available online: https://greenspaceamdsb.pbworks.com/f/BMIKey2Edvmay05.pdf (accessed on 20 September 2016.).
- Zwick, P. Key to the West Palaearctic genera of stoneflies (Plecoptera) in the larval stage. Limnologica 2004, 34, 315–348. [Google Scholar] [CrossRef]
- Nilsson, A. Aquatic Insects of North Europe, A Taxonomic Handbook; Apollo Books: Steenstrup, Denmark, 1996; Volume 1. [Google Scholar]
- Nilsson, A. Aquatic Insects of North Europe, A Taxonomic Handbook; Apollo Books: Steenstrup, Denmark, 1997; Volume 2. [Google Scholar]
- Knoz, J. To identification of Czechoslovakian Black-flies (Diptera, Simuliidae). Folia Přírodovědecké Fakluty University J. E. Purkyně v Brně. Biologia 1965, 5, 1–54. [Google Scholar]
- Moog, O. Prefaces, Directory of authors and Introductions. In Fauna Aquatica Austriaca, 2nd ed.; Moog, O., Ed.; Wasserwirtschaftkataster, Bundesministerium für Land- und Forstwirtschaft: Vienna, Austria, 2002; Part I; pp. 1–92. [Google Scholar]
- Sertić Perić, M.; Miliša, M.; Matoničkin Kepčija, R.; Primc-Habdija, I.; Habdija, I. Seasonal and fine-scale spatial drift patterns in a tufa-depositing barrage hydrosystem. Arch. Hydrobiol. 2010, 178, 131–145. [Google Scholar] [CrossRef]
- Srdoč, D.; Horvatinčić, N.; Obelić, B.; Krajcar, I.; Sliepčević, A. Procesi taloženja kalcita u krškim vodama s posebnim osvrtom na Plitvička jezera. Krš Jugosl. 1985, 11, 1–104. [Google Scholar]
- Sironić, A.; Barešić, J.; Horvatinčić, N.; Brozinčević, A.; Vurnek, M.; Kapelj, S. Changes in the geochemical parameters of karst lakes over the past three decades—The case of Plitvice Lakes, Croatia. Appl. Geochem. 2017, 78, 12–22. [Google Scholar] [CrossRef]
- Belančić, A.; Matoničkin Kepčija, R.; Miliša, M.; Plenković Moraj, A.; Habdija, I. Flow Velocity Effect on Leaf Litter Breakdown in Tufa Depositing System (Plitvice Lakes, Croatia). Int. Rev. Hydrobiol. 2009, 94, 391–398. [Google Scholar] [CrossRef]
- Biondić, B.; Biondić, R.; Meaški, H. The conceptual hydrogeological model of the Plitvice Lakes. Geol. Croat. 2010, 63, 195–206. [Google Scholar] [CrossRef]
- Kempe, S.; Emeis, K.; Pegler, K.; Reimer, A.; Richnow, H.H.; Salge, U. Carbonate Chemistry and the Formation of Plitvice Lakes. Mitteilungen Aus Dem Geol.-Paläontologischen Inst. Universität Hamburg 1985, 58, 351–383. [Google Scholar]
- Barešić, J.; Horvatinčić, N.; Roller-Lutz, Z. Spatial and seasonal variations in the stable isotope composition of dissolved inorganic carbon and in physic-chemical water parameters in the Plitvice lakes system. Isot. Environ. Healt. S. 2011, 47, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Drysdale, R.; Taylor, M.P.; Ihlenfeld, C. Factors controlling the chemical evolution of travertine-depositing rivers of the Barkly karst, northern Australia. Hydrol. Process. 2002, 16, 2941–2962. [Google Scholar] [CrossRef]
- Habdija, I.; Stilinović, B.; Maloseja, Ž.; Primc, B.; Futač, N. Ekološka Istraživanja na Trajnim Plohama u Akvatičkom Dijelu Ekosistema Nacionalnog Parka Plitvička Jezera. Expert study, Faculty of Science, University of Zagreb, Zagreb, Croatia, 1987. [Google Scholar]
- Matoničkin Kepčija, R.; Habdija, I.; Primc-Habdija, B.; Miliša, M. The role of simuliid and trichopteran silk structures in tufa formation during the Holocene of the Plitvice Lakes (Croatia). In Proceedings of the 1st International Symposium on Travertine, Denizli, Turkey, 21–25 September 2005. [Google Scholar]
- Chen, J.; Zhang, D.D.; Wang, S.; Xiao, T.; Huang, R. Factors controlling tufa deposition in natural waters at waterfall sites. Sediment. Geol. 2004, 166, 353–366. [Google Scholar] [CrossRef]
- Francoeur, S.N.; Biggs, B.J.; Smith, R.A.; Lowe, R.L. Nutrient limitation of algal biomass accrual in streams: Seasonal patterns and a comparison of methods. J. N. Am. Benthol. Soc. 1999, 18, 242–260. [Google Scholar] [CrossRef]
- Beck, W.S.; Markman, D.W.; Oleksy, I.A.; Lafferty, M.H.; Poff, N.L. Seasonal shifts in the importance of bottom–up and top–down factors on stream periphyton community structure. Oikos 2019, 128, 680–691. [Google Scholar] [CrossRef]
- Dodds, W.K.; Smith, V.H.; Lohman, K. Nitrogen and phosphorus relationships to benthic algal biomass in temperate streams. Can. J. Fish. Aquat. Sci. 2002, 59, 865–874. [Google Scholar] [CrossRef]
- Francoeur, S.N. Meta-analysis of lotic nutrient amendment experiments: Detecting and quantifying subtle responses. J. N. Am. Benthol. Soc. 2001, 20, 358–368. [Google Scholar] [CrossRef]
- Pentecost, A.; Whitton, B.A. Limestones. In The Ecology of Cyanobacteria; Whitton, B.A., Potts, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 257–279. [Google Scholar]
- Plenković-Moraj, A.; Horvatinčić, N.; Primc-Habdija, B. Periphyton and its role in tufa deposition in karstic waters (Plitvice Lakes, Croatia). Biologia 2002, 57, 423–431. [Google Scholar]
- Davies-Colley, R.J.; Quinn, J.M. Stream lighting in five regions of North Island New Zealand: Control by channel size and riparian vegetation. N. Z. J. Mar. Fresh. 1998, 32, 391–605. [Google Scholar] [CrossRef]
- Schiller Von, D.; Marti, E.; Riera, J.L.; Sabater, F. Effects of nutrients and light on periphyton biomass and nitrogen uptake in Mediterranean streams with contrasting land uses. Freshwater Biol. 2007, 52, 891–906. [Google Scholar] [CrossRef]
- Vilenica, M.; Gattolliat, J.-L.; Ivković, M.; Kučinić, M.; Mičetić Stanković, V.; Mihaljević, Z.; Sartori, M. The mayfly fauna (Insecta, Ephemeroptera) of the Plitvice Lakes National Park. Nat. Croat. 2014, 23, 349–363. [Google Scholar]
- Sviben, S.; Matoničkin Kepčija, R.; Vilenica, M.; Krulik, I.; Mičetić Stanković, V.; Kružić, P.; Kučinić, M. Benthic macroinvertebrate communities change along a karstic barrage-lake system. Nat. Croat. 2024, 33, 105–121. [Google Scholar] [CrossRef]
- Habdija, I.; Radanović, I.; Matoničkin, R. Functional feeding structure of benthic macroinvertebrates in travertine barrier biotopes. Verh. Internat. Verein Limnol. 2000, 27, 2594–2599. [Google Scholar] [CrossRef]
- Williams, D.D.; Feltmate, B.W. Aquatic Insects; CAB International: Wallingford, Oxfordshire, UK, 1994; pp. 215–232. [Google Scholar]
- Čmrlec, K.; Ivković, M.; Šemnički, P.; Mihaljević, Z. Emergence phenology and microhabitat distribution of aquatic diptera community at the outlets of barrage lakes: Effect of temperature, substrate and current velocity. Pol. J. Ecol. 2013, 16, 135–144. [Google Scholar]




| Effect | d.f. | F | p |
|---|---|---|---|
| Season | 3, 204 | 51.21 | 0.00000 |
| Microhabitat | 3, 204 | 8.80 | 0.00002 |
| Treatment | 4, 204 | 36.23 | 0.00000 |
| Season*Microhabitat | 9, 204 | 5.93 | 0.00000 |
| Season*Treatment | 12, 204 | 1.86 | 0.04010 |
| Microhabitat*Treatment | 12, 204 | 1.36 | 0.18855 |
| Season*Microhabitat*Treatment | 36, 204 | 1.19 | 0.21977 |
| Effect | d.f. | F | p |
|---|---|---|---|
| Season | 3, 228 | 84.47 | 0.00000 |
| Microhabitat | 3, 228 | 50.70 | 0.00000 |
| Treatment | 4, 228 | 12.42 | 0.00000 |
| Season*Microhabitat | 9, 228 | 15.53 | 0.00000 |
| Season*Treatment | 12, 228 | 2.50 | 0.00430 |
| Microhabitat*Treatment | 12, 228 | 1.52 | 0.11845 |
| Season*Microhabitat*Treatment | 36, 228 | 0.87 | 0.68741 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vurnek, M.; Matoničkin Kepčija, R. Influence of Experimental Eutrophication on Macrozoobenthos in Tufa-Depositing System of Plitvice Lakes National Park, Croatia. Limnol. Rev. 2025, 25, 14. https://doi.org/10.3390/limnolrev25020014
Vurnek M, Matoničkin Kepčija R. Influence of Experimental Eutrophication on Macrozoobenthos in Tufa-Depositing System of Plitvice Lakes National Park, Croatia. Limnological Review. 2025; 25(2):14. https://doi.org/10.3390/limnolrev25020014
Chicago/Turabian StyleVurnek, Maja, and Renata Matoničkin Kepčija. 2025. "Influence of Experimental Eutrophication on Macrozoobenthos in Tufa-Depositing System of Plitvice Lakes National Park, Croatia" Limnological Review 25, no. 2: 14. https://doi.org/10.3390/limnolrev25020014
APA StyleVurnek, M., & Matoničkin Kepčija, R. (2025). Influence of Experimental Eutrophication on Macrozoobenthos in Tufa-Depositing System of Plitvice Lakes National Park, Croatia. Limnological Review, 25(2), 14. https://doi.org/10.3390/limnolrev25020014







