Abstract
A green synthesis of Ag/AgCl with an exceptional SPR and photocatalysis property is greatly benefit to the environmental application especially pollutant removal. In this work, a novel green plasmonic photocatalysis of Ag/AgCl nanocatalyst using aqueous garlic extract (Allium Sativum L.) was successfully synthesized. The allicin and organosulfur compounds in the garlic can act as reducing agents in the green synthesis process. The nanocatalyst properties were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray diffractometer. The light-harvesting property was investigated by UV-vis absorption spectra which reveals its visible light absorption capability owing surface plasmon resonance behavior of Ag nanoparticles. The degradation and mineralization of methylthioninium chloride (MC) using this photocatalyst were evaluated under visible light and natural solar irradiation. Surface plasmon resonance of Ag nanoparticles and the presence of organosulfur from the garlic extract facilitated adsorption of MC onto the particle surface, promoting greater degradation. The photocatalytic reaction under visible light can be explained by the pseudo first-order pattern with the highest reaction rate of 0.5829 mg L−1 min−1 at pH 10. The photocatalytic activity of the Ag/AgCl under the natural sunlight reached 90% and 75% for MC and total organic carbon (TOC), respectively. The intermediate products detected during MC degradation under sunlight irradiation before final transformation to CO2, H2O, HNO3, and H2SO4 were also reported. The simplicity of Ag/AgCl green synthesis with the photocatalysis properties under visible light and sunlight can offer the convenience of applying these nanoparticles for pollutant removal in water treatment processes.
1. Introduction
The death of people caused by water and wastewater pollution is an alarming signal that humanity is facing a global crisis. Water pollution with organic pollutants is no longer an issue only for scientists; it calls for attention from the public and policymakers. To improve water quality, there are at least two issues that must be considered. First is the remediation of polluted water without causing secondary pollution. Second is a commitment to green and sustainable chemistry by the use of renewable materials and eco-friendly processes.
It is well known that noble metals such as gold (Au) and silver (Ag) possess great antibacterial properties. Recent studies have shown that the unique surface plasmon resonance (SPR) property of Ag nanoparticle photocatalysts gives them enhanced absorption coefficients in a broad UV/Vis/NIR spectral range [1,2,3]. For instance, Awazu et al. [4] showed that Ag nanoparticles deposited on TiO2 or SiO2 take on a substantially enhanced photocatalytic activity; however, this effect could be obtained only under UV illumination, creating both technical and economical challenges. Interestingly, this major roadblock was solved when the nanoparticles comprised a combination of Ag and AgCl (Ag/AgCl). This composite was activated in the visible light range due to the SPR effect of metallic Ag [5]. Various approaches have been proposed for synthesizing the Ag/AgCl composite, such as an ion-exchange reaction [6], ionic liquid process [7], a two-step route [8], or a microwave-assisted process [9]. Most methods produced micrometer-scale particles, resulting in reduced surface area and catalytic performance compared to nanometer-scale particles because SPR increases as size decrease in plasmonic photocatalysts. In addition, these processes are complicated, time-consuming, and, notably, require hazardous chemical reagents such as hydrochloric acid. This unavoidable ingredient prevents high volume production and is not eco-friendly.
The emergence of green chemistry to replace hazardous chemicals with naturally available biomaterials has provoked the scientist and engineers for the eco-friendly synthesis method. A variety of nanoparticles have been synthesized using natural biomaterial such as honey [10], extract of soybean [11], green tea [12], coffee [13], and seashell [14] for the improvement of physico-chemical properties of the green nanocatalysts. Garlic extract (Allium Sativum L.) is one of green biomaterials for visible light triggered antibacterial activity of Ag/AgCl nanoparticles [15]. The organosulfur compounds and allicin (diallyl thiosulfinate) in the solvent-extracted garlic are the biologically active compound. With strong antioxidant properties, the allicin reacts with hydroxyl radicals and, simultaneously, suppresses the formation of oxygen radicals. The application of the nanocatalyst synthesized from this plant for antibacterial has been reported recently [15]. However, less work demonstrated the influence of organosulfur constituents from garlic extract on the enhancement of plasmonic photocatalytic activities of Ag/AgCl.
In this work, the ability of Ag/AgCl nanoparticles obtained from garlic extract (Allium Sativum L.) in mineralizing the methylthioninium chloride (MC) is illustrated. The methylthioninium chloride (MC) is a salt used as a medication and dye. For the medical purpose, it is used for the diagnosis of fungal keratitis and to treat methemoglobinemia [16]. As a dye, it is widely used to examine RNA or DNA for biological staining. It is reported as a safe drug when used in therapeutic doses (<2 mg kg−1) [17]. However, in a high dose, it can cause toxicity, including cardiac arrhythmias, coronary vasoconstriction, decreased cardiac output, renal blood flow, and mesenteric blood flow; increased pulmonary vascular pressure and pulmonary vascular resistance and gas exchange deterioration [17].
The objective of this work was to demonstrate a feasible technique using a green synthesis approach with environmentally friendly benefit to obtain Ag/AgCl nanoparticles while maintaining the exceptional SPR and photocatalysis properties. The Ag/AgCl synthesis inherits its economically and environmentally friendly features from garlic extracts because allicin and organosulfur compounds can act as reducing agents. The influence of employing Ag/AgCl nanoparticles synthesized using Allium Sativum L. and the obtained physico-chemical characteristics related to SPR property were discussed. The efficiency of Ag/AgCl-G nanoparticles in decomposing the methylthioninium chloride (MC) at room temperature and sunlight under visible light was evaluated and reported.
2. Materials and Methods
2.1. Materials
The chemicals for Ag/AgCl synthesis and photocatalysis experiment were analytical grade and used without further purification. AgNO3 (≥99.0%), methylthioninium chloride (MC, >99.5%), and glucose (>99.5%) are obtained from Merck. Polyvinyl pyrrolidone or PVP (MW = 58,000, >99.0%), NaOH (≥98%), NaCl (≥99.0%), and other chemicals or reagents were purchased from Sigma-Aldrich. The ultrapure water (Milli-Q with 18.2 MΩ. cm at 25 °C was used in all experiments.
2.2. Synthesis of Ag/AgCl Catalyst
The silver nanoparticles without garlic extract (Ag-chem) were synthesized following previous methods by reducing AgNO3 in aqueous PVP [18,19]. In detail, 3.4 g of AgNO3 was added to 20 mL ultrapure water. The AgNO3 solution was dropwise to the mixture solution of PVP, glucose, and NaOH. After 10 min stirring, the precipitated Ag-chem can be separated by centrifugation. For the Ag/AgCl synthesis in the absence of organosulfur compounds from aqueous garlic extract (Ag/AgCl-chem), 2 mL NaCl solution was dropped to the AgNO3 solution at room temperature. The opaque white solution was delivered to a 10-mL test tube, then at 100 °C for 20 min. The precipitated Ag/AgCl-chem were centrifuged and separated from the solution.
For the green synthesis, the garlic extract was obtained from the twice-blending 50 g of garlic in deionized water (100 mL) and filtrate through the Whatman filter (No. 41). The ‘Green’ Ag (Ag-G) nanoparticles were obtained from the dilution of 0.5 g AgNO3 solution in deionized water (20 mL). The aqueous garlic extract was mixed with the AgNO3 solution under 100 °C for 15 min. Deionized water was used to wash the obtained Ag-G nanoparticles.
Synthesized ‘Green’ AgCl (AgCl-G) chemicals were obtained by the addition of garlic extract to the AgNO3 solution. The formation of AgCl-G nanoparticles occurred from this mixture under the dark at room temperature. ‘Green’ Ag/AgCl (Ag/AgCl-G) nanoparticles were derived from the dropwise of 0.5 M NaCl to the dilution of AgNO3 in deionized water under vigorous magnetic stirring at room temperature. The precipitated Ag/AgCl-G nanoparticles were obtained from the addition of 2 mL garlic extract to the AgNO3 solution, which was performed at 100 °C for 20 min. After separated by centrifugation, the Ag/AgCl-G nanoparticles were thoroughly washed with ultrapure water.
2.3. Material Characterization
Scanning electron microscopy (SEM; Leo1455VP, Carl Zeiss, Oberkochen, Germany) was used to determine the physical morphology of the Ag/AgCl nanoparticle surface. The investigated nanoparticles were mounted on a copper grid and analyzed by transmission electron microscopy (TEM; Philips Tecnai 12, Hillsboro, OR, USA) to determine the crystal size. Before the examination, all samples were sputter-coated with an Au thin layer. The crystal structure of Ag/AgCl nanoparticles was determined using X-ray diffraction (XRD; JDX-3530, Jeol Ltd., Akishima, Tokyo, Japan) using a graphite mono-chromator and Cu Kα radiation at 20–70 W at wavelength of 1.54060 Å. The nanoparticles were scanned at the range (10–70°) with a scanning rate of 2.0/min. The zeta potentials of nanoparticles (pHpzc) were measured using a Zetasizer Nano series instrument (Malvern, UK) with a He-Ne laser operating at an incident wavelength of 633 nm and a 173° backscattering angle. The samples of 50 µL 5 wt% dispersion mixed with ethanol and homogenized well using ultrasonicate for 10 s. The clear disposable zeta-potential cells were rinsed with ethanol and deionized water prior to measurement. The values of zeta potential for each Ag nanoparticle sample were obtained from the average arithmetic mean of the 5 measurements of each sample.
The optical absorption spectra of Ag/AgCl nanoparticles were conducted using Perkin Elmer UV/Vis Lambda 35 spectrophotometer equipped with an integrating sphere (Lambda 35 (P/N C6951014), Norwalk, CT, USA) to capture the scattered light from nanoparticles in solid sample holder set. The absorbance mode was performed with the scanning range 200–800 nm using a 2-nm slit to measure the visible light absorbance of the Ag/AgCl nanoparticles. For the analysis of functional groups on Ag/AgCl nanoparticles and garlic extract, the Fourier-transform infrared (FTIR) spectroscopy (Frontier, PerkinElmer, Waltham, MA, USA) was operated in the wavelength range of 400–4000 cm−1 by 128 scans at 4 cm−1 resolution.
2.4. Photocatalytic Activities of Ag/AgCl Nanoparticles
The decomposing MC under visible light at room temperature or under sunlight was conducted to evaluate the photocatalytic activities of the Ag/AgCl nanoparticles. The visible light source was a 10 W Hitachi fluorescent daylight lamp that provided UV-free light. The Ag/AgCl nanoparticles were suspended in 10 mg L−1 MC in a beaker with a magnetic stirrer. Prior to irradiation, the aqueous solution was agitated in the dark (30 min) to establish the adsorption/desorption equilibrium between the Ag/AgCl nanoparticles, MC, and water. The reaction temperature was controlled at 25 ± 2 °C by an external cooling jacket. During the experiment, the aqueous solution was stirred constantly by a magnetic stirrer.
The photocatalysis experiment was conducted in the same way but using an initial MC concentration of 20 mg L−1 under direct sunlight exposure. Actual direct sunlight irradiation was used between 1100 h and 1300 h on a sunny day in June at latitude 18.7954° North and longitude 98.9585° East (Chiang Mai, Thailand). The average solar intensity was found to be 1900 W m−2. The temperature inside the beakers ranged from 30 to 35 °C. Analytical samples were withdrawn at various time intervals and analyzed by UV/Vis spectrophotometer (Lambda 35, Perkin Elmer). The MC removal was calculated as follows:
where C0 and C are the concentrations of MC in solution at time 0 and t, respectively.
After the photocatalysis experiment, the Ag/AgCl-G photocatalyst was separated from the reaction mixture by centrifugation. The used photocatalyst was regenerated by stirring in water (dark) for 1 h and irradiated for 1 h before using it in the next cycle under the sunlight.
3. Results and Discussion
3.1. Characteristics of Ag/AgCl-G Nanoparticles
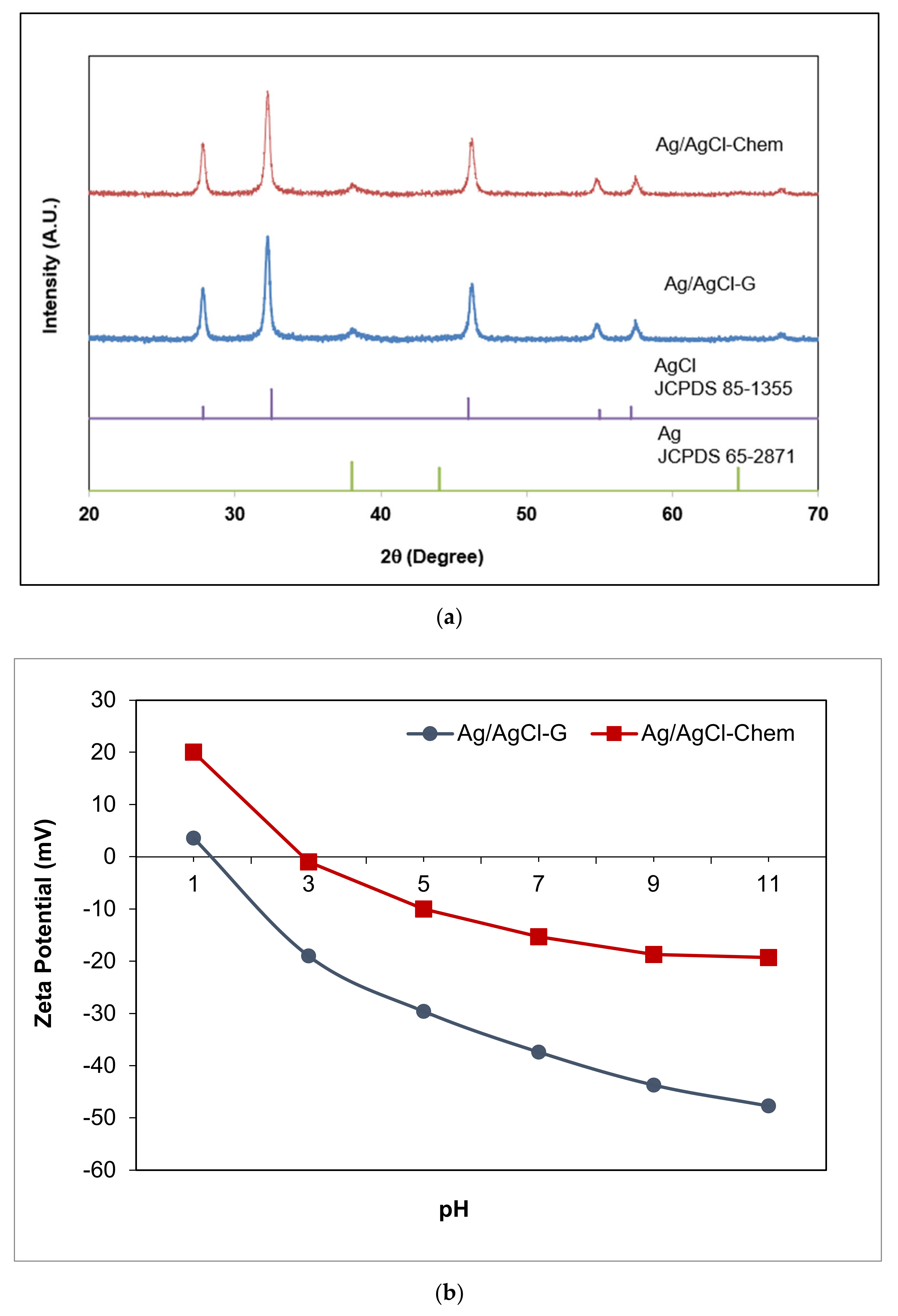
The XRD analysis of Ag/AgCl-G nanoparticles was shown in Figure 1. The crystallographic planes of cubic AgCl (ICDD PDF no. 31-1238) were identified at 2θ values of 27.9°, 32.3°, 46.3°, 54.9°, 57.5°, and 67.2° which were assigned respectively to the (111), (200), (220), (311), (222), and (400). The peaks at 2θ values of 37.83 and 64.24 were evidenced by the small quantity of Ag [20]. It notes that the reasonably lower intensity of Ag diffraction peaks results from the low content of metallic Ag in the sample. The average size, using the Debye-Scherrer equation from the XRD pattern, was found to be 22 nm. The SPR effect of Ag nanoparticles could be seen under visible light.
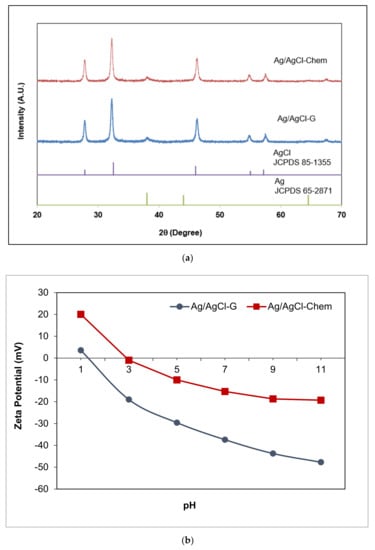
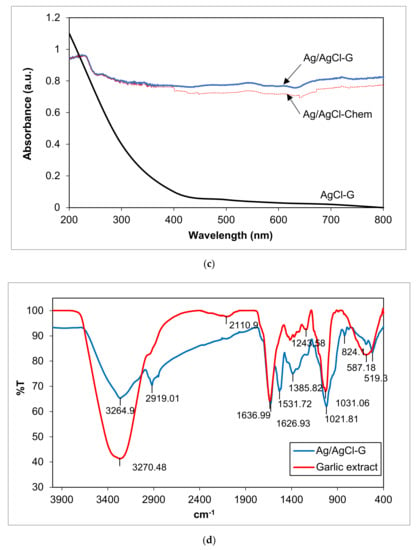
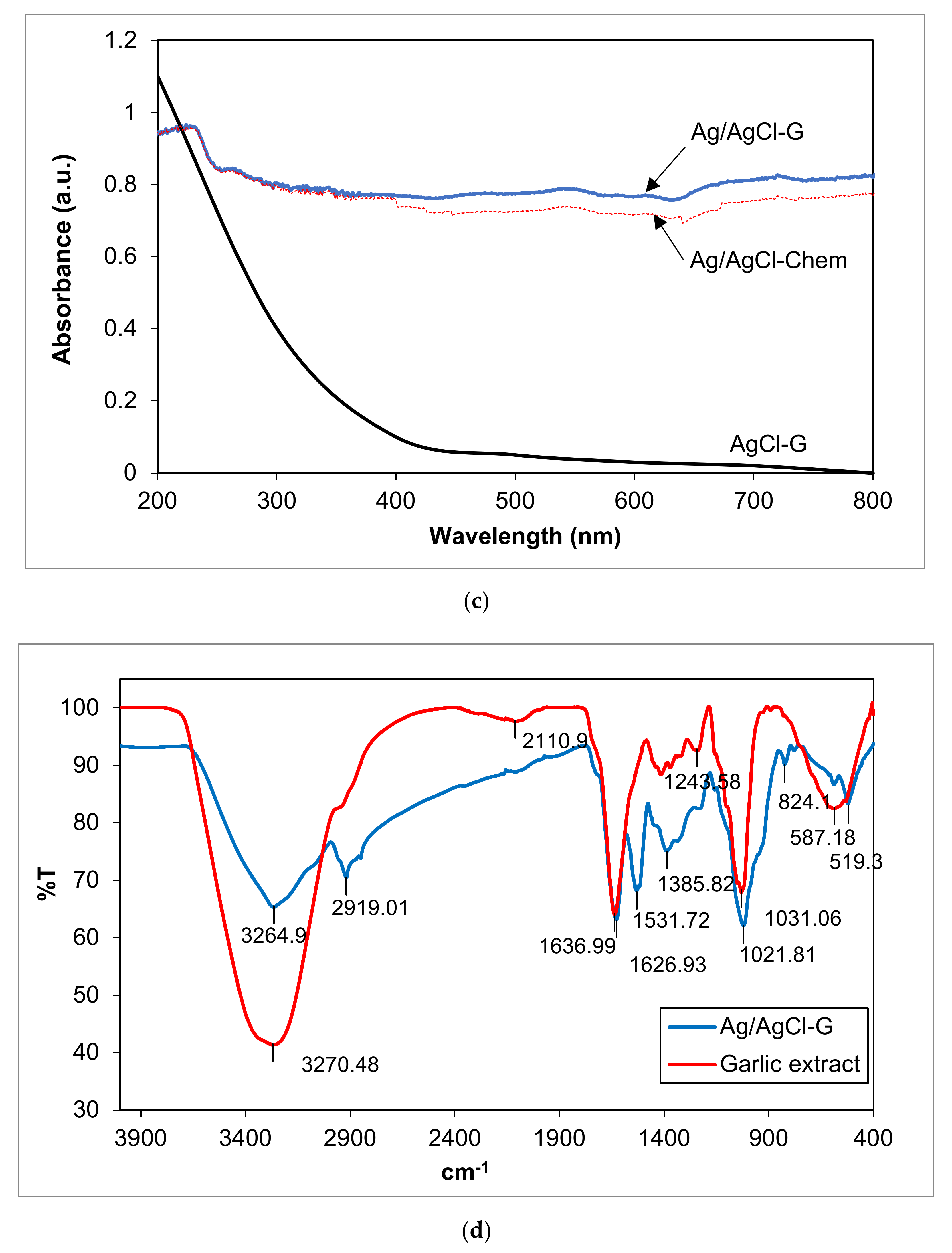
Figure 1.
The XRD patterns of Ag/AgCl-G nanoparticles (a); zeta potential of Ag/AgCl-G (b); absorption spectra of Ag/AgCl-G, Ag/AgCl-Chem, and AgCl-G nanoparticles (c); and FTIR spectra of Ag/AgCl-G and garlic extract (d).
To compare surface properties, zeta potentials were determined for Ag-chem, Ag-G, Ag/AgCl-chem, and Ag/AgCl-G at pH 7 and 25 °C. They were found to be −7.65, −30.13, −15.3, and −35.37 mV, respectively, showing a negative charge distribution on the surface of Ag nanoparticles. Interestingly, nanoparticles synthesized using green chemistry had higher negative charges than those synthesized using classical chemical methods. The zeta potential of Ag/AgCl-G (Figure 1b) also revealed that the negative charge on the surface increased with increasing pH. The surface was fully covered with a negative surface charge at high pH, enhancing adsorption of cationic ions such as MC onto its surface. This zeta potential of Ag-G was consistent with that found in previous work [21] and for other Ag and Au nanoparticles stabilized with sugars such as citrate [22,23], maltose [24], gum acacia (a polysaccharide) [25], glucose [26], and galactose [27].
The UV–Vis absorption spectra of the Ag/AgCl-chem and Ag/AgCl-G nanoparticles are compared in Figure 1c. The Ag/AgCl-G nanoparticles showed strong absorption in both the UV and visible regions, in good agreement with previous work [28]. A characteristic absorption spectrum of AgCl-G particles is shown in the same figure. It is obvious that the spectrum of AgCl-G has relatively low absorbance in the visible light region of 400–800 nm. From the previous works, the Ag nanoparticles with the SPR property exhibited a strong light absorption in a range of broad UV/Vis/NIR spectral [1,2,3]. Thus, the strong absorption of the visible lights of both Ag/AgCl-G and Ag/AgCl-Chem as shown in 400 to 800 nm could be ascribed to the local SPR of Ag nanoparticles. Also, the Ag/AgCl-G nanoparticles were gray, whereas the AgCl particles were white. The apparent light adsorption for Ag/AgCl-G nanoparticles in visible light and sunlight could be enhanced as a result of the metallic Ag deposited on the surface of AgCl particles. The band gap (Eg) of both nanoparticles was estimated by the Kubelka-Munk equation [29] and the Eg values for Ag/AgCl-chem and Ag/AgCl-G nanoparticles were 2.8 eV and 2.5 eV, respectively. Thus, the Eg value of Ag/AgCl-G nanoparticles were smaller than Ag/AgCl-chem.
FTIR spectra for the composition of Ag/AgCl-G and the active species in the garlic extract were analyzed (Figure 1d). Comparatively, the spectra of Ag/AgCl-G displays many absorption peaks reflecting the active species of garlic composition on its surface. The significant functional groups derived from the garlic extract and coated on the Ag/AgCl-G included the peaks of O–H, C=C, S–O, and PO43− which were detected at 3264.90, 1626.93, 1243.58, and 1021.8 cm−1, respectively. These peaks confirm the presence of allicin, diallyl disulfide, and other active species of the extract garlic in the Ag/AgCl-G nanoparticles.
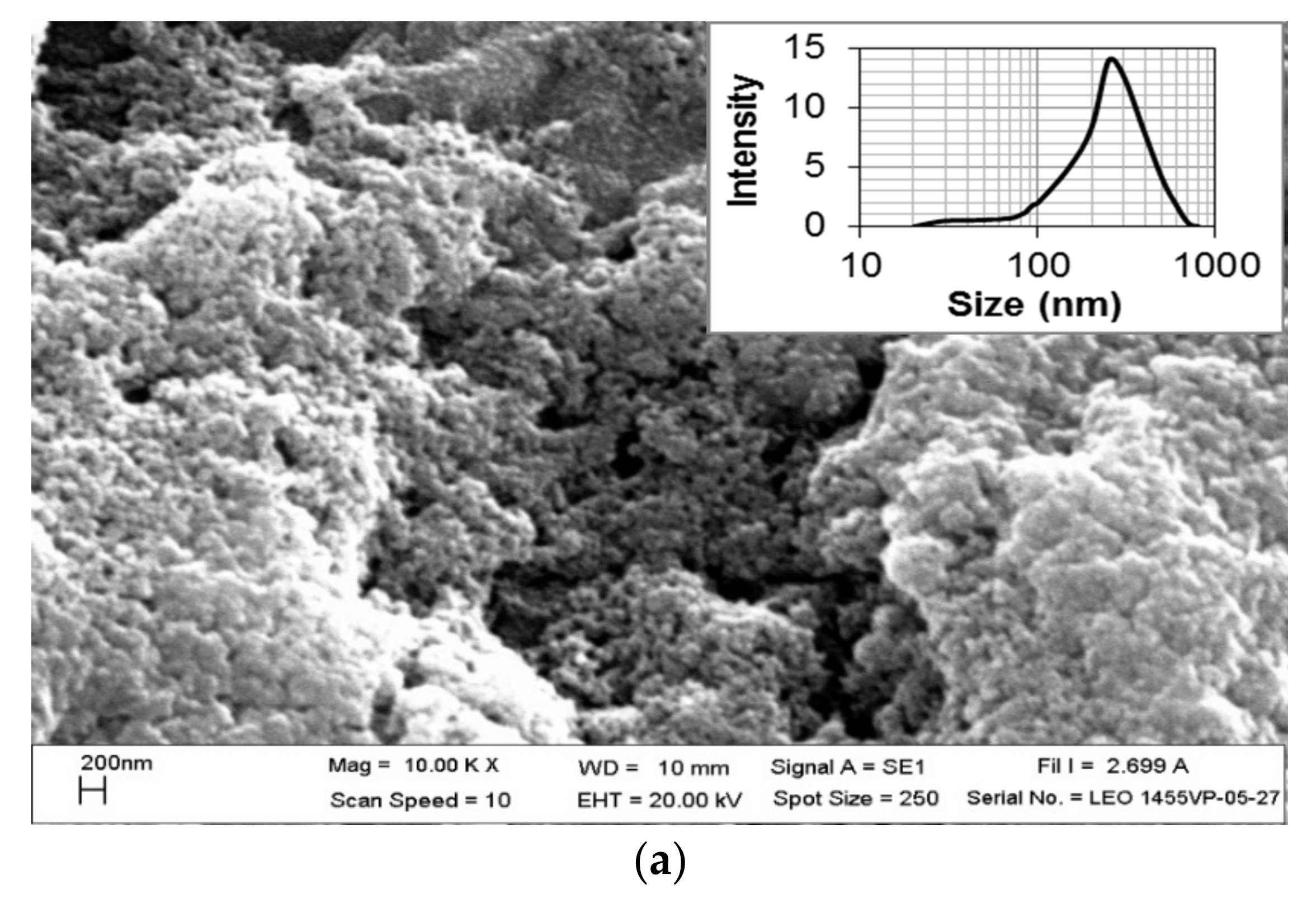
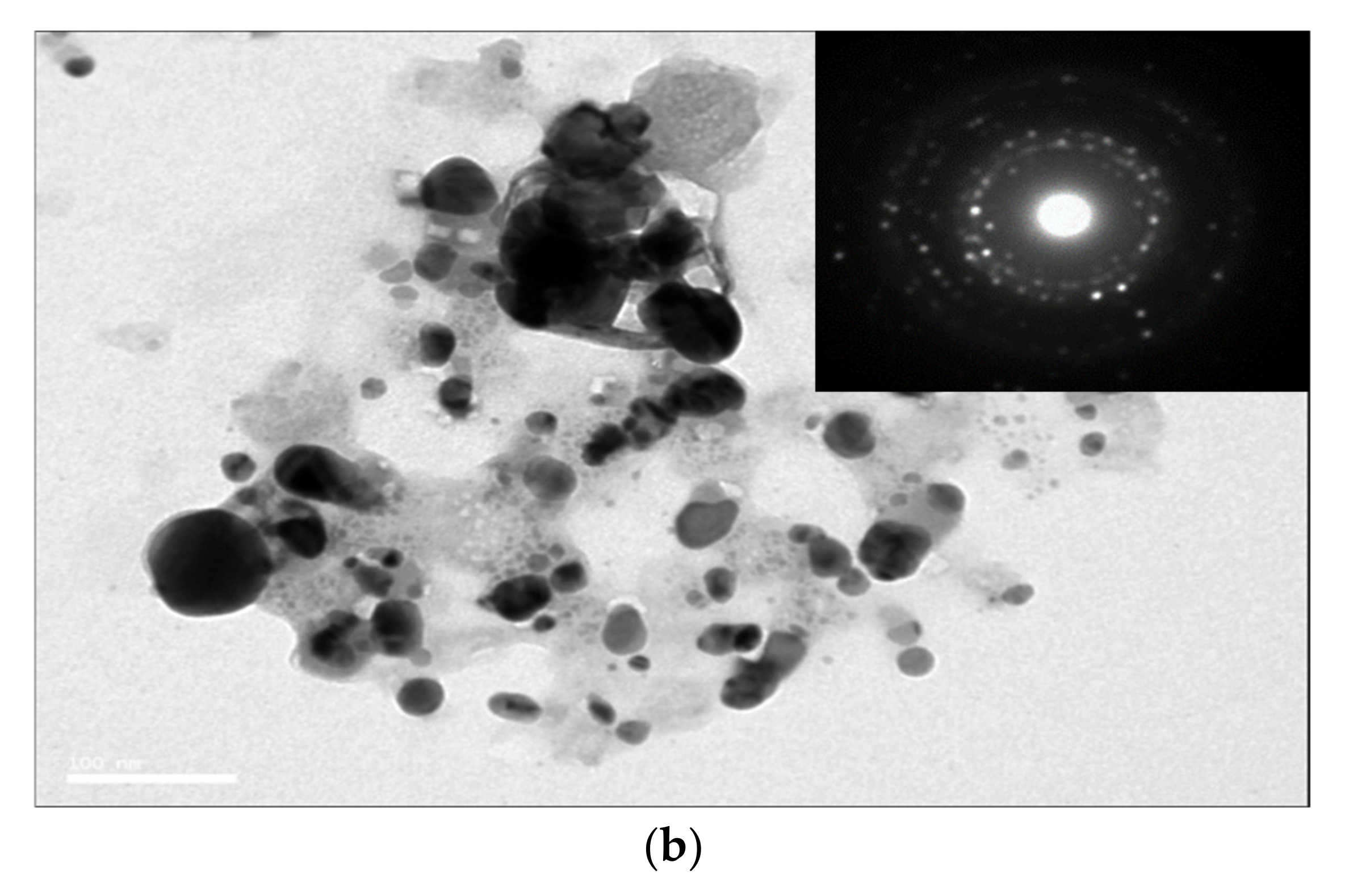
The SEM and TEM images also exhibited the size and morphological features of the Ag/AgCl-G nanoparticles, as shown in Figure 2a,b, respectively. The SEM image shows a spherical morphology, and the TEM image shows the average crystal size of Ag/AgCl-G was 17.13 ± 6.25 nm which is not significantly different from XRD results (22 nm). The average crystal size of the Ag/AgCl-G is slightly smaller than that of Ag/AgCl-Chem (33.12 ± 9.21 nm).
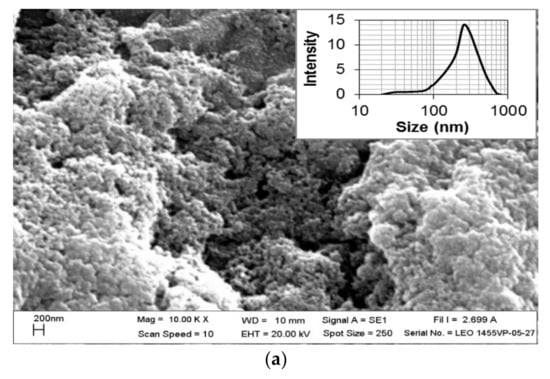
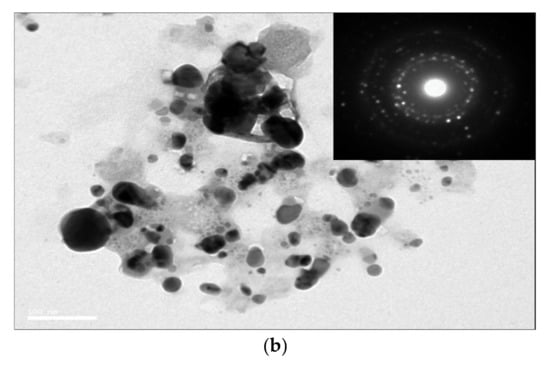
Figure 2.
Scanning electron microscopy (a); and transmission electron microscopy images of Ag/AgCl-G (b).
The selected-area electron diffraction (SAED) pattern of Ag/AgCl-G demonstrates the randomly oriented diffraction spots, representing that these synthesized nanoparticles are polycrystallinity in nature. The SEM image exhibits aggregation among the obtain nanoparticles. The agglomerate particle size of Ag/AgCl-G was about 250.8 ± 24.1 nm as shown in Figure 2a (inset). The agglomeration formation of the investigated nanoparticles is possibly occurred from the high ionic content of Na, Cl, and NO3 from the electrolyte concentration as discussed in the previous work [30].
3.2. Plasmonic Photocatalytic Activity of Ag/AgCl in MC Removal under Visible Light and Sunlight
3.2.1. Effect of Ag/AgCl-G Dosages on MC Removal and Kinetics
The plasmonic photocatalysts in the form Ag/AgX where X is Cl, Br, or I, have demonstrated their excellent visible-light photocatalytic performance in many previous works [31,32,33]. However, the performance and reaction kinetics of Ag/AgX obtained using green chemistry methods are rarely reported. In this work, the photocatalytic activity of Ag/AgCl-G synthesized from aqueous garlic extract has been investigated by evaluating the photocatalytic degradation of MC aqueous solutions under visible light. Major factors affecting the SPRs of noble metal nanoparticles strongly influencing the photocatalytic activities of plasmonic photocatalysts are reported. These parameters were the Ag/AgCl-G nanoparticle dosages, solution pH, and initial concentrations of pollutants.
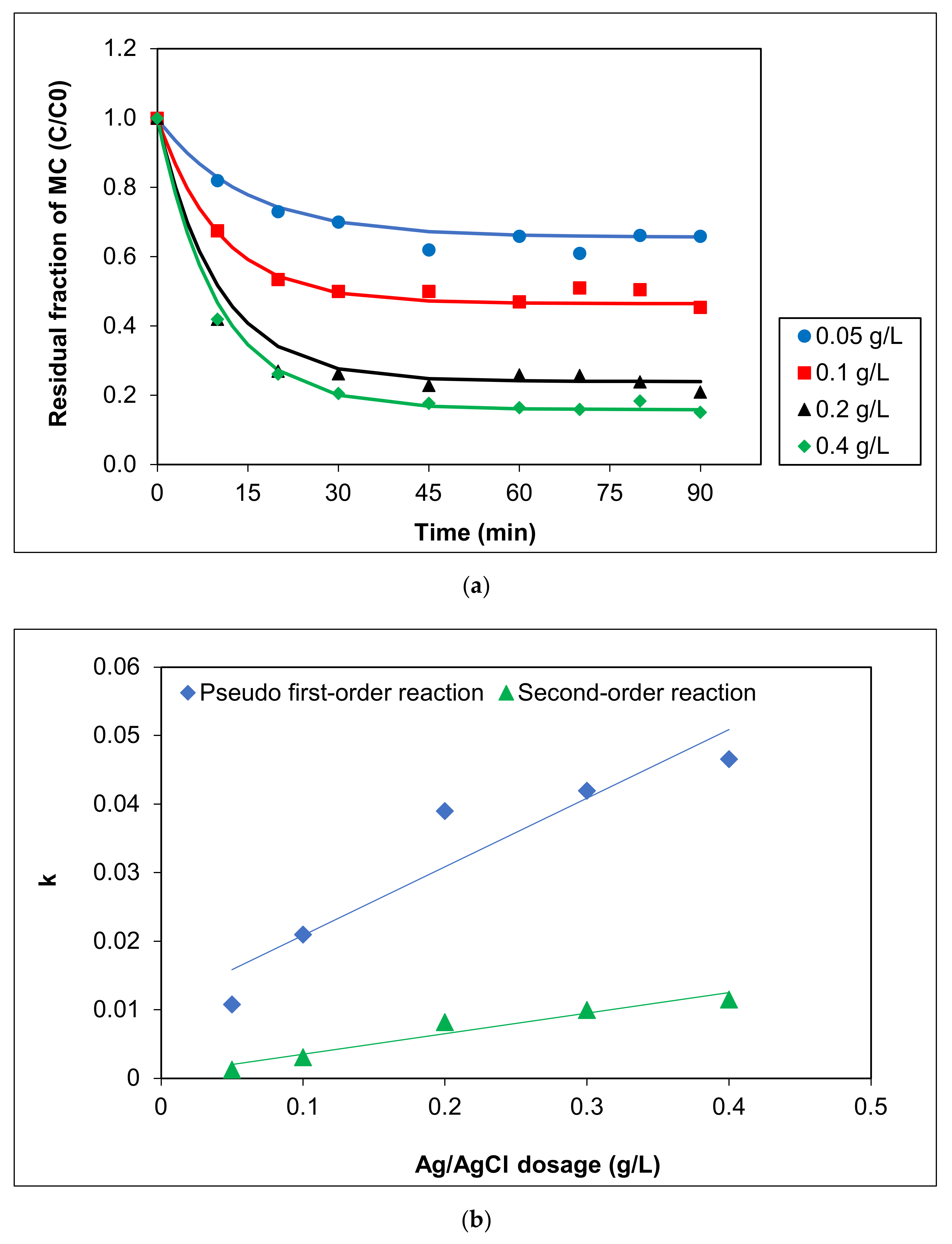
The effect of Ag/AgCl-G dosages on MC removal was investigated, and the results are shown in Figure 3a. Increasing the dosage of Ag/AgCl-G from 0.05 to 0.4 g L−1 led to a pronounced increase in photocatalytic degradation of MC (from 34.07% to 77.52%). A further increase (to 0.4 g L−1) afforded a slight increase in photocatalytic efficiency in MC discolorization, but identical activities were observed after 90 min exposure to visible light. This phenomenon may be explained by the difficulty of permeating light through the solution when the catalyst was at higher concentrations. Alternatively, as reported previously, an increase in the catalyst can decrease photoabsorption, reducing the pollutant adsorption onto the catalyst surface, thus reducing reaction rates [33,34].
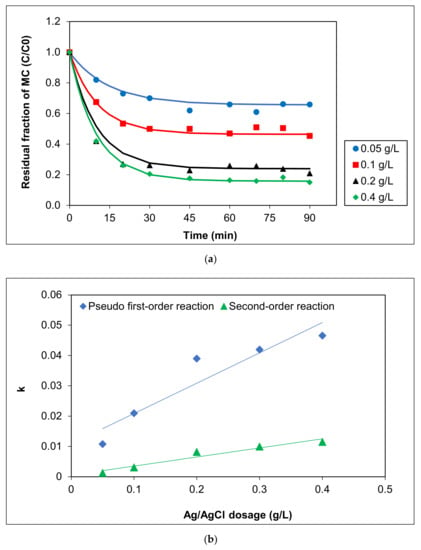
Figure 3.
Effect of Ag/AgCl-G dosages on (a) residual fraction of MC with time using Ag/AgCl-G and (b) relation of kinetic constants (k) with Ag/AgCl dosage (pH = 10 and MC concentration = 10 mg L−1).
To investigate the kinetics of photocatalytic discolorization reactions between MC and Ag/AgCl-G, experimental results for the photodegradation of MC on the Ag/AgCl-G were applied to pseudo-first-order and second-order kinetic models using Equations (2) and (3):
and
where C0 and C are the initial and residual concentrations of MC in aqueous solution at time t, respectively. The reaction rate constant is k. The half-life of a first-order reaction is expressed as t1/2 = 0.693/k (ln2 = 0.693).
Kinetic parameters, including the initial rate (r), kinetic constant (k), and half-life of MC (t1/2), based on the plasmonic photocatalytic reaction of MC using various Ag/AgCl dosages under visible light from both pseudo-first order and second-order reactions, are reported in Table 1. All the correlation coefficients (R2) for second order kinetic models were greater than those for first-order kinetic models (0.8726–0.9508 vs. 0.7189–0.8218, respectively). Thus, the MC photocatalytic degradation using Ag/AgCl-G was a second-order reaction. The kinetic values were greatly dependent on the photocatalyst dosage, as shown in Figure 3b. Degradation of MC was enhanced with increasing dosage: the reaction rate at 0.4 g L−1 (r = 0.5291 mg L−1 min−1) was threefold that at 0.05 g L−1 (r = 0.1799 mg L−1 min−1). The rate constant of the photocatalytic reaction using 0.05 g L−1 was only 1.30 × 10−3 min−1, whereas using 0.4 g L−1 was up to 1.15 × 10−2 min−1. These increases clearly show the influence of the dosage of Ag/AgCl-G on its photocatalytic activity.

Table 1.
Kinetic Parameters Based on the Plasmonic Photocatalytic Reaction of Ag/AgCl with Methylthioninium Chloride Under Visible Light Using Various Ag/AgCl Dosages 1.
3.2.2. Effect of pH on the Degradation of MC by a Photocatalytic Process under Visible Light
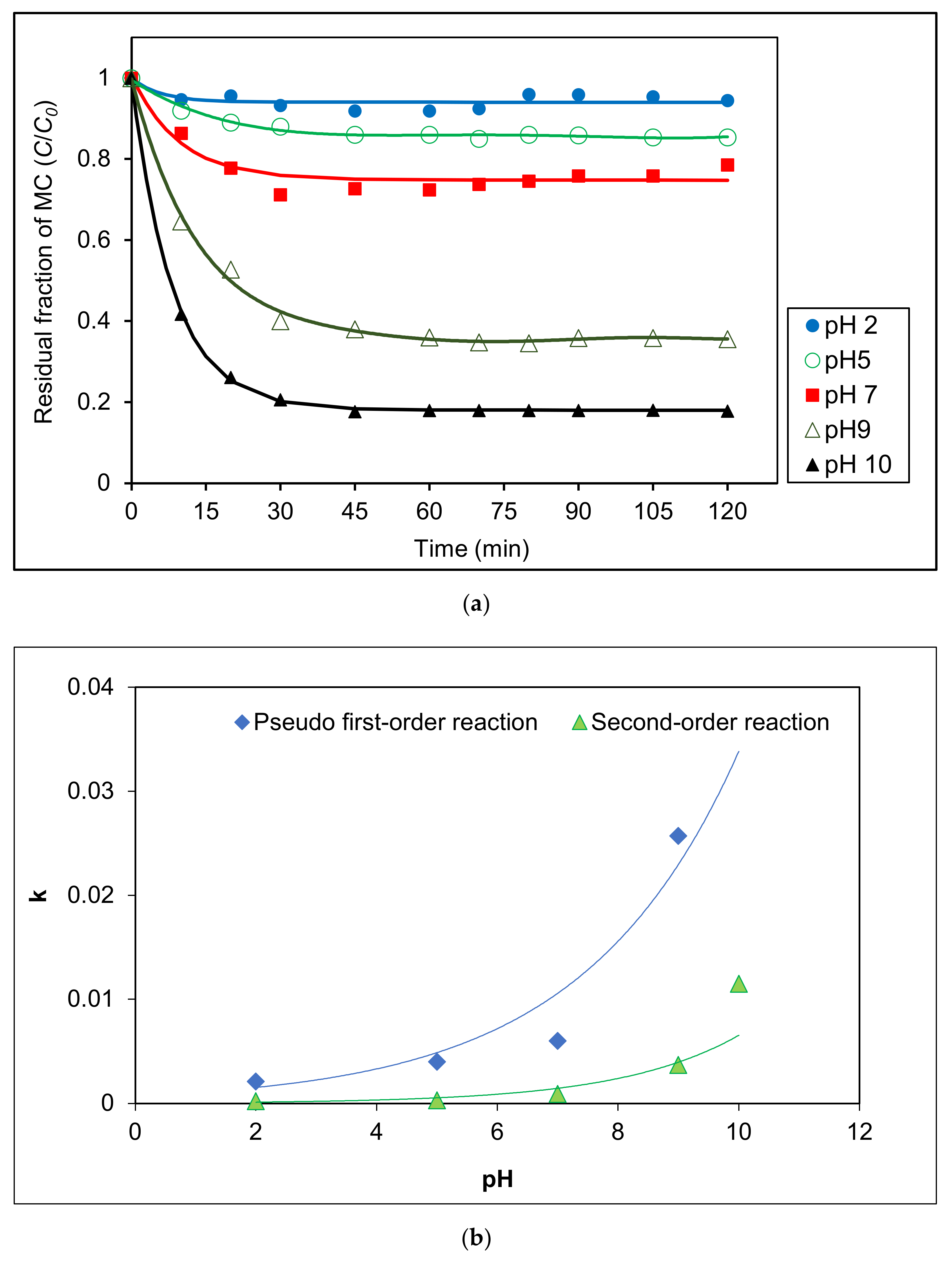
The effect of pH on the degradation of MC by a photocatalytic process under visible light was analyzed in the range of pH 2–pH 10 using 0.4 g L−1 Ag/AgCl-G. Figure 4a shows that degradation was highly effective at higher pH values especially at pH 10. Photocatalytic degradation kinetics of MC in various pH solutions were measured using the change in MC concentration as shown in Figure 4b. Kinetic parameters at various pH are reported in Table 2. Based on correlation coefficients, photocatalytic reactions were well-described by pseudo-first order model. Reaction rate enhancement at pH 10 was much higher than that at pH 2, likely attributable to the high adsorption of MC on the surface of Ag/AgCl-G resulting from the negative zeta potential of the photocatalyst in alkaline pH. The reaction rate at pH 10 (r = 0.5829 mg L−1 min−1) was elevenfold that at pH 2 (r = 0.0530 mg L−1 min−1). The rate constant of the photocatalytic reaction (explained by first-order model) at pH 2 was only 2.10 × 10−3 min−1, whereas pH 10 was up to 4.70 × 10−2 min−1. These increases clearly show the influence of the pH on the photocatalytic activity of Ag/AgCl-G.
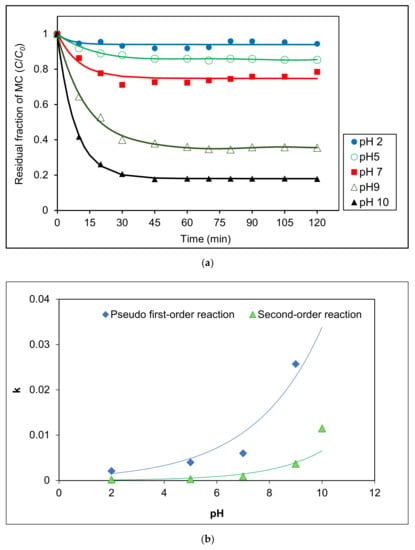
Figure 4.
Effect of different pH solutions on (a) residual fraction of MC with time using Ag/AgCl-G and (b) relation of kinetic constants (k) with different pH solutions (Ag/AgCl-G loading = 0.4 g L−1 and MC concentration = 10 mg L−1).

Table 2.
Kinetic parameters based on the plasmonic photocatalytic reaction of Ag/AgCl with methylthioninium chloride under visible light at various pH 1.
3.2.3. Influence of Initial MC Concentration on the Photocatalytic Activity of Ag/AgCl-G under Visible Light
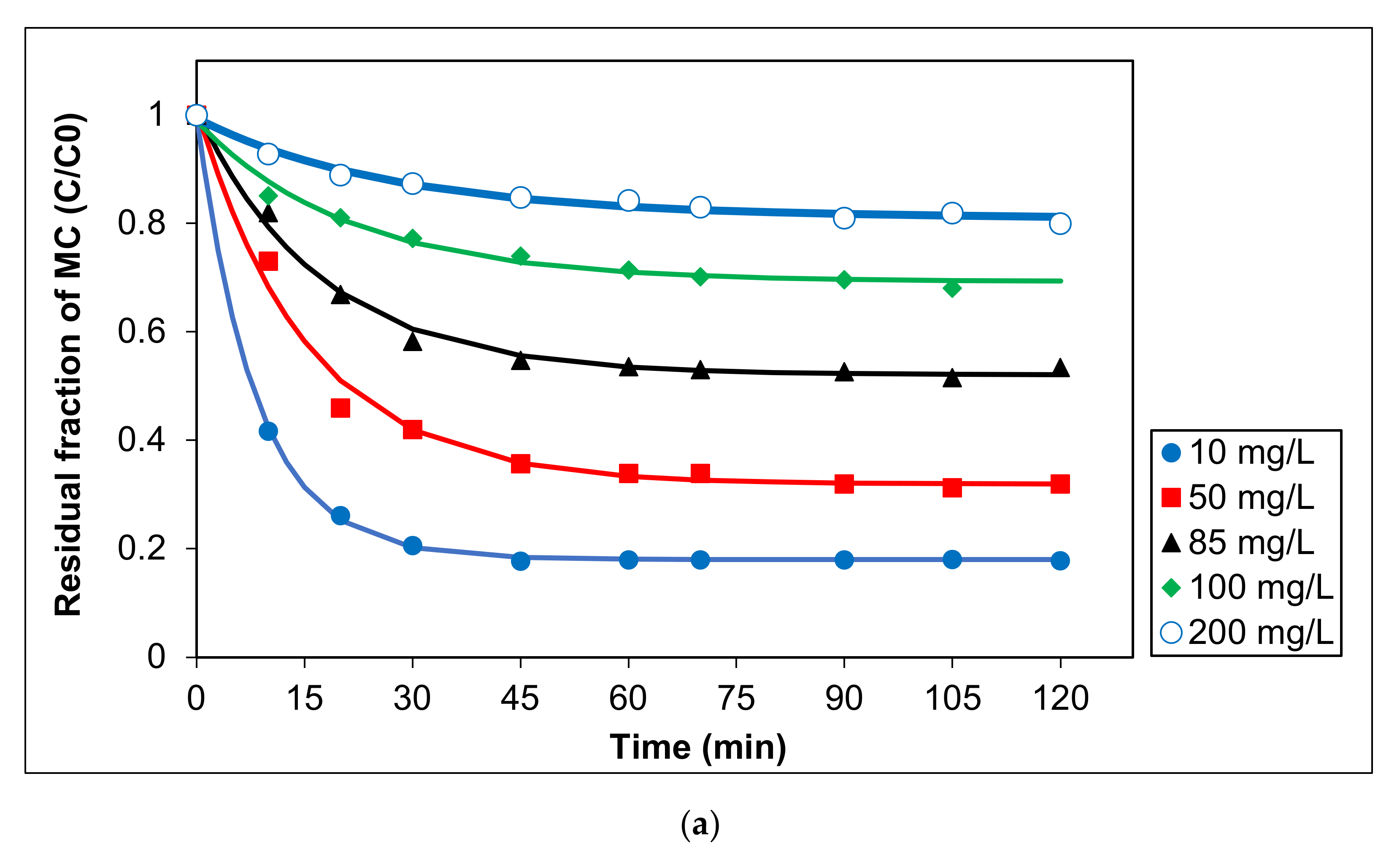
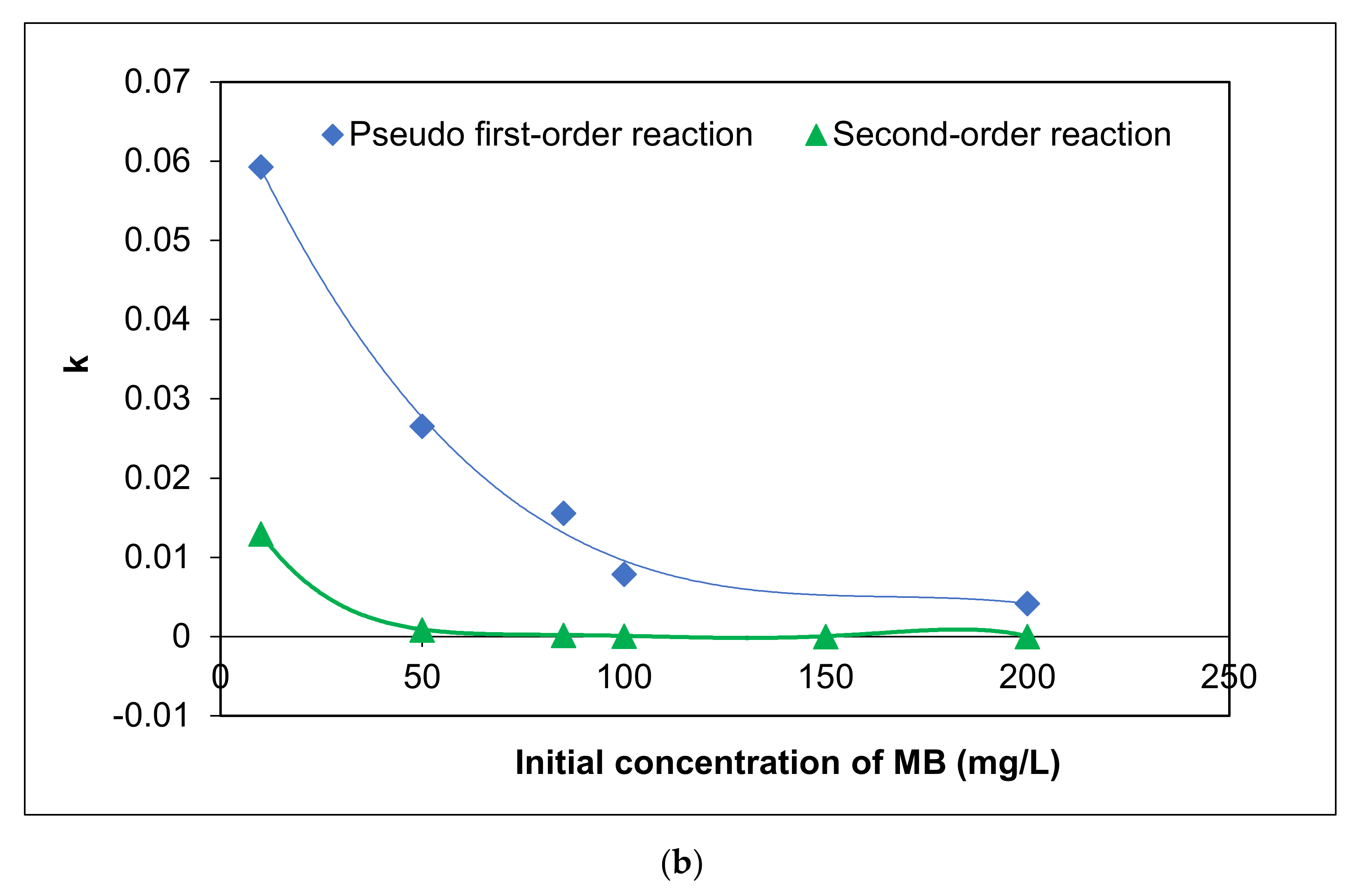
To elucidate the influence of initial MC concentration on the photocatalytic activity of Ag/AgCl-G under visible light, five concentrations were tested between 10 and 200 mg L−1. In all cases, pH was 10, and Ag/AgCl-G was dosed at 0.2 g L−1. Experimental results are shown in Figure 5a, and the relation of kinetic constants with a different initial concentration of MC was exhibited in Figure 5b. The kinetic parameters are also reported in Table 3. The MC degradation is followed a pseudo first-order reaction. This reveals that the concentrations of MC and the carriers (electron and hole) on the surface of the catalyst play essential roles in chemical reactions. The kinetic constants decrease with increasing initial MC concentration. The kinetic constants using a pseudo-first order model for the MC degradation were in the range of 5.93 × 10−2 to 7.9 × 10−5 mg L−1 min−1. Obviously, the reaction rate and kinetic constants are greatly dependent on the initial MC concentration.
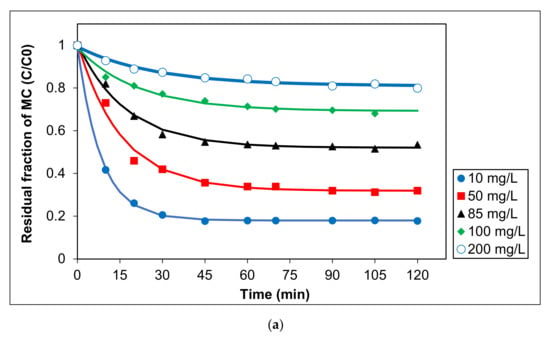
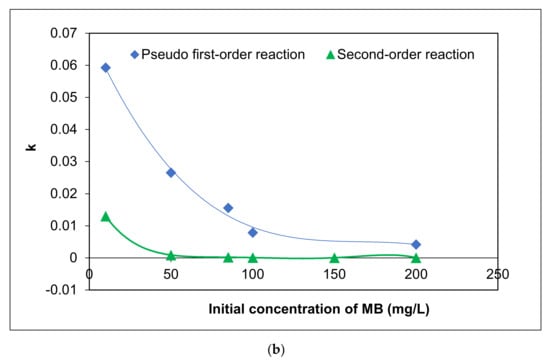
Figure 5.
Effect of different initial concentrations of MC on (a) residual fraction of MC with time using Ag/AgCl-G and (b) relation of kinetic constants (k) with different initial concentration of MC (pH = 10, and Ag/AgCl-G loading = 0.4 g L−1).

Table 3.
Kinetic parameters based on the plasmonic photocatalytic reaction of Ag/AgCl under visible light using various initial concentrations of methylthioninium chloride 1.
3.2.4. Plasmonic Photocatalytic Activity of Ag/AgCl-G for MC Removal under Sunlight
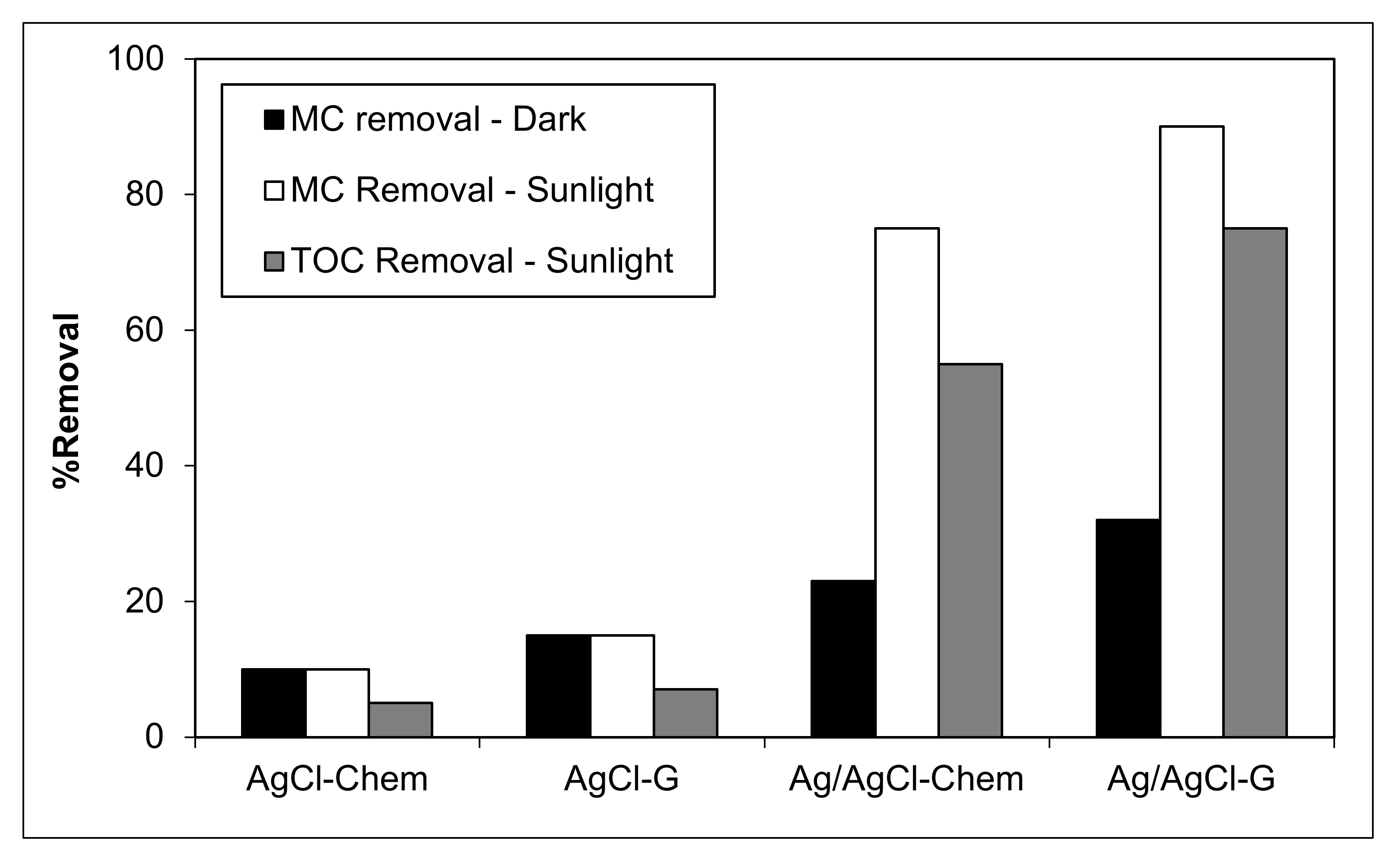
To demonstrate plasmonic photocatalytic activity in natural conditions, MC removal was investigated under sunlight using AgCl-chem, AgCl-G, Ag/AgCl-chem, and Ag/AgCl-G. Photocatalytic activity, represented by the percent decrease in MC concentration and the mineralization ability, represented by the percent decrease in total organic carbon (TOC), is shown in Figure 6. In all cases, pH was 10, catalyst dosage was 0.4 g L−1, initial MC concentration was 20 mg L−1, and the exposure time was 60 min. Prior to illumination, all mixtures were equilibrated in the dark for 30 min. The measured quantity of MC was inversely proportional to the color loss of the chemical during photocatalysis. Results show that both AgCl-chem and AgCl-G had minimal effect on removing MC (15–17% degradation) from an aqueous solution. During the dark experiment, the Ag/AgCl-G had the highest percentage (32.2%) of MC adsorption on its surface. The rising of both MC and TOC removal percentages occurred during natural sunlight irradiation. Both Ag/AgCl catalysts provided a high percentage of MC and TOC removal. Between them, Ag/AgCl-G had the higher activity, removing MC and TOC by 90% and 75%, respectively. Comparatively, Ag/AgCl-chem can remove only 75% and 55%, respectively. The order of reactivity for the removal of MC and TOC in the aqueous solution was as follows: Ag/AgCl-G > Ag/AgCl-chem > AgCl-G > AgCl-chem.
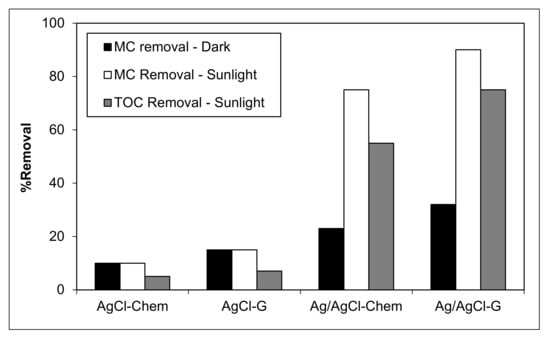
Figure 6.
Plasmonic photocatalytic removal of methylthioninium chloride (MC) and total organic carbon (TOC) in sunlight or darkness using classically-prepared nanoparticles (AgCl-chem and Ag/AgCl-chem) and those synthesized using garlic extract (AgCl-G and Ag/AgCl-G).
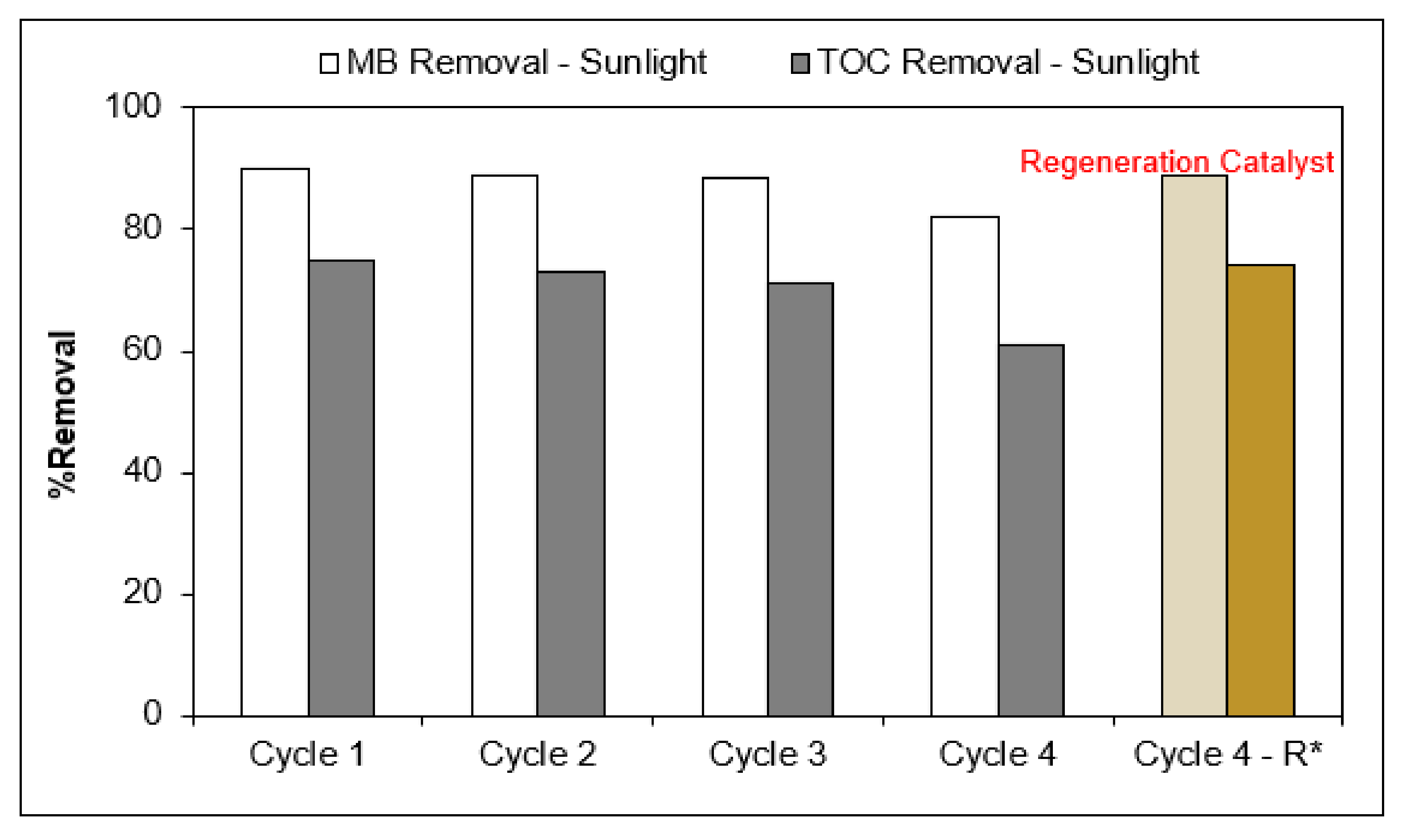
The long-term stability of the photocatalysts was determined over multiple cycles of MC by Ag/AgCl-G under sunlight. As shown in Figure 7, under the same experimental conditions, it was found that the degradation percentage MC and TOC slightly decreased after three cycles of photocatalytic. However, the MC and TOC removal efficiency of Ag/AgCl-G decreased significantly in the fourth cycle from 90% MC removal in the first cycle to 82% and from 75% TOC removal in the first cycle to 61%. These results expressed a significant long-term stability of the Ag/AgCl-G photocatalysts for the degradation of MC and TOC under the sunlight.
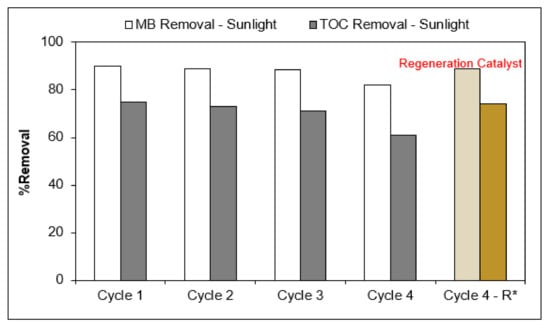
Figure 7.
Long-term stability and regeneration performance test of Ag/AgCl-G for MC degradation under the sunlight. * Regeneration Catalyst.
A regeneration experiment was also carried out to exhibit the recyclability of the Ag/AgCl-G photocatalyst and the results are shown in Figure 7. It was found that the Ag/AgCl-G nanoparticles still kept ~89% regeneration efficiency of MC and 74% of TOC at the end of the fourth cycle, indicating a relatively high regeneration potential of this photocatalyst.
3.3. Mechanism and Degradation Pathway of MC Removal Using Ag/AgCl-G
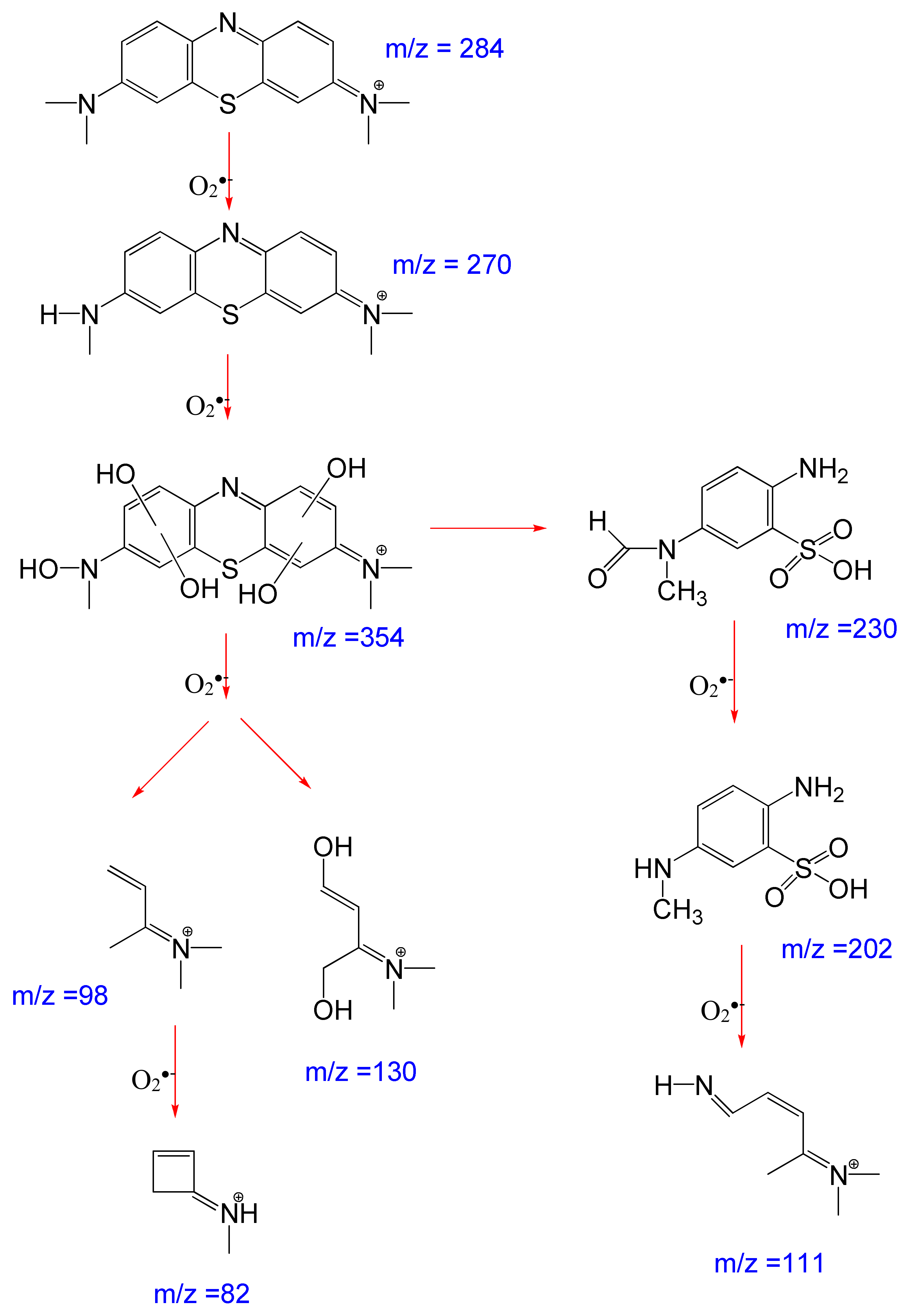
Performing GC/MS of residual MC after sunlight irradiation, a degradation pathway was proposed and is shown in Figure 8. The MC (m/z = 284) can be degraded into several intermediate products before mineralization. The spectrum obtained after 60 min reaction time revealed the presence of major intermediates N-methyl-N-(7-(methylamino)-3H-phenothiazin-3-ylidene) methanaminium (m/z = 270) at 20.1 min retention and N-(7-(hydroxyl(methyl)amino)-3H-phenothiazin-3-ylidene)-N-methylmethanaminium (m/z = 354) at 40.1 min retention, suggesting oxygen is incorporated into the structure of MC. The single bonds between N and CH3 and N and C are also broken. Peaks at lower masses identify 2-amino-5-(N-methylformamido) benzenesulfonic acid (m/z = 230) and 2-amino-5-(methylamino) benzenesulfonic acid (m/z = 202), suggesting that the photocatalytic oxidation process led to degradation into smaller molecular fragments (at m/z 82, 98, 111, and 130), which were further oxidized until finally transformed into CO2, H2O, HNO3, and H2SO4 [35,36,37].
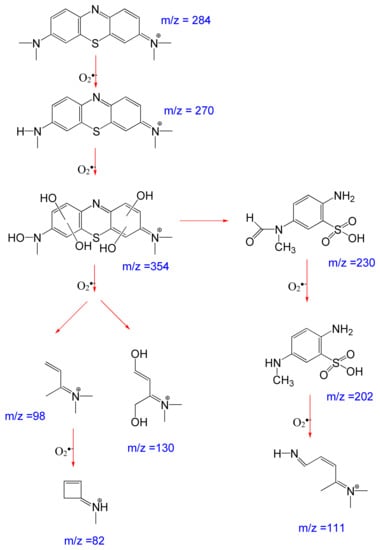
Figure 8.
Degradation pathway of methylthioninium chloride (MC+) by Ag/AgCl-G under sunlight.
4. Conclusions
The Ag/AgCl-G nanoparticles demonstrated clearly enhanced photocatalytic activity, relative to Ag-chem and Ag/AgCl-chem, which may have come from allicin and diallyl disulfide, the principal bioactive compounds present in aqueous garlic extract. The strong SPR effect from metallic Ag nanoparticles accompanied by the negative surface charge on Ag/AgCl-G nanoparticles induced by organosulfur in garlic promoted adsorption of MC, leading to better performance in MC degradation under visible light. The zeta potential showed an increased negative charge on the surface of Ag/AgCl-G from the garlic capping agent, enhancing the adsorption of MC in visible light. With the high adsorption of MC on the surface of Ag/AgCl-G, the oxidation of MC can readily occur by both the holes on the surface of Ag nanoparticles and the superoxide radicals generated from the transferred electrons. The MC can be successfully degraded into several intermediate products, including N-methyl-N-(7-(methylamino)-3H-phenothiazin-3-ylidene) methanaminium, N-(7-(hydroxyl (methyl) amino) -3H-phenothiazin-3-ylidene)-N-methylmethanaminium, 2-amino-5-(N-methylformamido) benzenesulfonic acid, and 2-amino-5-(methylamino) benzenesulfonic acid before mineralization. Finally, the photocatalytic oxidation using the Ag/AgCl-G led to the transformation of MC into CO2, H2O, HNO3, and H2SO4. The simplicity of Ag/AgCl-G synthesis with the synchronous functions in photocatalysis under visible light and sunlight can offer the convenience in applying these nanoparticles for pollutant removal in water treatment processes.
Author Contributions
Conceptualization, P.K.; methodology, P.U. and P.K.; investigation, T.B.; writing—original draft preparation, P.U. and T.B.; writing—review and editing, P.U. and P.K.; supervision, P.K.; funding acquisition, P.K. and S.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Council of Thailand under the International Research Network-Green Technologies Network for Sustainable Environment: Food-Water-Energy Nexus (Grant No. IRN62W0005) and the Postdoctoral Fellowship, CMU Presidential Scholarship support, Chiang Mai University. The work was partially supported by the Sustainable Engineering Research Center for Pollution and Environmental Management, Faculty of Engineering, Chiang Mai University, Thailand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tao, A.; Sinsermsuksakul, P.; Yang, P. Polyhedral silver nanocrystals with distinct scattering signatures. Angew. Chem. Int. Ed. Engl. 2006, 45, 4597–4601. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.J.; Barbic, M.; Smith, D.R.; Schultz, D.A.; Schultz, S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys. 2002, 116, 6755–6759. [Google Scholar] [CrossRef]
- Zhang, Q.; Ge, J.; Pham, T.; Goebl, J.; Hu, Y.; Lu, Z.; Yin, Y. Reconstruction of silver nanoplates by UV irradiation: Tailored optical properties and enhanced stability. Angew. Chem. Int. Ed. Engl. 2009, 48, 3516–3519. [Google Scholar] [CrossRef] [PubMed]
- Awazu, K.; Fujimaki, M.; Rockstuhl, C.; Tominaga, J.; Murakami, H.; Ohki, Y.; Yoshida, N.; Watanabe, T. A plasmonic photocatalyst consisting of silver nanoparticles embedded in titanium dioxide. J. Am. Chem. Soc. 2008, 130, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ye, J. Heteroepitaxial growth of platinum nanocrystals on AgCl nanotubes via galvanic replacement reaction. Chem. Commun. 2010, 46, 1532–1534. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Wei, J.; Whangbo, M.H. Ag@AgCl: A highly efficient and stable photocatalyst active under visible light. Angew. Chem. Int. Ed. 2008, 47, 7931–7933. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, H.; Xia, J.; Yin, S.; Luo, Z.; Liu, L.; Xu, L. One-pot synthesis of visible-light-driven plasmonic photocatalyst Ag/AgCl in ionic liquid. ACS Appl. Mater. Interfaces 2011, 3, 22–29. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y. Porous AgCl/Ag nanocomposites with enhanced visible light photocatalytic properties. J. Phys. Chem. C 2010, 114, 3175–3179. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, L. Rapid microwave-assisted nonaqueous synthesis and growth mechanism of AgCl/Ag, and its daylight-driven plasmonic photocatalysis. Chem. Eur. J. 2011, 17, 3710–3717. [Google Scholar] [CrossRef]
- Philip, D. Honey mediated green synthesis of gold nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 650–653. [Google Scholar] [CrossRef]
- Shukla, R.; Nune, S.K.; Chanda, N.; Katti, K.; Mekapothula, S.; Kulkarni, R.R.; Welshons, W.V.; Kannan, R.; Katti, K.V. Soybeans as a phytochemical reservoir for the production and stabilization of biocompatible gold nanoparticles. Small 2008, 4, 1425–1436. [Google Scholar] [CrossRef]
- Smuleac, V.; Varma, R.; Sikdar, S.; Bhattacharyya, D. Green synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for reductive degradation of chlorinated organics. J. Membr. Sci. 2011, 379, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Phuinthiang, P.; Kajitvichyanukul, P. Degradation of paraquat from contaminated water using green TiO2 nanoparticles synthesized from Coffea arabica L. In photocatalytic process. Water Sci. Technol. 2019, 79, 905–910. [Google Scholar] [CrossRef]
- Wang, W.; Lin, F.; Yan, B.; Cheng, Z.; Chen, G.; Kuang, M.; Yang, C.; Hou, L. The role of seashell wastes in TiO2/Seashell composites: Photocatalytic degradation of methylene blue dye under sunlight. Environ. Res. 2020, 188, 109831. [Google Scholar] [CrossRef]
- Boonupara, T.; Kajitvichyanukul, P. Facile synthesis of plasmonic Ag/AgCl nanoparticles with aqueous garlic extract (Allium sativum L.) for visible-light triggered antibacterial activity. Mater. Lett. 2020, 277, 128362. [Google Scholar] [CrossRef]
- Lan, L.; Wang, F.; Zeng, G. Staining with methylthioninium chloride for the diagnosis of fungal keratitis. Exp. Ther. Med. 2013, 6, 1229–1232. [Google Scholar] [CrossRef] [Green Version]
- Ginimuge, P.R.; Jyothi, S.D. Methylene Blue: Revisited. J. Anaesthesiol. Clin. Pharmacol. 2010, 26, 517–520. [Google Scholar] [CrossRef]
- Chou, K.S.; Ren, C.Y. Synthesis of nanosized silver particles by chemical reduction method. Mater. Chem. Phys. 2000, 64, 241–246. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Preparation of silver nanoparticles by chemical reduction method. Colloids Surf. A Physicochem. Eng. Asp. 2005, 256, 111–115. [Google Scholar] [CrossRef]
- Shu, J.; Wang, Z.; Xia, G.; Zheng, Y.; Yang, L.; Zhang, W. One-pot synthesis of AgCl at Ag hybrid photocatalyst with high photocatalytic activity and photostability under visible light and sunlight irradiation. Chem. Eng. J. 2014, 252, 374–381. [Google Scholar] [CrossRef]
- Von White, G.; Kerscher, P.; Brown, R.M.; Morella, J.D.; McAllister, W.; Dean, D.; Kitchens, C.L. Green synthesis of robust, biocompatible silver nanoparticles using garlic extract. J. Nanomater. 2012, 2012, 730746. [Google Scholar] [CrossRef]
- Kittler, S.; Greulich, C.; Diendorf, J.; Köller, M.; Epple, M. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Massich, M.D.; Giljohann, D.A.; Schmucker, A.L.; Patel, P.C.; Mirkin, C.A. Cellular response of polyvalent oligonucleotide—Gold nanoparticle conjugates. ACS Nano 2010, 4, 5641–5646. [Google Scholar] [CrossRef]
- Köth, A.; Joachim, K.; Appelhans, D.; Voit, B. “Sweet” gold nanoparticles with oligosaccharide-modified poly(ethyleneimine). Colloid Polym. Sci. 2008, 286, 1317–1327. [Google Scholar] [CrossRef]
- Rao, Y.N.; Banerjee, D.; Datta, A.; Das, S.K.; Guin, R.; Saha, A. Gamma irradiation route to synthesis of highly re-dispersible natural polymer capped silver nanoparticles. Radiat. Phys. Chem. 2010, 79, 1240–1246. [Google Scholar] [CrossRef]
- Sur, I.; Cam, D.; Kahraman, M.; Baysal, A.; Culha, M. Interaction of multi-functional silver nanoparticles with living cells. Nanotechnology 2010, 21, 175104. [Google Scholar] [CrossRef]
- Losso, J.N.; Nakai, S. Molecular size of garlic fructooligosaccharides and fructopolysaccharides by matrix-assisted laser desorption ionization mass spectrometry. J. Agric. Food Chem. 1997, 45, 4342–4346. [Google Scholar] [CrossRef]
- Zheng, Y.; Shu, J.; Wang, Z. AgCl@Ag composites with rough surfaces as bifunctional catalyst for the photooxidation and catalytic reduction of 4-nitrophenol. Mater. Lett. 2015, 158, 339–342. [Google Scholar] [CrossRef]
- Wang, S.; Tang, S.; Gao, H.; Chen, X.; Liu, H.; Yu, C.; Yin, Z.; Zhao, X.; Pan, X.; Yang, H. Microstructure, optical, photoluminescence properties and the intrinsic mechanism of photoluminescence and photocatalysis for the BaTiO3, BaTiO3/TiO2 and BaTiO3/TiO2/CeC2 smart composites. Opt. Mater. 2021, 118, 111273. [Google Scholar] [CrossRef]
- Valenti, L.E.; Giacomelli, C.E. Stability of silver nanoparticles: Agglomeration and oxidation in biological relevant conditions. J. Nanopart. Res. 2017, 19, 156. [Google Scholar] [CrossRef]
- Tian, J.; Liu, R.; Wang, G.; Xu, Y.; Wang, X.; Yu, H. Dependence of metallic Ag on the photocatalytic activity and photoinduced stability of Ag/AgCl photocatalyst. Appl. Surf. Sci. 2014, 319, 324–331. [Google Scholar] [CrossRef]
- Epling, G.A.; Lin, C. Photoassisted bleaching of dyes utilizing TiO2 and visible light. Chemosphere 2002, 46, 561–570. [Google Scholar] [CrossRef]
- An, C.; Peng, S.; Sun, Y. Facile synthesis of sunlight-driven AgCl: Ag plasmonic nanophotocatalyst. Adv. Mater. 2010, 22, 2570–2574. [Google Scholar] [CrossRef] [PubMed]
- Madhu, G.M.; Raj, M.A.L.A.; Pai, K.V.K.; Rao, S. Photodegradation of methylene blue dye using UV/BaTiO3, UV/H2O2 and UV/H2O2/BaTiO3 oxidation processes. Indian J. Chem. Technol. 2007, 14, 139–144. [Google Scholar]
- Sriskandakumar, T.; Opembe, N.; Chen, C.H.; Morey, A.; King’ondu, C.; Suib, S.L. Green decomposition of organic dyes using octahedral molecular sieve manganese oxide catalysts. J. Phys. Chem. A. 2009, 113, 1523–1530. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Gonçalves, M.; Guerreiro, M.C.; Ramalho, T.C.; Fabris, J.D.; Pereira, M.C.; Sapag, K. A new catalyst material based on niobia/iron oxide composite on the oxidation of organic contaminants in water via heterogeneous Fenton mechanisms. Appl. Catal. A Gen. 2007, 316, 117–124. [Google Scholar] [CrossRef]
- Liu, T.; Yang, G.; Wang, W.; Wang, C.; Wang, M.; Sun, X.; Xu, P.; Zhang, J. Preparation of C3N5 nanosheets with enhanced performance in photocatalytic methylene blue (MB) degradation and H2-evolution from water splitting. Environ. Res. 2020, 188, 109741. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).