Abstract
Microsamples are collections usually less than 50 µL, although all devices that we have captured as part of this review do not fit within this definition (as some can perform collections of up to 600 µL); however, they are considered microsamples that can be self-administered. These microsamples have been introduced in pre-clinical, clinical, and research settings to overcome obstacles in sampling via traditional venepuncture. However, venepuncture remains the sampling gold standard for the metabolic phenotyping of blood. This presents several challenges in metabolic phenotyping workflows: accessibility for individuals in rural and remote areas (due to the need for trained personnel), the unamenable nature to frequent sampling protocols in longitudinal research (for its invasive nature), and sample collection difficulty in the young and elderly. Furthermore, venous sample stability may be compromised when the temperate conditions necessary for cold-chain transport are beyond control. Alternatively, research utilising microsamples extends phenotyping possibilities to inborn errors of metabolism, therapeutic drug monitoring, nutrition, as well as sport and anti-doping. Although the application of microsamples in metabolic phenotyping exists, it is still in its infancy, with whole blood being overwhelmingly the primary biofluid collected through the collection method of dried blood spots. Research into the metabolic phenotyping of microsamples is limited; however, with advances in commercially available microsampling devices, common barriers such as volumetric inaccuracies and the ‘haematocrit effect’ in dried blood spot microsampling can be overcome. In this review, we provide an overview of the common uses and workflows for microsampling in metabolic phenotyping research. We discuss the advancements in technologies, highlighting key considerations and remaining knowledge gaps for the employment of microsamples in metabolic phenotyping research. This review supports the translation of research from the ‘bench to the community’.
1. Introduction
Healthcare has undergone a paradigm shift in recent years, evolving from a reactive disease care focus to one that is predictive, preventive, personalised, and participatory (P4 medicine) [1]. This shift has relied on advances in systems medicine platforms such as metabolic phenotyping, whereby the sensitive detection and measurement of circulating metabolites provides researchers with detailed descriptors of the metabolic perturbations that occur in response to stimuli such as disease, thereby offering mechanistic insights into pathological processes [2,3]. Furthermore, through the biochemical classification of an individual’s physiological or pathological state, such approaches have facilitated the stratification of populations, assisting in disease diagnosis, prognosis, response to therapeutic interventions, and the identification of disease risk factors at the population level [2,3].
Gas chromatography– and liquid chromatography–mass spectrometry (GC–, LC–MS) techniques are routinely employed in metabolic phenotyping. Of the two techniques, LC–MS is the most beneficial for its ability to be set up for discovery and targeted pathway analysis. Through the use of high-resolution, accurate MS, the individual identification and quantification of metabolites can be performed [3,4]. Additionally, triple-quadrupole MS can be used for targeted applications [3,4]. MS is performed following the preparation of samples using different extraction solvents, which are selected on the basis of their ability to perform the optimal extraction of metabolites of interest based on their molecular properties.
When considering applications of metabolic phenotyping, the gold standard for measurement is venous whole blood, which is commonly taken from the anti-cubital fossa, and then separated into either plasma or serum [5]. This entails time-consuming protocols for a venepuncture blood collection to occur in an approved clinical space, scheduling an appointment in advance with a trained phlebotomist, and, depending on the nature of research, it may require a patient to abstain from food for >8 h and travel to a laboratory for blood collection. Furthermore, the lengthy post-collection protocols that venepuncture necessitates include sample processing via centrifugation, splitting (into smaller aliquots), and freezing at −80 °C prior to cold-chain transport, which further add to the burden of sample collection and economic costs, which impact budgets [6,7]. In the laboratory, if the sample is intended for metabolic phenotyping technologies, whole blood from the collection then needs to be separated into either serum or plasma and frozen to ensure the long-term integrity of the sample. Venepuncture presents two main issues: reduced study sizes and the inability to perform frequent sampling [8]. As such, venepuncture may limit the scope for metabolic phenotyping research, particularly in research areas that include remote or rural populations, patients with limited mobility, or those who are immunocompromised. This diminishes opportunities for research teams and population-based studies [9,10,11].
The invasive nature of venepuncture and its resultant frontend sample yields an excess of 400–2000-times the volumes required for metabolic phenotyping analyses (for typical phlebotomy collections of 2–10 mL) [2]. However, metabolic phenotyping assays that employ LC–MS are amenable to low sample volumes; for example, LC–MS assays routinely require <25 µL to perform metabolic phenotyping for both discovery profiling analysis and targeted pathway analysis [2,12,13,14,15,16,17,18,19,20]. Such methods have been applied to metabolic phenotyping experiments that have assessed outcomes of health and disease including inflammation [17], cardiometabolic diseases [21], dementia [17], and nutrition [22]; they are commonly targeted for many clinical and human physiology studies. This move towards sample miniaturisation in the space of metabolic phenotyping has led research teams to begin the evaluation of microsamples over traditional venepuncture for sample collection [23].
To advance the field of metabolic phenotyping, there is a growing demand for biospecimen collections to move beyond laboratory collections and into the community (i.e., patient self-sampling). The use of microsamples, particularly in the form of dried blood spots (DBSs), began back in the 1960s, where Guthrie and Susi spotted neonate blood onto filter paper for the screening of genetic conditions [24]. Since the DBS conception, other applications in research and routine laboratories have commenced, including therapeutic drug monitoring [25], routine illicit drug and alcohol monitoring [26], sport and anti-doping [27], nutrition [28], and the detection of inborn errors of metabolism [29]. Microsamples facilitate self-sampling and are ideal candidates for biospecimen collections as small volumes (typically < 50 µL). Although all devices that we have captured as part of this review do not fit within this definition (as some can perform collections of up to 600 µL), they are considered microsamples that can be self-administered. These microsamples can be obtained using a range of specific devices and collection protocols [30]. Common collection technologies include traditional dried samples such as DBSs and advanced microsampling devices (commercially available devices optimised to collect a variety of biological media, including whole blood [31,32], whole blood separated into plasma [33] or serum [34], and urine [35]). Most of these devices require a small amount of capillary blood from a minor skin incision such as a prick [28], usually from a fingertip; however, other sites have been utilised, such as the arm [36].
The employment of microsamples in metabolic phenotyping workflows thus far has been predominantly in the pre-clinical space. These applications have the potential to provide researchers with a widely applicable, less invasive sampling workflow that can be easily implemented in challenging environments to improve accessibility [9,11,30,37]. This can include those in underserved rural and remote areas without access to cold storage and trained personnel. Microsampling also allows for safe sampling on those who are immunocompromised, facilitates amenable blood collections for elderly or infant participants, and improves access for individuals where travelling presents a barrier to their participation [9,11]. Furthermore, the use of microsampling devices could ease sampling constraints, allowing researchers greater frequency of sampling and increased overall population sample size in epidemiology and clinical studies, thereby improving statistical sample power [10,38]. The benefits of microsamples derived from our reading of the literature have been illustrated (Figure 1). We have also illustrated their comparison to phlebotomy when used in metabolic phenotyping pipelines (Figure 2).
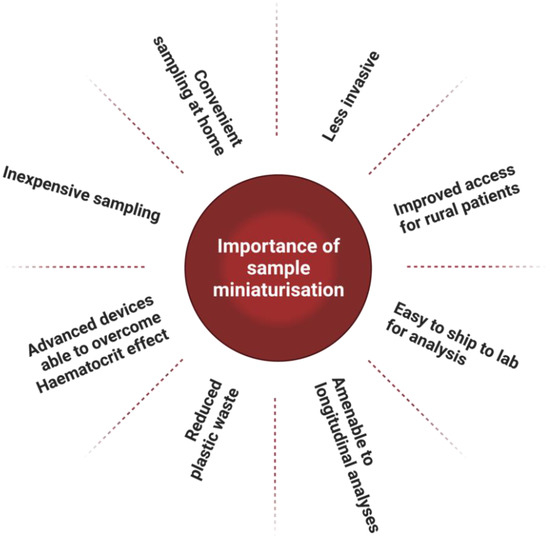
Figure 1.
Illustration of the importance of sample miniaturisation for clinical applications.
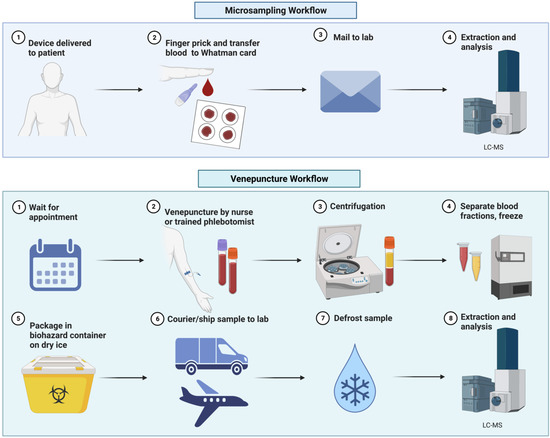
Figure 2.
Metabolic phenotyping pipeline comparison for microsampling and venepuncture.
This review of microsamples, to our knowledge, is the first of its kind to summarise applications to the metabolic phenotyping literature, wherein we highlight where applications have been successful/advantageous. Importantly, we also provide thoughtful consideration for where future research must further be developed in the metabolic phenotyping community, to overcome some of the barriers faced by research groups currently using microsampling. We discuss the available literature on microsampling on DBS in metabolic phenotyping where papers were readily available from PubMed, Google Scholar, and our university library portal.
2. Approaches to Microsample Collections
2.1. Traditional Dried Samples: Dry Blood Spots (DBSs)
Historically, DBSs have been the flagbearer for microsampling. The concept for the preservation of dried human biological samples as a spot is attributed to Ivar Christian Bang in 1913 [39,40]. Typically, these microsamples can be collected via a skin prick with a lancet or created by transferring from a phlebotomy tube with a micro-pipette [30,41]. Currently, such samples are collected on a specialised filter paper, which can contain anywhere from 15 to 50 µL of blood, and take approximately three to four hours to completely dry at room temperature [39]. This can make DBSs amenable to collections where cold-chain shipping protocols may not be feasible. However, it should be noted that below-zero temperatures (−20 °C, −80 °C) are still recommended for the long-term storage of DBSs [30,39,41].
DBS samples have been evaluated in MS-based metabolic phenotyping and have been used in applications of cancer diagnostics [42], cancer treatments [43], air pollution [44], drug discovery [45], acidemia [46], and pyruvate kinase deficiency [47]. Despite this wide range of applications of DBS samples, there still remain concerns around their volumetric accuracy, particularly in metabolic phenotyping, where the standardisation of sample volume is required. For example, the uneven spread of collected blood across the spot can introduce metabolite variation due to differences in viscosity/haematocrit (hct %; the fraction of blood made up of red blood cells). This has been observed in DBS studies using liquid scintillation analysis, where sub-punches from an individual sample were taken and the number of metabolites measured varied vastly from sample to sample [48]. DBS collections are remarkably susceptible to variation based on an individual’s haematocrit. Varying haematocrit levels are of particular concern due to its propensity to undergo rapid changes in the body. This is a widely known phenomenon in DBS microsampling and is commonly referred to as the ‘haematocrit effect’ [49], where external factors such as dehydration, polycythaemia, anaemia, overhydration, kidney failure, or chronic inflammatory conditions can cause plasma volume perturbations. Additionally, pregnancy may also cause slightly decreased hct due to an increase in blood volume [30,50]. For reference, the normal range of hct is 36–48% for women and 42–52% for men [51]. Put simply, increases in hct beyond the normal range affect blood viscosity and therefore reduce the spread of the blood spotted on the carrier material, whereas decreases in hct (i.e., reduced viscosity) can create greater spread [52]. As such, the sample’s spotted area has a linear, inverse relationship with hct [49,50].
In MS-based studies, normalisation techniques have been employed in order to address the ‘haematocrit effect’ [53]. However, it remains one of the most prevalent challenges faced for translating the wide use of microsamples in metabolic phenotyping workflows. To date, studies have successfully performed DBS haematocrit normalisation through potassium content [54], using near-infrared (NIR) spectroscopy [55], haemoglobin measurement using non-contact diffuse reflectance spectroscopy [56], and using wax barriers on DBSs [57]. Despite the development of these normalisation techniques for DBSs, they are yet to be widely adopted in metabolic phenotyping workflows, with many literature examples not implementing a normalisation step in their protocol descriptions [43,44,46,47,55,58,59,60]. Only two studies have openly reported hct normalisation of their DBS samples as part of their metabolic phenotyping workflow. The first was by Koulman et al., who analysed infant heel-prick DBS samples, utilising a volumetric and hct-independent LC–MS method [61]. This was performed by relatively expressing the extracted lipid intensity of a given DBS sub-punch to its summed intensity. Interestingly, this method revealed that lipid profiles in DBSs showed comparable or better precision to plasma and whole blood samples, which the authors propose could be attributed to the halting of the oxidative process in dried samples compared to traditional venous whole blood and plasma samples [61]. Another study successfully employed an automated haematology analyser for the analysis of hct in serum to normalise steroid concentrations obtained from traditional DBSs [36]. This correction, by Salamin et al., used the following equation [36]:
Another biological factor that affects DBS sample quality includes the nature of the analyte(s) of interest, because blood cells can cause variations in the amount of analyte that is extractable from the surface of the DBS card itself [62]. The reason for this is that analyte partitioning can occur between plasma and blood cells, which significantly influences the concentrations of analytes in plasma or whole blood samples taken from a DBS, although this is most commonly seen in monoclonal antibodies for pharmacokinetic studies [25,63]. Additionally, prominent sources of variation in DBS homogeneity have been attributed to the paper substrate used [49]; inconsistencies in the storage, packaging, and transport of samples [42,43]; and contact of DBSs with other surfaces [6].
The circulating blood metabolome is a tightly controlled homeostatic system, where preanalytical variation (paper substrate, storage, packaging, transport) can unavoidably lead to inaccurate and possibly misleading results [64]. This is a current limitation in pre-analytical workflows for DBS microsamples, particularly when considering the inherently heterogenous nature of biological samples, where physiological conditions (i.e., hct) already contribute dynamic changes [65]. As such, efforts seeking to enhance accuracy, sensitivity, and specificity during the analytical phase are in vain if the technical aspects that underpin microsample workflows are not reproducible. Thus, a lack of reproducibility and accuracy is detrimental to achieving outcomes of P4 medicine using DBS samples in metabolic phenotyping pipelines [1].
2.2. Improving Microsample Collection: Are Advanced Devices the Future for Metabolic Phenotyping?
Advancements in microsampling devices have allowed for improvements in blood collections by removing some of the inconsistencies experienced in DBS collections whilst maintaining the convenience of microsampling (Table 1). These devices can be classified into three broad classes: advanced dried samples; passive separation devices; and whole biofluid collectors, which will be discussed in the context of metabolic phenotyping below (Section 3).

Table 1.
Microsampling devices.
Advanced dried sample devices collect samples as a fluid and produce a dried sample either with a polymer tip (volumetric absorptive microsampling, “VAMS”—Neoteryx; Torrance, CA, USA) or carriers akin to DBS cards, such as the hemaPEN (Trajan; Melbourne, VIC, Australia), HemaXis DB10 (DBS System SA; Gland, Switzerland), Capitainer qDBS (quantitative dried blood spot) and B-Vanadate (Capitainer AB; Solna, Sweden), TASSO-M20 (HemoLink; Seattle, WA, USA), and HemaSpot HD and HF (Spot on Sciences; San Francisco, CA, USA) [27,28,36,66,67,68,69,70,71,72,73]. These technologies improve upon standard DBS cards by providing accurate/volumetric aspiration and thus heamatocrit-independent sample collection over a wide range of microsample sizes (2.74–30 µL).
Passive separation devices allow for the in situ separation of whole blood into its sub-components. e.g., serum or plasma, which can then store the resultant product in liquid form, e.g., TASSO+ (HemoLink; Seattle, WA, USA); as dried serum, e.g., HemaSpot SE (Spot on Sciences; San Francisco, CA, USA), or as dried plasma, e.g., Tellimmune Plasma Separation Cards (Novilytic; West Lafayette, IN, USA). Passive separation devices represent an expanding area commercially, with many dried plasma spot (DPS) devices currently in development, including the DPS (Capitainer AB; Solna, Sweden), Book-Type DPS (Q2 Solutions; Morrisville, NC, USA), and the Hemaxis DX (DBS System SA; Gland, Switzerland) [74,75,76]. It is not known how these devices will perform in metabolic phenotyping. However, as they produce samples akin to those obtained from venous whole blood separations, which are commonly used in metabolic phenotyping, they warrant further investigation in the field.
Whole biofluid collectors are advanced devices with the ability to collect and produce samples as liquid samples without the need for cellulose material. These devices can collect sample volumes as small as 23 µL, e.g., the MSW2 (Shimadzu; Kyoto, Japan), and extend to 100 µL, e.g., the TAP (Yourbio Health; Medford, MA, USA), and up to 600 µL, e.g., the TASSO+ and TASSO-SST (HemoLink; Seattle, WA, USA) [10,77,78].
All three broad classes of advanced microsampling devices are well positioned for direct implementation in metabolic phenotyping workflows in clinical and epidemiology studies. For example, they are already commercially available and therefore have advanced manufacturing consistency; they have been designed to counter specific challenges in DBS and traditional microsampling workflows, including the ‘haematocrit effect’ [30,71], and have already achieved translation to non-metabolic phenotyping analytical chemistry protocols [27,79]. Despite these benefits, the translation of microsampling devices to metabolic phenotyping research is yet to be widely adopted by the field, as extensive studies investigating comparability to venipucture and metabolite stability are lacking [80]. This is important as validated microsampling methods that leverage the advances in device design have the potential to enhance metabolic phenotyping studies in clinical and epidemiology settings, facilitating greater sampling frequency and sample size, and thereby providing valuable gains in statistical power [80,81].
3. Advanced Microsamples: Current Applications in Metabolic Phenotyping
Recently, the implementation of advanced microsampling devices in metabolic phenotyping workflows has been reported in the literature; however, collection and analytical protocols have not been extensively evaluated and optimised for the given devices available. These reported advanced microsampling devices cover a range of technologies for capillary blood collection, including advanced dried samples, passive separation devices, and whole biofluid collectors (Table 2). However, by no means do they encompass all devices currently available, commercially or for research purposes. Moreover, although the literature presents a large variety of applications and pre-analytical sample preparation techniques, all studies primarily utilised LC–MS based platforms for analysis.

Table 2.
Comparison of microsamples in mass spectrometry-based analytical chemistry and metabolic phenotyping methodologies: Extraction and analytical methods.
3.1. Advanced Dried Microsamples
Advanced dried microsamples represent the largest portion of published literature in the scope of metabolic phenotyping, for which metabolite coverage and metabolite stability have been the key focus. A variety of devices of this technology type have been used, with findings detailed below. Some studies additionally performed analysis of haematocrit to identify the ability of the devices to perform haematocrit-independent sample collection, which are also detailed below, separately. A summary of applications of advanced dried microsamples in metabolic phenotyping has also been provided below.
3.1.1. Metabolite Coverage and Stability of Different Advanced Dried Microsample Devices
The Capitainer B-Vanadate (Capitainer AB; Solna, Sweden) was used for the quantification of caffeine and paraxanthine using venous blood from a healthy, caffeine-abstinent female (n = 1) [73]. This research was limited to simulated blood collections using venous blood transferred to the microsampling device with a micropipette, and stability testing was performed in triplicate for each of the two time points. Caffeine and paraxanthine concentrations were examined as stable at 4 days (60 °C) and at 3 months (room temperature or −20 °C).
The VAMS Mitra (Neoteryx; Torrance, CA, USA) device has been employed on two published accounts in metabolic phenotyping for the assessment of metabolite coverage and stability. In a study performed by Kok et al., the 10 µL VAMS Mitra (Neoteryx; Torrance, CA, USA) device was assessed using reversed-phase chromatography and hydrophilic interaction liquid chromatography on plasma taken from one individual [99]. From the two methodologies, 36 analytes were recovered (24 amino acids and 12 organic acids) and assessed for stability over 15 days, with measures being repeated at 2 h, and 1, 4, 7, and 15 days. No stability issues were observed during the first four days when stored at room temperature. However, at 15 days, increases and decreases were observed in some amino acids (a decrease of 19.2% and 15.4%, respectively, for methionine and tyrosine). These changes were seen more markedly in organic acids, highlighting that they are less stable than amino acids. Specifically, these were malic acid (−21.8%), glutathione (−31.8%), uric acid (−22.9%), glyoxylic acid (+36.8%), pyruvic acid (+26.0%), 3-hydroxypropionic acid (+37.9), and succinic acid (+46.3%). These stability findings contrast the known instability of organic acids and amino acids stored in liquid blood for the same time [99]. In another study, an untargeted methodology was used by Volani et al., to analyse an unknown number of pooled EDTA venous blood samples using 69 VAMS Mitra (Neoteryx) devices. The results of this study contrasted those of Kok et al., who demonstrated that metabolites were stable for up to 4 days when stored at room temperature [100]. Specifically, differences were observed in ~75% (77/103) putatively identified metabolites at 2 h, 24 to 48 h, and 4 days, when visualised with principal component analysis (PCA), and in fact up to 6 months (one-way ANOVA, p < 0.005), with 36% of these differences in metabolites manifesting as decreases over time (e.g., histidine, glutamine, and asparagine), and 31% of these metabolites increasing over time (e.g., glutamic acid, glyceric acid, and methionine) [100]. Reasons for metabolite increases were not postulated, but could potentially include the conversion of one metabolite to another or a relative concentration effect.
In a study of 20 healthy participants, the hemaPEN (Trajan; Melbourne, VIC, Australia) was used to collect blood samples for the analysis of 13 metabolites influenced by exercise, at training intervals, including nine amino acids and four organic acids [72]. Nine metabolite concentrations had variation <15% upon storage at −20 °C for five months. However, concentration decreases were observed in 2-oxoglutaric acid (35.1%) and methionine (56.5%), and concentrations increased for creatine (17.6%), and taurine (15.7%) [72].
Venous-derived serum, cited as the World Anti-Doping Agency (WADA) gold standard for sample collection in steroid profiling, was compared to a variety of advanced dried microsamples by Salamin et al. [36]. The researchers analysed 11 free and 8 conjugated steroids following testosterone gel administration in 14 healthy, eumenorrheic women. Testosterone concentrations were first measured in traditional DBSs (created with venous blood) and were found to highly correlate with those in serum (r > 0.84 with Passing–Bablok regression analysis). Following these positive results, Salamin et al. also investigated the TASSO-M20 (HemoLink; Seattle, WA, USA) (placed on the upper arm) for steroid profiles using capillary blood from 14 healthy volunteers (7 females, 7 males) compared to traditional Whatman Protein Saver Card DBSs (Cytiva; Marlborough, MA, USA) (via finger prick). Finger pick DBSs and TASSO-M20 (HemoLink; Seattle, WA, USA) dried samples demonstrated strong correlations for all quantified steroids using a Spearman’s correlation. These steroids included testosterone (r = 0.97, p < 0.0001), androstenedione (r = 0.85, p < 0.0001), DHEA (r = 0.93, p < 0.0001), 17a-OH-progesterone (r = 0.98, p < 0.0001), progesterone (r = 0.93, p < 0.01), and cortisol (r = 0.91, p < 0.0001). The researchers caveat their capillary DBS findings with the likelihood of high capillary testosterone concentrations being attributed to the topical application of the testosterone gel, although the use of advanced devices such as the TASSO-M20 (HemoLink; Seattle, WA, USA) can overcome this pitfall due to its independence from the sample collection site (i.e., can be applied to upper arm) [36,77]. Furthermore, Salamin et al. highlight the potential for DBS storage of steroids for 1 and 3 weeks at room temperature with minimal risk of degradation (contrary to serum samples) [36]. This translation is important for future applications of microsamples in the context of anti-doping (i.e., WADA) [36,77].
3.1.2. Analysis of Haematocrit
Evaluation of hct across studies using advanced dried microsamples has shown promising results. Results by Velghe et al. demonstrated the ability of the Capitainer B-Vanadate (Capitainer AB; Solna, Sweden) device to eliminate hct bias over a range of 18.8–55.0 [73]. Linear regression of Capitainer samples revealed that caffeine and paraxanthine were not affected by hct when compared to whole blood. Interestingly, when repeating the analysis using a traditional DBS sub-punch (again, simulated with a micropipette from venous blood), linear regression revealed the presence of a negative hct bias. Here, there were reduced analyte concentrations witnessed in DBSs compared to whole blood [73]. Accurate and precise collection of samples collected from a single drop of blood with minimal influence by hematocrit is similarly demonstrated by the hemaPEN (Trajan; Melbourne, VIC, Australia) [27,72,79]. This has been examined in the scope of analytical chemistry for caffeine and paraxanthine, where comparing hemaPEN (Trajan; Melbourne, VIC, Austrtalia) concentrations and those in whole blood revealed a 6.90% and 5.40% difference in mean concentrations, respectively [27]. Although statistically significant, the researchers concluded that this variation was negligible, when comparing hemaPEN (Trajan; Melbourne, VIC, Australia) to conventional sub-punch DBS results, and sub-punch DBS results to whole blood. In the therapeutic drug monitoring of fluoxetine and sertraline (and their metabolites), hemaPEN (Trajan; Melbourne, Victoria, Australia) samples revealed a range of 3–9% variation in analyte recovery for different values of hct [79]. Conversely, other advanced dried sample technologies purported (and marketed) to overcome the ‘haematocrit effect’, such as the VAMS Mitra (Neoteryx; Torrance, CA, USA), are yet to investigate hct in metabolic phenotyping workflows.
3.1.3. Summary of Advanced Dried Microsample Findings
Generally, the metabolic phenotyping of advanced dried microsamples performed analysis within a few days of microsample collection, with infrequent longitudinal stability assessment, up to a maximum of six months [99]. This is in contrast to traditional DBSs, which have been performed up to 21 years following hct correction (a factor that may not be necessary for the future use of archived advanced samples that are hct-independent) [56]. Storage conditions typically employed those readily available in laboratory settings (room temperature, −20 °C, −80 °C), which may not reflect the reality of multi-site clinical studies, where microsamples are likely to undergo temperature variations or may encounter extreme climates (high heat and humidity) [101]. Pre-analytical workflows for these samples mainly relied on manufacturer protocols and demonstrated great variability in drying time and analytical turnaround. Furthermore, 3/6 studies were limited to an n = 1 [36,73,99], with only one study reaching an n = 20 [72], which further research should seek to expand upon to increase the statistical power and ensure the reproducibility of results.
3.2. Passive Separation Devices
Passive separation devices with the ability to obtain blood fractions such as plasma and sera currently represent the area of greatest focus in microsampling technology innovation. Many plasma separation devices are yet to be made commercially available, including the Capitainer DPS (Capitainer AB; Solna, Sweden), Book-Type DPS Card (Q2 Solutions; Morrisville, NC, USA), and the HemaXis DX (DBS System SA; Gland, Switzerland), and thus have yet to be translated to metabolic phenotyping.
Telimmune plasma cards (formerly Noviplex plasma prep cards) are currently the only plasma separation technology that has been translated to metabolic phenotyping research [53]. This was performed by Cvetko et al., who compared the glycoprofiles of 10 participants. This study utilised self-sampling, to compare Telimmune plasma cards (Novilytic), DBSs, and the VAMS Mitra (Neoteryx) devices to plasma. Both Telimmune plasma cards (Novilytic) and the VAMS Mitra (Neoteryx) devices managed to adequately replicate venous-derived plasma glycoprofiles. Their comparability was calculated using the relative deviance between the advanced microsampling devices and traditional DBSs, to plasma, for each of the 39 glycan peaks. Interestingly, the Telimmune plasma cards (Novilytic) had the least relative deviance to plasma (0.069), followed by VAMS Mitra (Neoteryx) (0.092), with the most deviance to plasma being displayed in traditional DBS samples (0.674).
A smaller subset of five participants was utilised to perform self-sampling in hexaplicate to assess the analytical reproducibility of different microsample platforms. These were calculated as average %CV (coefficient of variation) for all glycan peaks. Telimmune plasma cards (Novilytic) displayed the least variation (4.831%), followed by the VAMS Mitra (Neoteryx) (7.098%), with the most variation being seen in DBSs (14.305%). Cvetko et al. demonstrated the ability of passive plasma separation devices such as the Telimmune plasma cards (Novilytic) to show reproducibility with venous-derived plasma, and analytical reproducibility when sampling is self-administered by participants, thus highlighting the great potential for the use of this advanced microsampling technology in larger cohort studies [53]. Importantly, although acknowledged as a limitation, variations in sample volume and hct from self-sampling are factors that were not addressed in this research. Furthermore, haemolysis (from inappropriate capillary lancing techniques) and under-sampling by participants may interfere with mass spectrometry-based metabolic phenotyping pipelines, introducing erroneous results [53].
The only serum passive separation device in existence is the HemaSpot SE (Spot On Sciences; San Francisco, CA, USA). Currently, applications are limited to manufacturer publications, highlighting a gap in the translation of serum separating technologies to metabolic phenotyping [34].
3.3. Whole Biofluid Collectors
Presently, only one reported account of whole biofluid collection technology has been used in metabolic phenotyping [78]. This was a pre-clinical study performed using the MSW2 (Shimadzu; Kyoto, Japan) by Hotta et al., who investigated the pharmacokinetic effects of administering a cocktail of antiepileptic drugs (carbamazepine, lamotrigine, and phenytoin) in four rats, and performed qualitative metabolite identification in rats (n = 2) using carbamazepine (100 mg/kg) as a model drug. In the pharmacokinetic study, blood samples were collected via the tail vein, using a glass capillary to inoculate the device with blood before centrifuging to obtain the respective separated blood fragment ‘chips’. Drug stability testing was then performed for a variety of storage conditions on the different chips. The stability data of the drugs revealed percent biases within the acceptable range (≤±15.0%) for all conditions. In a pre-clinical context, this highlights the stability of these antiepileptic drug samples over a wide range of storage conditions. These conditions included plasma at ambient temperature for 24 h, whole blood at ambient temperature for 1 h, and plasma at −20 °C for 140 days (the total storage duration from sample collection to analysis). Additionally, freeze/thaw (−20 °C/ambient temperature) was investigated for two cycles, as were processed plasma samples at 6 °C for 42 h.
For the latter metabolite identification study, blood samples were collected at 1, 2, 4, 8, and 24 h post-carbamazepine dose. The contents of two device ‘chips’ at the same time points were pooled in a tube (to total 5.6 µL of plasma). This plasma was then diluted 10-fold with blank rat plasma. Following this, LC–MS/MS analysis allowed for the characterisation of seven metabolites of carbamazepine. This allowed for the development of a proposed metabolic pathway of carbamazepine in rat plasma. Hotta et al. concluded that the MSW2 (Shimadzu; Kyoto, Japan) is technically easy to use with minimal training, and their findings suggest that whole biofluid collection technologies can be useful for the assessment of metabolite safety and to perform metabolite identification [78].
4. Considerations for Future Application of Advanced Microsampling in Metabolic Phenotyping Workflows
The use of advanced microsampling devices (of each technology type) demonstrated within the literature has shown promise for bridging the gap in the routine adoption of microsamples in metabolic phenotyping and within clinical research. However, there remain crucial shortcomings associated with microsample collections that need to be addressed. For the successful metabolic phenotyping of microsamples, crucial additional validation steps are required yet often not always considered within the scope of the assessed literature. Dependent on the analysis, targeted or untargeted standardised methods can be used [20,60,102]. However, prior to achieving this, the assessment of device accuracy, optimal extraction method, and optimal temperature and storage conditions would need to be performed prior to their implementation, routinely. Additionally, the stability of the sample is another important factor as different conditions can affect the recovery of metabolites in a sample. Considerations for stability are further discussed in this section, with particular focus given to the shortcomings of assessments of the long-term storage and stability of advanced microsamples, pre-analytical reproducibility in microsample preparation, and analyte concentration variations between whole blood, plasma, and serum. This is needed before the successful identification, validation, and quantification of novel biomarkers can occur. Indeed, if microsampling is going to be successful in population-based metabolic phenotyping research, patient self-variability/error is an additional area of contention that needs to be addressed. These are important considerations for the future adoption of advanced microsampling devices in longitudinal metabolic phenotyping studies with regular participant follow-up.
4.1. Microsample Collection and Stability
DBSs have traditionally been used in a variety of applications due to their ability to provide a low-volume, fast, and minimally invasive collection. In fact, multiple protocols and guidelines exist for the collection of traditional dried microsamples (i.e., DBSs), such as those for the proper sampling of capillary blood [103], selection of filter paper [104], sample application to the carrier [104], and packaging and transport of samples [105], thus ensuring some level of sampling reproducibility in current applications of DBSs in the field of metabolic phenotyping. However, these sampling protocols do not apply to advanced microsamples, which are limited to manufacturer SOPs. A research gap therefore exists in assessing optimal collection protocols that consider different drying times, temperatures, and storage conditions, which is inherently linked to microsample stability.
Assessment of the long-term stability and optimal storage conditions of advanced devices is still necessary for best practice in metabolic phenotyping pipelines. Accurate detection of metabolite concentrations in biological samples naturally requires sample stability, as fluctuations in environmental conditions, such as temperature and humidity, may have a deleterious effect on biological samples in the pre-analytical phase [6,8]. For example, plasma and serum samples are known to be affected by storage conditions such as increased temperatures and repeated freeze/thaw cycles [78]. Microsamples are no different, and thus considerations from the literature have included ensuring airtight and leak-proof packaging with desiccant to prevent deterioration from heat and moisture accumulation, as well as providing protocols for temperate conditions during transport (in multi-site studies), and timeframes for sample turnaround [100,106].
Recent microsample applications have implemented the use of data loggers (EL-USB2+; Lascar Electronics, Erie, PA, USA) to monitor the temperature, dew point, and humidity of DBS samples during shipment. In one study, temperature and humidity were measured at 30 min intervals in DBS samples that travelled 11,600 miles in six days [6,8]. Samples were observed to range from below freezing to over 25 °C throughout the course of the journey. Although the compound analysed by Bowen et al. was not disclosed, the results of their in-house stability assessment (based on temperatures that their DBSs encountered previously) were performed by setting the low QC level to −20 °C and high QC level to 40 °C. Taken together, the study by Bowen et al. indicated that temperature extremes had no deleterious effects on the stability of a single compound in analytical replicates, with %CV ≤ 8% on average, following ambient storage for 4 months, and at −20 °C and 40 °C storage for 48 h [7,8].
Another advantage of dried sample matrices is enhanced metabolite stability over a range of temperatures during storage and transportation. Most research concludes that analytes appear to be stable (for a variety of matrices) when stored in conditions with a low temperature and humidity (~30%, akin to a lyophilised environment) [107]. Indeed, in a study by Strnadová et al., who examined the long-term stability of amino acids and acylcarnitines, it was found that, of the analysed amino acids, valine was stable for up to 14 years in DBS samples stored at room temperature. However, other amino acids and acylcarnitines degraded more rapidly ‘per year’ [108]. In 2017, similar results were demonstrated in a lipidomic analysis of air-dried DBS cards stored with desiccants at different temperatures (4 °C to 37 °C) by Gao et al. [109]. Significant changes were noted for diacylglycerides in cards stored at 4 °C and room temperature for up to two weeks, and in most lipids stored at 37 °C. In addition, in 2017, Drolet et al. attributed increased variation in 350 DBS metabolites to storage temperatures up to 37 °C in comparison to those at room temperature and −20 °C [82]. The highest level of variability that Drolet et al. observed was at 37 °C on Day 14 of the storage conditions [82]. Future research must consider translating these findings to advanced microsampling devices, as this is yet to be performed beyond temperatures commonly encountered in a laboratory setting (−80 °C, −20 °C, 4 °C, and room temperature).
A variety of procedures and environmental conditions have been investigated for their impact on the metabolite stability of advanced microsamples. However, in the current literature, most research has not provided meaningful conclusions relevant to long-term storage outcomes for metabolites. For instance, ‘variation’ in metabolites (classes, panels, or even individually) was often reported without an associated direction (increase or decrease). Additionally, analytical reference points for assessing stability were often weak, and time points spanned relatively short periods (or were infrequent if conducted over a longer time). The interpretations of these stability data were inconsistent, mainly focusing on analyte recoveries and concentrations for the classification of disease outcomes. Future research should seek to identify the reproducibility of purported metabolite stabilities based on storage duration and temperature. Furthermore, these should be over a greater period, with more frequent time points. This will allow for meaningful comparisons between studies, and address the limited research published on advanced microsampling devices’ long-term stability.
4.2. Microsample Preparation
Consistent microsample preparation including sub-punches (if using traditional DBSs), extraction, and automation is important for reproducible analyte extraction. Similarly, consistent enrichment is important, should detection limits need to be improved for quantification (preconcentration) and the selective removal of interfering substances (sample clean-up) [110]. These processes are integral in metabolic phenotyping workflows as they determine the molecular concentrations in complex biological matrices (including blood, urine, and body tissues) used to derive clinical and biological conclusions. As such, sample preparation optimisation (specific to the advanced microsample device in use, i.e., advanced dried microsample, passive separation device, or whole biofluid collector) is vital for the accurate interpretation of results.
In DBS microsample preparation workflows, a sub-punch (also referred to as a partial punch), usually 3 mm in diameter, is taken. Theoretically, this is to reduce volumetric inaccuracies by using a fixed-diameter punch, similar to taking a fixed volume of plasma with a pipette. However, the literature disagrees on the usefulness of this, largely due to sources of error related to the potential effect of hct and punch location bias when the whole spot is not analysed. Importantly, the size of DBS sub-punches can also artificially inflate values of metabolites compared to venous blood (when DBSs are simulated with venous blood as a result of inaccurate sample delivery) [48]. Accuracy and precision can be improved by including the fixed or individual hct values into the models that relate plasma and blood concentrations of analytes [49]. Following a sub-punch, the microsample is then ready for extraction. In metabolic phenotyping, extractions are normally performed using a variety of organic solvents to perform a protein precipitation prior to metabolic phenotyping [64,65,100,111].
The move towards automating pre-analytical processes, such as microsample preparation, has the potential to improve throughput and decrease manual error in metabolic phenotyping pipelines. In fact, this consideration has recently been adopted in the design of advanced microsampling devices such as the VAMS (Neoteryx; Torrance, CA, USA), which are also available in 96-well plate format (VAMS 96-Autorack) (Neoteryx; Torrance, CA, USA) [89]. Such designs have the potential for the automation of extraction in advanced microsampling device metabolic phenotyping pipelines; however, this kind of use is yet to be reported on a large scale. Future applications of advanced microsampling devices in automated metabolic phenotyping workflows offer the potential to conduct large epidemiological studies. Derivatisation is often used in metabolic phenotyping pipelines to improve analyte volatility (i.e., in GC) and analytical sensitivity [112]. However, it may affect the detection and reproducibility of certain classes of metabolites and hamper the detection of compounds [58]. Currently, derivatisation has been automated for fatty acid analysis of the phospholipid fraction of human plasma, and, to date, has been successfully applied to more than 28,000 samples of the InterAct project, measuring plasma–phospholipid profiles in over 12,000 diabetes cases and over 16,000 sub-cohort participants as part of a cancer study [112]. Scales of this size have not yet been achieved in automated workflows for the metabolic phenotyping of microsamples.
4.3. Determining an Equivalent Concentration Factor
The type of blood collection performed to obtain a microsample (venous, arterial, or capillary) will influence the metabolite content of the sample [103,104]. These differences mainly arise between capillary and venous samples (e.g., when comparing their sera or plasma). Differences are primarily due to capillary samples being derived from a dermal puncture, which results in contamination of venous blood with arterial blood, interstitial fluid, and intracellular fluid [103]. One study comparing capillary and venepuncture samples found that spot size is the most significant factor when applying a correction formula to account for differences in metabolite concentrations [113]. The formula derived by the researchers was
which calculates the estimated standard value (Y) from DBS treatment (X), the treatment conditions such as temperature, humidity, drying time, shipment time, spot size (T), and donor characteristics such as health, age, and sex (H) reflecting potential interaction effects [113]. Use of newly developed microsampling devices that collect a fixed volume of blood may eliminate this consideration of spot size and punching [27].
Recently, correlations between DBS and dried serum spot (DSS) samples for the analysis of 25-hydroxyvitamin D (25OHD) were performed using LC–MS/MS technology [114]. Karvaly and colleagues observed that the mean biases showed no correlation with the 25OHD levels between DBS and DSS matrices (r ≤ 0.0671) [114]. In another study, DBS HbA1c (commonly used for diabetes monitoring) demonstrated over 95% correlation with standard venous samples; however, results from DBS cards older than 7 days had to be adjusted, indicating that metabolite concentrations will vary between sampling sites and matrices on which microsamples are collected [115]. Bridging studies should ideally be performed to validate that the concentrations obtained from capillary microsamples are equivalent to venous whole blood. Specifically, research is needed in the use of anticoagulants in venepuncture collections compared to advanced microsamples where anticoagulants are not included [107]. This is to overcome analytical and physiological issues associated with miniaturised volumes and complex collection matrices, which demand method development to focus on robust extraction procedures and the selection of sensitive analytical platforms.
4.4. Self-Sampling
The bulk of the literature on the metabolic phenotyping of microsamples has utilised samples collected prior to the study (excess/remnant samples) or has utilised trained personnel to collect microsamples (primarily DBSs). Alternatively, pipelines that adopt self-sampling offer the potential for improved participant accessibility and retention in research studies. Such patient-centric benefits are exemplified by the established use of blood glucose monitoring devices by diabetics [28]. However, the routine adoption of self-sampling (with advanced microsampling devices) is currently limited to a few accounts, with only a single published application in metabolic phenotyping (to our knowledge) [53]. Currently, only Cvetko et al. have investigated the use of self-sampling for dried samples (DBS) (VAMS) (Neoteryx; Torrance, CA, USA) and a passive plasma separation device (Telimmune plasma cards) (Novilytic; West Lafayette, IN, USA) for metabolic phenotyping [53]. They reported issues in undersampling, hct variation, and haemolysis. Importantly, this study acknowledged its relatively small sample size (n = 10), who were ‘familiar with self-sampling procedures’, for which only five participants participated in sample collection reproducibility testing. Interestingly, the reproducibility testing required the collection of hexaplicates for each of the three devices (i.e., 18 microsamples collected per participant in a single sitting), which potentially introduced repeated lancing pain. Furthermore, this account does not specify if these findings were device-specific [53]. Beyond metabolic phenotyping, advanced microsampling devices such as the HemaSpot HF (Spot on Sciences; San Francisco, CA, USA) have shown promising results in improving health engagement in men with HIV-1 [28]. This study had a return rate of 75.5%, with 418/554 enrolled participants returning a microsample. Of those, 80.6% (337/418 participants) returned kits that contained enough blood for testing. Of those who received a kit, interestingly, 49 required a second kit due to losing the kit or difficulties collecting blood (11 of whom attributed this to problems with the lancet because of calloused fingertips). Kocher et al. concluded that home collection with these devices for the assessment of viral load could be utilised as a monitoring tool between clinical visits for patients who struggle with antiretroviral therapy adherence [28]. Since the COVID-19 pandemic, the general public are much more adapted to home sampling, albeit with immunoassay kits, but the practice of self-sampling is now more accepted for health monitoring in the home. Advanced microsampling devices have the potential to leverage this familiarity of self-sampling for future research applications in the home setting.
5. Conclusions
Microsamples are collections usually less than 50 µL, although all devices that we have captured as part of this review do not fit within this definition (as some can perform collections of up to 600 µL); however, they are considered microsamples that can be self-administered. These microsamples have demonstrated applicability in the field of metabolic phenotyping. This is evidenced in the literature by the use of both traditional dried microsamples as well as advanced microsample devices (advanced dried microsamples, passive separation devices, and whole biofluid collectors) for sample collections in mass spectrometry-based metabolic phenotyping. In particular, the advance in microsampling technology has provided promising avenues to overcome some of the traditional challenges associated with microsampling, and paves the way for wider application in the field. Early adoption of microsampling in the metabolic phenotyping literature indicates the vast potential for their integration into larger-scale clinical/epidemiology applications. However, much evaluation and optimisation is still required from future studies to support the wide adoption of microsamples in replacing routine venepuncture for metabolic phenotyping workflows.
Author Contributions
J.L.R.: writing, editing, conceptualisation, reviewing; N.G.L., L.W., N.G. and M.G.: editing, reviewing, conceptualisation. All authors have read and agreed to the published version of the manuscript.
Funding
At the time the review was written JLR was a recipient of a joint-postgraduate scholarship from Murdoch University and Bruker Daltonics. The APC was funded by the Australian National Phenome Centre.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Alanah Grant-St James, Nicholas Morrison.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 25OHD | 25-hydroxyvitamin D |
| ACN | Acetonitrile |
| CV | Coefficient of variation |
| DBS | Dried blood spot |
| DPS | Dried plasma spot |
| DSS | Dried serum spot |
| DUS | Dried urine spot |
| EDTA | Ethylenediaminetetraacetic |
| GC–MS | Gas chromatography–mass spectrometry |
| h | Hours |
| hct | Haematocrit |
| LC–MS | Liquid chromatography–mass spectrometry |
| MeOH | Methanol |
| mL | Millilitre |
| MS | Mass spectrometry |
| NIR | Near-infrared |
| P4 medicine | Predictive, preventive, personalised, and participatory medicine |
| QC | Quality control |
| qDBS | Quantitative dried blood spot |
| TAP | Touch-activated phlebotomy |
| UHPLC–MS | Ultra-high-performance liquid chromatography–mass spectrometry |
| VAMS | Volumetric absorptive microsampling |
| WADA | World Anti-Doping Agency |
| WB | Whole blood |
| µL | Microlitre |
References
- Flores, M.; Glusman, G.; Brogaard, K.; Price, N.D.; Hood, L. P4 medicine: How systems medicine will transform the healthcare sector and society. Pers. Med. 2013, 10, 565–576. [Google Scholar] [CrossRef] [Green Version]
- Gray, N.; Lawler, N.G.; Yang, R.; Morillon, A.-C.; Gay, M.C.L.; Bong, S.-H.; Holmes, E.; Nicholson, J.K.; Whiley, L. A simultaneous exploratory and quantitative amino acid and biogenic amine metabolic profiling platform for rapid disease phenotyping via UPLC-QToF-MS. Talanta 2021, 223, 121872. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.M.; Darzi, A.W.; Takats, Z.; Lindon, J.C. Metabolic phenotyping in clinical and surgical environments. Nature 2012, 491, 384–392. [Google Scholar] [CrossRef]
- Rainville, P.D.; Theodoridis, G.; Plumb, R.S.; Wilson, I.D. Advances in liquid chromatography coupled to mass spectrometry for metabolic phenotyping. TrAC Trends Anal. Chem. 2014, 61, 181–191. [Google Scholar] [CrossRef]
- Holen, T.; Norheim, F.; Gundersen, T.E.; Mitry, P.; Linseisen, J.; Iversen, P.O.; Drevon, C.A. Biomarkers for nutrient intake with focus on alternative sampling techniques. Genes Nutr. 2016, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Evans, C.A.; Bowen, C.L.; Filali-Ansary, A. Dried blood spots: Challenges. Future Sci. Group (FSG) 2013, 1, 30–46. [Google Scholar] [CrossRef]
- Bowen, C.L.; Licea-Perez, H.; Karlinsey, M.Z.; Jurusik, K.; Pierre, E.; Siple, J.; Kenney, J.; Stokes, A.; Spooner, N.; Evans, C.A. A novel approach to capillary plasma microsampling for quantitative bioanalysis. Bioanalysis 2013, 5, 1131–1135. [Google Scholar] [CrossRef]
- Bowen, C.L.; Dopson, W.; Kemp, D.C.; Lewis, M.; Lad, R.; Overvold, C. Investigations into the environmental conditions experienced during ambient sample transport: Impact to dried blood spot sample shipments. Bioanalysis 2011, 3, 1625–1633. [Google Scholar] [CrossRef]
- Thomas, S.L.; Wakerman, J.; Humphreys, J.S. Ensuring equity of access to primary health care in rural and remote Australia-what core services should be locally available? Int. J. Equity Health 2015, 14, 111. [Google Scholar] [CrossRef] [Green Version]
- Xing, J.; Loureiro, J.; Patel, M.T.; Mikhailov, D.; Gusev, A.I. Evaluation of a novel blood microsampling device for clinical trial sample collection and protein biomarker analysis. Bioanalysis 2020, 12, 919–935. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare (AIHW). Rural & Remote Health; AIHW: Canberra, Australia, 2019.
- Ahmetaj-Shala, B.; Olanipekun, M.; Tesfai, A.; Maccallum, N.; Kirkby, N.; Quinlan, G.J.; Shih, C.-C.; Kawai, R.; Mumby, S.; Paul-Clark, M.; et al. Development of a novel UHPLC–MS/MS-based platform to quantify amines, amino acids and methylarginines for applications in human disease phenotyping. Sci. Rep. 2018, 8, 13987. [Google Scholar] [CrossRef]
- Want, E.J.; Wilson, I.; Gika, H.G.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.P.; Holmes, E.; Nicholson, J. Global metabolic profiling procedures for urine using UPLC–MS. Nat. Protoc. 2010, 5, 1005–1018. [Google Scholar] [CrossRef]
- Want, E.J. LC–MS Untargeted Analysis. In Metabolic Profiling: Methods and Protocols; Theodoridis, G.A., Gika, H.G., Wilson, I.D., Eds.; Springer: New York, NY, USA, 2018; pp. 9–116. [Google Scholar]
- Contrepois, K.; Mahmoudi, S.; Ubhi, B.K.; Papsdorf, K.; Hornburg, D.; Brunet, A.; Snyder, M. Cross-Platform Comparison of Untargeted and Targeted Lipidomics Approaches on Aging Mouse Plasma. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Climaco Pinto, R.; Karaman, I.; Lewis, M.R.; Hällqvist, J.; Kaluarachchi, M.; Graça, G.; Chekmeneva, E.; Durainayagam, B.; Ghanbari, M.; Ikram, M.A.; et al. Finding Correspondence between Metabolomic Features in Untargeted Liquid Chromatography–Mass Spectrometry Metabolomics Datasets. Anal. Chem. 2022, 94, 5493–5503. [Google Scholar] [CrossRef]
- Whiley, L.; Chappell, K.E.; D’Hondt, E.; Lewis, M.R.; Jiménez, B.; Snowden, S.G.; Soininen, H.; Kłoszewska, I.; Mecocci, P.; Tsolaki, M.; et al. Metabolic phenotyping reveals a reduction in the bioavailability of serotonin and kynurenine pathway metabolites in both the urine and serum of individuals living with Alzheimer’s disease. Alzheimer’s Res. Ther. 2021, 13, 20. [Google Scholar] [CrossRef]
- Sands, C.J.; Gómez-Romero, M.; Correia, G.; Chekmeneva, E.; Camuzeaux, S.; Izzi-Engbeaya, C.; Dhillo, W.S.; Takats, Z.; Lewis, M.R. Representing the Metabolome with High Fidelity: Range and Response as Quality Control Factors in LC–MS-Based Global Profiling. Anal. Chem. 2021, 93, 1924–1933. [Google Scholar] [CrossRef]
- Letertre, M.P.; Myridakis, A.; Whiley, L.; Camuzeaux, S.; Lewis, M.R.; Chappell, K.E.; Thaikkatil, A.; Dumas, M.-E.; Nicholson, J.K.; Swann, J.R.; et al. A targeted ultra performance liquid chromatography–Tandem mass spectrometric assay for tyrosine and metabolites in urine and plasma: Application to the effects of antibiotics on mice. J. Chromatogr. B 2020, 1164, 122511. [Google Scholar] [CrossRef]
- Whiley, L.; Nye, L.C.; Grant, I.; Andreas, N.J.; Chappell, K.E.; Sarafian, M.H.; Misra, R.; Plumb, R.S.; Lewis, M.R.; Nicholson, J.K.; et al. Ultrahigh-Performance Liquid Chromatography Tandem Mass Spectrometry with Electrospray Ionization Quantification of Tryptophan Metabolites and Markers of Gut Health in Serum and Plasma—Application to Clinical and Epidemiology Cohorts. Anal. Chem. 2019, 91, 5207–5216. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Jin, X.; Wu, Y.; Yang, M.; Xu, T.; Li, X.; Ren, J.; Yan, L.L. A Novel Dried Blood Spot Detection Strategy for Characterizing Cardiovascular Diseases. Front. Cardiovasc. Med. 2020, 7, 542519. [Google Scholar] [CrossRef]
- Brignardello, J.; Holmes, E.; Garcia-Perez, I. Chapter Seven-Metabolic Phenotyping of Diet and Dietary Intake. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 231–270. [Google Scholar]
- Morgan, P.E. Microsampling Devices for Routine Therapeutic Drug Monitoring-Are We There Yet? Ther. Drug Monit. 2021, 43, 322–334. [Google Scholar] [CrossRef]
- Constantinou, M.; Papakonstantinou, E.; Benaki, D.; Spraul, M.; Shulpis, K.; Koupparis, M.; Mikros, E. Application of nuclear magnetic resonance spectroscopy combined with principal component analysis in detecting inborn errors of metabolism using blood spots: A metabonomic approach. Anal. Chim. Acta 2004, 511, 303–312. [Google Scholar] [CrossRef]
- Mingas, P.-D.; Zdovc, J.; Grabnar, I.; Vovk, T. The Evolving Role of Microsampling in Therapeutic Drug Monitoring of Monoclonal Antibodies in Inflammatory Diseases. Molecules 2021, 26, 1787. [Google Scholar] [CrossRef]
- Beck, O.; Mellring, M.; Löwbeer, C.; Seferaj, S.; Helander, A. Measurement of the alcohol biomarker phosphatidylethanol (PEth) in dried blood spots and venous blood—Importance of inhibition of post-sampling formation from ethanol. Anal. Bioanal. Chem. 2021, 413, 5601–5606. [Google Scholar] [CrossRef]
- Deprez, S.; Paniagua-González, L.; Velghe, S.; Stove, C.P. Evaluation of the Performance and Hematocrit Independence of the HemaPEN as a Volumetric Dried Blood Spot Collection Device. Anal. Chem. 2019, 91, 14467–14475. [Google Scholar] [CrossRef] [Green Version]
- Kocher, S.; Tshiananga, J.K.T.; Koubek, R. Comparison of Lancing Devices for Self-Monitoring of Blood Glucose regarding Lancing Pain. J. Diabetes Sci. Technol. 2009, 3, 1136–1143. [Google Scholar] [CrossRef]
- Jacob, M.; Malkawi, A.; Albast, N.; Al Bougha, S.; Lopata, A.; Dasouki, M.; Rahman, A.M.A. A targeted metabolomics approach for clinical diagnosis of inborn errors of metabolism. Anal. Chim. Acta 2018, 1025, 141–153. [Google Scholar] [CrossRef]
- Delahaye, L.; Veenhof, H.; Koch, B.; Alffenaar, J.; Linden, R.; Stove, C. Alternative Sampling Devices to Collect Dried Blood Microsamples: State-of-the-Art. Ther. Drug Monit. 2021, 43, 310–321. [Google Scholar] [CrossRef]
- Tasso, Inc. Tasso+. 2022. Available online: https://www.tassoinc.com/tasso-plus (accessed on 10 March 2022).
- Shimadzu. MSW² Type Udck. 2022. Available online: https://www.shimadzu.eu/msw2 (accessed on 10 March 2022).
- Telimmune. Telimmune Plasma Separation Cards (Formerly Noviplex). 2022. Available online: https://www.telimmune.com/plasma-separation-cards (accessed on 10 March 2022).
- Spot On Sciences. HEMASPOT SE. 2022. Available online: https://www.spotonsciences.com/hemaspot-se/ (accessed on 10 March 2022).
- Protti, M.; Marasca, C.; Cirrincione, M.; Sberna, A.E.; Mandrioli, R.; Mercolini, L. Dried Urine Microsampling Coupled to Liquid Chromatography—Tandem Mass Spectrometry (LC–MS/MS) for the Analysis of Unconjugated Anabolic Androgenic Steroids. Molecules 2020, 25, 3210. [Google Scholar] [CrossRef]
- Salamin, O.; Nicoli, R.; Xu, C.; Boccard, J.; Rudaz, S.; Pitteloud, N.; Saugy, M.; Kuuranne, T. Steroid profiling by UHPLC–MS/MS in dried blood spots collected from healthy women with and without testosterone gel administration. J. Pharm. Biomed. Anal. 2021, 204, 114280. [Google Scholar] [CrossRef]
- Hemmati, M.; Nix, C.; Crommen, J.; Servais, A.-C.; Fillet, M. Benefits of microsampling and microextraction for metabolomics studies. TrAC Trends Anal. Chem. 2020, 127, 115899. [Google Scholar] [CrossRef]
- Bictash, M.; Ebbels, T.M.; Chan, Q.; Loo, R.L.; Yap, I.K.; Brown, I.J.; de Iorio, M.; Daviglus, M.L.; Holmes, E.; Stamler, J.; et al. Opening up the "Black Box": Metabolic phenotyping and metabolome-wide association studies in epidemiology. J. Clin. Epidemiol. 2010, 63, 970–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpentieri, D.; Colvard, A.; Petersen, J.; Marsh, W.; David-Dirgo, V.; Huentelman, M.; Pirrotte, P.; Sivakumaran, T. Mind the Quality Gap When Banking on Dry Blood Spots. Biopreservation Biobanking 2021, 19, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Grüner, N.; Stambouli, O.; Ross, R.S. Dried Blood Spots-Preparing and Processing for Use in Immunoassays and in Molecular Techniques. J. Vis. Exp. 2015, 97, e52619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, S.; Delaby, C.; Vialaret, J.; Ducos, J.; Hirtz, C. Current And future use of “dried blood spot” analyses in clinical chemistry. Clin. Chem. Lab. Med. CCLM 2013, 51, 1897–1909. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Q.; Gao, P.; Dong, J.; Zhu, Z.; Fang, Y.; Fang, Z.; Sun, X.; Sun, T. A dried blood spot mass spectrometry metabolomic approach for rapid breast cancer detection. Onco Targets Ther. 2016, 9, 1389–1398. [Google Scholar] [CrossRef] [Green Version]
- Hasanah, Y.I.F.; Harahap, Y.; Purwanto, D.J. Phenotyping Study of Cyclophosphamide 4-Hydroxylation in Malay Cancer Patients. Drug Des. Dev. Ther. 2021, 15, 305–313. [Google Scholar] [CrossRef]
- Loo, R.L.; Lu, Q.; Carter, E.M.; Liu, S.; Clark, S.; Wang, Y.; Baumgartner, J.; Tang, H.; Chan, Q. A feasibility study of metabolic phenotyping of dried blood spot specimens in rural Chinese women exposed to household air pollution. J. Expo. Sci. Environ. Epidemiol. 2020, 31, 328–344. [Google Scholar] [CrossRef]
- Lang, W.; Qi, J.; Caldwell, G.W. Drug, Lipid, and Acylcarnitine Profiling Using Dried Blood Spot (DBS) Technology in Drug Discovery. In Optimization in Drug Discovery; Humana Press: Totowa, NJ, USA, 2013; pp. 461–475. [Google Scholar] [CrossRef]
- Haijes, H.A.; Jans, J.; Van Der Ham, M.; Van Hasselt, P.M.; Verhoeven-Duif, N.M. Understanding acute metabolic decompensation in propionic and methylmalonic acidemias: A deep metabolic phenotyping approach. Orphanet J. Rare Dis. 2020, 15, 68. [Google Scholar] [CrossRef]
- Van Dooijeweert, B.; Broeks, M.H.; Verhoeven-Duif, N.M.; Van Beers, E.J.; Nieuwenhuis, E.E.S.; Van Solinge, W.W.; Bartels, M.; Jans, J.J.M.; van Wijk, R. Untargeted metabolic profiling in dried blood spots identifies disease fingerprint for pyruvate kinase deficiency. Haematologica 2020, 106, 2720. [Google Scholar] [CrossRef]
- O’Mara, M.; Hudson-Curtis, B.; Olson, K.; Yueh, Y.; Dunn, J.; Spooner, N. The effect of hematocrit and punch location on assay bias during quantitative bioanalysis of dried blood spot samples. Bioanalysis 2011, 3, 2335–2347. [Google Scholar] [CrossRef]
- Watson, P.; Maughan, R.J. Artifacts in Plasma Volume Changes due to Hematology Analyzer-Derived Hematocrit. Med. Sci. Sports Exerc. 2014, 46, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundrapu, S.; Noguez, J. Chapter Six-Laboratory Assessment of Anemia. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 197–225. [Google Scholar]
- Fischbach, F.T.; Fischbach, M.A. Fischbach’s Manual of Laboratory and Diagnostic Tests, 10th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Hematocrit. Nursing Critical Care. 2020, 15, 38. [CrossRef]
- Cvetko, A.; Tijardović, M.; Bilandžija-Kuš, I.; Gornik, O. Comparison of self-sampling blood collection for N-glycosylation analysis. BMC Res. Notes 2022, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Petrick, L.; Edmands, W.; Schiffman, C.; Grigoryan, H.; Perttula, K.; Yano, Y.; Dudoit, S.; Whitehead, T.; Metayer, C.; Rappaport, S. An untargeted metabolomics method for archived newborn dried blood spots in epidemiologic studies. Metabolomics 2017, 13, 27. [Google Scholar] [CrossRef] [Green Version]
- Palmer, E.A.; Cooper, H.J.; Dunn, W.B. Investigation of the 12-Month Stability of Dried Blood and Urine Spots Applying Untargeted UHPLC–MS Metabolomic Assays. Anal. Chem. 2019, 91, 14306–14313. [Google Scholar] [CrossRef]
- Yu, M.; Dolios, G.; Yong-Gonzalez, V.; Björkqvist, O.; Colicino, E.; Halfvarson, J.; Petrick, L. Untargeted metabolomics profiling and hemoglobin normalization for archived newborn dried blood spots from a refrigerated biorepository. J. Pharm. Biomed. Anal. 2020, 191, 113574. [Google Scholar] [CrossRef]
- Baillargeon, K.R.; Brooks, J.C.; Miljanic, P.R.; Mace, C.R. Patterned Dried Blood Spot Cards for the Improved Sampling of Whole Blood. ACS Meas. Sci. Au 2022, 2, 31–38. [Google Scholar] [CrossRef]
- Kong, S.T.; Lin, H.-S.; Ching, J.; Ho, P.C. Evaluation of Dried Blood Spots as Sample Matrix for Gas Chromatography/Mass Spectrometry Based Metabolomic Profiling. Anal. Chem. 2011, 83, 4314–4318. [Google Scholar] [CrossRef]
- Michopoulos, F.; Theodoridis, G.; Smith, C.J.; Wilson, I.D. Metabolite profiles from dried blood spots for metabonomic studies using UPLC combined with orthogonal acceleration ToF-MS: Effects of different papers and sample storage stability. Bioanalysis 2011, 3, 2757–2767. [Google Scholar] [CrossRef]
- Ward, C.; Nallamshetty, S.; Watrous, J.D.; Acres, E.; Long, T.; Mathews, I.T.; Sharma, S.; Cheng, S.; Imam, F.; Jain, M. Nontargeted mass spectrometry of dried blood spots for interrogation of the human circulating metabolome. Biol. Mass Spectrom. 2021, 56, e4772. [Google Scholar] [CrossRef]
- Koulman, A.; Prentice, P.; Wong, M.C.; Matthews, L.; Bond, N.J.; Eiden, M.; Griffin, J.; Dunger, D. The development and validation of a fast and robust dried blood spot based lipid profiling method to study infant metabolism. Metabolomics 2020, 10, 1018–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Naviaux, J.C.; Monk, J.M.; Wang, L.; Naviaux, R.K. Improved Dried Blood Spot-Based Metabolomics: A Targeted, Broad-Spectrum, Single-Injection Method. Metabolites 2020, 10, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, K.C.; Hall, C.; Baker-Lee, C.; Soto, M.; Scott, G.; Prince, P.J.; Retter, M.W. Measurement of in vivo therapeutic mAb concentrations: Comparison of conventional serum/plasma collection and analysis to dried blood spot sampling. Bioanalysis 2013, 5, 1979–1990. [Google Scholar] [CrossRef] [PubMed]
- Stevens, V.L.; Hoover, E.; Wang, Y.; Zanetti, K.A. Pre-Analytical Factors that Affect Metabolite Stability in Human Urine, Plasma, and Serum: A Review. Metabolites 2019, 9, 156. [Google Scholar] [CrossRef] [Green Version]
- Yin, P.; Lehmann, R.; Xu, G. Effects of pre-analytical processes on blood samples used in metabolomics studies. Anal. Bioanal. Chem. 2015, 407, 4879–4892. [Google Scholar] [CrossRef] [Green Version]
- Mandrioli, R.; Mercolini, L.; Protti, M. Blood and Plasma Volumetric Absorptive Microsampling (VAMS) Coupled to LC–MS/MS for the Forensic Assessment of Cocaine Consumption. Molecules 2020, 25, 1046. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, C.M.; Wagmann, L.; Meyer, M.R. Development, validation, and application of a quantitative volumetric absorptive microsampling–based method in finger prick blood by means of LC-HRMS/MS applicable for adherence monitoring of antipsychotics. Anal. Bioanal. Chem. 2021, 413, 1729–1737. [Google Scholar] [CrossRef]
- Protti, M.; Mandrioli, R.; Mercolini, L. Tutorial: Volumetric absorptive microsampling (VAMS). Anal. Chim. Acta 2018, 1046, 32–47. [Google Scholar] [CrossRef]
- Gao, X.; Chen, C.; Geng, D.; Bateman, K.P.; Shi, S.; Woolf, E.J.; Xu, Y. Volumetric absorptive microsampling (VAMS®) in therapeutic protein quantification by LC–MS/MS: Investigation of anticoagulant impact on assay performance and recommendations for best practices in method development. J. Pharm. Biomed. Anal. 2021, 196, 113895. [Google Scholar] [CrossRef]
- Denniff, P.; Spooner, N. Volumetric Absorptive Microsampling: A Dried Sample Collection Technique for Quantitative Bioanalysis. Anal. Chem. 2014, 86, 8489–8495. [Google Scholar] [CrossRef]
- Kok, M.G.; Fillet, M. Volumetric absorptive microsampling: Current advances and applications. J. Pharm. Biomed. Anal. 2018, 147, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Nix, C.; Hemmati, M.; Cobraiville, G.; Servais, A.-C.; Fillet, M. Blood Microsampling to Monitor Metabolic Profiles During Physical Exercise. Front. Mol. Biosci. 2021, 8, 681400. [Google Scholar] [CrossRef] [PubMed]
- Velghe, S.; Stove, C.P. Evaluation of the Capitainer-B Microfluidic Device as a New Hematocrit-Independent Alternative for Dried Blood Spot Collection. Anal. Chem. 2018, 90, 12893–12899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, J.; Lenk, G.; Ullah, S.; Beck, O.; Stemme, G.; Roxhed, N. An Autonomous Microfluidic Device for Generating Volume-Defined Dried Plasma Spots. Anal. Chem. 2019, 91, 7125–7130. [Google Scholar] [CrossRef] [PubMed]
- Ryona, I.; Henion, J. A Book-Type Dried Plasma Spot Card for Automated Flow-Through Elution Coupled with Online SPE-LC–MS/MS Bioanalysis of Opioids and Stimulants in blood. Anal. Chem. 2016, 88, 11229–11237. [Google Scholar] [CrossRef]
- Forchelet, D.; Béguin, S.; Sajic, T.; Bararpour, N.; Pataky, Z.; Frias, M.; Grabherr, S.; Augsburger, M.; Liu, Y.; Charnley, M.; et al. Separation of blood microsamples by exploiting sedimentation at the microscale. Sci. Rep. 2018, 8, 14101. [Google Scholar] [CrossRef]
- Roadcap, B.; Hussain, A.; Dreyer, D.; Carter, K.; Dube, N.; Xu, Y.; Anderson, M.; Berthier, E.; Vazvaei, F.; Bateman, K.; et al. Clinical application of volumetric absorptive microsampling to the gefapixant development program. Bioanalysis 2020, 12, 893–904. [Google Scholar] [CrossRef]
- Hotta, K.; Ishida, T.; Noritake, K.-I.; Kita, K.; Mano, Y. Quantitative and qualitative application of a novel capillary microsampling device, Microsampling Wing™ (MSW), using antiepileptic drugs in rats. J. Pharm. Biomed. Anal. 2020, 194, 113788. [Google Scholar] [CrossRef]
- Protti, M.; Marasca, C.; Cirrincione, M.; Cavalli, A.; Mandrioli, R.; Mercolini, L. Assessment of capillary volumetric blood microsampling for the analysis of central nervous system drugs and metabolites. Analyst 2020, 145, 5744–5753. [Google Scholar] [CrossRef]
- Wilson, I. Global metabolic profiling (metabonomics/metabolomics) using dried blood spots: Advantages and pitfalls. Bioanalysis 2011, 3, 2255–2257. [Google Scholar] [CrossRef]
- Amsterdam, P.V.; Waldrop, C. The application of dried blood spot sampling in global clinical trials. Bioanalysis 2010, 2, 1783–1786. [Google Scholar] [CrossRef] [Green Version]
- Drolet, J.; Tolstikov, V.; Williams, B.A.; Greenwood, B.P.; Hill, C.; Vishnudas, V.K.; Sarangarajan, R.; Narain, N.R.; Kiebish, M.A. Integrated Metabolomics Assessment of Human Dried Blood Spots and Urine Strips. Metabolites 2017, 7, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cytiva. Whatman 903 Proteinsaver Card. 2022. Available online: https://www.cytivalifesciences.com/en/us/shop/whatman-laboratory-filtration/whatman-dx-components/blood-collection-cards-and-accessories/903-proteinsaver-card-p-01011 (accessed on 10 March 2022).
- PerkinElmer Inc. PerkinElmer 226 Spot Saver RUO Card. 2022. Available online: https://www.perkinelmer.com/uk/product/pki-ruo-spot-saver-card-pack-of-100-gr2261005 (accessed on 10 March 2022).
- Ahlstrom-Munksjö. Biosamples Collection Cards. 2022. Available online: https://www.ahlstrom-munksjo.com/products/medical-life-sciences-and-laboratory/specimen-collection-cards/Biosamples-collection-cards/ (accessed on 10 March 2022).
- Tasso, Inc. Tasso-M20. 2022. Available online: https://www.tassoinc.com/tasso-m20 (accessed on 10 March 2022).
- Capitainer. Capitainer®B Vanadate. 2022. Available online: https://capitainer.se/capitainer-b-vanadate/ (accessed on 10 March 2022).
- Capitainer. Capitainer®qDBS. 2022. Available online: https://capitainer.se/capitainer-qdbs/ (accessed on 10 March 2022).
- Neoteryx. The Mitra 96-Autorack. 2022. Available online: https://www.neoteryx.com/mitra-high-throughput-rack-systems?hsLang=en (accessed on 10 March 2022).
- Neoteryx. The Mitra Cartridge. 2022. Available online: https://www.neoteryx.com/mitra-cartridge-blood-sampling-device-dbs?hsLang=en (accessed on 10 March 2022).
- Neoteryx. The Mitra Clamshell. 2022. Available online: https://www.neoteryx.com/mitra-clamshell-blood-collection-device?hsLang=en (accessed on 10 March 2022).
- Medical TSa. Hemapen. 2022. Available online: https://www.trajanscimed.com/pages/hemapen (accessed on 10 March 2022).
- HemaXis. HemaXis DB10 Whole Blood Collection Device. 2022. Available online: https://hemaxis.com/products/hemaxis-db10/ (accessed on 10 March 2022).
- Spot On Sciences. HEMASPOT HD. Available online: https://www.spotonsciences.com/hemaspot-hd/ (accessed on 10 March 2022).
- Spot On Sciences. HEMASPOT HF. Available online: https://www.spotonsciences.com/hemaspot-hf/ (accessed on 10 March 2022).
- YourBioHealth. Touch Activated Phlebotomy (TAP). 2022. Available online: https://company.yourbiohealth.com/products/tap/ (accessed on 10 March 2022).
- YourBioHealth. Touch Activated Phlebotomy II (TAP II). 2022. Available online: https://company.yourbiohealth.com/products/tap-ii/ (accessed on 10 March 2022).
- Tasso, Inc. Tasso-SST. 2022. Available online: https://www.tassoinc.com/tasso-sst (accessed on 10 March 2022).
- Kok, M.G.; Nix, C.; Nys, G.; Fillet, M. Targeted metabolomics of whole blood using volumetric absorptive microsampling. Talanta 2019, 197, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Volani, C.; Caprioli, G.; Calderisi, G.; Sigurdsson, B.B.; Rainer, J.; Gentilini, I.; Hicks, A.A.; Pramstaller, P.P.; Weiss, G.; Smarason, S.V.; et al. Pre-analytic evaluation of volumetric absorptive microsampling and integration in a mass spectrometry-based metabolomics workflow. Anal. Bioanal. Chem. 2017, 409, 6263–6276. [Google Scholar] [CrossRef] [Green Version]
- Crimmins, E.M.; Zhang, Y.S.; Kim, J.K.; Frochen, S.; Kang, H.; Shim, H.; Ailshire, J.; Potter, A.; Cofferen, J.; Faul, J. Dried blood spots: Effects of less than optimal collection, shipping time, heat, and humidity. Am. J. Hum. Biol. 2020, 32, e23390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiley, L.; Godzien, J.; Ruperez, F.J.; Legido-Quigley, C.; Barbas, C. In-Vial Dual Extraction for Direct LC–MS Analysis of Plasma for Comprehensive and Highly Reproducible Metabolic Fingerprinting. Anal. Chem. 2012, 84, 5992–5999. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines on Drawing Blood: Best Practices in Phlebotomy, Capillary Sampling; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Hannon, W. Blood Collection on Filter Paper for Newborn Screening Programs; Clinical and Laboratory Standards Institute: Wayne, IL, USA, 2013. [Google Scholar]
- CDC. Laboratory Quality Assurance and Standardization Programs. In Guidelines for the Shipment of Dried Blood Spot Specimens; Centers for Disease Control and Prevention: Atlanta, GA, USA, 1993. [Google Scholar]
- Kirwan, J.A.; Brennan, L.; Broadhurst, D.; Fiehn, O.; Cascante, M.; Dunn, W.B.; Schmidt, M.A.; Velagapudi, V. Preanalytical Processing and Biobanking Procedures of Biological Samples for Metabolomics Research: A White Paper, Community Perspective (for “Precision Medicine and Pharmacometabolomics Task Group”—The Metabolomics Society Initiative). Clin. Chem. 2018, 64, 1158–1182. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Lee, M.S. Dried Blood Spots: Applications and Technique, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Strnadová, K.A.; Holub, M.; Mühl, A.; Heinze, G.; Ratschmann, R.; Mascher, H.; Stöckler-Ipsiroglu, S.; Waldhauser, F.; Votava, F.; Lebl, J.; et al. Long-Term Stability of Amino Acids and Acylcarnitines in Dried Blood Spots. Clin. Chem. 2007, 53, 717–722. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; McDaniel, J.; Chen, E.Y.; Rockwell, H.E.; Drolet, J.; Vishnudas, V.K.; Tolstikov, V.; Sarangarajan, R.; Narain, N.R.; Kiebish, M.A. Dynamic and temporal assessment of human dried blood spot MS/MSALL shotgun lipidomics analysis. Nutr. Metab. 2017, 14, 28. [Google Scholar] [CrossRef] [Green Version]
- Grecsó, N.; Zádori, A.; Baráth, Á.; Galla, Z.; Rácz, G.; Bereczki, C.; Monostori, P. Comparison of different preparation techniques of dried blood spot quality controls in newborn screening for congenital adrenal hyperplasia. PLoS ONE 2021, 16, e0252091. [Google Scholar] [CrossRef]
- González-Domínguez, R.; González-Domínguez, Á.; Sayago, A.; Fernández-Recamales, Á. Recommendations and Best Practices for Standardizing the Pre-Analytical Processing of Blood and Urine Samples in Metabolomics. Metabolites 2020, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Summerhill, K.; Rodriguez-Canas, C.; Mather, I.; Patel, P.; Eiden, M.; Young, S.; Forouhi, N.G.; Koulman, A. Development and validation of a robust automated analysis of plasma phospholipid fatty acids for metabolic phenotyping of large epidemiological studies. Genome Med. 2013, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Börsch-Supan, A.; Weiss, L.M.; Börsch-Supan, M.; Potter, A.J.; Cofferen, J.; Kerschner, E. Dried blood spot collection, sample quality, and fieldwork conditions: Structural validations for conversion into standard values. Am. J. Hum. Biol. 2020, 33, e23517. [Google Scholar] [CrossRef] [PubMed]
- Karvaly, G.; Molnár-Világos, G.; Kovács, K.; Mészáros, K.; Patócs, A.; Vásárhelyi, B. Evaluation of the Analytical and Clinical Concordance of 25-Hydroxyvitamin D Levels in Dried Blood Spots, Dried Serum Spots, and Serum as Potential Biorepository Specimens. Biopreservation Biobanking 2017, 15, 285–292. [Google Scholar] [CrossRef]
- Mastronardi, C.A.; Whittle, B.; Tunningley, R.; Neeman, T.; Paz-Filho, G. The use of dried blood spot sampling for the measurement of HbA1c: A cross-sectional study. BMC Clin. Pathol. 2015, 15, 13. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).