Abstract
The accumulation of rare-earth and heavy metal ions from wastewater is important for industrial technology. However, practical accumulators of metal ions are expensive with respect procurement of raw materials, synthesis, and preparation. Therefore, it is preferable to accumulate metal ions using sustainable resources, such as natural polymers. Fucoidan, a water-soluble natural polymer, is a sulfated polysaccharide from the cell-wall of brown algae. Therefore, fucoidan behaves as an acidic polysaccharide in an aqueous solution. We prepared a fucoidan-inorganic composite material by mixing fucoidan and a silane coupling reagent, bis(3-(trimethoxysilyl)propyl)amine (SiNSi). This fucoidan-SiNSi (F-SiNSi) composite material showed a water-insoluble property. This is due to the encapsulation of fucoidan into a three-dimensional network of SiNSi with siloxane bonding. When the F-SiNSi composite material is immersed in a metal ion-containing aqueous solution, the composite material accumulated the metal ions. The binding affinity of each metal ion was Ca(II), Mg(II) << Nd(III) < Cu(II), Zn(II), Ni(II), La(III) < In(III) < Y(III). Additionally, the maximum-accumulated amounts of the Nd(III), Cu(II), Zn(II), Ni(II), La(III), In(III), and Y(III) ions were 140, 200, 190, 200, 200, 230, and 270 nmol per mg of fucoidan, respectively. Furthermore, the molar ratios of the acidic groups (the sulfate and carboxyl groups) in the fucoidan and accumulated metal ions, were 0.081–0.156. Therefore, the F-SiNSi composite material showed a selectivity for rare-earth and heavy metal ions. The accumulation mechanism of the rare-earth and heavy metal ions was related to the carboxyl groups in the fucoidan.
1. Introduction
Wastewater from factories dealing with metals may contain harmful heavy metal ions such as mercury, cadmium, and lead ions [1]. Since even a small amount of heavy metal ions has an adverse effect on the human body, these heavy metal ions have to be removed from wastewater [1]. Rare-earth metal ions, such as yttrium, lanthanum, and neodymium ions, are used in the field of electronics, magnetics, and optics [2,3]. Additionally, rare-earth ions are found in catalysts for synthetic chemistry, fluorescent substances, and optical glasses [2,3]. Since the content of these rare-earth ions in the earth is low, and these metal ions are expensive, it is desirable to recover them from the wastewater of a factory that uses rare-earth metals. Therefore, accumulating and recovering rare-earth and heavy metal ions from wastewater or groundwater is an important technology in environmental science, industrial chemistry, and separation technology [4,5]. At present, inorganic materials [5,6], such as single-walled or multi-walled carbon nanotubes, biological materials [5,7] such as leaf powder or sawdust, bioadsorbants [5,8] such as bacteria or fungi, and polymer materials [5] such as hyper-crosslinked polystyrene or ethylenediaminetetraacetic acid (EDTA)-functionalized polymers, have been reported as viable tools for metal accumulation. Polymer materials have been used for various applications [3,4,5,9,10], such as ion-exchange resins, filters, metal ion flocculants, electroplating, and wastewater treatment method, among others. However, since these materials are complicated to procure and synthesize, and take time to prepare, the accumulation of rare-earth and heavy metal ions using these materials is costly. Therefore, it is desirable that accumulation of metal ions is done using sustainable resources, such as natural polymers.
Fucoidan, a water-soluble natural polymer, is a sulfated polysaccharide from the cell-wall of brown algae [11,12]. Fucoidan has a viscous property, and this property plays a role in protecting against tidal flow, as well as microorganisms that eat the algae. Chemically, fucoidan has several different structures among brown seaweed species [12]. Scheme 1a shows the partial molecular structure of fucoidan. Although fucoidan mainly consists of fucose, from which its name originates, and sulfate, it also contains monosaccharides such as mannose, galactose, glucose, and xylose. Additionally, fucoidan possesses uronic acid with a carboxyl group. Therefore, fucoidan behaves as an acidic polysaccharide in aqueous solution. Various bioactive functions, such as anti-cancer, anti-viral, anti-inflammation, immunity, gut and digestive health, wound healing, and anti-aging, have been reported for fucoidan [13]. Therefore, the study of fucoidan has mainly occurred in the medical and pharmaceutical fields. Fucoidan contains various functional groups, such as sulfate, carboxyl, and hydroxy groups, in its structure, and these functional groups can interact with metal ions or react with other organic molecules. Therefore, fucoidan may be used not only in the field of biomedicine, such as for drug delivery and tissue regeneration, but also in the field of engineering and environmental protection [13,14,15]. However, the utilization of fucoidan as a material for accumulating and recovery of rare-earth and heavy metal ions has been rarely reported to the best of our knowledge [15]. This is due to the high water solubility of fucoidan. Since fucoidan possesses many hydroxy groups in its molecular structure, it cannot be separated even if it interacts with other molecules or ions. However, since fucoidan possesses sulfate and carboxyl groups, which strongly interact with metal ions, water-insolubilized fucoidan might be used as an accumulation and a recovery material for rare-earth and heavy metal ions.
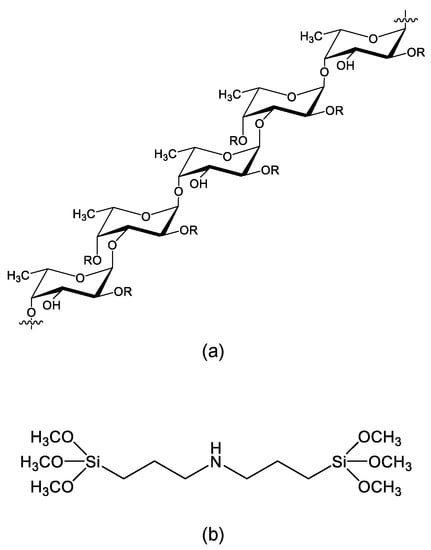
Scheme 1.
Molecular structure of (a) fucoidan and (b) SiNSi. The R indicates carbohydrate (α-L-fucopyranose and α-D-glucuronic acid) or non-carbohydrate (sulfate and acetyl groups).
Insolubilization of biopolymers is an important method for using biopolymers in engineering and environmental protection. The insolubilization of a biopolymer includes its immobilization on an insoluble substrate, cross-linking, encapsulation, and composition, among other factors. Especially, e combination or gelation of a biopolymer with other organic compounds may be achieved by electrostatic, hydrogen, or hydrophobic interactions, as well as by cross-linking reactions [16,17]. However, these biopolymer-organic composites may cause destruction of the biopolymer, decreasing functional groups by electrostatic interaction, and the disappearance of function by hydrophobic interaction. Additionally, since materials consisting only of organic substances have low mechanical strength, they cannot be used for a long time under practical condition. As a result, the biopolymer-organic composite material cannot fully perform its function. In contrast, biopolymer-inorganic composite materials have attracted much attention due to insolubilization methods applied to the biopolymer [18,19,20,21]. Since the biopolymer-inorganic composite materials are prepared by a soft process, such as the sol-gel reaction using a silane coupling reagent, the structural destruction of a biopolymer does not occur. Additionally, the function of a biopolymer can be maintained by the encapsulation of the biopolymer in the network of a silane coupling reagent. Furthermore, biopolymer-inorganic composite materials have properties of both the organic and inorganic components, such as flexibility and strength. Therefore, biopolymer-inorganic composite materials have been used as sensing materials, energy materials, and biomaterials [18,19,20,21]. Recently, we prepared various biopolymer-inorganic composite materials, such as DNA-inorganic [22], RNA-inorganic [23], pectin-inorganic [24], and gellan gum-inorganic [25] composite materials. These biopolymer-inorganic composite materials were able to accumulate harmful compounds such as dibenzo-p-dioxin and polychlorinated biphenyl (PCB) derivatives, and harmful metal ions, such as heavy metal ions [22,24,25]. In addition, the biopolymer-inorganic composite materials showed approximately twice the mechanical strength of biopolymers without inorganic components [22,24,25]. Therefore, the preparation of a biopolymer-inorganic composite material by the sol-gel reaction with a silane coupling reagent can be used for insolubilizing fucoidan.
In this study, we prepared a fucoidan-inorganic composite material by mixing fucoidan (F) and a silane coupling reagent, bis(3-(trimethoxysilyl)propyl)amine (SiNSi). This fucoidan-SiNSi (F-SiNSi) composite material was water-insoluble due to the encapsulation of fucoidan into the three-dimensional network of SiNSi with siloxane bonding. When the F-SiNSi composite material was immersed in an aqueous solution containing metal ions, the composite material accumulated the metal ions. The binding affinity of each metal ion was Ca(II), Mg(II) << Nd(III) < Cu(II). Zn(II), Ni(II), La(III) < In(III) < Y(III), and the maximum-accumulated amounts of the Y(III) ion, which was the most accumulated ion by F-SiNSi, was 270 nmol per mg of fucoidan. Therefore, the F-SiNSi composite material showed a selectivity for rare-earth and heavy metal ions. The accumulative mechanism of the rare-earth and heavy metal ions was related to the carboxyl groups.
2. Materials and Methods
2.1. Material
Fucoidan (from Fucus serratus L.) was purchased from Biosynth Carbosynth, Berkshire, UK. The silane coupling reagent, bis(3-trimethoxysilylpropyl)amine (SiNSi) was obtained from Tokyo Kasei Industries Ltd., Tokyo, Japan. Scheme 1b shows the molecular structure of SiNSi. Yttrium(III) chloride hexahydrate, lanthanum(III) chloride heptahydrate, indium(III) chloride tetrahydrate, neodymium(III) chloride hexahydrate, copper(II) chloride dihydrate, zinc(II) chloride, nickel(II) chloride hexahydrate, magnesium(II) chloride hexahydrate, and calcium(II) chloride dihydrate were obtained from Fujifilm Wako Pure Chemical Industries Ltd., Tokyo, Japan or Kanto Chemical Co., Inc., Tokyo, Japan. A standard solution of Y(III), La(III), In(III), Nd(III), Cu(II), Zn(II), Ni(II), Mg(II), and Ca(II) ions was obtained from Fujifilm Wako Pure Chemical Industries. Xylenol orange (XO) and methylthymol blue (MTB) were purchased from Dojindo Co., Kumamoto, Japan and Fujifilm Wako Pure Chemical Industries Ltd., respectively. The strongly acidic ion-exchange resin, Amberlite IR120(H), was obtained from Supelco, Bellefonte, PA. Analytical grade solvents were used in all the experiments described. Ultra-pure water (Merck KGaA, Darmstadt, Germany) was used in experiments.
2.2. Preparation of Fucoidan-Inorganic Composite Material
The fucoidan-SiNSi (F-SiNSi) composite film was prepared as follows. A fucoidan aqueous solution (200 μL, 50 mg/mL) and 1 M HCl solution (30 μL) were mixed in microtube. The SiNSi solution was added to the solution and immediately mixed using a vortex mixer. The fucoidan and SiNSi mixed solution (50 μL) was placed on a Teflon® plate and reacted at 90 °C for 20 min. Composite films were stripped from the Teflon® plate and stored in a desiccator. The mixing ratio (wt%) of the fucoidan and SiNSi was determined by ((weight of SiNSi)/{(weight of fucoidan) + (weight of SiNSi)}) × 100. The weight of fucoidan and weight of SiNSi in the equations were the weights of the fucoidan and SiNSi in the mixed solution, respectively. The mixing ratio of the fucoidan and SiNSi varied from 0 to 20 wt%.
The amounts of the sulfate and the carboxyl groups in the fucoidan were evaluated as follows. Since natural fucoidan has sodium and potassium salts, these metal ions were substituted by H+ using an ion exchange column with a strongly acidic ion exchange resin [24,25]. Ion exchange was confirmed by pH measurements. The amount of sulfate and carboxyl groups in the fucoidan was estimated by neutralization titration. The amount of the sulfate and the carboxyl groups in the fucoidan was 1.72 × 10−3 mol g−1.
A photograph of F-20 wt% SiNSi composite material stripped from the Teflon® plate, was taken under the dried condition.
2.3. Water Stability of F-SiNSi Composite Material
The water stability of F-SiNSi composite material was evaluated from the eluted amount of composite film to water. The eluted amount of composite material was measured as follows. The dried F-SiNSi composite film, which was quantified by a precision balance, was immersed in pure water (10 mL) and incubated at room temperature for various times. The immersed composite films were dried in a desiccator for more than 12 h, then weighed using the precision balance once more. The eluted amount of the composite film to water was evaluated from the weight difference before and after immersion.
2.4. Structural and Thermal Analyses of F-SiNSi Composite Material
The molecular structure of F-SiNSi composite material was analyzed by infrared (IR) absorption spectra using an FT-IR 8400 Fourier transform infrared spectrometer (Shimadzu Corp., Kyoto, Japan) and an IR spectrophotometer FT/IR-4700 (JASCO Corporation, Tokyo, Japan) equipped with a diamond attenuated total reflection (ATR) prism. The IR spectrum was measured at a resolution of 4 cm−1.
The thermal stability of the F-SiNSi composite film was analyzed by thermogravimetric-differential thermal analysis (TG-DTA) (DTG-60, Shimadzu Corp.). The TG-DTA measurement was carried out at a heating rate of 10 °C min−1 from room temperature to 300 °C in flowing dry nitrogen. The sample weights of the composite materials were normalized at 1 mg.
2.5. Accumulation of Metal Ions by F-SiNSi Composite Film
YCl3∙6H2O, LaCl3∙7H2O, InCl3∙4H2O, NdCl3∙6H2O, CuCl2∙2H2O, ZnCl2, NiCl2∙6H2O, MgCl2∙6H2O, and CaCl2∙2H2O were dissolved separately in pure water. The concentrations of the aqueous metal ion solutions ranged from 0–100 ppm. The accumulation of metal ions by the F-SiNSi composite film was demonstrated as follows. Ten F-SiNSi composite films were added to an aqueous solution (10 mL) containing metal ions. These solutions were stirred at room temperature for 6 h. The concentrations of metal ions were estimated from a calibration curve with the metal indicator XO and MTB [25]. The accumulated amounts of metal ions were calculated from the concentration of the metal ions in the absence or presence of the F-SiNSi composite film.
2.6. IR Measurements of Metal Ion-Accumulated F-SiNSi Composite Material
The metal ion-accumulated F-SiNSi composite material was prepared as follows. The composite film was immersed in an aqueous solution (10 mL) containing metal ions cat room temperature for 24 h. The metal ion-accumulated composite film was washed using pure water (20 mL × 3 times) and dried at room temperature for more than 12 h. The concentration of metal ion was 1000 ppm. The metal ion-accumulated composite films were analyzed using IR spectrometer.
3. Results and Discussion
3.1. Preparation of F-SiNSi Composite Material
The fucoidan-SiNSi (F-SiNSi) composite material was prepared by mixing fucoidan (F), bis(trimethoxysilylpropyl)amine (SiNSi), and hydrochloric acid. The F-SiNSi composite material was obtained by reacting the mixed solution at 90 °C for 20 min. The F-SiNSi composite material formed a film at a mixing ratio of <30 wt% SiNSi. In contrast, when the mixing ratio of SiNSi was more than 30 wt%, the F-SiNSi composite material did not form a film and was a lumpy material. Figure 1 shows a photograph of the F-20 wt% SiNSi composite film, which was stripped from the Teflon® plate, under a dried condition. The thickness of F-20 wt% SiNSi composite film was approximately 100 μm. This brown composite film was transparent and it as possible to read letters under the film. Additionally, the composite film was homogeneous. The dried F-SiNSi composite film was not flexible under a 50% relative humidity condition and easily cracked when bent.

Figure 1.
Photograph of F-20 wt% SiNSi composite film stripped from the Teflon® plate under a dried condition. The thickness of composite film was approximately 100 μm. The scale bar represents 5 mm.
Next, we demonstrated the immersion of the F-SiNSi composite film in water. The fucoidan film without the mixing of SiNSi or hydrochloric acid dissolved in water. The composite film without heat treatment did not show water stability. Therefore, we evaluated the water-stability of heat-treated F-SiNSi composite film at different mixing ratios of SiNSi. Figure 2 shows the water stability of the F-SiNSi composite film with mixing of 5 wt% SiNSi (closed squares), 10 wt% SiNSi (closed triangles), and 20 wt% SiNSi (closed circles). When the F-5 wt% SiNSi composite film was incubated in pure water, the eluted amount of the composite film increased with the incubation time and reached a constant value at 2 h. This value was approximately 75%. The eluted amount decreased with an increase in the mixing ratio of SiNSi and had a constant value at 20 wt% (see closed circle in Figure 2). These values were approximately 50%. As a result, the water stability of the composite material increased with the mixing amount of SiNSi. The composite film with mixing of 20 wt% SiNSi had the highest water stability. Additionally, the composite film, which was incubated in water for 2 h, did not subsequently dissolve in water. Therefore, we used the F-20 wt% SiNSi composite material in the following experiments. The F-SiNSi composite films were stored in pure water for more than 2 h to remove the water-soluble components, then used in the experiments.
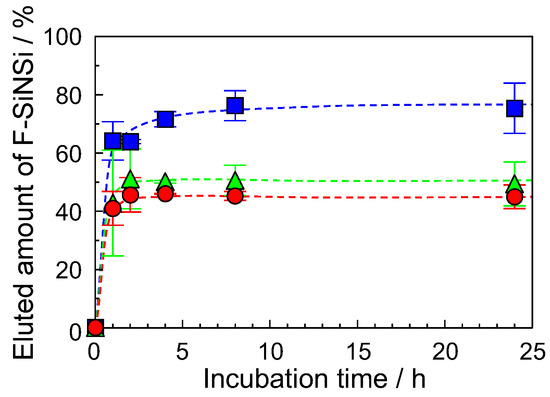
Figure 2.
Water stability of F-SiNSi composite film: F-5 wt% SiNSi (closed squares), F-10 wt% SiNSi (closed triangles), and F-20 wt% SiNSi (closed circles) composite film. Each of the values represents the mean of three separate determinations ± standard deviations.
In contrast, although the F-SiNSi composite film was incubated in water at room temperature for more than 1 week, these composite films did not dissolve in water. These incubated composite films in water could be removed from a vessel by pinching with tweezers and collapse of the composite material after a long incubation in water did not occur. Furthermore, the swollen composite film showed flexibility.
3.2. Molecular Structure of F-SiNSi Composite Material
Figure 3 shows the IR spectra of (a) fucoidan without the composite of SiNSi, (b) F-5 wt% SiNSi, (c) F-10 wt% SiNSi, (d) F-20 wt% SiNSi, and (e) the SiNSi molecule without combination with fucoidan. SiNSi without combination with fucoidan was used as a sample that was condensed by heat treatment. The IR spectra of the SiNSi molecule without combination with fucoidan showed absorption bands at ca. 1000 cm−1 and 1100 cm−1, related to the stretching vibration of the linear O-Si-O and macrocyclic O-Si-O, respectively [26,27]. These absorption bands also appeared in the F-SiNSi composite material. These results indicated that the SiNSi molecules in the composite material formed a three-dimensional structure by siloxane bonding. On the other hand, the fucoidan molecule showed the absorption bands at 1212 cm−1 and 1620 cm−1, attributed to the stretching vibration of S-O [28,29] and the symmetric stretching vibration of -COO− [28,29,30], respectively. Generally, carboxylate and sulfate groups that interact with other molecules show a shift of their absorption bands. Although the intensity of these absorption bands decreased with increase in the mixing ratio of the SiNSi molecule, it did not show any shift. These phenomena indicated that the carboxylate and sulfate groups in fucoidan did not strongly interact with the SiNSi molecule in the composite material. These results suggested that fucoidan is encapsulated in a three-dimensional siloxane network of SiNSi, and the structure and property of the fucoidan were retained in the composite material.
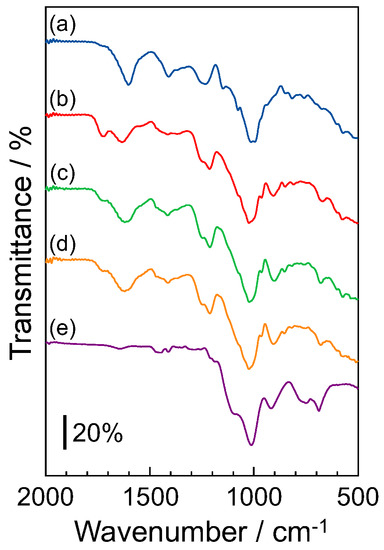
Figure 3.
IR spectra of (a) fucoidan without combination with SiNSi, (b) F-5 wt% SiNSi composite, (c) F-10 wt% SiNSi composite, (d) F-20 wt% SiNSi composite, and (e) SiNSi without combination with fucoidan. The SiNSi without the composite of fucoidan was used as a sample that was condensed by heat treatment. Triplicate experiments gave similar results.
3.3. Accumulation of Metal Ions by the F-SiNSi Composite Material
Figure 4 shows the accumulation of Cu(II) ions by the F-20 wt% SiNSi composite material at various times. The accumulated amount of Cu(II) ions increased with incubation time and reached a constant value at 6 h. The accumulated amount of Cu(II) ions at 6 h was approximately 180 nmol. Similar accumulative behavior was seen with the accumulation of Zn(II) ions (data not shown). Therefore, we demonstrated the accumulation of other metal ions, such as rare-earth metal and light metal ions, at 6 h.
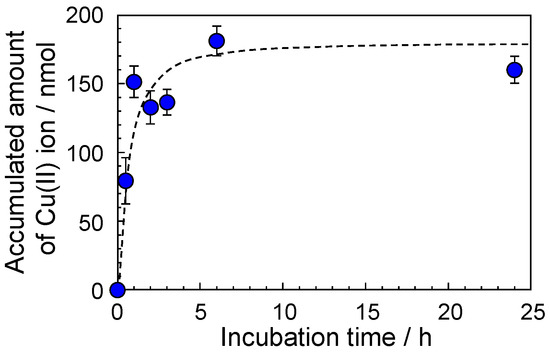
Figure 4.
Accumulated amount of Cu(II) ion by the F-20 wt% SiNSi composite material at various times. The concentration of the Cu(II) ion was 50 ppm. The concentration of the Cu(II) ion was estimated from a calibration curve with the metal indicator. Each of the values represents the mean of three separate determinations ± standard deviations.
Figure 5 shows the accumulated amount of Cu(II) ions (closed circles), Y(III) ions (closed squares), and Ca(II) ions (closed triangles). The dotted lines indicate assumed accumulated amounts. The accumulated amount of the Cu(II) and Y(III) ions increased with the initial concentration of these metal ions and reached a constant value at approximately 100 ppm. We defined the constant amount of accumulation as the maximum-accumulated amount of metal ions. As a result, the maximum-accumulated amounts of the Cu(II) and Y(III) ions were 200 nmol and 270 nmol, respectively. Therefore, we demonstrated the accumulation of various metal ions, such as rare-earth and heavy metal ions. The maximum-accumulated amounts of In(III), La(III), Ni(II), Zn(II), and Nd(III) ions were 230, 200, 200, 190, and 140 nmol, respectively (data not shown). In contrast, when the accumulation of Ca(II) ions, as light metal ions, by the F-20 wt% SiNSi composite material was demonstrated, the concentration of the Ca(II) ions was almost the same as the initial concentration (see closed triangles in Figure 5). As a result, the F-SiNSi composite material could not accumulate the Ca(II) ions. A similar result was obtained for Mg(II) ions (data not shown). Therefore, the F-SiNSi composite material could not accumulate light metal ions, such as the Ca(II) and Mg(II) ions.
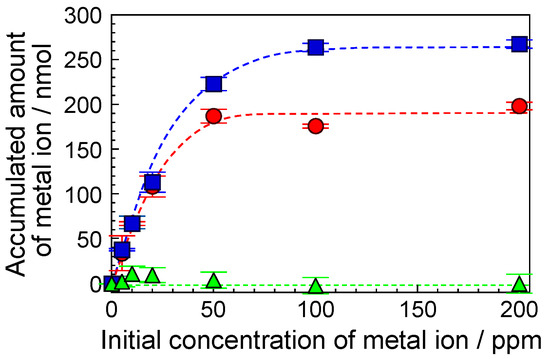
Figure 5.
Accumulated amounts of Cu(II) ions (closed circle), Y(III) ions (closed square), and Ca(II) ions (closed triangle). The incubation time was 6 h. Each of the values represents the mean of three separate determinations ± standard deviations. Dotted lines indicate the assumed accumulated amount.
Figure 6 shows the molar ratio of acidic groups (sulfate and carboxyl groups) in the fucoidan and metal ions. The moles of the accumulated metal ions were estimated from the maximum-accumulated amount calculated from the assumed line in Figure 5. The concentration of acid groups, such as the sulfate and carboxyl groups, in the fucoidan was determined from the neutralization titration at 1.72 × 10−3 mol g−1. The molar ratios of the acidic groups and metal ions were different for the different types of metal ions. The molar ratios of the accumulated metal ions, such as the rare-earth and heavy metal ions, were 0.081–0.156, and the molar ratio of Y(III) ion was approximately twice that of the Nd(III) ion. In this case, one Y(III) ion and one Nd(III) ion were surrounded by approximately six and twelve acidic groups, respectively, and the other rare-earth and heavy metal ions were surrounded by approximately ten acidic groups on average. In contrast, the molar ratios of the light metal ions, such as the Ca(II) and Mg(II) ions, were almost zero and the F-20 wt% SiNSi composite material could not accumulate the light metal ions. Since the molar ratio indicates the tendency of interaction with a metal ion, the binding affinity becomes higher when the molar ratio increases. As a result, the binding affinity of each metal ion was Ca(II), Mg(II) << Nd(III) < Cu(II), Zn(II), Ni(II), La(III) < In(III) < Y(III). These results suggested that the F-20 wt% SiNSi composite material showed a selectivity for the rare-earth and heavy metal ions.
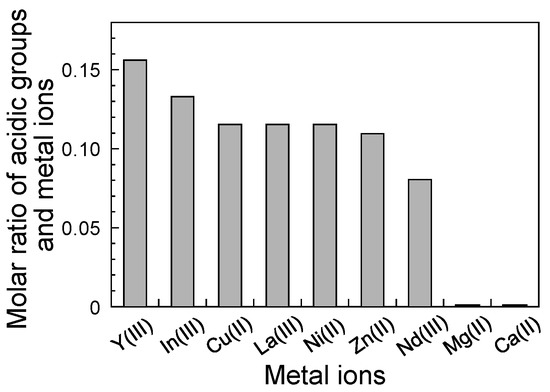
Figure 6.
Molar ratios of acidic groups (sulfate and carboxylate groups) and metal ions. The amount of acid groups, such as the sulfate and carboxyl groups, in the fucoidan was determined by neutralization titration. The moles of the accumulated metal ions were estimated from the maximum-accumulated amount calculated from the assumed line in Figure 5.
3.4. Accumulative Mechanism of Metal Ions by F-SiNSi Composite Material
The F-20 wt% SiNSi composite material could selectively accumulate the rare-earth and heavy metal ions. The selective accumulation of metal ions was evaluated by IR measurements. Figure 7 shows the IR spectra of (a) Y(III) ion-accumulated, (b) Cu(II) ion-accumulated, (c) Mg(II) ion-accumulated, and (d) non-accumulated F-20 wt% SiNSi composite materials. The non-accumulated F-20 wt% SiNSi composite material was prepared by immersion in an aqueous solution for 24 h. The F-20 wt% SiNSi composite material showed absorption bands at 1212 cm−1 and 1620 cm−1, attributed to the stretching vibration of S-O [28,29] and the symmetric stretching vibration of -COO− [28,29,30], respectively. The absorption band of -COO− shifted to a lower wavenumber when the composite material accumulated the metal ions. The order of this shift value was Mg(II) < Cu(II) < Y(III) and depended on the accumulative amount. For the Y(III) ion, which showed the highest accumulative amount, the shift value was ca. 30 cm−1. In contrast, the stretching vibration of S-O did not show any shift. These results suggest that the accumulation of metal ions occurs at the site of the -COO− group, while the sulfate group does not affect the accumulation of the metal ions. Furthermore, the different in affinity between the metal ion and the -COO− group affected the selectivity of the metal ion.
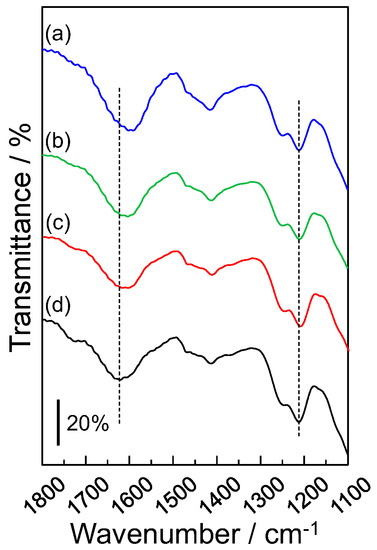
Figure 7.
IR spectra of (a) Y(III) ion accumulated, (b) Cu(II) ion accumulated, (c) Mg(II) ion accumulated, and (d) non-accumulated F-20 wt% SiNSi composite materials. Triplicate experiments gave similar results.
The order of distance between the hydrated divalent ion and the oxygen atom in a water molecule, which was hydrated to a metal ion, was Cu(II) < Ni(II) < Zn(II) < Mg(II) << Ca(II) [31,32]. This order coincided with the order of the accumulated amount, such as Cu(II) > Ni(II) > Zn(II) >> Mg(II), Ca(II). These phenomena can be explained as follows. A metal ion with a large hydrated radius cannot penetrate into the three-dimensional network of the F-SiNSi composite material. As a result, since a metal ion with a large hydrated radius, such as the Mg(II) and Ca(II) ions, interacted with the -COO− group only on the surface of the composite material, the accumulated amounts of Mg(II) and Ca(II) ions were low. Since divalent heavy metal ions with a small hydrated radius, such as the Cu(II), Ni(II), and Zn(II) ions, penetrated into the F-SiNSi composite material, these metal ions were effectively accumulated.
On the other hand, trivalent metal ions, such as rare-earth ions, were present in high accumulated amounts. This is due to coordination number. Generally, rare-earth ions such as Y(III), La(III), In(III), and Nd(III) ions, possess various coordination numbers and form various coordination structures [33]. The rare-earth ions are stabilized in a three-dimensional network since the -COO− groups of the F-SiNSi composite material behave as a ligand that can coordinate metal ions in the network. As a result, the accumulated amount of the rare-earth ions increased compared to heavy and light metal ions. In addition, according to hard and soft acids and bases (HSAB) theory [33], the -COO− groups of the F-SiNSi composite material and the rare-earth ions are classified as hard bases and hard acids, respectively. Based on the HSAB theory, the hard (or soft) bases and hard (or soft) acids strongly interact [33]. Furthermore, the distance between the rare-earth ion and the oxygen atom in a water molecule, which is hydrated to metal ion, is In(III), Y(III) < La(III), Nd(III) [31,34,35,36]. Therefore, the hardness of the rare-earth ion in HSAB theory is assumed to be La(III), Nd(III) < In(III), Y(III). As a result, the F-SiNSi composite material accumulated the In(III) and Y(III) ions more than the La(III) and Nd(III) ions.
4. Conclusions
We prepared a water-insoluble fucoidan-inorganic composite material by mixing fucoidan (F) with a silane coupling reagent (SiNSi). The water-stability property of the composite was due to the encapsulation of fucoidan into the three-dimensional network of the SiNSi molecule. These F-SiNSi composite materials effectively accumulated metal ions, and the binding affinity of each metal ion was Ca(II), Mg(II) << Nd(III) < Cu(II), Zn(II), Ni(II), La(III) < In(III) < Y(III). Therefore, the F-SiNSi composite material showed selectivity for rare-earth and heavy metal ions. The metal ion selectivity of the F-SiNSi composite material is related to the -COO− groups in the fucoidan. Since fucoidan is a sustainable resource, fucoidan-containing materials can be used industrially at extremely low cost. Additionally, the preparation of F-SiNSi composite materials though the sol-gel method is simpler than that of other reported materials. Therefore, the F-SiNSi composite material may have potential use for accumulation of heavy metal ions and recovery of rare-earth ions from industrial liquid waste, ground water, river water, or sea water. Furthermore, since fucoidan is a material that is friendly to the human body, fucoidan-containing materials can also be used to purify drinking water in an emergency.
Author Contributions
Conceptualization, M.Y.; methodology, M.Y.; software, M.Y. and Y.S.; validation, M.Y. and Y.S.; formal analysis, M.Y. and Y.S; investigation, M.Y. and Y.S.; resources, M.Y.; data curation, M.Y. and Y.S.; writing—original draft preparation, M.Y.; writing—review and editing, M.Y.; visualization, M.Y. and Y.S.; supervision, M.Y.; project administration, M.Y.; funding acquisition, M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI Grant Number JP22K12451.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated and analyzed during this study are included in this published article.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number JP22K12451.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lim, A.P.; Aris, A.Z. A review on economically adsorbents on heavy metals removal in water and wastewater. Rev. Environ. Sci. Biotechnol. 2014, 13, 163–181. [Google Scholar] [CrossRef]
- Khan, A.M.; Bakar, N.K.A.; Bakar, A.F.A.; Ashraf, M.A. Chemical speciation and bioavailability of rare earth elements (REEs) in the ecosystem: A review. Environ. Sci. Pollut. Res. 2017, 24, 22764–22789. [Google Scholar] [CrossRef] [PubMed]
- Gorbatenko, A.A.; Revina, E.I. A review of instrumental methods for determination of rare earth elements. Inorg. Mater. 2015, 51, 1375–1388. [Google Scholar] [CrossRef]
- Naila, A.; Meerdink, G.; Jayasen, V.; Sulaiman, A.Z.; Aji, A.B.; Berta, G. A review on global metal accumulators—Mechanism, enhancement, commercial application, and research trend. Environ. Sci. Pollut. Res. 2019, 26, 26449–26471. [Google Scholar] [CrossRef]
- Fei, Y.; Hu, Y.H. Design, synthesis, and performance of adsorbents for heavy metal removal from wastewater: A review. J. Mater. Chem. A 2022, 10, 1047–1085. [Google Scholar] [CrossRef]
- Borji, H.; Ayoub, G.M.; Bilbeisi, R.; Nassar, N.; Malaeb, L. How effective are nanomaterials for the removal of heavy metals from water and wastewater? Water Air Soil Pollut. 2020, 231, 330. [Google Scholar] [CrossRef]
- Dhir, B. Potential of biological materials for removing heavy metals from wastewater. Environ. Sci. Pollut. Res. 2014, 21, 1614–1627. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Dao, V.H.; Cameron, N.R.; Saito, K. Synthesis, properties and performance of organic polymers employed in flocculation applications. Polym. Chem. 2016, 7, 11–25. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Seaweed polysaccharide-based nanoparticles: Preparation and applications for drug delivery. Polymers 2016, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Voleskya, B.; Mucci, A. A review of the biochemistry of heavy metalbiosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Seidi, F.; Shamsabadi, A.A.; Amooghin, A.E.; Saeb, M.R.; Xiao, H.; Jin, Y.; Rezakazemi, M. Biopolymer-based membranes from polysaccharides for CO2 separation: A review. Environ. Chem. Lett. 2022, 20, 1083–1128. [Google Scholar] [CrossRef]
- Titirici, M.M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; Monte, F.; Clark, J.H.; MacLachlang, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef] [PubMed]
- Samiey, B.; Cheng, C.H.; Wu, J. Organic-inorganic hybrid polymers as adsorbents for removal of heavy metal ions from solutions: A review. Materials 2014, 7, 673–726. [Google Scholar] [CrossRef]
- Erigoni, A.; Diaz, U. Porous silica-based organic-inorganic hybrid catalysts: A review. Catalysts 2021, 11, 79. [Google Scholar] [CrossRef]
- Pandey, S.; Mishra, S.B. Sol-gel derived organic–inorganic hybrid materials: Synthesis, characterizations and applications. J. Sol-Gel Sci. Technol. 2011, 59, 73–94. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Yamada, M.; Aono, H. DNA-inorganic hybrid material as selective absorbent for harmful compounds. Polymer 2008, 49, 4658–4665. [Google Scholar] [CrossRef]
- Yamada, M.; Tohyama, C.; Yamada, T. Preparation of water-insoluble and biochemically-stable RNA hybrid material. Polym. Adv. Technol. 2018, 29, 2890–2898. [Google Scholar] [CrossRef]
- Yamada, M.; Ogino, T. Anhydrous proton conductor consisting of pectin-inorganic composite material. J. Appl. Polym. Sci. 2015, 132, 42433–42439. [Google Scholar] [CrossRef]
- Yamada, M.; Kametani, Y. Preparation of gellan gum-inorganic composite film and its metal ion accumulation property. J. Compos. Sci. 2022, 6, 42. [Google Scholar] [CrossRef]
- Takano, N.; Fukuda, T.; Ono, K. Change in the structure of heat-treated siloxane oligomers as observed by FT-IR. Kobunshi Ronbunshu 2000, 57, 743–750. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Silane Coupling Agents, 2nd ed.; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Silverstein, R.M.; Webster, F.X. Spectrometric Identification of Organic Compounds; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Ptak, S.H.; Sanchez, L.; Fretté, X.; Kurouski, D. Complementarity of Raman and infrared spectroscopy for rapid characterization of fucoidan extracts. Plant Methods 2021, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Gunasekaran, S. Preparation of pectin–ZnO nanocomposite. Nanoscale Res. Lett. 2008, 3, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Ohtaki, H. Preparation and characterization of heteropolytungstates containing group 3a elements. Rev. Inorg. Chem. 1982, 4, 103. [Google Scholar]
- Caminiti, R.; Licheri, G.; Piccaluga, G.; Pinna, G.; Magini, M. Structural properties of lead-iron phosphate glasses by X-ray diffraction. Rev. Inorg. Chem. 1979, 1, 333. [Google Scholar]
- Cotton, F.A.; Wilkinson, G.; Gaus, P.L. Basic Inorganic Chemistry; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Smith, L.S.; Wertz, D.L. Solute structuring in aqueous lanthanum(III) chloride solutions. J. Am. Chem. Soc. 1975, 97, 2365–2368. [Google Scholar] [CrossRef]
- Habenschuss, A.; Spedding, F.H. The coordination (hydration) of rare earth ions in aqueous chloride solutions from X-ray diffraction. II. LaCl3, PrCl3, and NdCl3. J. Chem. Phys. 1979, 70, 3758–3763. [Google Scholar] [CrossRef]
- Lindqvist-Reis, P.; Lamble, K.; Pattanaik, S.; Persson, I.; Sandström, M. Hydration of the yttrium(III) ion in aqueous solution. An X-ray diffraction and XAFS structural study. J. Phys. Chem. B 2000, 104, 402–408. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).