Abstract
Water pollution, resulting from the degradation of plastics into microplastics, exposes humans and other living organisms to contaminated drinking water. Microplastics are capable of adsorbing toxic heavy metals which are carcinogenic and may affect the reproductive functions of living organisms. Hence, this study focuses on the characterization and quantification of microplastics in water to raise the awareness and propose a method of dealing with this emerging pollutant in various aqueous environments. The microplastics were separated from water using polyvinylidene difluoride (PVDF) and PVDF modified with carbon nano-onions (CNOs). The PVDF exhibited the highest concentration of microplastics in the wastewater influent (140 ± 1.85 MP/L) compared to the effluent (8.8 ± 2.10 MP/L), tap water (6.5 ± 5.77 MP/L), and lake water (10 ± 2.65 MP/L). The stereo microscope displayed red, blue, and black colored plastics. The morphological properties were determined using SEM. ATR-FTIR, equipped with Spectrum 10 Spectroscopy Software was used to establish the presence of high-density polyethylene (50%), poly(1,4-butylene terephthalate) (16.6%), nylon 12 (16.6%), and cellulose (16.6%) in the influent. The quantification of heavy metals extracted from the microplastics indicated that the concentrations of As (1.759 to 8.699 mg/L), Cu (83.176 mg/L) and Zn (0.610 mg/L) were above the acceptable limits. Our work is beneficial for the development of a microplastics monitoring protocol for various municipalities. Water treatment plants may also include the treatment of microplastics in the influent and monitor the effluent before the water is released back into the environment.
1. Introduction
Microplastics are emerging nonbiodegradable pollutants in fresh water and wastewater that can be detrimental to humans and aquatic-living organisms [1,2,3]. Recent studies have shown that the ingestion of microplastics (MPs) by marine species can cause chronic poisoning such as reproductive damage, internal abrasion, and obstruction of organs [2,3]. The MPs ingested by fish can be carried over to humans through the food chain, and thus toxic additives used in plastic manufacturing also find their way into the human body [1]. Recent studies have reported MPs in human stool, affirming that humans are part of the food chain and that they are directly impacted by MPs [2]. Uheida et al., 2021 stated that plastics with a size of <130 µm have been found to trigger immune responses by translocating into human tissues [3]. Microplastics are characterized with a size between 5 mm to 5 µm [4]. They are composed of toxic materials such as high- and low-density polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), polyurethane (PUR) and polyethylene terephthalate (PET) [2]. Microplastics are divided into a primary and secondary category. Primary microplastics include personal care products (hand or facial cleaners and toothpaste), drilling fluids and abrasives in the oil and gas sectors that contain microplastics with amorphous shapes and a size ranging from 74–420 µm [4]. Polyethylene is the most prevalent type of microplastic used in personal care cleaning products [5,6]. Secondary microplastics result from the breaking down of plastic waste that is exposed to harsh solar radiation and abrasion from the action of wind and water waves [4]. This results in small flakes and fibers from plastics.
A growing concern is that microplastics contain other toxic components which are added during the production of plastic materials for specific applications. For example, bisphenol is added to harden plastic water bottles and food plates. If bisphenol is ingested, it affects the brain and the development of the fetus in humans [7]. Additives such as calcium carbonate, and lubricants such as sodium stearate, are added to plastics to reduce costs. After consumption of microplastics by humans, the calcium carbonate and sodium stearate can leach out of the microplastic and negatively affect the human body by causing cancer or corrosion of the internal organs [7]. Plasticizers, stabilizers, pigments, fillers and flame retardants may leak into the environment and may be exposed to humans and animals. These chemicals are not only toxic, but they can also be carcinogenic or endocrine-active, affecting the reproductive functions of organisms [8,9]. Microplastics are also capable of absorbing numerous contaminants such as heavy metals, chlorinated and aromatic chemicals, and possibly persistent organic pollutants due to their hydrophobic nature [10,11,12]. Heavy metals in plastics can be unintentionally introduced by mixed waste streams or be intentionally introduced as catalytic chemicals that aid in the polymerization reaction for plastics synthesis [11]. Heavy metals are naturally occurring compounds that are found in lower concentrations in the environment, but they can accumulate over time as a result of industrial and manufacturing processes such as the combustion of fossil fuels. Heavy metal exposure can cause cancer, kidney failure, skin problems, lung disease, neurological disorders, schizophrenia, and artery and liver damage [13]. High levels of manganese can cause low hemoglobin, neurotoxicity and gastrointestinal build-up, while high levels of zinc and copper can cause loss of appetite, muscle stiffness, irritability, and nausea. High concentrations of microplastics in oceans and rivers can be hazardous to aquatic species since they are poisonous and can cause damage to bodily tissue [7,12]. Fish, for example, can get microplastics stuck in their gills which can make them feel full even though their digestive tracts are devoid of nourishment, causing them to starve to death [12].
Microplastics enter freshwater systems through surface runoffs, treated wastewater, industrial effluent, contaminated plastic debris and atmospheric deposition. The effluent produced from wastewater treatment plants, sewer leakage during heavy rain events, and sludge runoff added to agricultural land are the three principal contamination routes for river systems [10].
About 80% of microplastics in the oceans are expected to come from land-based sources, with the remaining 20% coming from aquaculture or fishing sectors [7]. Land-based sources include urban runoff and effluent discharge from wastewater treatment plants (WWTPs) [14]. Throughout the previous few decades, wastewater treatment has been required to increase the consistency of the final effluents. Unfortunately, wastewater treatment is not explicitly designed to completely remove microplastics from the effluent due to the lack of quality enhancement technologies in the infrastructure [10,14]. Hence microplastics are detected in wastewater, fresh water, food, air and drinking water [3]. In studies conducted in Northern Europe, the United States and Australia, microplastic concentrations in pre-treated and treated wastewater were reported to range between 1–3160 and 0.0007–125 particles per liter [7,15]. To the best of our knowledge there has been limited research in South Africa on microplastics in fresh water and wastewater.
There are standard techniques for removing microplastics such as agglomeration into biological flocs, combination of oxidation and fluorescent staining, polycarbonate filters as well as filtration with 0.45 μm filter paper. The disadvantage of the above-mentioned methods is the production of secondary waste by the additional chemicals used and lower stability of the materials used for the filtration of microplastics [15,16,17,18]. Membrane technology is an alternative method that can be used in the separation of microplastics from different sources of water. Membrane separation is currently highly sought as a potential technique due to its ease of scale up, low energy consumption, operational flexibility and ability to handle large volumes of water [19]. Researchers have used membrane-based filtration methods for the rejection of 99% microplastics affirming that membrane-based technology is the most efficient method that can be employed in the tertiary treatment stage of WWTP [20].
Polyvinylidene fluoride (PVDF) is commonly used in membrane systems due to its crystalline and amorphous characteristics. Its amorphous nature gives flexibility, while the crystallinity improves mechanical strength and impact resistance [21]. PVDF is frequently used in wastewater treatment, solid phase assays, amino acid or protein studies, aqueous and organic solution filtration, analytical sample preparation, chromatography, clarifying, and protein chemistry. This is possible due to its exceptional properties such as superior strength that can withstand aggressive handling or automated equipment without breaking or tearing, ensuring clean tests with consistent results; exceptional sensitivity that detects low-level components; a hydrophobic character for high protein binding; and allowing high consistency quality checks that ensure consistent binding for dependable results every time [22].
In our study the PVDF membrane was modified with carbon nano-onions (CNOs) to enhance the performance of the PVDF. The coating of CNOs on PVDF is possible since PVDF is a piezoelectric and pyroelectric semicrystalline thermoplastic and electroactive polymer with heat stability, flexibility, and chemical resistance [23]. PVDF is available in five different phases (α, β, δ, γ, and ε-phase). The zigzag carbon atoms on the CNO surface match the all-trans conformation of β-phase PVDF, potentially causing PVDF crystallization in the β-polymorphic structure [5]. A positively charged layer of CNOs was introduced into the polymer matrix, while the pure PVDF membrane is negatively charged. The CNOs are most likely bound to the PVDF membrane matrix by a strong interfacial hydrogen bonding interaction. This interaction develops between the CNOs’ enhanced hydroxyl groups (single bond OH) and the PVDF polymer’s chain carbon-fluorine (single bond CF) [24].
Our current study aims to offer an analytical protocol to evaluate the presence of microplastics in fresh water and wastewater by utilizing polyvinylidene fluoride membranes as filter, as well as to extract and characterize any heavy metals that may be attached onto the microplastics to ensure the safety of the water.
2. Methodology
This study involves water sampling from a wastewater treatment plant (influent and effluent), local lake, and tap water. The polyvinylidene difluoride (PVDF) and polyvinylidene difluoride modified with carbon nano-onions (PVDF/CNOs) membranes were characterized and used to separate microplastics from the water samples. The microplastics were quantified by weighing by difference and characterized by stereo microscope, scanning electron microscopy (SEM) and attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) and heavy metals attached to them were extracted and detected by inductively coupled plasma optical emission spectroscopy (ICP-OES). The methodology is summarized in Scheme 1.
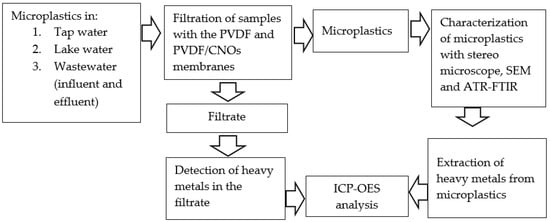
Scheme 1.
Summary of the methodology for detecting microplastics and any attached heavy metals.
2.1. Chemicals and Reagents
Analytical grade acids and chemicals used in sample preparation such as iron sulphate heptahydrate (FeSO4·7H2O), sodium chloride (NaCl), hydrochloric acid (HCl), 30% hydrogen peroxide (H2O2), ethanol (C2H5OH), nitric acid (HNO3), and the heavy metal multielement standard solution, were purchased from Sigma Aldrich (Pty) Ltd., Kempton Park, South Africa. Deionized water, purified by a Milli-Q-RO4 system (Millipore, Bedford, MA, USA), was used for the preparation of standards solutions for heavy metal analysis in Inductively coupled plasma optical emission spectroscopy (ICP-OES), dilutions and for cleaning containers before storing the samples. The polyvinylidene fluoride membranes (0.45 µM pore size) for filtering microplastics was purchased from Sigma Aldrich (Pty) Ltd. (Johannesburg, South Africa). Carbon nano-onions were synthesized using the Sikeyi et al. method [22].
2.2. Sample Sites and Collection
Water samples were collected from the Daspoort Wastewater Treatment Plant Daspoort in Pretoria (South Africa). The plant receives wastewater containing sewage from hotels, houses, schools, shopping malls and other commercial properties as well as water without sewage from mines and abattoirs [25]. The water at the plant is screened to remove solid materials, coagulated to precipitate finer materials, and is then biologically treated to degrade the bacteria that originated from sewage and abattoir water that contains blood, fat etc. Thereafter mechanical filtration of the water is carried out to produce the effluent that can be used in cooling towers, irrigation, and swimming pools. The influent was sampled at the primary water entrance point before pre-treatment while the effluent was taken from the exit point after water treatment. 5 L samples were collected in brand new plastic bottles which were washed with tap water and rinsed several times with deionized water [16,26].
Lake water was also collected in plastic bottles from a stream leading out of the Sterkfontein Lake (Johannesburg) which is situated in an urban area. Lastly, 3 L of tap water were collected in a plastic bottle from Doornfontein (Johannesburg) after the tap was run for 1 min.
The sampling was performed every third month in 2021, from March to November, which included our autumn, winter and spring seasons as shown in Table S1. All samples were stored in the fridge at 3 °C during the analysis period. In April 2022, the influent was sampled again to confirm the presence of microplastics on the surface of the PVDF membrane.
2.3. Sample Preparation
The wet peroxide method was used to separate microplastics from water samples that contained organic matter (turbidity) such as the influent from the wastewater treatment plant. For these samples, 80 mL of aqueous 0.05 M Fe2+ and 80 mL of 30% H2O2 were added to each 400 mL of water to remove the matrix in the wastewater. The samples were covered and allowed to stand for 5 min and were then heated to 75 °C for 30 min while stirring. NaCl (6 g) was added to each sample and heated until the salt dissolved. The mixture was transferred to a separating funnel and allowed to stand overnight. The NaCl increases the density of the water which allows the plastic particles to float.
2.4. Preparation of PVDF Membrane Modified with Carbon Nano-Onions
10 mg CNOs were weighed into a beaker containing 50 mL of deionized water and 2 mL of ethanol. The mixture was allowed to sonicate for 20 min. The CNOs were then dispersed on top of the PVDF membrane through filtration as indicated in Scheme 2.
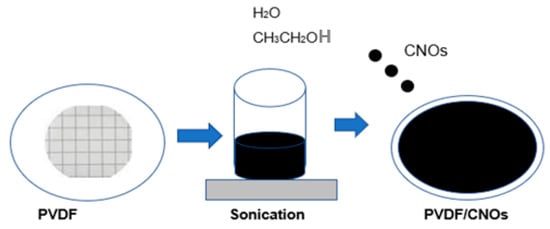
Scheme 2.
Modification of PVDF membrane with CNOs.
2.5. Separation of Microplastics from Water Samples
The samples were filtered through weighed PVDF (47 mm diameter) and PVDF/CNOs (47 mm diameter) membranes with the assistance of a vacuum pump to separate the microplastics. With PVDF membranes, 400 mL water samples were filtered while only 200 mL water samples could be filtered through PVDF/CNOs membranes due to the slowness of the process which was caused by CNOs clots on the surface of the membranes. After filtration the membranes were allowed to dry for 24 h and weighed again to determine the mass of the microplastics in each sample. Using tweezers, visible microplastics were picked up from the membranes and these were analyzed.
2.6. Extraction of Heavy Metals Attached to the Microplastics Obtained from Wastewater Influent
Only the PVDF membrane used for filtering wastewater influent trapped visible microplastics that could be removed for the extraction of heavy metals. The microplastics thus obtained were weighed into a glass beaker to extract the microplastics. Acid extraction was performed by adding 9 mL of 12 M HCl and 3 mL of 16M HNO3. To digest the microplastics, each sample was heat to 50 °C. The solutions were sampled after 30, 60 and 90 min to determine the concentration of heavy metals extracted from the microplastics after different time intervals. After heating the solutions were allowed to cool, diluted with deionized water, and filtered into 50 mL centrifuge tubes. The extraction was performed in triplicate. The samples were stored in the fridge and the heavy metals were detected with ICP-OES Avio 200 from PerkinElmer, Utah, USA. The same procedure was also followed by using deionized water as the extraction solvent.
2.7. Quality Control Analysis
For monitoring the quality of the analysis, the Sigma-Aldrich traceable multielement standard for ICP containing arsenic, mercury, aluminum, cadmium, calcium, chromium, cobalt, copper, iron, lead, magnesium, nickel, silver and zinc was utilized. A stock solution was used for preparing working standards of the following concentrations: 2, 4, 6, 8 and 10 mg/L. Deionized water was acidified with 1% nitric acid and used as the blank for calibration.
2.8. Characterization Techniques
2.8.1. Membrane Performance
The porosity of the PVDF and PVDF/CNOs membranes was characterized by measuring the dry-weight and wet-weight of the membranes using an analytical balance. Porosity was calculated using Equation (1) [27]:
where, 1 is the wet-weight of the membrane (g), 2 is the dry-weight of the membrane (g), A is the effective area of the membrane (m2) and l is the membrane thickness (m) and dw is the water density (0.998 g cm−3).
Membrane mean pore radius (rm) was determined by Equation (2) [27]
where is the water viscosity (8.9 × 10−4 Pas), is the volume of the permeate pure water per unit time (m3 s−1) and is the transmembrane pressure (Pa).
The water permeability of the membranes was determined using deionized water in a dead-end cell. The pressure was adjusted using compressed nitrogen gas. The membranes were pressurized at 40 KPa. The system was subjected to the ultrafiltration test at 40 KPa, and the pure water flux was recorded every 20 s. Permeation flux was calculated using Equation (3) [27]
where is the pure water flux of the membrane (L m−2 h−1), is the volume of permeate water (L), is the effective area of membrane sample (m2), and is the permeation time (h).
2.8.2. Characterization of Microplastics
The shapes and colors of the microplastics filtered through PVDF and PVDF/CNOs membranes were viewed using the Zeiss Discovery stereo microscope (Germany) equipped with AxioCam HRC digital camera at 1000 µm resolution and 4.5× magnitude. The surface morphology of the microplastics was visually detected by scanning electron microscope A FEI XL40 ESEM equipped with two EDAX Sapphire Si(Li) EDS detectors as well as up to date MLA software. The composition of the polymeric material in microplastics obtained from influent samples was confirmed by ATR-FTIR spectroscopy with ATR sample base plate diamond, at wavelength 450–4000 cm−1 purchased from Perkin Elmer, Johannesburg in South Africa. Spectrum 10 Spectroscopy Software was used to determine the exact composition of the microplastics [28].
2.8.3. Analytical Figures of Merit
The limit of detection (LOD) and limit of quantification (LOQ) for each element were calculated using Equations (4) and (5):
where is the mean concentration of the blank (mg/L) and is the standard deviation of the blank.
3. Results and Discussion
3.1. Evaluation of the PVDF and PVDF/CNOs Performance
The pore size of the purchased PVDF membrane was 0.4500 µm with a porosity of 78%. The obtained porosity for the PVDF/CNOs membranes was 41% and the pore size was 0.2182 µm. The reduced pore size of the PVDF/CNOs membrane is due to the surface layer of nanoparticles (CNOs) which clog the membrane [29]. The flux analysis in Figure 1 shows that, due to the larger pore size, the PVDF membrane had a higher water flux than the PVDF/CNOs membrane. The hydrophobic nature of CNOs, as mentioned by Sikeyi et al., show poor water solubility hence a smaller volume was filtered [22].
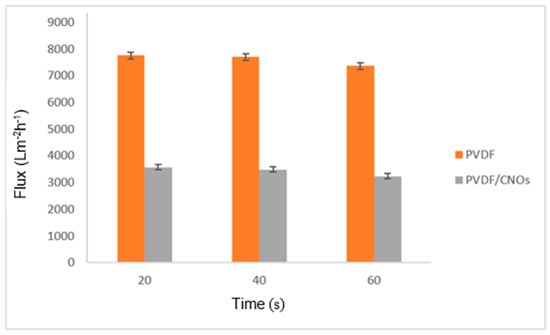
Figure 1.
Pure water flux of PVDF and PVDF/CNOs.
3.2. Quantification of Microplastics in Water Samples
Table 1 provides the mass and concentration of microplastics in tap water, lake water, influent and effluent filtered through the PVDF membrane. The highest mass of microplastics was obtained from the influent. The concentration of microplastics was determined in microplastic per litre (MP/L) since the microplastics are insoluble in water. A high concentration and a fair precision of the measurement (140 ± 1.85) was obtained for the influent sample (Table 1). This result was confirmed by the low relative standard deviation (1.3%) for the influent samples. The reason for the high concentration of microplastics in the influent is due to the fact that sampling was carried out before any treatment at the wastewater treatment plant.

Table 1.
Mass (g) and concentration (MP/L) of microplastics filtered with a PVDF membrane from 400 mL water samples.
As shown in Table 2, lower masses of microplastics were obtained from PVDF/CNOs filtration due to the reduced water flux [29]. The decrease in water flux was due to the formation of clots that blocked the membrane pores and resulted in lower adsorption of microplastics from the water samples [30].

Table 2.
Mass (g) and concentration (MP/L) of microplastics filtered with PVDF/ CNOs membrane from 200 mL water samples.
The concentration of microplastics obtained by using the PVDF membrane is compared to other methods in literature as listed in Table 3. The concentration range of the microplastics removed by the above-mentioned methods was between 5.23 and 288.50 MP/L. Consequently, our method also has the potential of removing microplastics.

Table 3.
Comparison of microplastics concentration removed from wastewater influent by different methods in literature.
3.3. Characterization of the Filtered Microplastics
3.3.1. Visual Identification of Microplastics Using a Stereo Microscope
The stereo microscope images of microplastics filtered with PVDF membranes are displayed in Figure 2. Figure 2a–c display blue and black fibers in tap water, while Figure 2d,e show blue fibers and sediments in the lake water sample. The plastics shine under the microscope hence it was easy to separate them from sediments. Figure 2f,g contain mostly blue and black fibers while Figure 2g also has a visible white plastic that can be seen with a naked eye. The wastewater effluent samples show small visible plastics which are blue and red in color (Figure 2h,I).
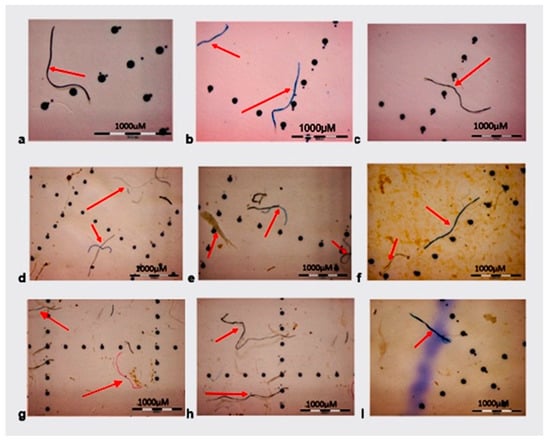
Figure 2.
Stereo microscope images of microplastics filtered with PVDF membranes from (a–c) tap water, (d,e) lake water, (f,g) influent, and (h,I) effluent.
The PVDF/CNOs modified membranes in Figure 3 show more shiny images of microplastics compared to the PVDF membranes (Figure 2). This is attributed to the fact that carbon-based materials are highly conductive, and hamper sample charging thus reducing the image resolution and so upon CNOs modification, the membrane exhibited enhanced image resolution. Tap water contained mostly small pieces of shinny plastics in a film shape (Figure 3a–c), while lake water and wastewater contained long red fibers and clear shiny string fibers (Figure 3d–j). Curled up black and blue fibers are observed in Figure 3i,j. The images for lake water and wastewater (influent and effluent) confirm the presence of a large amount of microplastics.
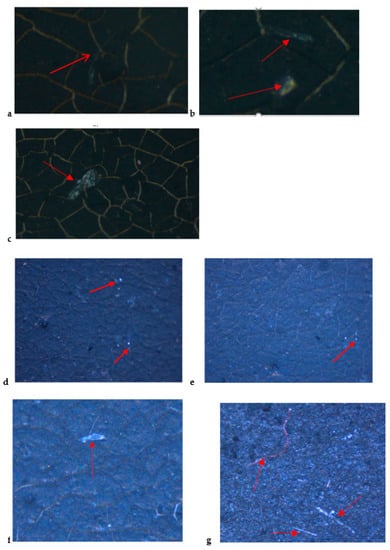
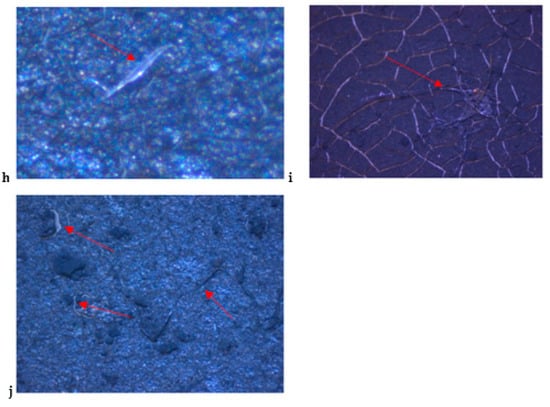
Figure 3.
Stereo microscope images of microplastics filtered with PVDF/CNOs membranes from (a–c) tap water, (d–f) lake water, (g,h) influent, and (i,j) effluent.
3.3.2. Morphology of Microplastics Determined by SEM
The morphology of microplastics filtered by PVDF membranes is represented in Figure 4. The SEM images show that the microplastics present in tap water exhibit long fibers in a three-dimensional (3D) film shapes (Figure 4a) while those in lake water show the presence of long fibers and threads (Figure 4b). The 3D film shapes of microplastics present in the influent are also observed in Figure 4c while curled-up fibers with film shapes are observed for the effluent in Figure 4d.
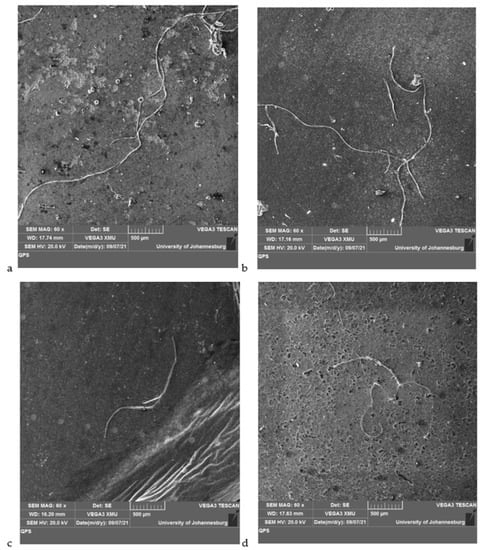
Figure 4.
SEM images of microplastics filtered through PVDF membranes from (a) tap water, (b) lake water, (c) influent, and (d) effluent.
The images obtained from microplastics filtered through the modified membrane are shown in Figure 5. A string-like fiber, film shapes, and flake-like fragments are observed for tap water samples (Figure 5a) while, in the lake water, a lot of film shapes, fragments and threads are observed (Figure 5b). For the influent, long fibers and thin film shapes are observed in Figure 5c, while fibers, long string fibers and big flakes-like fragments are seen for the effluent (Figure 5d). The images for lake water and wastewater effluent confirm the presence of lots of microplastics.
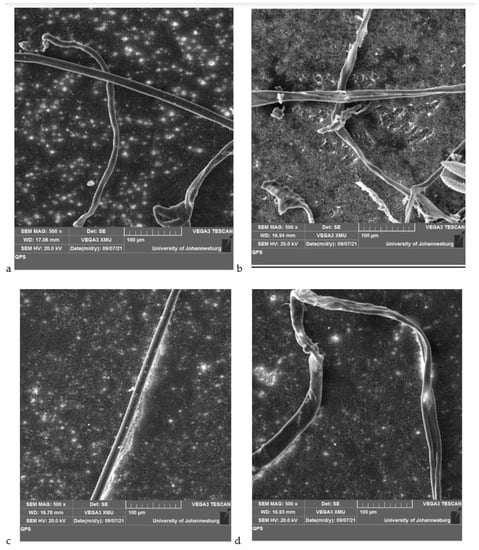
Figure 5.
SEM images of microplastics filtered through PVDF/CNOs membranes from (a) tap water, (b) lake water, (c) influent, and (d) effluent.
3.3.3. Composition of Microplastics Determined by ATR-FTIR
The composition of the microplastics filtered from the influent is represented by the spectrum in Figure 6. The ATR-FTIR is equipped with Spectrum 10 Spectroscopy Software and was able to classify the functional groups of the microplastics into various polymers. The yellow spectrum displayed peaks of C-O stretch at 3300 cm−1, C-H bend at 2830 cm−1 and aromatic ring stretch at 900 cm−1, which confirmed the presence of 16% cellulose. The cellulose originates from cotton in laundry which is introduced into the water system [31]. In the green spectrum, the following peaks are observed: C-H stretch at 2920 cm−1, CH2 bend at 1490 and 780 cm−1 confirming the presence of high-density polyethylene (50%). High density polyethylene (HDPE) is extremely prevalent in the environment since it is used in large amounts for consumer packaging such as plastic bags, storage containers, banners, bottle caps and so forth [26,32,33,34]. The red spectrum displays O-H stretch at 2915 cm−1, C=O symmetrical stretch at 1730 cm−1, C-O-C stretch at 1280 cm−1 and O-H bend at 750 cm−1 confirming the presence of 16.6% poly(1,4-butylene terephthalate) which originates from toothbrushes and false eyelashes. These fibers can be classified as primary microplastics and get introduced into the water system easily [31]. The blue spectrum shows C-H stretch at 2908 cm−1 C=O stretch at 1780 cm−1, C-N stretch at 1450 cm−1 and N-H bend at 900 cm−1 which confirmed the presence of 16.6% Nylon-12. Nylon-12 is mainly used for films for packaging material in the food industry, sterilized films and bags for the pharmaceutical and medical fields. It is also commonly used for cosmetics [35].
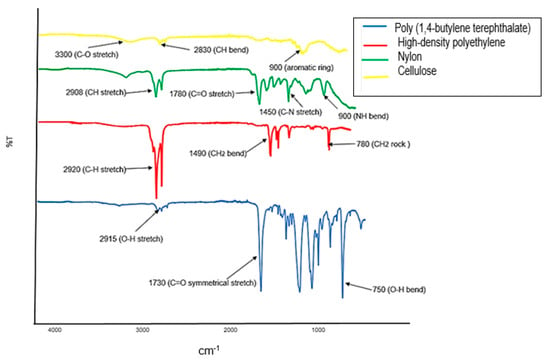
Figure 6.
ATR-FTIR spectra of microplastics obtained from a wastewater influent sample.
3.3.4. Characterization of Membranes before and after Filtration
The spectra in Figure 7 were obtained from analysis of the PVDF and PVDF/CNOs membranes before and after filtration of microplastics from wastewater influent which was sampled in April 2022. The green spectrum is that of a PVDF membrane and shows a band at 3060 cm−1 corresponding to the asymmetric and symmetric CH2 vibrations of PVDF. The absorption peak at 1790 cm−1 was attributed to CH2 vibrations, while the C-C band is observed at 1460 cm−1. The peaks at 1156 and 950 cm−1 are related to C-C-C asymmetrical stretch vibrations and CF stretching vibrations, respectively. After filtration of microplastics with the PVDF membrane, the C-H stretch was observed at 2998 cm−1, C=O stretch at 1730 cm−1 and N-H bend at 1490 cm−1, which confirmed the presence of Nylon-12 [35]. The red spectrum is that of a PDVF/CNOs membrane and displays a band at 3060 cm−1 (CH2) and small peaks at 1790 cm−1 (CH2), 1460 cm−1 (C-C), and 1150 cm−1 (C-C-C). The blue spectrum is for the PDVF/CNOs membrane after filtering the microplastics. The C-H stretch is observed at 2998 cm−1 and the N-H bend at 1400 and 1200 cm−1. It was thus observed that the characteristic absorption peaks of the Nylon-12 were retained in the spectrum [35].
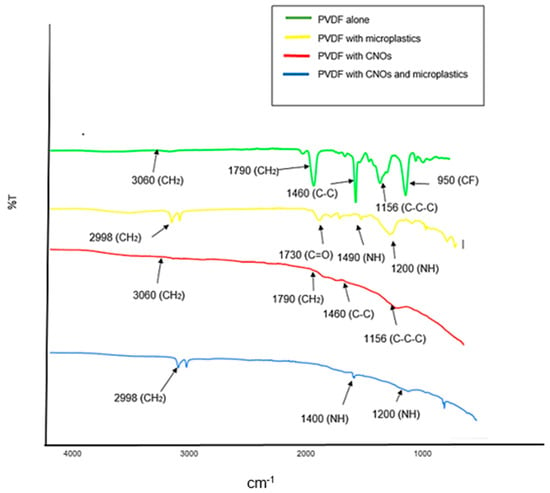
Figure 7.
ATR-FTIR spectra of PVDF and PVDF/CNOs membranes with microplastics obtained from a wastewater influent sample.
3.4. Analysis of Heavy Metals Extracted from Microplastics Using ICP-OES
3.4.1. Quality Control in Analysis
The calibration was performed with a heavy metal multielement standard solution and the correlation coefficient for all heavy metal calibration curves was R > 0.999 at the specific wavelengths that are listed in Table 4. The routine inclusion of a reference material and reagent blank ensured quality assurance throughout the analytical process. The accuracy of the measurements was determined using standard samples (Table 4). The relative standard deviations (RSD) of magnesium (0.7%), iron (1.1%), copper (0.9%), cadmium (1.7%), and aluminum (1.7%) were all lower than 2% which means that the data was closer to the mean concentration. For arsenic, chromium, cobalt, lead, mercury, nickel, silver and zinc the obtained RSD was above 2% which indicates that some of the concentration values were spread from of the mean concentration. The limit of detection was below 1 mg/L for most of the elements except for arsenic, copper and zinc. The limit of quantification was higher than the limit of detection which ensures the accuracy of the analysis.

Table 4.
Standard samples used for the analysis of heavy metals by ICP-OES.
3.4.2. Concentrations of Heavy Metals in the Filtrates Obtained by ICP-OES
Figure 8 shows the concentrations of heavy metals in the filtrate after effluent, influent, lake water and tap water samples were filtered through PVDF and PVDF/CNOs membranes. The highest concentrations of heavy metals were obtained in most samples filtered by PVDF. The magnesium concentrations were 367.645, 12.958, 915.581 and 1099.582 mg/L in tap water, lake water, effluent and influent, respectively. For silver the concentrations were 0.053, 0.043, 0.064, and 0.058 mg/L in tap water, lake water, effluent and influent, respectively. The filtrates from the samples filtered by PVDF/CNOs membranes also displayed high magnesium concentrations in tap water (366.279 mg/L), lake water (511.414 mg/L), effluent (954.47 mg/L) and influent (1067.62 mg/L). The silver concentrations were also low: tap water (0.054 mg/L), lake water (0.046 mg/L), influent (0.061 mg/L), and effluent (0.057 mg/L).
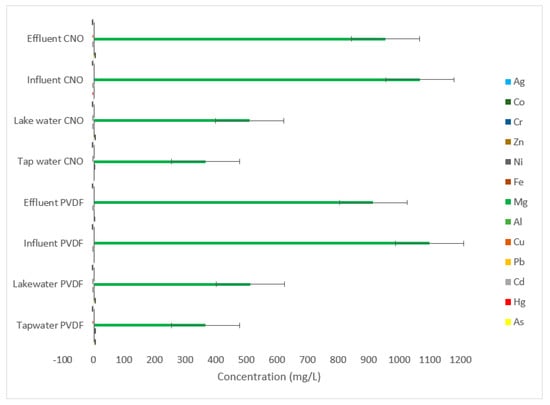
Figure 8.
Concentrations of heavy metals from the filtrates of effluent, influent, tap water, and lake water using ICP-OES.
The concentrations of heavy metals in the filtrates after removing microplastics confirm that only magnesium and silver were not attached to the microplastics.
3.4.3. Concentrations of Heavy Metals Extracted from Microplastics in Wastewater Influent
Acid and deionized water digestions were used to extract heavy metals that might have been adsorbed by the microplastics that were filtered from influent samples using PVDF membranes. The results are shown in Figure 9. During the extraction using deionized water, magnesium had the highest concentration. The concentrations were 154.924, 216.062, and 197.338 mg/L when sampled after 30, 60, and 90 min, respectively. Although these concentrations were high, they were considerably lower than the magnesium that was obtained in the filtrates. The maximum permissible limit of Mg in drinking water is <30 mg/L [36,37] (Table 5). Copper had the second highest concentration, with values of 4.327, 3.37, and 2.712 mg/L after 30, 60, and 90 min, respectively. Arsenic (4.743 mg/L) and zinc (2.117 mg/L) had high concentrations after 30 min of extraction. When acid extraction was applied to remove heavy metals from microplastics, copper had the highest concentrations. These were 34.139, 55.717, and 83.176 mg/L after 30, 60, and 90 min, respectively. Copper levels in drinking water are tolerated to a maximum of 1.3 mg/L [38]. Arsenic was the metal with the second highest concentrations: 5.036, 8.699, and 5.676 mg/L after 30, 60 and 90 min respectively. According to the Environmental Protection Agency (EPA), the federal limit for arsenic in drinking water is 0.010 mg/L [39] (Table 5). The third metal that had relatively high concentrations after acid extraction was zinc. The concentrations were 2.538, 1.388, and 1.859 mg/L after 30, 60 and 90 min, respectively. The maximum amount of zinc that should be present in drinking water is 5 mg/L [40,41] (Table 5). Although the presence of Zn in food is not always harmful, unusually high levels of Zn can signal the existence of metal contamination [40].
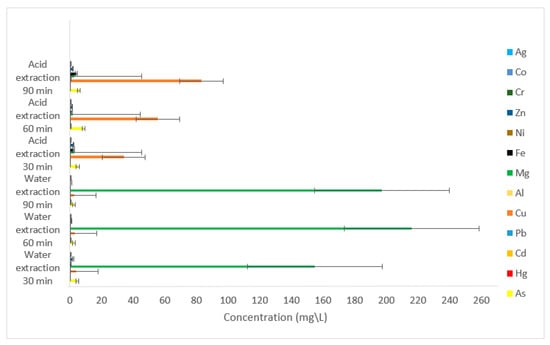
Figure 9.
Concentrations of heavy metals obtained by acid and water extraction of microplastics filtered from influent samples using PVDF membranes.

Table 5.
Comparison between the concentrations of heavy metals extracted from microplastics filtered from influent samples using PVDF membranes and the acceptable limits in drinking water.
When comparing the concentrations of the heavy metals obtained after extraction to those obtained in the filtrates (Section 3.4.2), it can be concluded that toxic heavy metals such as copper, arsenic and zinc can be adsorbed by microplastics.
4. Conclusions
The PVDF membrane successfully removed microplastics from wastewater and freshwater systems. The microplastics in the influent are composed of high density polyethylene, poly(1,4-butylene terephthalate), Nylon 12 and cellulose which resulted from hotels, houses, schools, industries and commercial businesses surrounding the treatment plant. High concentrations of toxic heavy metals such as arsenic, copper and zinc were attached to the microplastics. The heavy metals originated from the local mines in the area. The presence of microplastics and attached heavy metals in the influent indicate that the wastewater treatment infrastructure requires improvement by incorporating a microplastic trap before releasing the water to municipalities or back into the environment to avoid pollution. Thus, the research described in this paper can be applied as a microplastic analytical monitoring protocol to ensure adherence to water quality and management practices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations9070166/s1, Table S1: Sampling procedure of the influent, effluent, lake water and tap water from areas in Daspoort wastewater treatment plant, Sterkfontein lake and tap water in Doornfontein, South Africa.
Author Contributions
S.D.N. was responsible for investigation, methodology, validation, formal analysis, writing the manuscript, data curation and visualization S.P.M. was responsible for a student bursary, reviewing the manuscript and co-supervision. N.M. was responsible for conceptualization, supervision, project administration, resources, funding acquisition for chemicals and reviewing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation, grant number SRUG200326510622 and TTK180427324698; the Centre for Nanomaterials Science Research, University of Johannesburg (UJ) South Africa and the Faculty of Science, University of Johannesburg, South Africa.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data of this research can be found in Google Drive in the following link: https://drive.google.com/drive/folders/1qA5accf0tJPBNqB-G3VBRYm1Q64ZUY6G?usp=shar-605ing (accessed on 23 May 2022).
Acknowledgments
We would like to acknowledge Perkin Elmer, Midrand, South Africa for allowing us to use their equipment (FTIR and ICP-OES). We would like to thank Ludwe Sikeyi and Luthando Tshwenya from Electrochemistry Laboratory 3315, UJ for the carbon nano onions that were applied in this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nabi, I.; Bacha, A.U.; Li, K.; Cheng, H.; Wang, T.; Liu, Y.; Ajmal, S.; Yang, Y.; Feng, Y.; Zhang, L. Complete photocatalytic mineralization of microplastic on TiO2 nanoparticle film. iScience 2020, 23, 101326. [Google Scholar] [CrossRef]
- Wang, L.; Kaeppler, A.; Fischer, D.; Simmchen, J. Photocatalytic TiO2 micromotors for removal of microplastics and suspended matter. ACS Appl. Mater. Interfaces 2019, 11, 32937–32944. [Google Scholar] [CrossRef]
- Uheida, A.; Mejía, H.G.; Abdel-Rehim, M.; Hamd, W.; Dutta, J. Visible light photocatalytic degradation of polypropylene microplastics in a continuous water flow system. J. Hazard. Mater. 2021, 406, 124299. [Google Scholar] [CrossRef]
- Thompson, R.C.; Moore, C.J.; Vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Cheung, P.K.; Fok, L. Characterisation of plastic microbeads in facial scrubs and their estimated emissions in Mainland China. Water Res. 2015, 122, 53–61. [Google Scholar] [CrossRef]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Shi, J.; Wang, J.; Dai, Y.; Li, H.; Li, J.; Liu, X.; Chen, X.; Wang, Z.; Zhang, P. Interactions between microplastics and heavy metals in aquatic environments: A review. Front. Microbiol. 2021, 12, 730. [Google Scholar] [CrossRef]
- Santillo, D.; Miller, K.; Johnston, P. Microplastics as contaminants in commercially important seafood species. IEAM 2017, 13, 516–521. [Google Scholar] [CrossRef]
- Anderson, J.C.; Park, B.J.; Palace, V.P. Microplastics in aquatic environments: Implications for Canadian ecosystems. Environ. Pollut. 2016, 218, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Ivleva, N.P.; Wiesheu, A.C.; Niessner, R. Microplastic in aquatic ecosystems. Angew. Chem. Int. Ed. 2016, 56, 1720–1739. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, K.H.; Bansal, V.; Lazarides, T.; Kumar, N. Progress in the sensing techniques for heavy metal ions using nanomaterials. J. Ind. Eng. Chem. 2017, 54, 30–43. [Google Scholar] [CrossRef]
- Verster, C.; Minnaar, K.; Bouwman, H. Marine and freshwater microplastics research in South Africa. IEAM 2017, 13, 533–535. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.; Rintoul, L.; Leusch, F.D. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Raju, N.S.; Carbery, M.; Kuttykattil, A.; Senathirajah, K.; Lundmark, A.; Rogers, Z.; SCB, S.; Evans, G.; Palanisami, T. Improved methodology to determine the fate and transport of microplastics in a secondary wastewater treatment plant. Water Res. 2020, 173, 115549. [Google Scholar] [CrossRef]
- Franco, A.A.; Arellano, J.M.; Albendín, G.; Rodríguez-Barroso, R.; Quiroga, J.M.; Coello, M.D. Microplastic pollution in wastewater treatment plants in the city of Cádiz: Abundance, removal efficiency and presence in receiving water body. Sci. Total Environ. 2021, 776, 145795. [Google Scholar] [CrossRef]
- Yang, Z.; Li, S.; Ma, S.; Liu, P.; Peng, D.; Ouyang, Z.; Guo, X. Characteristics and removal efficiency of microplastics in sewage treatment plant of Xi’an City, northwest China. Sci. Total Environ. 2021, 771, 145377. [Google Scholar] [CrossRef]
- Malankowska, M.; Echaide-Gorriz, C.; Coronas, J. Microplastics in marine environment: A review on sources, classification, and potential remediation by membrane technology. Environ. Sci. Water Res. Technol. 2021, 7, 243–258. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Setala, O.; Heinonen, M.; Koistinen, A. How well is microlitter purified from wastewater?—A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Liu, F.; Hashim, N.A.; Abed, M.R.M.; Li, K. Poly(vinylidene fluoride) (PVDF) membranes for fluid separation. React. Funct. Polym. 2015, 86, 134–153. [Google Scholar] [CrossRef]
- Sikeyi, L.; Ntuli, T.; Mongwe, T.; Maxakato, N.; Carleschi, E.; Doyle, B.; Coville, N.; Maubane-Nkadimeng, M. Microwave assisted synthesis of nitrogen doped and oxygen functionalized carbon nano onions supported palladium nanoparticles as hybrid anodic electrocatalysts for direct alkaline ethanol fuel cells. Int. J. Hydrog. 2021, 46, 10862–10875. [Google Scholar] [CrossRef]
- Begum, S.; Kausar, A.; Ullah, H.; Siddiq, M. Potential of polyvinylidene fluoride/carbon nanotube composite in energy, electronics, and membrane technology: An overview. Polym.-Plast. Technol. Eng. 2016, 55, 1949–1970. [Google Scholar] [CrossRef]
- Salim, N.; Siddiqa, A.; Shahidda, S.; Qaisar, S. PVDF based Nanocomposite Membranes: Application towards Wastewater treatment. MJNN 2019, 4, 139–147. [Google Scholar] [CrossRef]
- Wastewater Treatment. Available online: https://www.wastewatertreatment.co.za/?gclid=Cj0KCQiAjJOQBhCkARIsAEKMtO2-d4UdyyJ1wci0gTLrOiUcJJNmuOuzJOE8tq2g3UZKitcuSLtiFJ8aAtkPEALw_wcB (accessed on 23 May 2022).
- Mintenig, S.M.; Int-Veen, I.; Loder, M.G.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of wastewater treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365e372. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, J.; Shan, M.; Li, Y.; Li, B.; Niu, J.; Zhou, B.; Qian, X. Organosilane—Functionalized graphene oxide for enhanced antifouling and mechanical properties of polyvinylidene fluoride ultrafiltration membranes. J. Membr. Sci. 2014, 458, 1–13. [Google Scholar] [CrossRef]
- Espinoza, B.; Maricela, R. Microplastics in Wastewater Treatment Systems and Receiving Waters. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2019. [Google Scholar] [CrossRef]
- Kamaz, M.; Sengupta, A.; Gutierrez, A.; Chiao, Y.H.; Wickramasinghe, R. Surface modification of PVDF membranes for treating produced waters by direct contact membrane distillation. Int. J. Environ. Res. Public Health 2019, 16, 685. [Google Scholar] [CrossRef] [Green Version]
- Bartkowski, M.; Giordani, S. Supramolecular chemistry of carbon nano-onions. Nanoscale 2020, 12, 9352–9358. [Google Scholar] [CrossRef]
- Andrady, A.L. Ultraviolet radiation and polymers. In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007; pp. 857–866. [Google Scholar] [CrossRef] [Green Version]
- Kershaw, P.J.; Turra, A.; Galgani, F. Guidelines or the Monitoring and Assessment of Plastic Litter in the Ocean; GESAMP Reports and Studies 99; GESAMP: London, UK, 2019. [Google Scholar] [CrossRef]
- Govender, J.; Naidoo, T.; Rajkaran, A.; Cebekhulu, S.; Bhugeloo, A.; Sershen, S. Towards characterising microplastic abundance, typology and retention in mangrove-dominated estuaries. Water 2020, 12, 2802. [Google Scholar] [CrossRef]
- Magnusson, K.; Norén, F. Screening of Microplastic Particles in and Down-Stream a Wastewater Treatment Plant. In Swedish; Environmental Protection Agency: Washington, DC, USA, 2014. Available online: http://urn.kb.se/resolve?urn=urn%3Anbn%3Ase%3Anaturvardsverket%3Adiva-2226 (accessed on 23 May 2022).
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef]
- Thomas, M.P. Calcium and magnesium in drinking-water: Public Health Significance. Int. J. Environ. Sci. 2010, 67, 612–613. [Google Scholar] [CrossRef]
- Gregory, M.R. Plastic “scrubbers” in hand cleansers: A further (and minor) source for marine pollution identified. Mar. Pollut. Bull. 1996, 32, 867–871. [Google Scholar] [CrossRef]
- Morritt, D.; Stefanoudis, P.V.; Pearce, D.; Crimmen, O.A.; Clark, P.F. Plastic in the Thames: A river runs through it. Mar. Pollut. Bull. 2014, 78, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Nedzivhe-Mqehe, K.C.; Makhado, K.; Olorundare, O.F.; Arotiba, O.A.; Makhatha, E.; Nomngongo, P.N.; Mabuba, N. Bio-Adsorbents for the Removal of Heavy Metals from Water. In Arsenic-Analytical and Toxicological Studies; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, F.; Guillaume, D.; Abdulwali, N.; Al-Hadrami, K.; Maqtari, M.A. ICP-OES assisted determination of the metal content of some fruit juices from Yemen’s market. Heliyon 2020, 6, e04908. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philosophical Transactions of the Royal Society of London. Ser. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).