Synergistic Effect of NiO-Ga2O2-Graphene Heterostructures on Congo Red Photodegradation in Water
Abstract
:1. Introduction
2. Materials and Methods
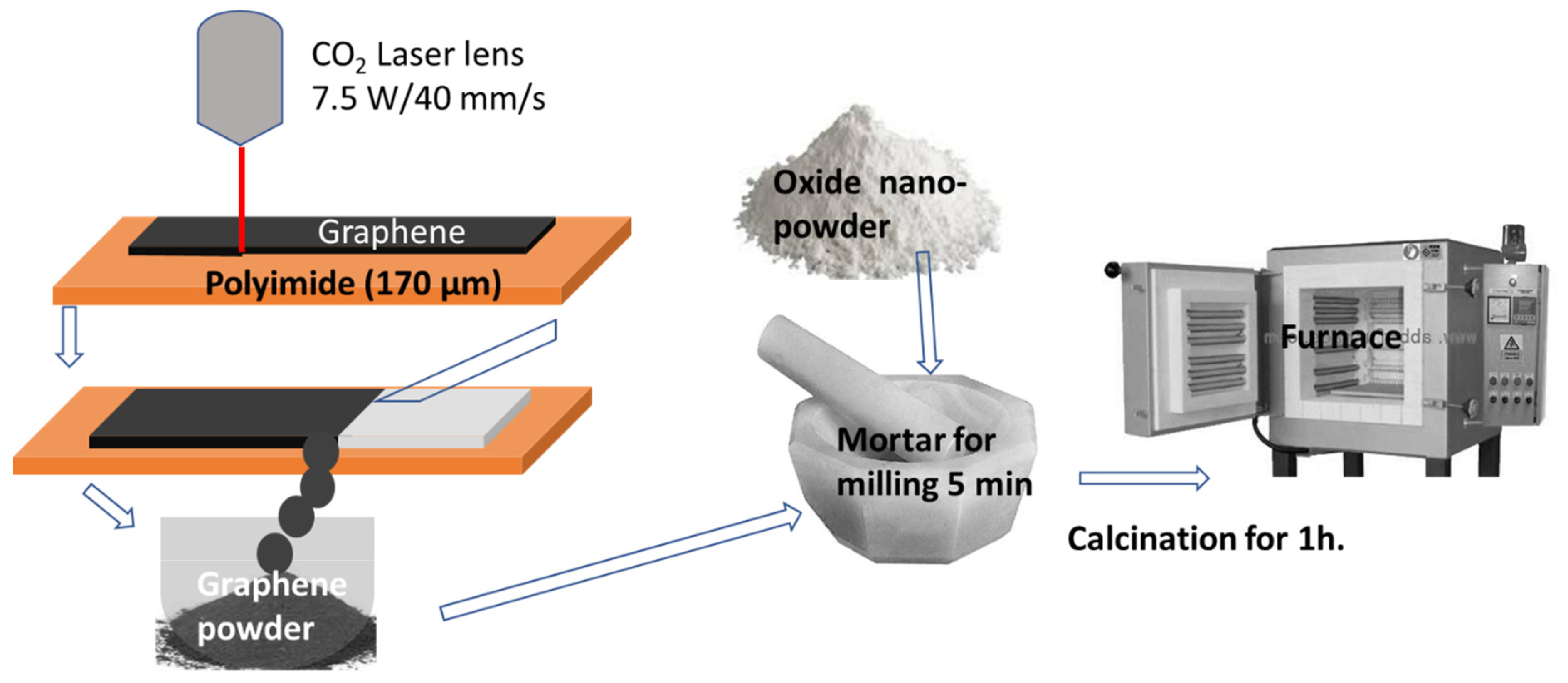
2.1. Materials Preparation
2.2. Characterizations
3. Results and Discussion
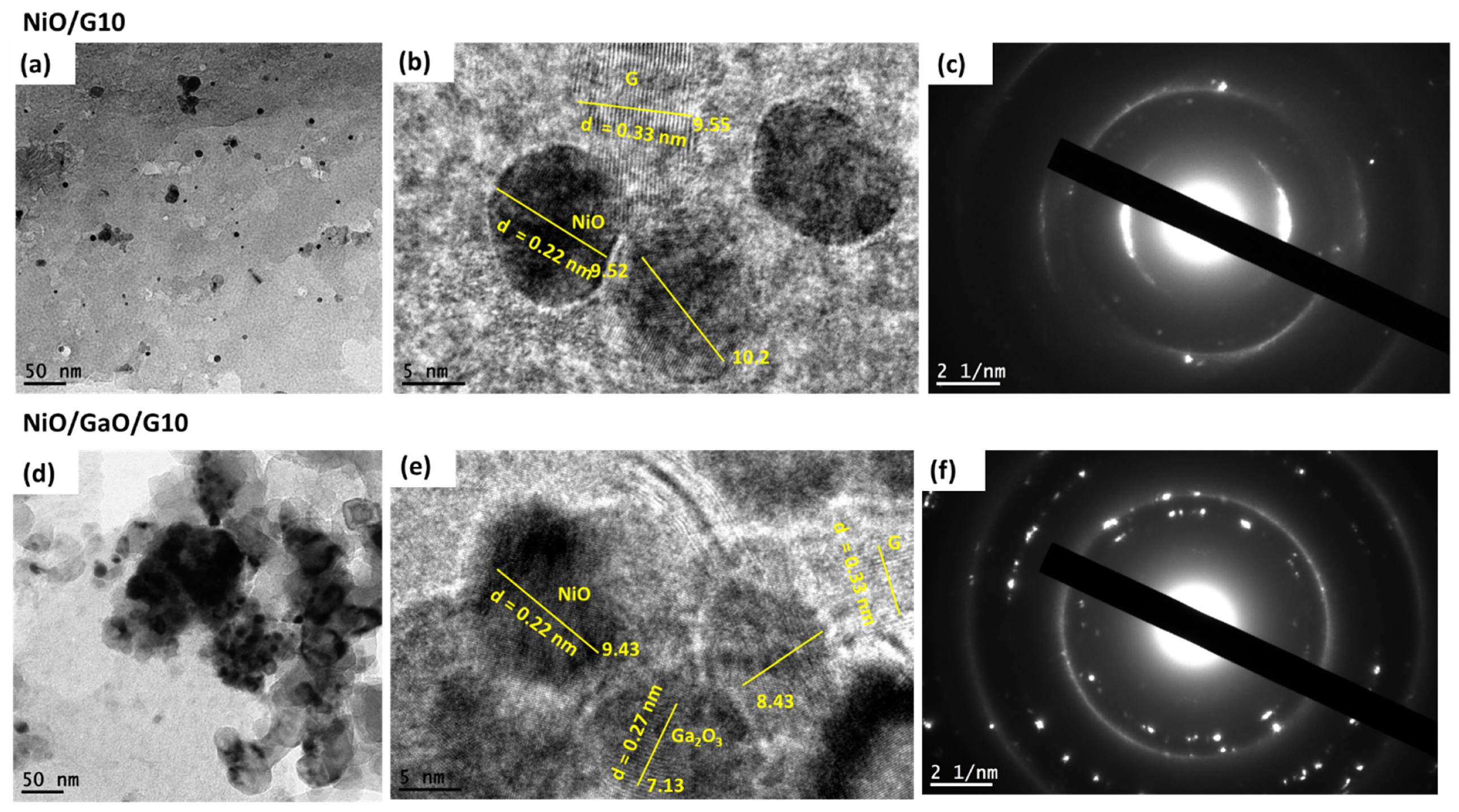
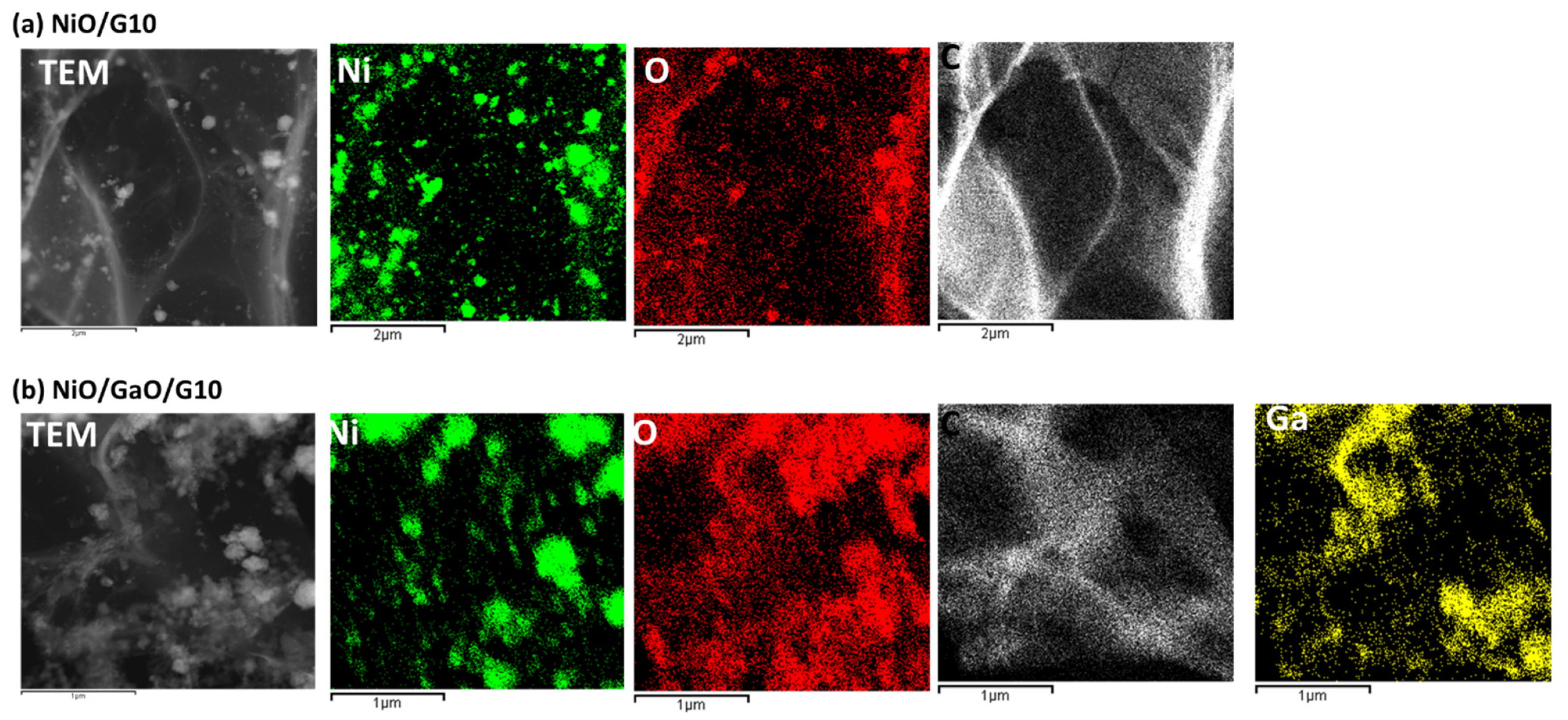
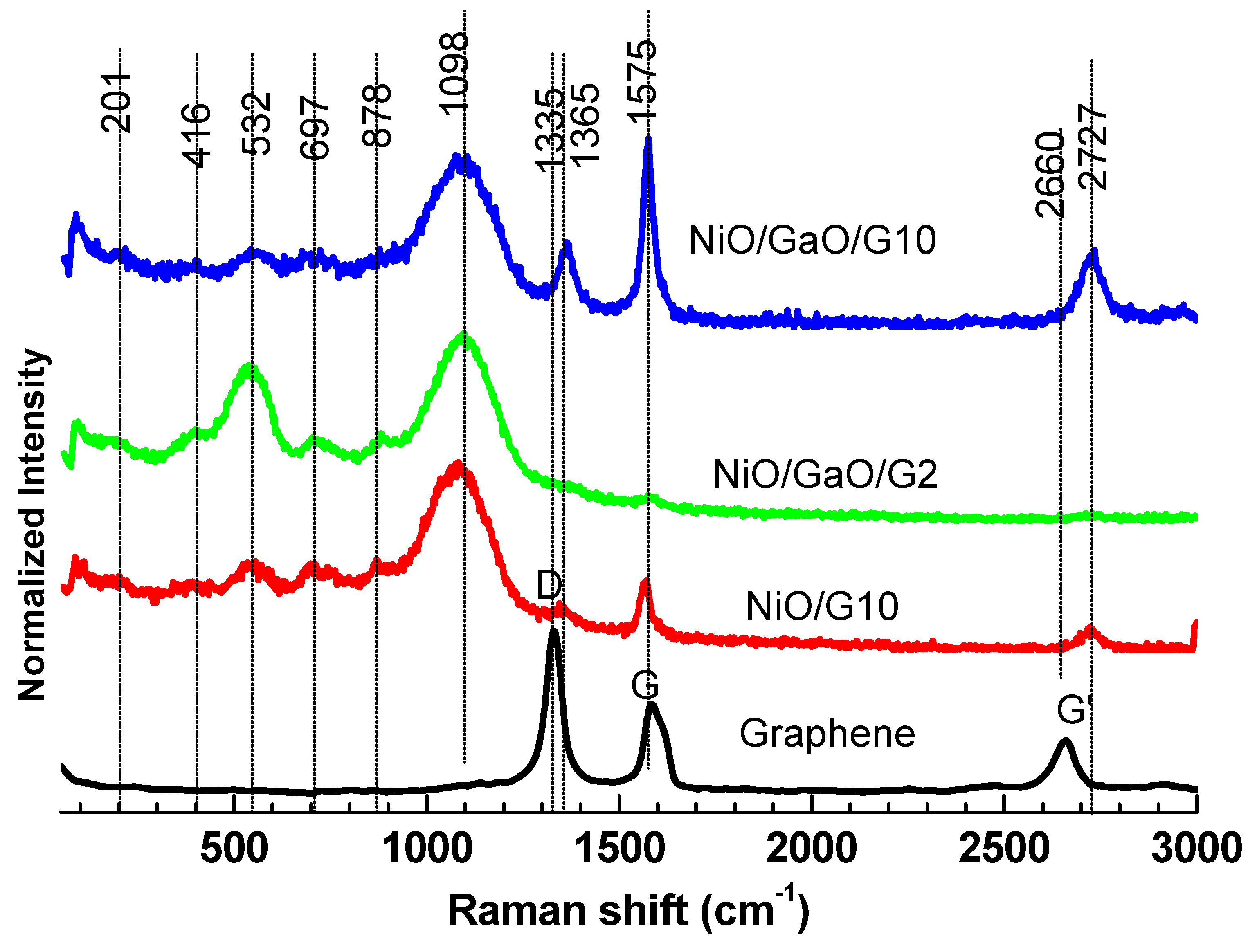
3.1. Structural Analysis
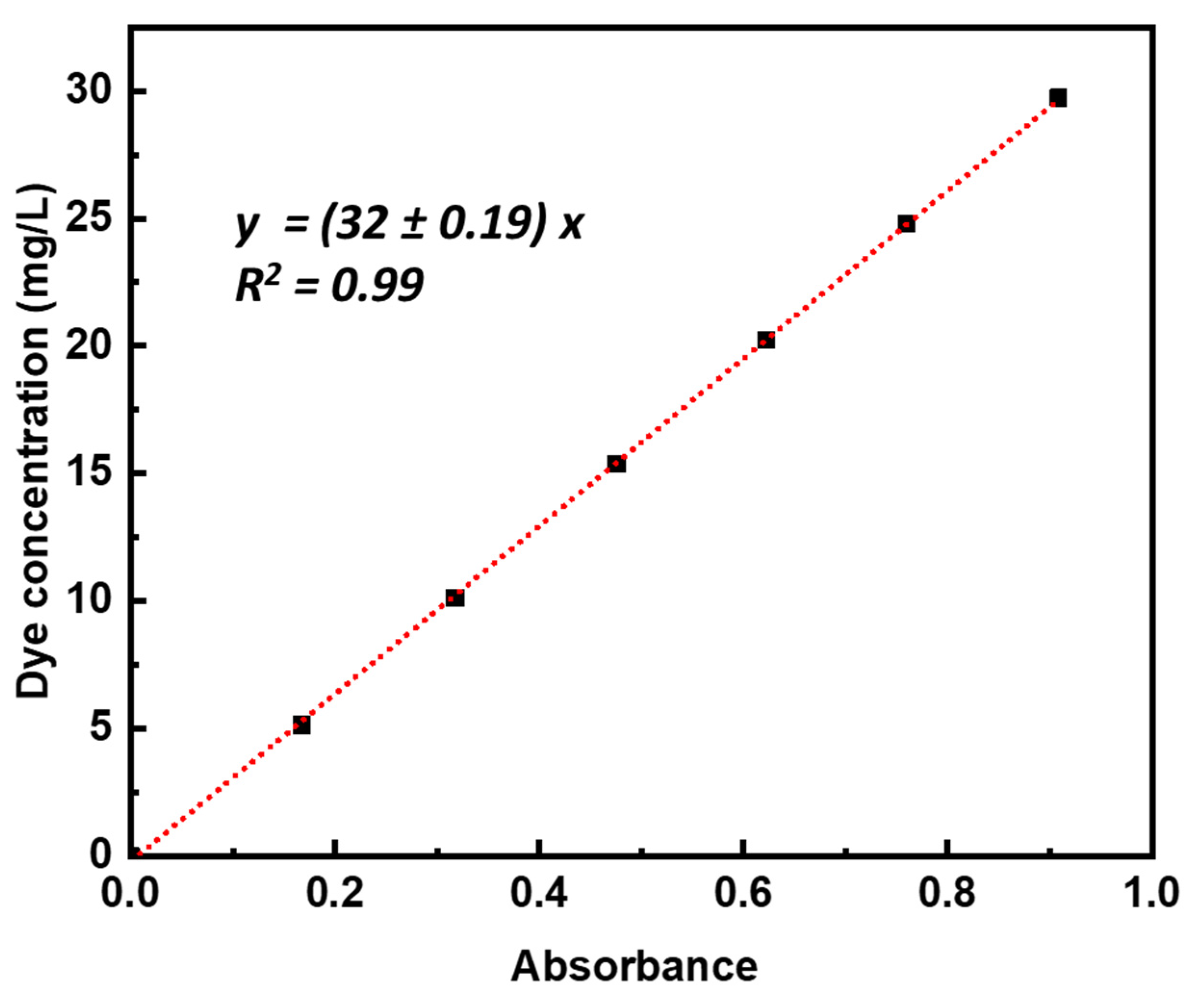
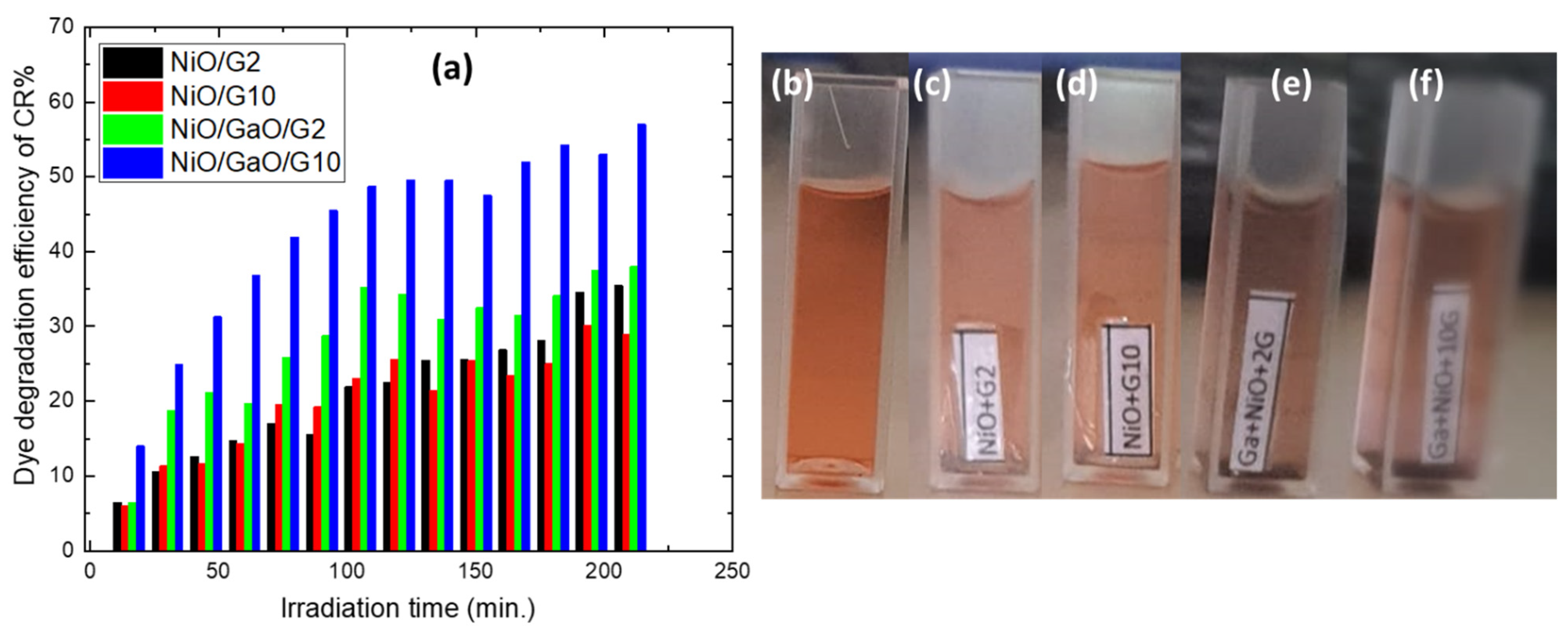
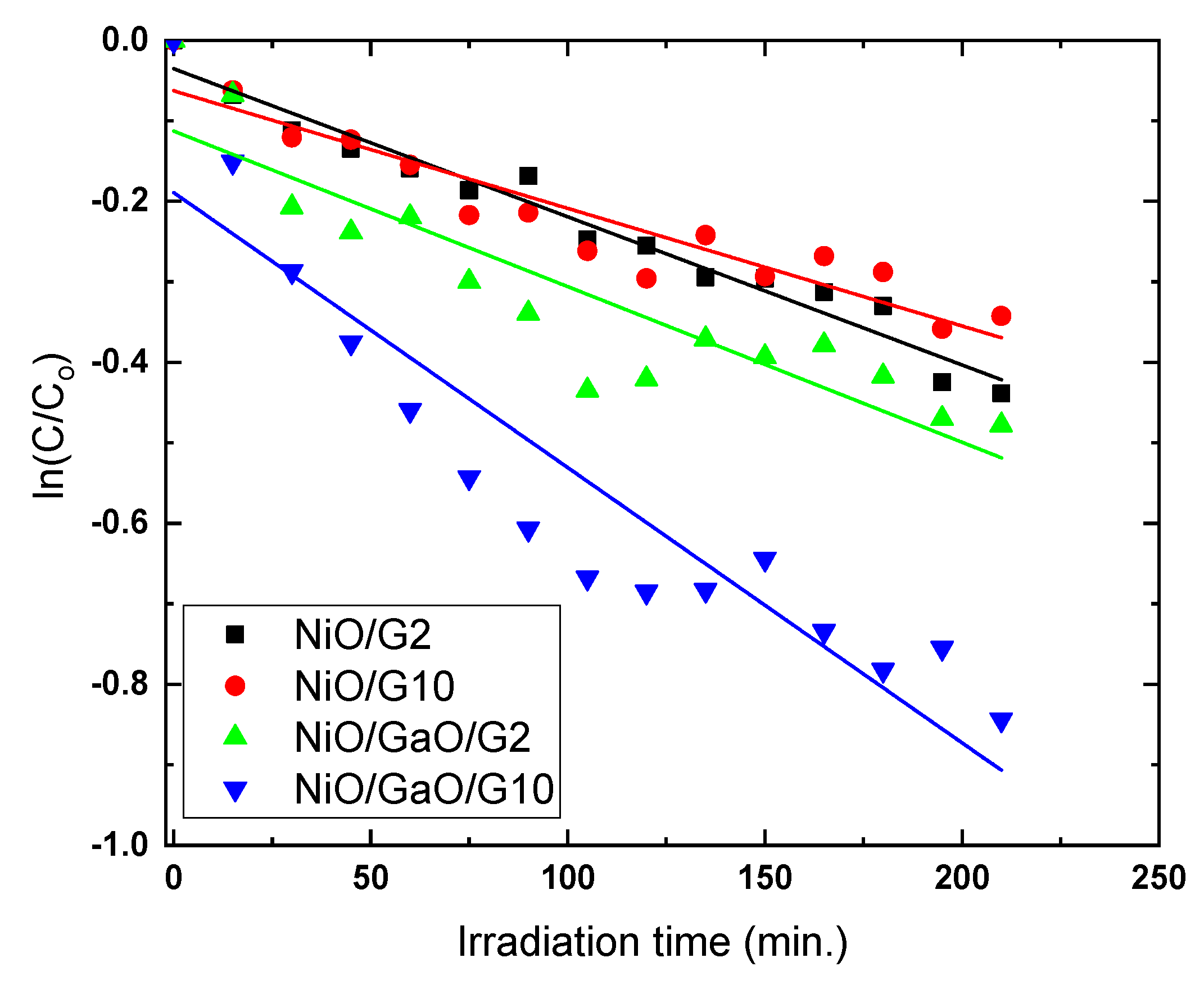
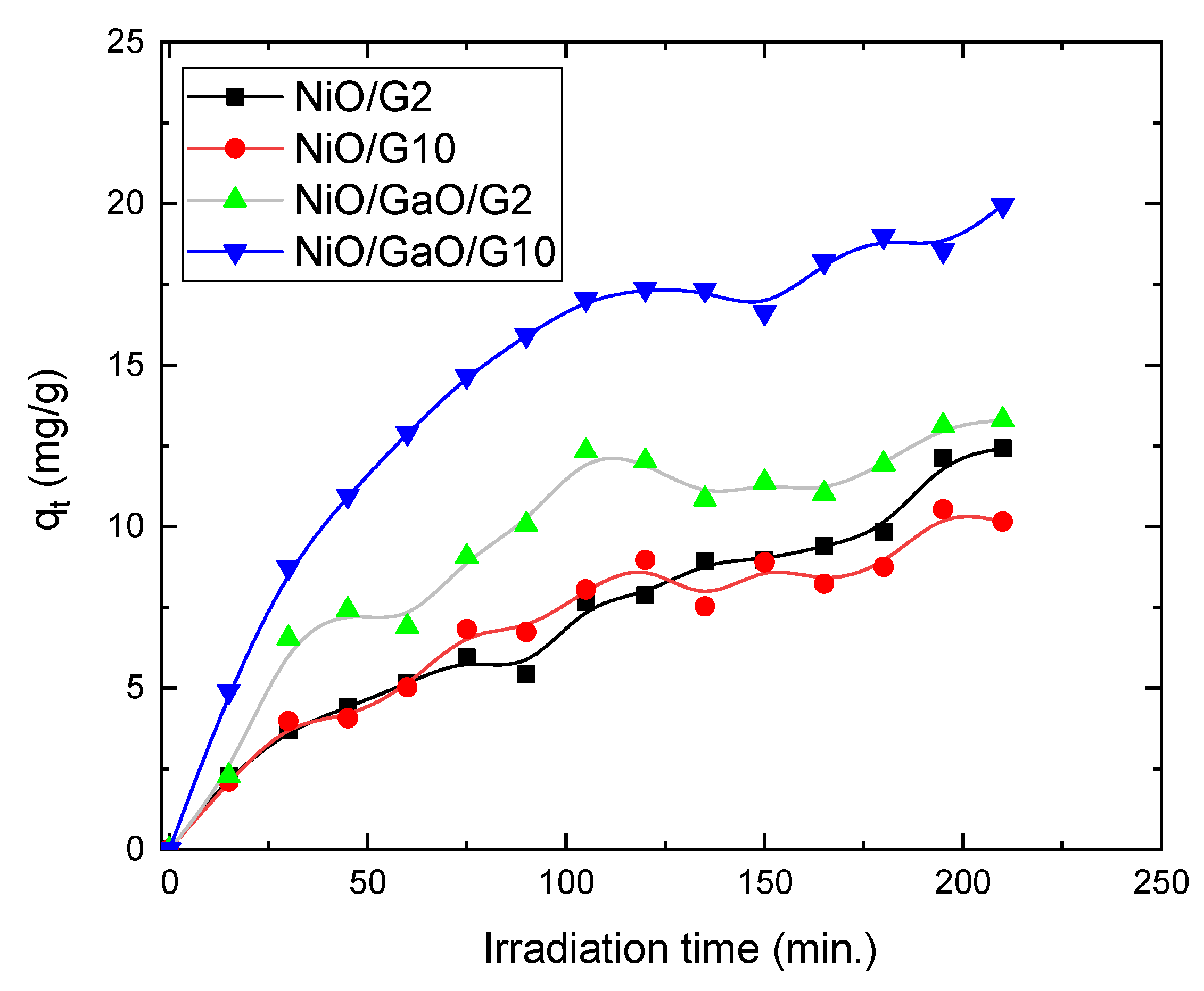
3.2. Photocatalytic Measurements
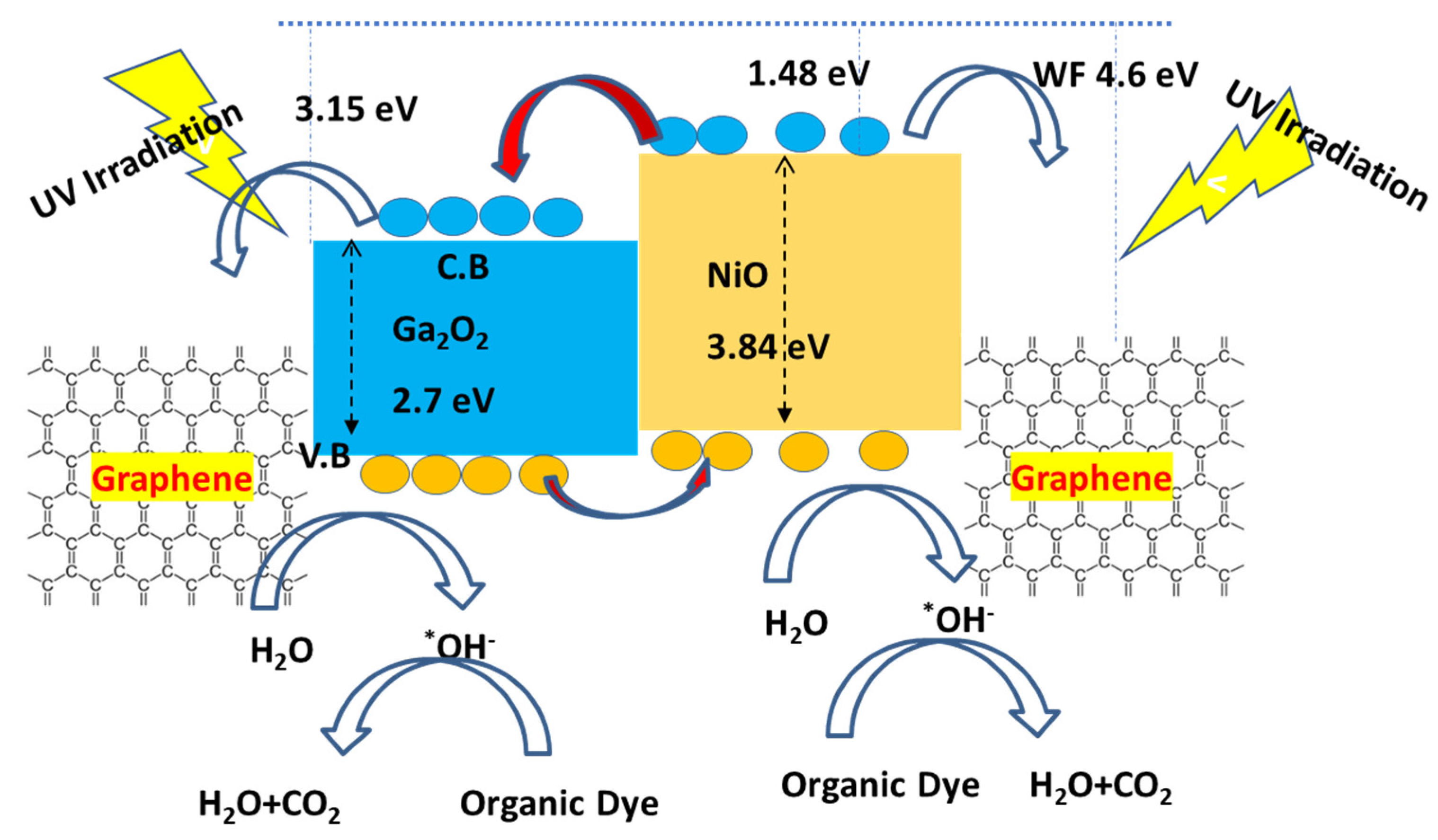
3.3. Photocatalytic Mechanism on Ternary Nanocomposite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raza, A.; Altaf, S.; Ali, S.; Ikram, M.; Gao, L. Recent advances in carbonaceous sustainable nanomaterials for wastewater treatments. Sustain. Mater. Technol. 2022, 32, e00406. [Google Scholar] [CrossRef]
- Rizzo, L.; Selcuk, H.; Nikolaou, A.D.; Pagano, S.M.; Belgiorno, V. A comparative evaluation of ozonation and heterogeneous photocatalytic oxidation processes for reuse of secondary treated urban wastewater. Desalin. Water Treat 2014, 52, 1414–1421. [Google Scholar] [CrossRef]
- Jamal, N.; Radhakrishnan, A.; Raghavan, R.; Bhaskaran, B.; Access, O. Efficient photocatalytic degradation of organic dye from aqueous solutions over zinc oxide incorporated nanocellulose under visible light irradiation. Main Group Met. Chem. 2020, 43, 84–91. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Wang, M.; Zhang, X.; Liao, L.; Fang, T.; Li, B. Photocatalytic degradation of Congo red by using the Cu2O/α-Fe2O3. Compos. Catal. 2021, 215, 222–231. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Li, J.; Li, L.; Lin, S. First-principles study on transition metal-doped anatase TiO2. Nanoscale Res. Lett. 2014, 9, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwish, A.A.A.; Rashad, M.; L-Aoh, H.A.A. Methyl orange adsorption comparison on nanoparticles: Isotherm, kinetics, and thermodynamic studies. Dye. Pigment. 2019, 160, 563–571. [Google Scholar] [CrossRef]
- El-Deen, A.G.; El-Shafei, M.H.; Hessein, A.; Hassanin, A.H.; Shaalan, N.M.; El-Moneim, A.A. High-performance asymmetric supercapacitor based hierarchical NiCo2O4@ carbon nanofibers//Activated multichannel carbon nanofibers. Nanotechnology 2020, 31, 365404. [Google Scholar] [CrossRef]
- Li, Y.; Xie, W.; Hu, X.; Shen, G.; Zhou, X.; Xiang, Y.; Zhao, X.; Fang, P. Comparison of dye photodegradation and its coupling with light-to-electricity conversion over TiO2 and ZnO. Langmuir 2010, 26, 591–597. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Hamad, D.; Aljaafari, A.; Abdel-Latief, A.Y.; Abdel-Rahim, M.A. Preparation and Characterization of Developed CuxSn1−xO2 Nanocomposite and Its Promising Methane Gas Sensing Properties. Sensors 2019, 19, 2257. [Google Scholar] [CrossRef] [Green Version]
- De Jongh, P.E.; Meulenkamp, E.A.; Vanmaekelbergh, D.; Kelly, J.J. Charge carrier dynamics in illuminated, particulate ZnO electrodes. J. Phys. Chem. B 2000, 104, 7686–7693. [Google Scholar] [CrossRef]
- Cui, Y.; Lieber, C.M. Functional nanoscale electronic devices assembled using silicon nanowire building blocks. Science 2001, 291, 851–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S.; Mondal, S.; Maity, J.; Mukherjee, R. Synthesis and characterization of ZnO nanoparticles using Moringa oleifera leaf extract: Investigation of photocatalytic and antibacterial activity. Int. J. Nanosci. Nanotechnol. 2018, 14, 111–119. [Google Scholar]
- Kotan, G.; Kardaş, F.; Yokuş, Ö.A.; Akyıldırım, O.; Saral, H.; Eren, T.; Yola, M.L.; Atar, N. A novel determination of curcumin via Ru@ Au nanoparticle decorated nitrogen and sulfur-functionalized reduced graphene oxide nanomaterials. Anal. Methods 2016, 8, 401–408. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N.; Eren, T.; Karimi-Maleh, H.; Wang, S. Correction: Sensitive and selective determination of aqueous triclosan based on gold nanoparticles on polyoxometalate/reduced graphene oxide nanohybrid. RSC Adv. 2015, 5, 72590–72591. [Google Scholar] [CrossRef] [Green Version]
- Atar, N.; Eren, T.; Yola, M.L.; Gerengi, H.; Wang, S. Fe@ Ag nanoparticles decorated reduced graphene oxide as ultrahigh capacity anode material for lithium-ion battery. Ionics 2015, 21, 3185–3192. [Google Scholar] [CrossRef]
- Atar, N.; Eren, T.; Yola, M.L. Ultrahigh capacity anode material for lithium ion battery based on rod gold nanoparticles decorated reduced graphene oxide. Thin Solid Film. 2015, 590, 156–162. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Kalaivani, S.; Joice, J.A.I.; Sivakumar, T. Photocatalytic activity of multielement doped TiO2 in the degradation of congo red. Appl. Surf. Sci. 2012, 258, 2515–2521. [Google Scholar] [CrossRef]
- Raza, A.; Zhang, X.; Ali, S.; Cao, C.; Rafi, A.A.; Li, G. Photoelectrochemical Energy Conversion over 2D Materials. Photochem 2022, 2, 272–298. [Google Scholar] [CrossRef]
- Rong, J.; Zhang, T.; Qiu, F.; Rong, X.; Zhu, X.; Zhang, X. Preparation of hierarchical micro/nanostructured Bi2S3-WO3 composites for enhanced photocatalytic performance. J. Alloy Compd. 2016, 685, 812–819. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Gong, Z.; Tao, J.; Sun, Z.; Lv, J.; Chen, X.; Jiang, X.; He, G.; Wang, P.; et al. Enhanced charge collection and photocatalysis performance of CdS and PbS nanoclusters co-sensitized TiO2 porous film. J. Alloy Compd. 2015, 649, 190–195. [Google Scholar] [CrossRef]
- Cui, W.; An, W.; Liu, L.; Hu, J.; Liang, Y. Novel PbS quantum dots sensitized flower-like BiOBr with enhanced photocatalytic properties under visible light. Mater. Lett. 2014, 132, 251–254. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, R.T.; Hong, L.F.; Ji, X.Y.; Lin, Z.D.; Li, Z.S.; Pan, W.G. A review of metal oxide-based Z-scheme heterojunction photocatalysts: Actualities and developments. Mater. Today Energy 2021, 21, 100829. [Google Scholar] [CrossRef]
- Li, F.; Cheng, L.; Xiang, J.F. Steering the behavior of photogenerated carriers in semiconductor photocatalysts: A new insight and perspective. J. Mater. Chem. A 2021, 9, 23765. [Google Scholar] [CrossRef]
- Cheng, L.; Li, B.; Yin, H.; Fan, J.; Xiang, Q. Cu clusters immobilized on Cd-defective cadmium sulfide nano-rods towards photocatalytic CO2 reduction. J. Mater. Sci. Technol. 2022, 118, 54. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Rashad, M.; Abdel-Rahim, M.A. CuO nanoparticles synthesized by microwave-assisted method for methane sensing. Opt. Quantum Electron. 2016, 48, 2–12. [Google Scholar] [CrossRef]
- Rashad, M.; Al-Ghamdi, S.A.; Alzahrani, A.O.M.; Al-Tabaa, K.; Al-Osemi, S.; Al-Atawi, O.; Al-Anzi, N.; Issa, S.A.M.; Abd-Elnaiem, A.M. Zinc oxide nanoparticles for adsorption of potassium permanganate from wastewater using shaking method. Desalin. Water Treat. 2021, 229, 227–234. [Google Scholar] [CrossRef]
- Rashad, M.; L-Aoh, H.A.A. Promising adsorption studies of bromophenol blue using copper oxide nanoparticles. Desalin. Water Treat. 2019, 139, 360–368. [Google Scholar] [CrossRef]
- Ponnusamy, P.M.; Agilan, S.; Muthukumarasamy, N.; Velauthapillai, D. Effect of Chromium and Cobalt Addition on Structural, Optical and Magnetic Properties of NiO Nanoparticles. Z. Fur. Phys. Chemie 2016, 230, 1185–1197. [Google Scholar] [CrossRef]
- Matassa, R.; Orlanducci, S.; Tamburri, E.; Guglielmotti, V.; Sordi, D.; Terranova, M.L.; Passeri, D.; Rossi, M. Characterization of carbon structures produced by graphene self-assembly. J. Appl. Crystallogr. 2014, 47, 222–227. [Google Scholar] [CrossRef]
- Azammi, A.M.N.; Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Atikah, M.S.N.; Asrofi, M.; Atiqah, A. 3—Characterization studies of biopolymeric matrix and cellulose fibres based composites related to functionalized fibre-matrix interface. In Woodhead Publishing Series in Composites Science and Engineering; Goh, K.L., Aswathi, M.K., De Silva, R.T., Thomas, S., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 29–93. [Google Scholar] [CrossRef]
- Kaburagi, Y.; Yoshida, A.; Hishiyama, Y. Chapter 7—Raman Spectroscopy. In Materials Science and Engineering of Carbon; Inagaki, M., Kang, F., Eds.; Butterworth-Heinemann: Oxford, UK, 2016; pp. 125–152. [Google Scholar] [CrossRef]
- Lucchese, M.M.; Stavale, F.; Ferreira, E.H.M.; Vilani, C.; Moutinho, M.V.O.; Capaz, R.B.; Achete, C.A.; Jorio, A. Quantifying ion-induced defects and Raman relaxation length in graphene. Carbon N. Y. 2010, 48, 1592–1597. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Jorio, A.; Saito, R. Raman spectroscopy for carbon nanotube applications. J. Appl. Phys. 2021, 129, 21102. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaalan, N.M.; Ahmed, F.; Kumar, S.; Melaibari, A.; Hasan, P.M.Z.; Aljaafari, A. Monitoring Food Spoilage Based on a Defect-Induced Multiwall Carbon Nanotube Sensor at Room Temperature: Preventing Food Waste. ACS Omega 2020, 5, 30531–30537. [Google Scholar] [CrossRef] [PubMed]
- Mironova-Ulmane, N.; Kuzmin, A.; Sildos, I.; Pärs, M. Polarisation dependent Raman study of single-crystal nickel oxide. Cent. Eur. J. Phys. 2011, 9, 1096–1099. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Steins, I.; Grabis, J.; Sildos, I.; Pärs, M. Raman scattering in nanosized nickel oxide NiO. J. Phys. Conf. Ser. 2007, 93, 012039. [Google Scholar] [CrossRef]
- Rao, R.; Rao, A.M.; Xu, B.; Dong, J.; Sharma, S.; Sunkara, M.K. Blueshifted Raman scattering and its correlation with the [110] growth direction in gallium oxide nanowires. J. Appl. Phys. 2005, 98, 094312. [Google Scholar] [CrossRef]
- Dohy, D.; Lucazeau, G.; Revcolevschi, A. Raman spectra and valence force field of single-crystalline β Ga2O2. J. Solid State Chem. 1982, 45, 180–192. [Google Scholar] [CrossRef]
- Onuma, T.; Fujioka, S.; Yamaguchi, T.; Itoh, Y.; Higashiwaki, M.; Sasaki, K.; Masui, T.; Honda, T. Polarized Raman spectra in β-Ga2O2 single crystals. J. Cryst. Growth 2014, 401, 330–333. [Google Scholar] [CrossRef]
- Rashad, M.; Shaalan, N.M.; Abd-Elnaiem, A.M. Degradation enhancement of methylene blue on ZnO nanocombs synthesized by thermal evaporation technique. Desalin. Water Treat. 2016, 57, 26267–26273. [Google Scholar] [CrossRef]
- Yan, H.; Hou, J.; Fu, Z.; Yang, B.; Yang, P.; Liu, K.; Wen, M.; Chen, Y.; Fu, S.; Li, F. Growth and photocatalytic properties of one-dimensional ZnO nanostructures prepared by thermal evaporation. Mater. Res. Bull. 2009, 44, 1954–1958. [Google Scholar] [CrossRef]
- Rashad, M. Performance efficiency and kinetic studies of water purification using ZnO and MgO nanoparticles for potassium permanganate. Opt. Quantum Electron. 2019, 51, 291. [Google Scholar] [CrossRef]
- Shao, L.; Duan, X.; Li, Y.; Zeng, F.; Ye, H.; Ding, P. Two-dimensional Ga2O2 monolayer with tunable band gap and high hole mobility. Phys. Chem. Chem. Phys. 2021, 23, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, N.M.; Hanafy, T.A.; Rashad, M. Dual optical properties of NiO-doped PVA nanocomposite films. Opt. Mater. 2021, 119, 111325. [Google Scholar] [CrossRef]
- Asiri, A.M.; Al-Amoudi, M.S.; Al-Talhi, T.A.; Al-Talhi, A.D. Photodegradation of Rhodamine 6G and phenol red by nanosized TiO2 under solar irradiation. J. Saudi Chem. Soc. 2011, 15, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
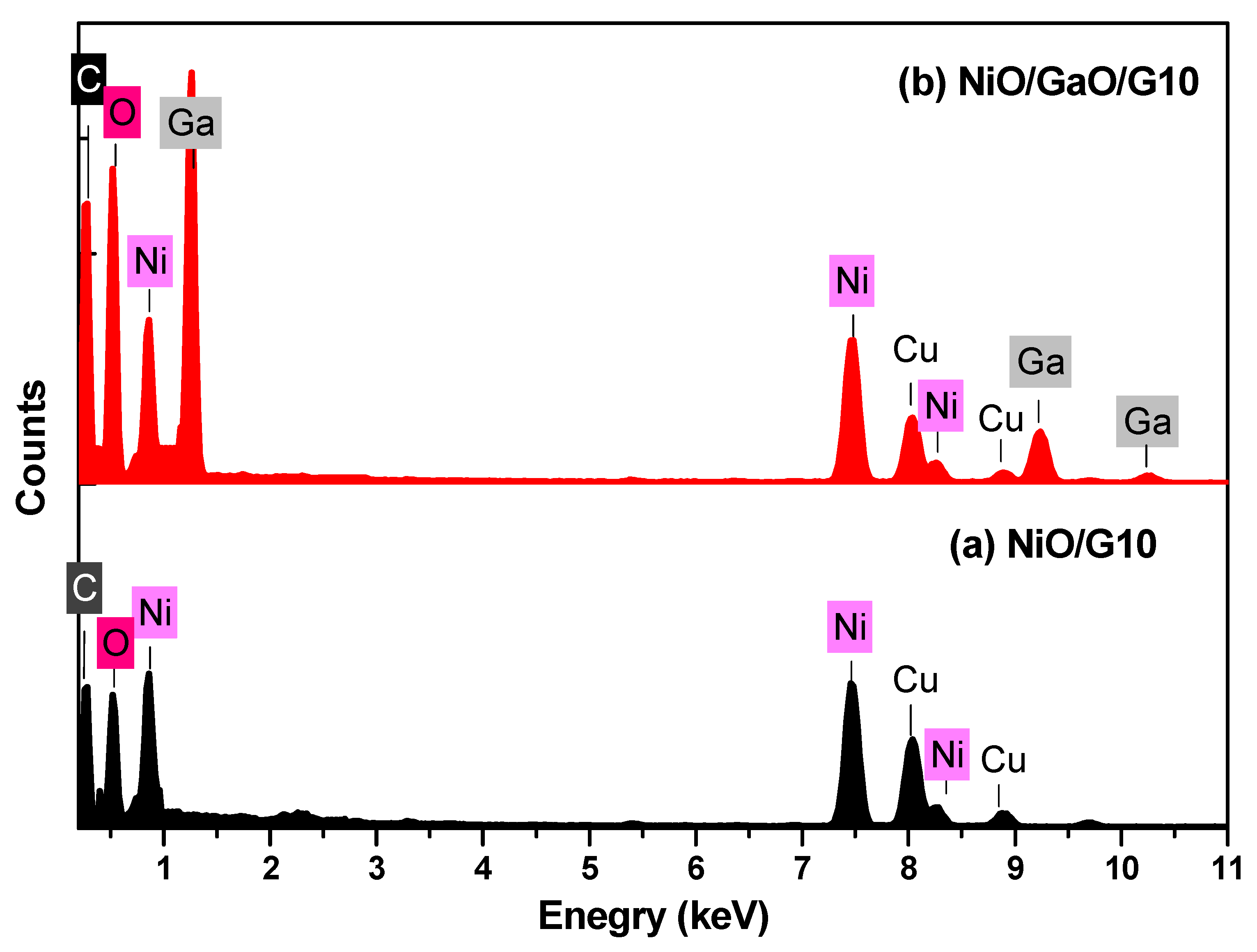

| Element | NiO/G10 | NiO/Ga2O2/G10 | ||
|---|---|---|---|---|
| wt.% | at.% | wt.% | at.% | |
| Ni | 54.07 | 21.53 | 27.43 | 12.83 |
| O | 22.59 | 33.00 | 11.46 | 19.67 |
| C | 23.34 | 45.47 | 22.92 | 52.45 |
| Ga | -- | -- | 38.20 | 15.05 |
| Vib. Mode (cm−1) | 201 | 416 | 532 | 697 | 878 | 1098 | 1335 | 1365 | 1575 | 2660 | 2727 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | |||||||||||
| Graphene | -- | -- | -- | -- | -- | -- | O | -- | O | O | -- |
| NiO/G10 | -- | -- | O | O | O | O | -- | O | O | -- | O |
| NiO/Ga2O2/G2 | O | O | O | O | O | O | -- | -- | O | -- | -- |
| NiO/Ga2O2/G10 | O | O | O | O | O | O | -- | O | O | -- | O |
| Vib. Mode (cm−1) | 201 | 416 | 532 | 697 | 878 | 1098 | 1335 | 1365 | 1575 | 2660 | 2727 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | |||||||||||
| Graphene | -- | -- | -- | -- | -- | -- | O | -- | O | O | -- |
| β-Ga2O2 | O | O | -- | O | -- | -- | -- | -- | -- | -- | -- |
| NiO | -- | -- | O | -- | O | O | -- | -- | -- | -- | -- |
| qe(exp) | k | K | |
|---|---|---|---|
| NiO/G2 | 12.40 | 0.2616 | 0.00703 |
| NiO/G10 | 10.15 | 0.1162 | 0.01256 |
| NiO/Ga2O2/G2 | 13.30 | 0.0857 | 0.02252 |
| NiO/Ga2O2/G10 | 19.95 | 0.0905 | 0.0378 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaalan, N.M.; Rashad, M.; Awada, C. Synergistic Effect of NiO-Ga2O2-Graphene Heterostructures on Congo Red Photodegradation in Water. Separations 2022, 9, 201. https://doi.org/10.3390/separations9080201
Shaalan NM, Rashad M, Awada C. Synergistic Effect of NiO-Ga2O2-Graphene Heterostructures on Congo Red Photodegradation in Water. Separations. 2022; 9(8):201. https://doi.org/10.3390/separations9080201
Chicago/Turabian StyleShaalan, Nagih M., Mohamed Rashad, and Chawki Awada. 2022. "Synergistic Effect of NiO-Ga2O2-Graphene Heterostructures on Congo Red Photodegradation in Water" Separations 9, no. 8: 201. https://doi.org/10.3390/separations9080201
APA StyleShaalan, N. M., Rashad, M., & Awada, C. (2022). Synergistic Effect of NiO-Ga2O2-Graphene Heterostructures on Congo Red Photodegradation in Water. Separations, 9(8), 201. https://doi.org/10.3390/separations9080201







