Development of a Novel HPLC-MS Method to Separate Polar and Non-Polar Compounds in Biodiesel/Petrodiesel Mixtures
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- BP. Statistical Review of World Energy 2020; BP p.l.c.: London, UK, 2020. [Google Scholar]
- Andruleit, H.; Meßner, J.; Pein, M.; Rebscher, D.; Schauer, M.; Schmidt, S.; von Goerne, G. Status, Daten und Entwicklungen der globalen Energieversorgung. Z. Energiewirtsch. 2018, 42, 179–191. [Google Scholar] [CrossRef]
- Bardi, U. Peak oil: The four stages of a new idea. Energy 2009, 34, 323–326. [Google Scholar] [CrossRef]
- Carr, P.H. Weather extremes from anthropogenic global warming—Special issue—Global warming. Nat. Sci. 2013, 5, 130–134. [Google Scholar] [CrossRef]
- Islam, B. Climate change, global warming and its impacts on oceans. Int. J. Chem. Sci. 2013, 11, 1426–1436. [Google Scholar]
- Kurane, I. The Effect of Global Warming on Infectious Diseases. Osong Public Health Res. Perspect. 2010, 1, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Coady, D.; Parry, I.; Sears, L.; Shang, B. How large are global fossil fuel subsidies? World Dev. 2017, 91, 11–27. [Google Scholar] [CrossRef]
- Warner, K.J.; Jones, G.A. The climate-independent need for renewable energy in the 21st century. Energies 2017, 10, 1197. [Google Scholar] [CrossRef]
- Eurostat. Energy, Transport and Environment Statistics 2020 Edition; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar] [CrossRef]
- Kraftfahrt-Bundesamt. Fahrzeugzulassungen (FZ) Bestand an Kraftfahrzeugen nach Umwelt-Merkmalen; FZ 13; Kraftfahrt-Bundesamt: Flensburg, Germany, 2021. [Google Scholar]
- Bundesministerium für Wirtschaft und Energie. Energiedaten: Gesamtausgabe; Bundesministerium für Wirtschaft und Energie: Berlin, Germany, 2019. [Google Scholar]
- Bundesministerium für Justiz. Gesetz zum Schutz vor Schädlichen Umwelteinwirkungen durch Luftverunreinigungen, Geräusche, Erschütterungen und ähnliche Vorgänge (Bundes-Immissionsschutzgesetz-BImSchG); Bundesministerium für Justiz: Berlin, Germany, 2021. [Google Scholar]
- Szybist, J.P.; Song, J.; Alam, M.; Boehman, A.L. Biodiesel combustion, emissions and emission control. Fuel Process. Technol. 2007, 88, 679–691. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Lubricity of components of biodiesel and petrodiesel. The origin of biodiesel lubricity. Energy Fuels 2005, 19, 1192–1200. [Google Scholar] [CrossRef]
- Monyem, A.; Van Gerpen, J.H. The effect of biodiesel oxidation on engine performance and emissions. Biomass Bioenergy 2001, 20, 317–325. [Google Scholar] [CrossRef]
- Chen, H.; Xie, B.; Ma, J.; Chen, Y. NOx emission of biodiesel compared to diesel: Higher or lower? Appl. Therm. Eng. 2018, 137, 584–593. [Google Scholar] [CrossRef]
- Karmakar, R.; Kundu, K.; Rajor, A. Fuel properties and emission characteristics of biodiesel produced from unused algae grown in India. Pet. Sci. 2018, 15, 385–395. [Google Scholar] [CrossRef]
- Varatharajan, K.; Cheralathan, M. Influence of fuel properties and composition on NOx emissions from biodiesel powered diesel engines: A review. Renew. Sustain. Energy Rev. 2012, 16, 3702–3710. [Google Scholar] [CrossRef]
- Pinzi, S.; Rounce, P.; Herreros, J.M.; Tsolakis, A.; Dorado, M.P. The effect of biodiesel fatty acid composition on combustion and diesel engine exhaust emissions. Fuel 2013, 104, 170–182. [Google Scholar] [CrossRef]
- Knothe, G.; Krahl, J.; Van Gerpen, J. The Biodiesel Handbook; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Bundesanstalt für Landwirtschaft und Ernährung. Evaluations- und Erfahrungsbericht für das Jahr 2018; Bundesanstalt für Landwirtschaft und Ernährung: Bonn, Germany, 2019. [Google Scholar]
- Lee, R.A.; Lavoie, J.-M. From first-to third-generation biofuels: Challenges of producing a commodity from a biomass of increasing complexity. Anim. Front. 2013, 3, 6–11. [Google Scholar] [CrossRef]
- Foteinis, S.; Chatzisymeon, E.; Litinas, A.; Tsoutsos, T. Used-cooking-oil biodiesel: Life cycle assessment and comparison with first- and third-generation biofuel. Renew. Energy 2020, 153, 588–600. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Jaffe, S.B. Detailed composition-based model for predicting the cetane number of diesel fuels. J. Ind. Eng. Chem. 2006, 45, 346–351. [Google Scholar] [CrossRef]
- Wang, F.C.Y.; Robbins, W.K.; Greaney, M.A. Speciation of nitrogen-containing compounds in diesel fuel by comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2004, 27, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Lu, M.; Keener, T.C.; Liu, Z.; Khang, S.-J. The organic composition of diesel particulate matter, diesel fuel and engine oil of a non-road diesel generator. J. Environ. Monit. 2005, 7, 983–988. [Google Scholar] [CrossRef]
- Alexandrino, G.L.; Malmborg, J.; Augusto, F.; Christensen, J.H. Investigating weathering in light diesel oils using comprehensive two-dimensional gas chromatography–High resolution mass spectrometry and pixel-based analysis: Possibilities and limitations. J. Chromatogr. A 2019, 1591, 155–161. [Google Scholar] [CrossRef] [PubMed]
- M’Peko, J.-C.; Reis, D.L.; De Souza, J.E.; Caires, A.R. Evaluation of the dielectric properties of biodiesel fuels produced from different vegetable oil feedstocks through electrochemical impedance spectroscopy. Int. J. Hydrogen Energy 2013, 38, 9355–9359. [Google Scholar] [CrossRef]
- Rüger, C.P.; Sklorz, M.; Schwemer, T.; Zimmermann, R. Characterisation of ship diesel primary particulate matter at the molecular level by means of ultra-high-resolution mass spectrometry coupled to laser desorption ionisation—Comparison of feed fuel, filter extracts and direct particle measurements. Anal. Bioanal. Chem. 2015, 407, 5923–5937. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Kafer, U.; Groger, T.; Wilharm, T.; Zimmermann, R.; Ruger, C.P. Investigation of Aging Processes in Bitumen at the Molecular Level with High-Resolution Fourier-Transform Ion Cyclotron Mass Spectrometry and Two-Dimensional Gas Chromatography Mass Spectrometry. Energy Fuels 2020, 34, 10641–10654. [Google Scholar] [CrossRef]
- Jones, H.E.; Palacio Lozano, D.C.; Huener, C.; Thomas, M.J.; Aaserud, D.J.; DeMuth, J.C.; Robin, M.P.; Barrow, M.P. Influence of Biodiesel on Base Oil Oxidation as Measured by FTICR Mass Spectrometry. Energy Fuels 2021, 35, 11896–11908. [Google Scholar] [CrossRef]
- Miettinen, I.; Kuittinen, S.; Paasikallio, V.; Mäkinen, M.; Pappinen, A.; Jänis, J. Characterization of fast pyrolysis oil from short-rotation willow by high-resolution Fourier transform ion cyclotron resonance mass spectrometry. Fuel 2017, 207, 189–197. [Google Scholar] [CrossRef]
- Hamacher, D.; Schrader, W. Investigating Molecular Transformation Processes of Biodiesel Components During Long-Term Storage Via High-Resolution Mass Spectrometry. ChemSusChem 2022, 15, e202200456. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Vetere, A.; Wisniewski, A.; Eberlin, M.N.; Schrader, W. Comparing Crude Oils with Different API Gravities on a Molecular Level Using Mass Spectrometric Analysis. Part 2: Resins and Asphaltenes. Energies 2018, 11, 2766. [Google Scholar] [CrossRef]
- Vetere, A.; Schrader, W. Mass spectrometric coverage of complex mixtures: Exploring the carbon space of crude oil. ChemistrySelect 2017, 2, 849–853. [Google Scholar] [CrossRef]
- Vetere, A.; Profrock, D.; Schrader, W. Quantitative and Qualitative Analysis of Three Classes of Sulfur Compounds in Crude Oil. Angew. Chem. Int. Ed. 2017, 56, 10933–10937. [Google Scholar] [CrossRef]
- Palacio Lozano, D.C.; Gavard, R.; Arenas-Diaz, J.P.; Thomas, M.J.; Stranz, D.D.; Mejía-Ospino, E.; Guzman, A.; Spencer, S.E.F.; Rossell, D.; Barrow, M.P. Pushing the analytical limits: New insights into complex mixtures using mass spectra segments of constant ultrahigh resolving power. Chem. Sci. 2019, 10, 6966–6978. [Google Scholar] [CrossRef] [PubMed]
- Adlof, R. Separation of conjugated linoleic acid methyl esters by silver-ion high performance liquid chromatography in semi-preparative mode. J. Chromatogr. A 2004, 1033, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Momchilova, S.M.; Nikolova-Damyanova, B.M. Advances in silver ion chromatography for the analysis of fatty acids and triacylglycerols—2001 to 2011. Anal. Sci. 2012, 28, 837–844. [Google Scholar] [CrossRef]
- Türkan, A.; Kalay, Ş. Monitoring lipase-catalyzed methanolysis of sunflower oil by reversed-phase high-performance liquid chromatography: Elucidation of the mechanisms of lipases. J. Chromatogr. A 2006, 1127, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Holčapek, M.; Jandera, P.; Fischer, J.; Prokeš, B. Analytical monitoring of the production of biodiesel by high-performance liquid chromatography with various detection methods. J. Chromatogr. A 1999, 858, 13–31. [Google Scholar] [CrossRef]
- de Matos, T.S.; dos Santos, R.C.; de Souza, C.G.; de Carvalho, R.C.; de Andrade, D.F.; D’ávila, L.A. Determination of the Biodiesel Content on Biodiesel/Diesel Blends and Their Adulteration with Vegetable Oil by High-Performance Liquid Chromatography. Energy Fuels 2019, 33, 11310–11317. [Google Scholar] [CrossRef]
- Schmitt-Kopplin, P.; Englmann, M.; Rossello-Mora, R.; Schiewek, R.; Brockmann, K.J.; Benter, T.; Schmitz, O.J. Combining chip-ESI with APLI (cESILI) as a multimode source for analysis of complex mixtures with ultrahigh-resolution mass spectrometry. Anal. Bioanal. Chem. 2008, 391, 2803–2809. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cho, Y.J.; Na, J.G.; Nho, N.S.; Kim, S.; Kim, S. Application of Saturates, Aromatics, Resins, and Asphaltenes Crude Oil Fractionation for Detailed Chemical Characterization of Heavy Crude Oils by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry Equipped with Atmospheric Pressure Photoionization. Energy Fuels 2012, 26, 2558–2565. [Google Scholar] [CrossRef]
- Gaspar, A.; Zellermann, E.; Lababidi, S.; Reece, J.; Schrader, W. Impact of Different Ionization Methods on the Molecular Assignments of Asphaltenes by FT-ICR Mass Spectrometry. Anal. Chem. 2012, 84, 5257–5267. [Google Scholar] [CrossRef]
- Arboleda, P.H.; Dettman, H.D.; Lucy, C.A. Hydrocarbon Group Type Separation of Gas Oil Resins by High Performance Liquid Chromatography on Hyper-Cross-Linked Polystyrene Stationary Phase. Energy Fuels 2015, 29, 6686–6694. [Google Scholar] [CrossRef]
- Jiang, P.; Lucy, C.A. Coupling normal phase liquid chromatography with electrospray ionization mass spectrometry: Strategies and applications. Anal. Methods 2016, 8, 6478–6488. [Google Scholar] [CrossRef]
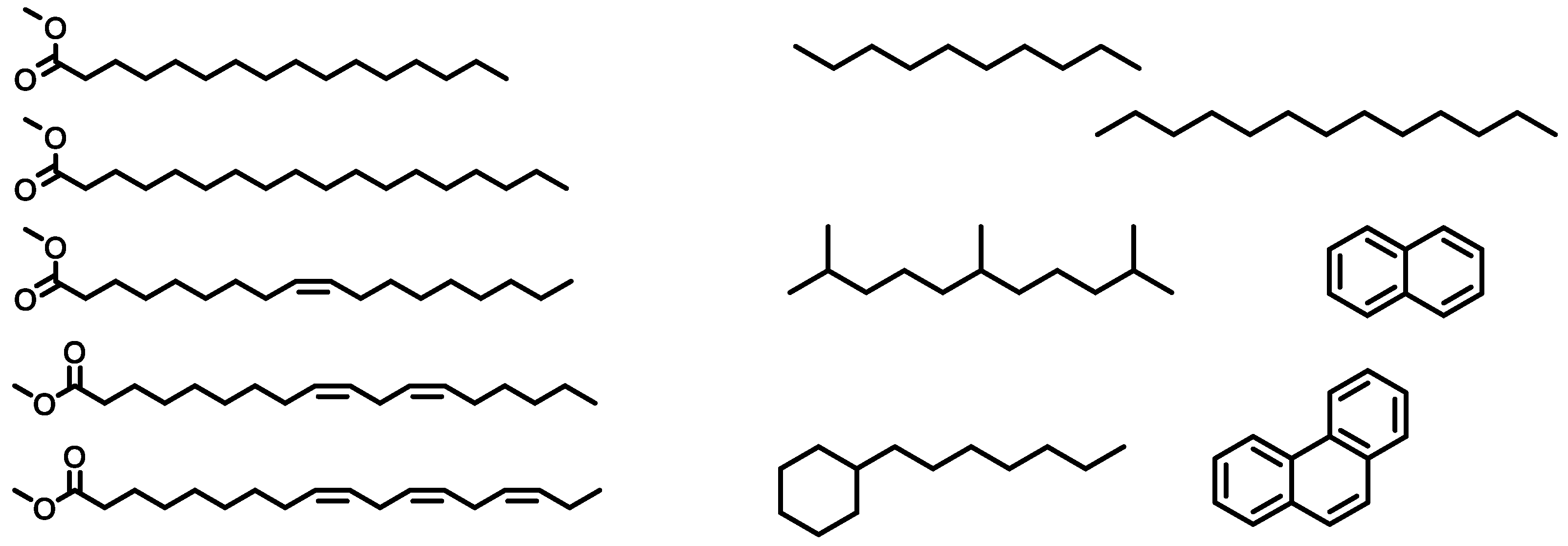
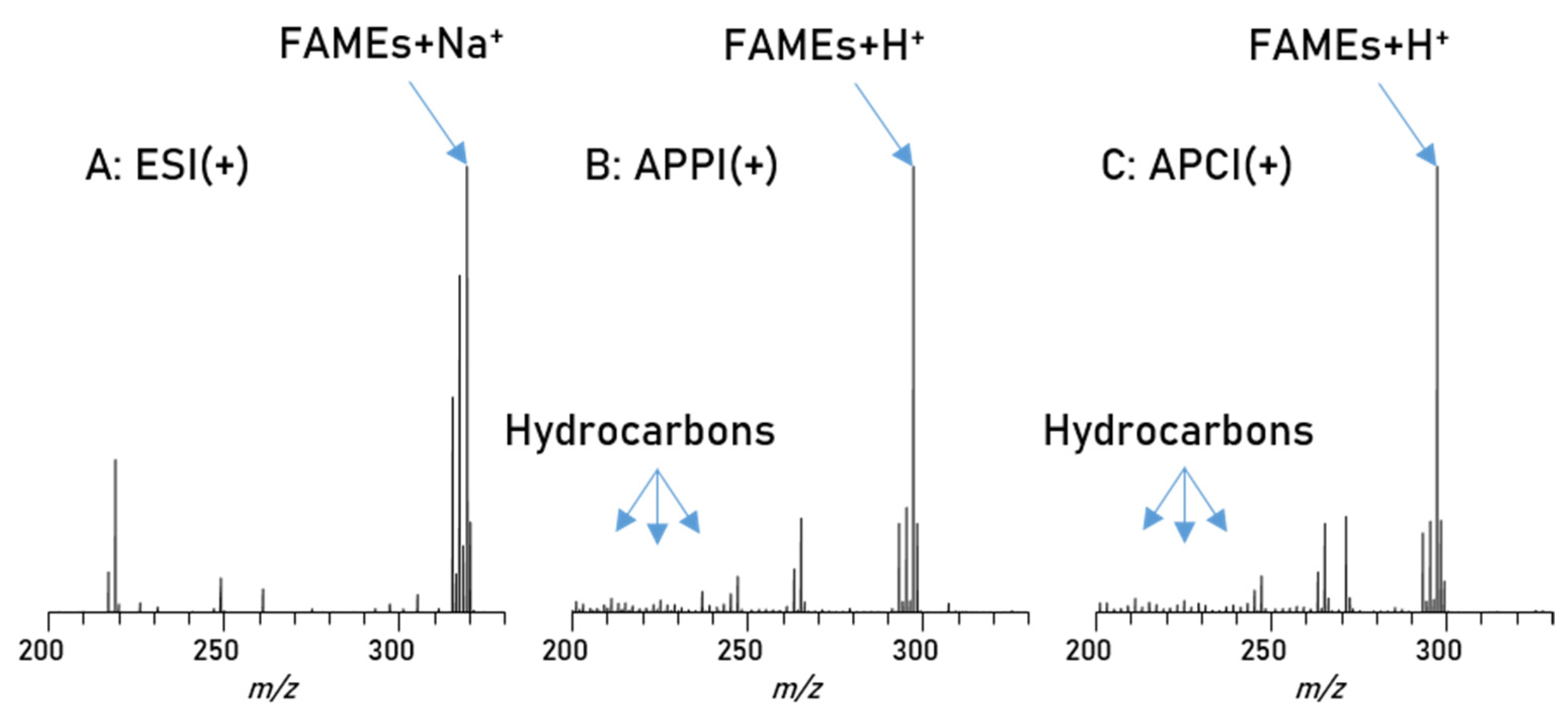
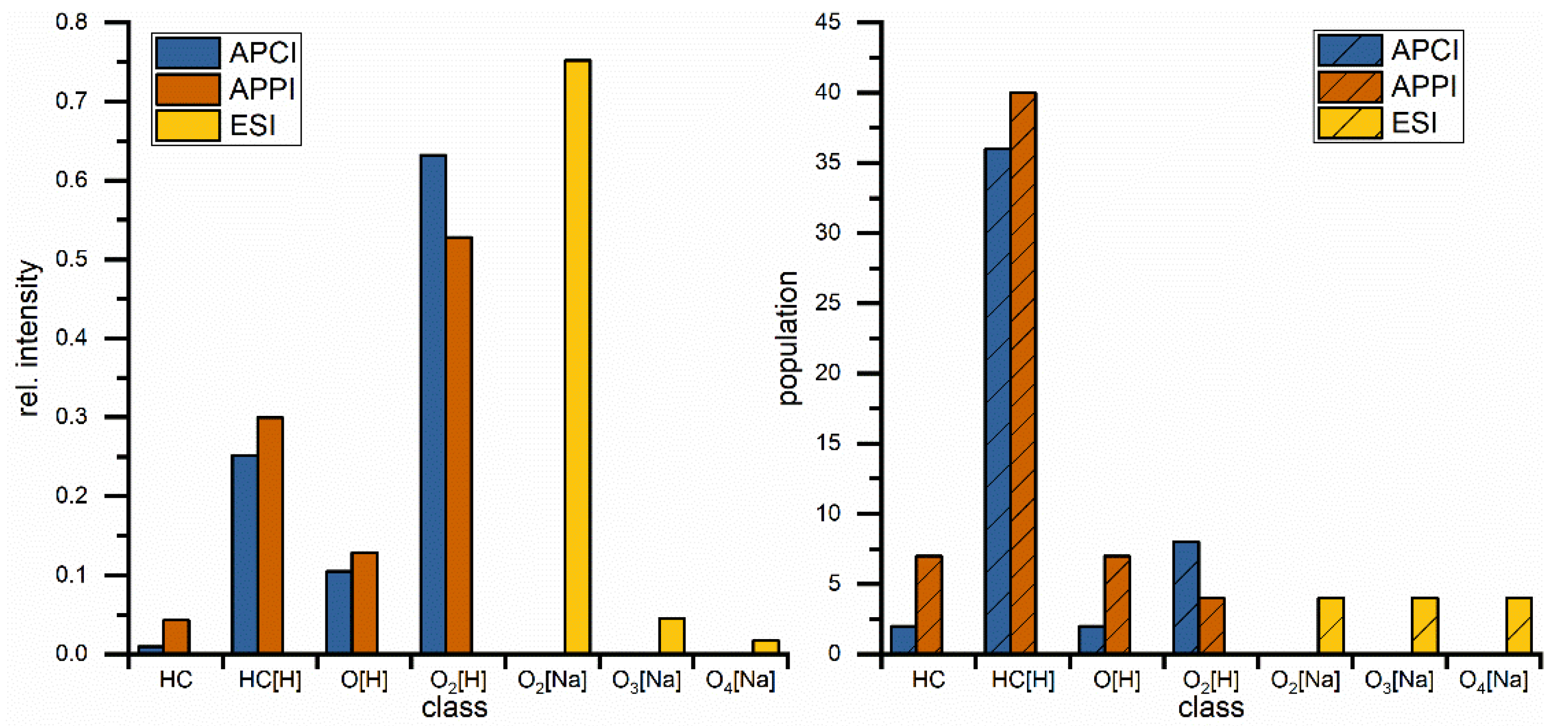
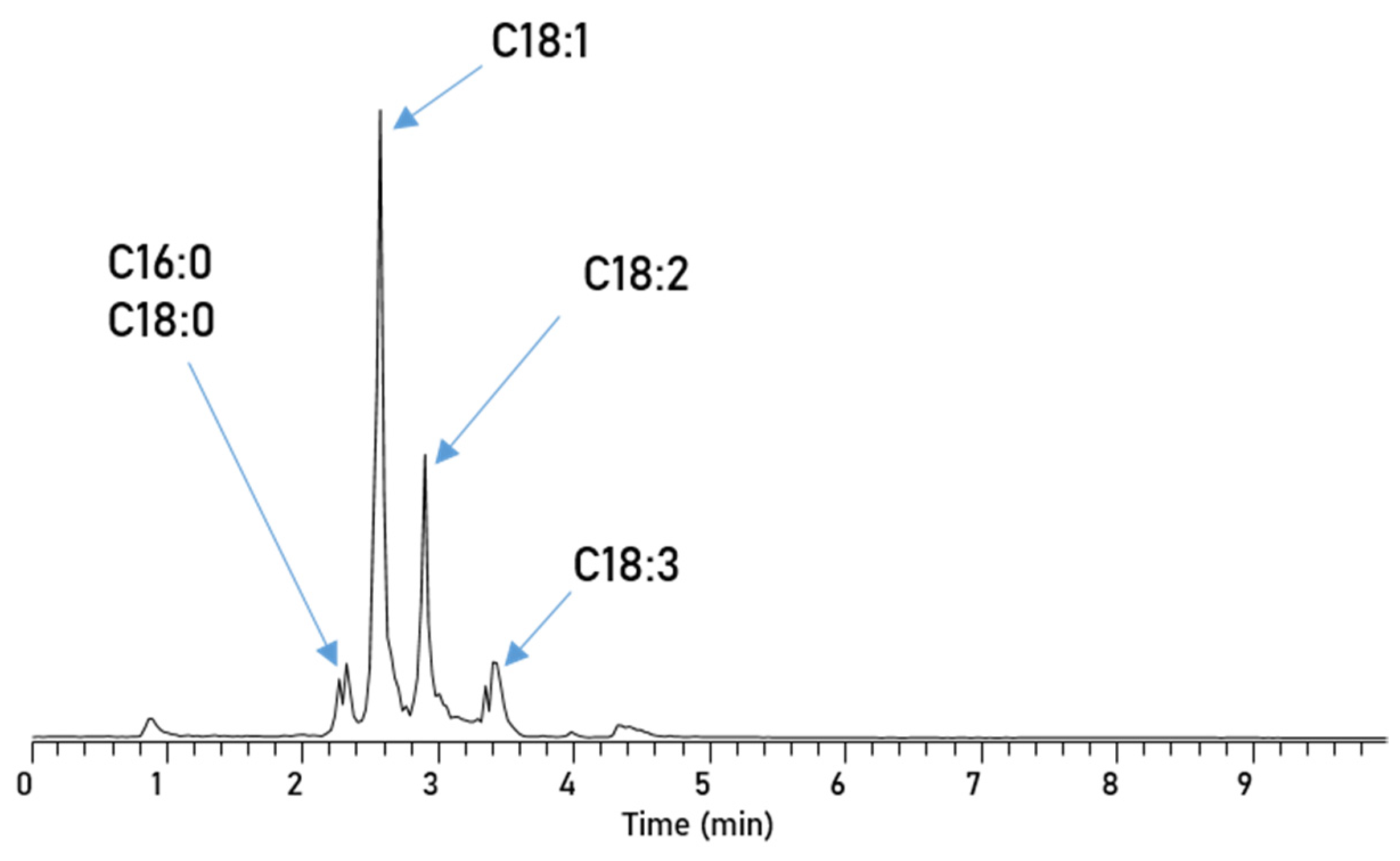
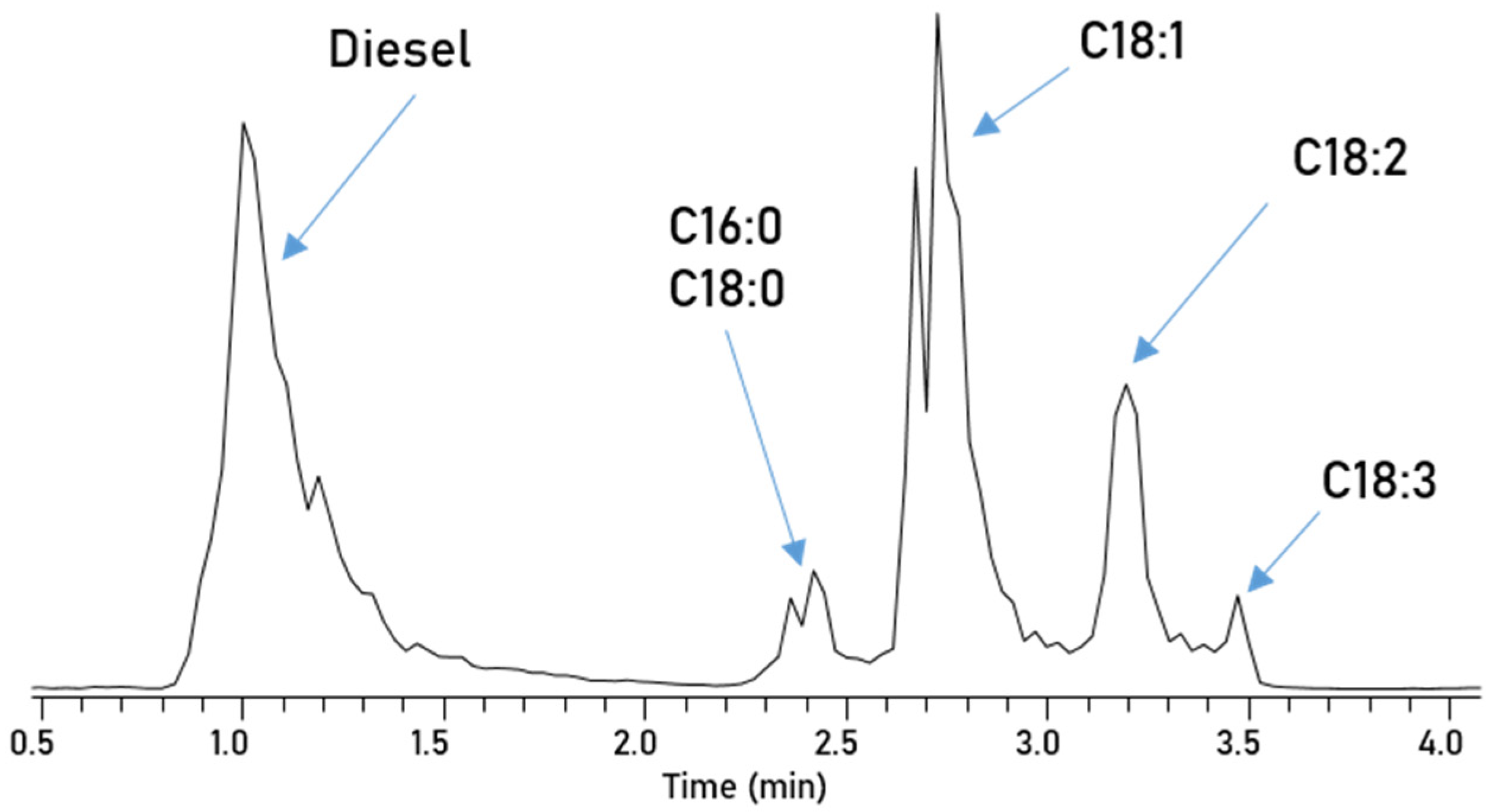
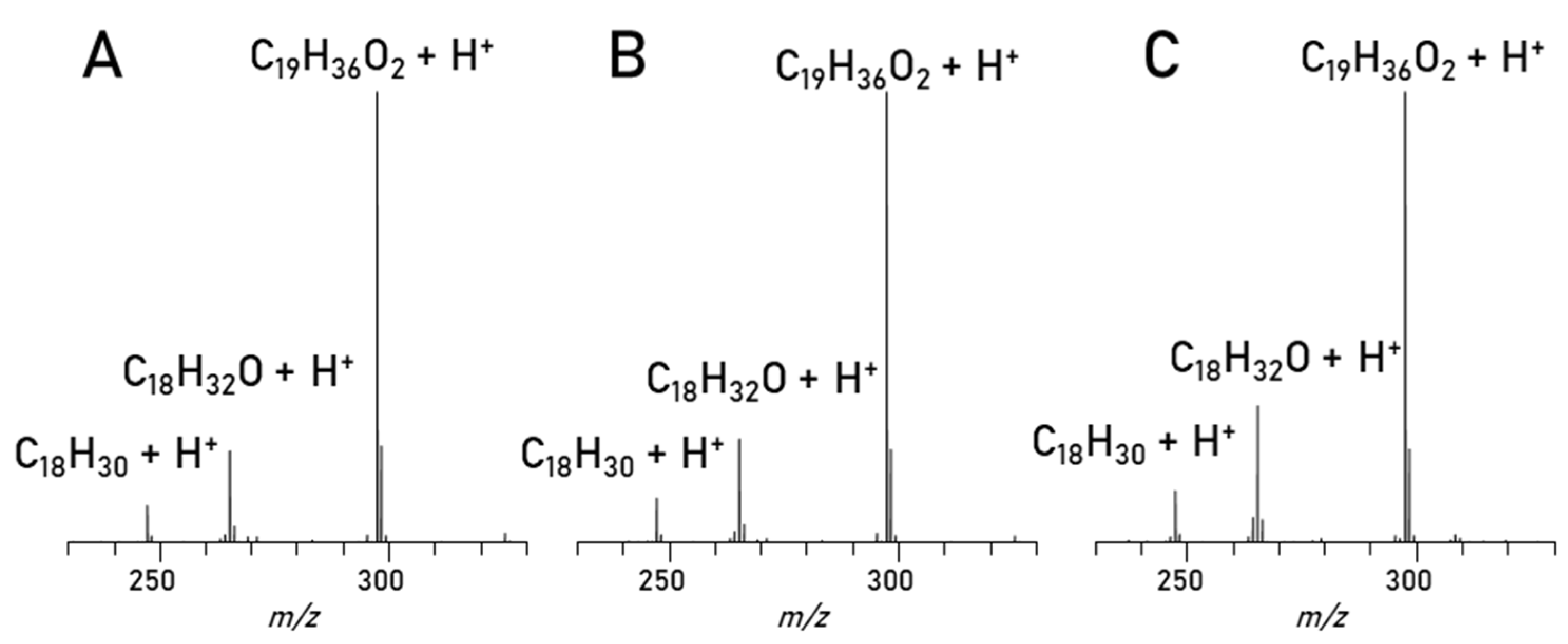
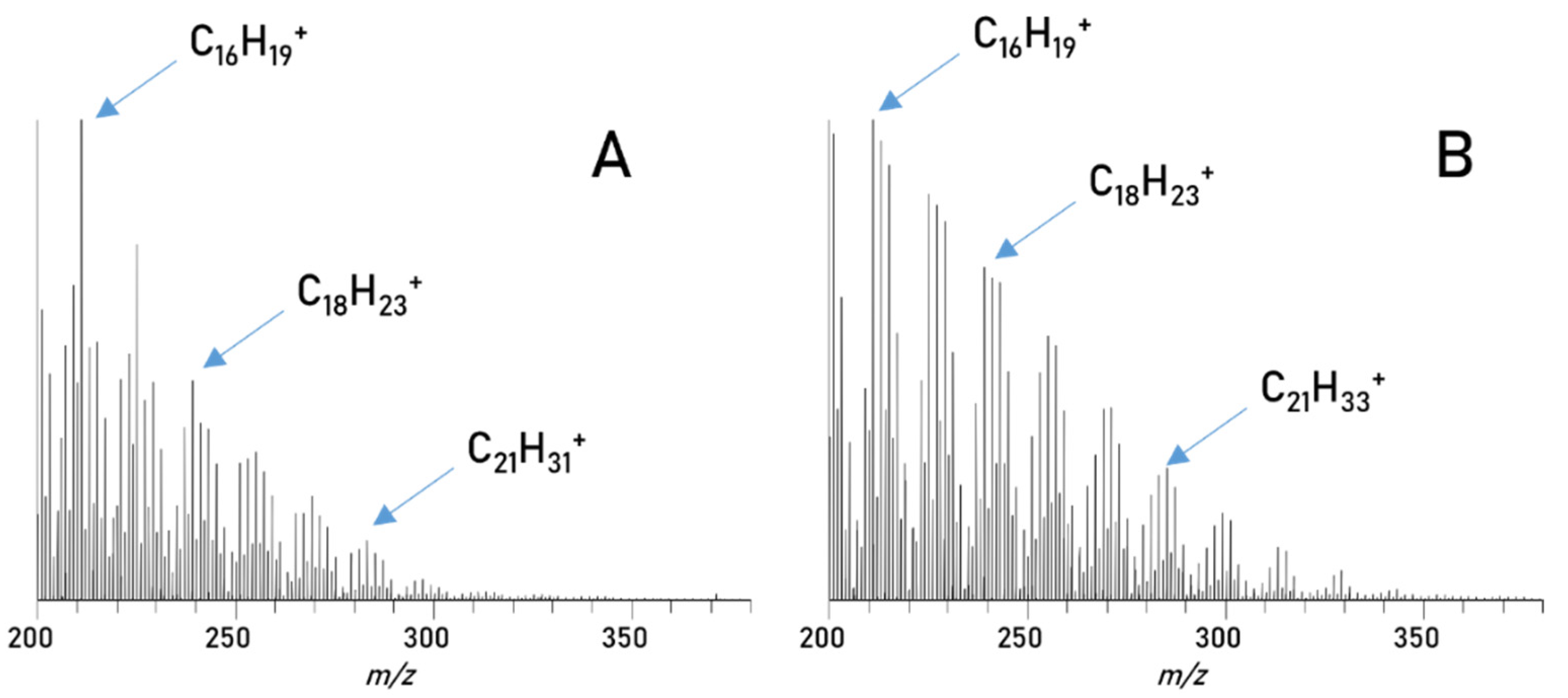
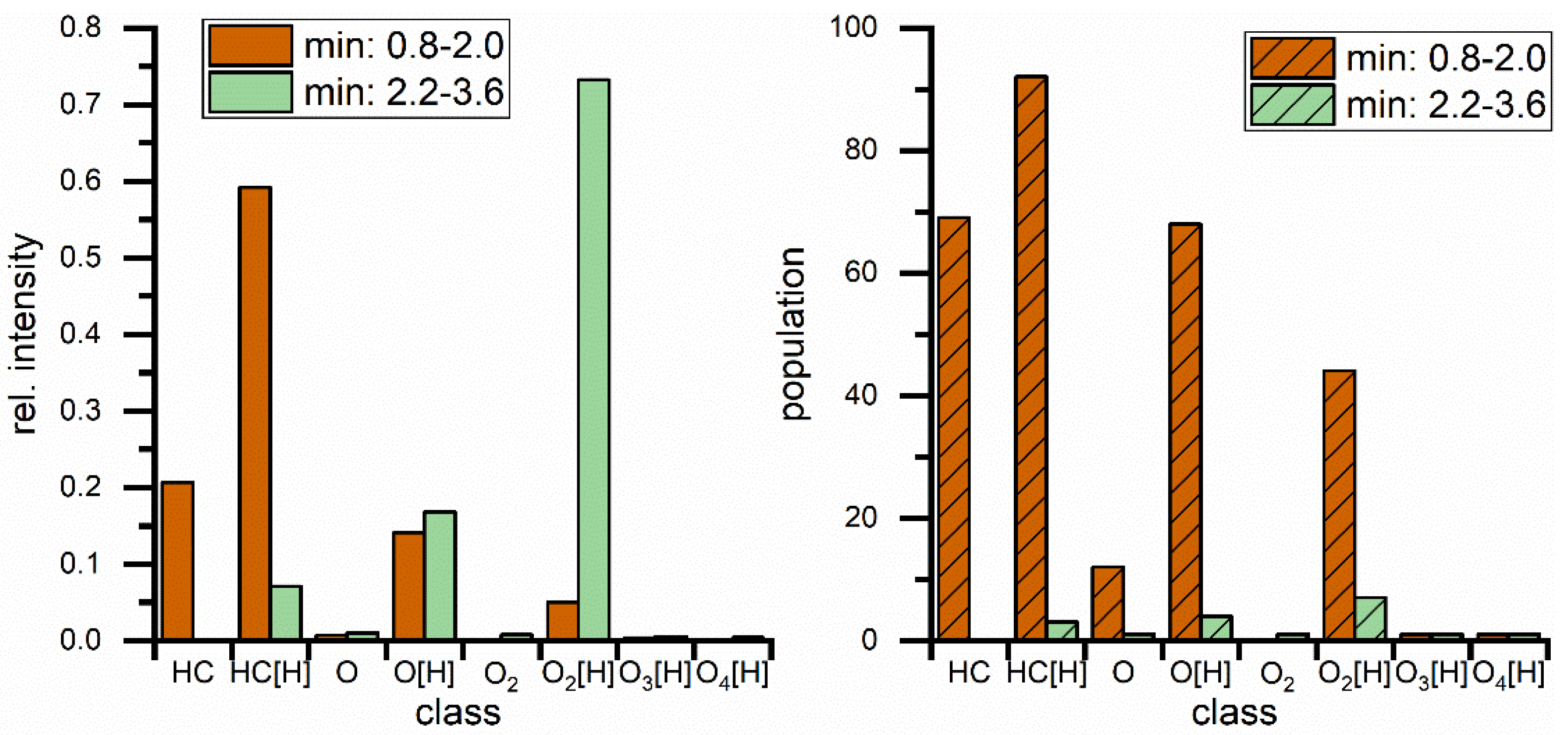
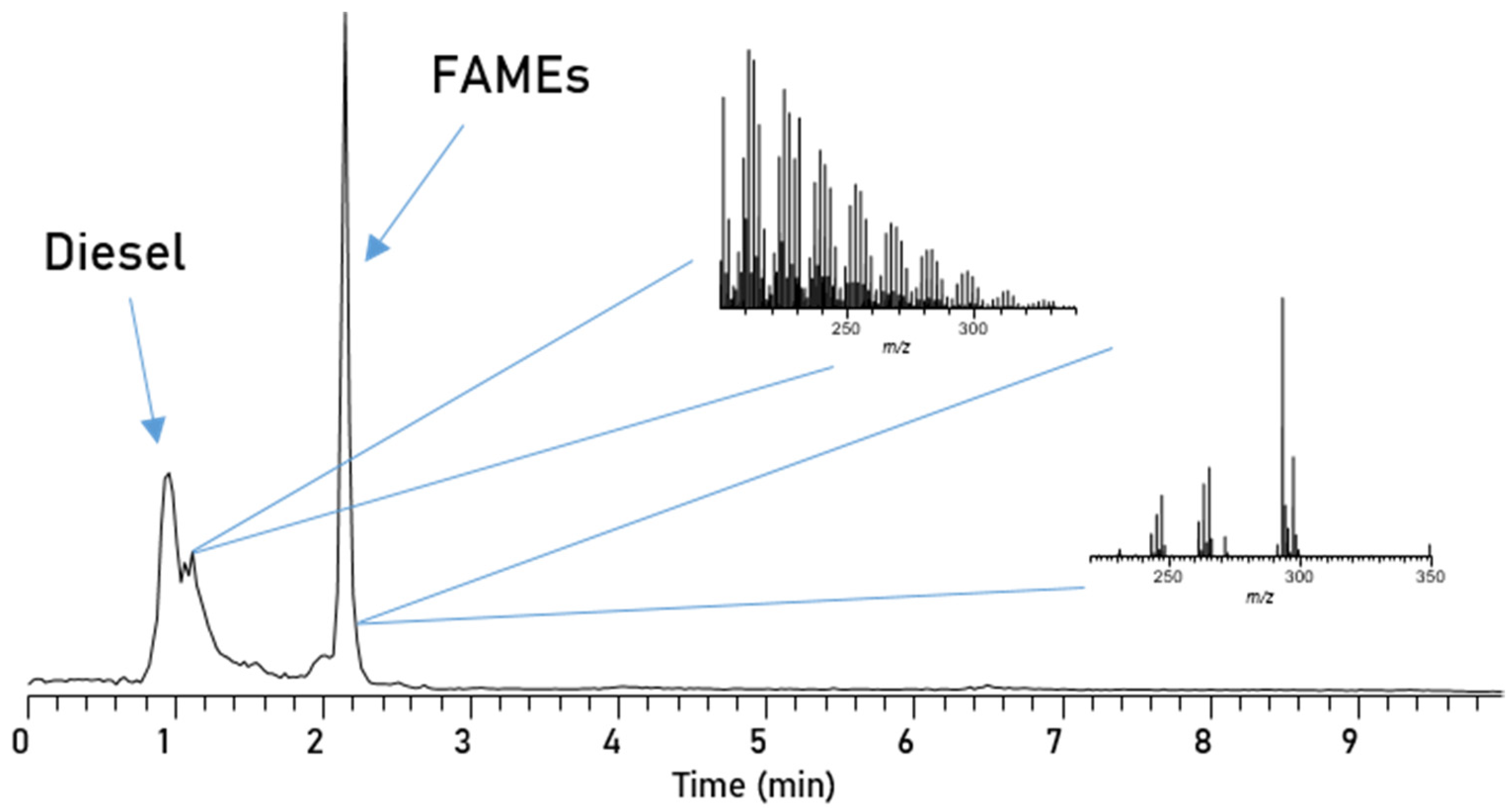
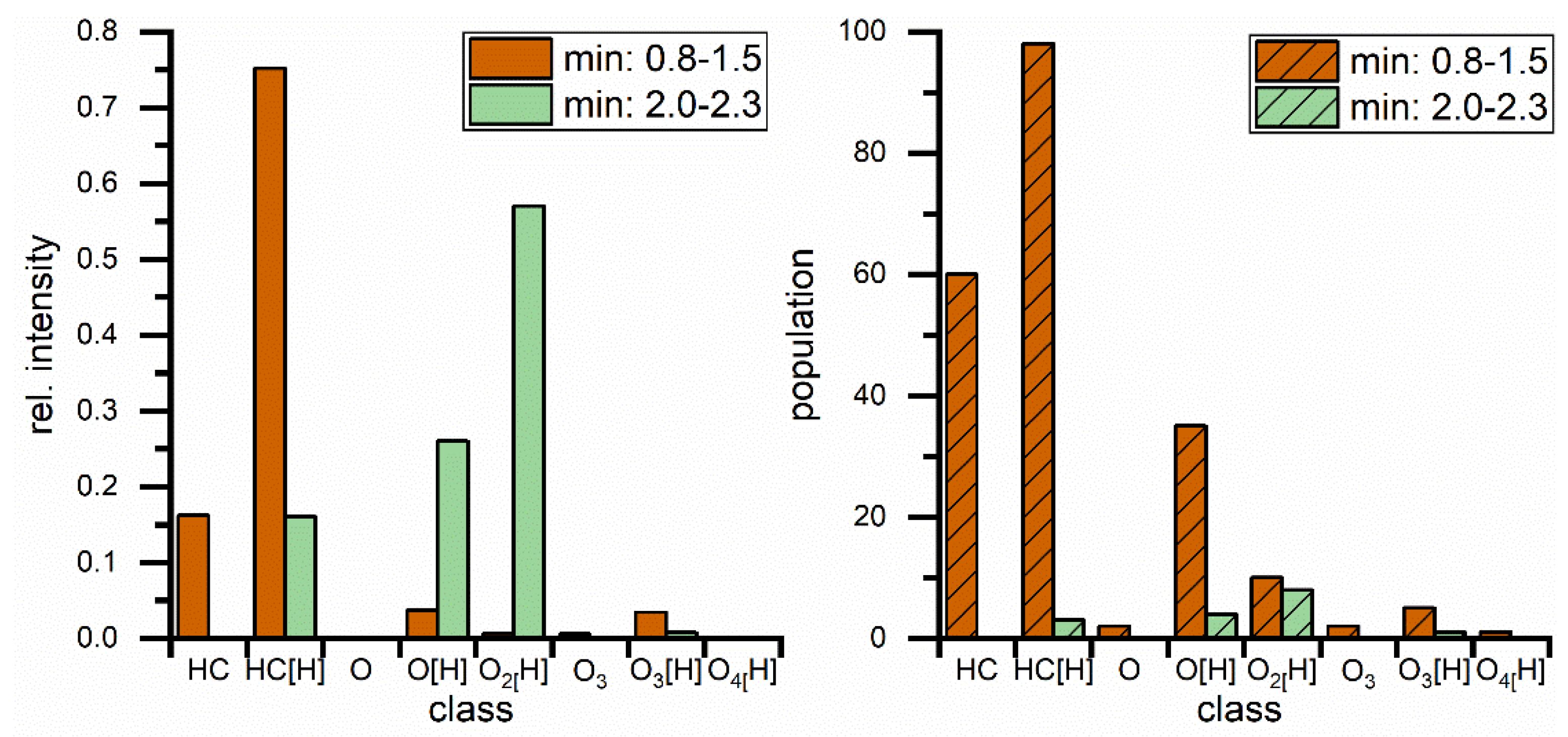
| Parameter | RME |
|---|---|
| Water content (mg/kg) | 264 |
| Ester content (%w/w) | |
| C16:0 | 4.1 |
| C18:0 | 1.8 |
| C18:1 | 56.2 |
| C18:2 | 19.7 |
| C18:3 | 10.0 |
| Method 1 | Method 2 | ||
|---|---|---|---|
| Time (min) | B (%) | Time (min) | B (%) |
| 0 | 0 | 0 | 0 |
| 1 | 0 | 1 | 20 |
| 1.5 | 10 | 8.5 | 20 |
| 5 | 30 | 9.5 | 0 |
| 8 | 30 | 19 | 0 |
| 10 | 0 | ||
| 29 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamacher, D.; Schrader, W. Development of a Novel HPLC-MS Method to Separate Polar and Non-Polar Compounds in Biodiesel/Petrodiesel Mixtures. Separations 2022, 9, 214. https://doi.org/10.3390/separations9080214
Hamacher D, Schrader W. Development of a Novel HPLC-MS Method to Separate Polar and Non-Polar Compounds in Biodiesel/Petrodiesel Mixtures. Separations. 2022; 9(8):214. https://doi.org/10.3390/separations9080214
Chicago/Turabian StyleHamacher, David, and Wolfgang Schrader. 2022. "Development of a Novel HPLC-MS Method to Separate Polar and Non-Polar Compounds in Biodiesel/Petrodiesel Mixtures" Separations 9, no. 8: 214. https://doi.org/10.3390/separations9080214
APA StyleHamacher, D., & Schrader, W. (2022). Development of a Novel HPLC-MS Method to Separate Polar and Non-Polar Compounds in Biodiesel/Petrodiesel Mixtures. Separations, 9(8), 214. https://doi.org/10.3390/separations9080214






