Recent Advances in Separation and Analysis of Saponins in Natural Products
Abstract
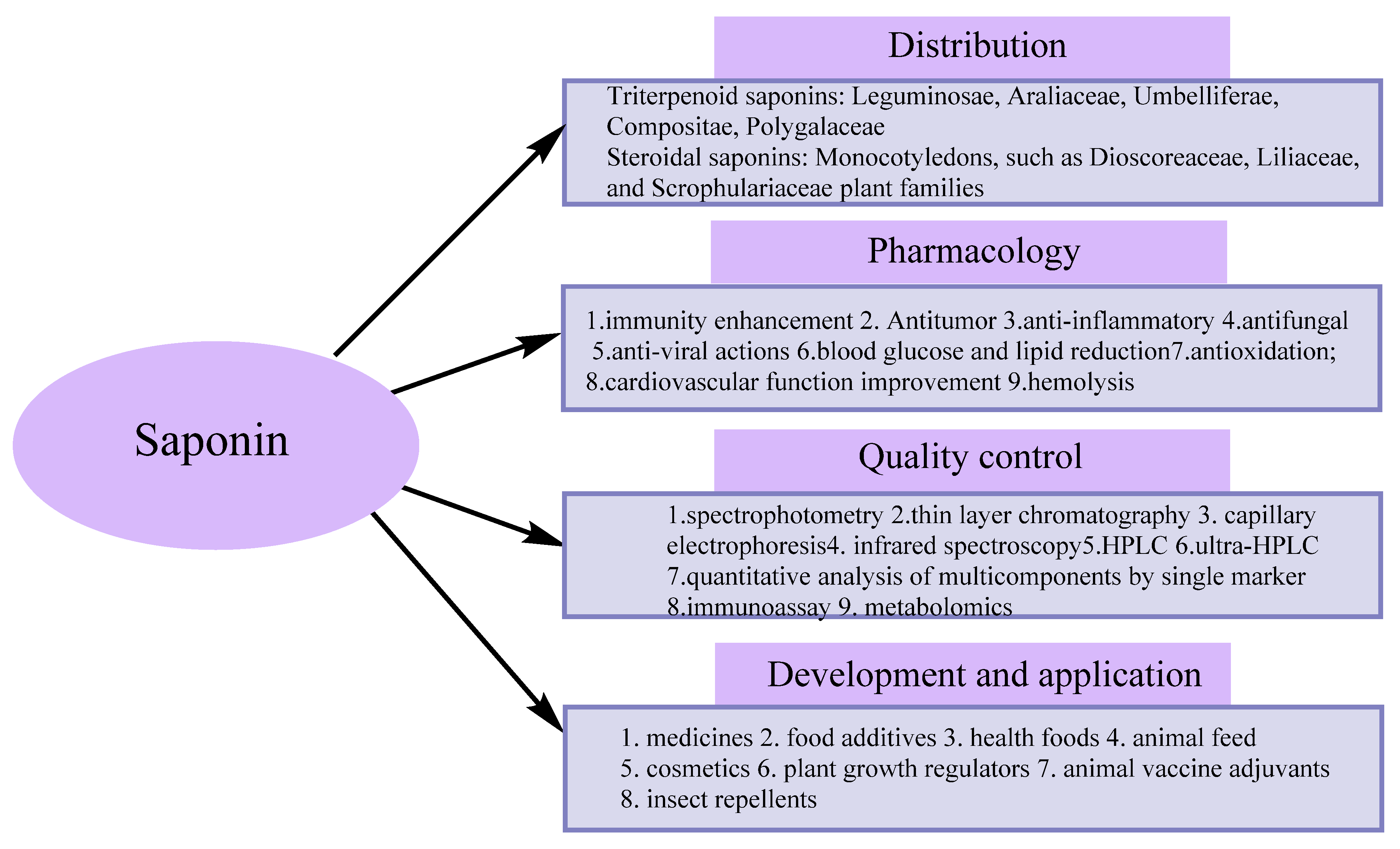
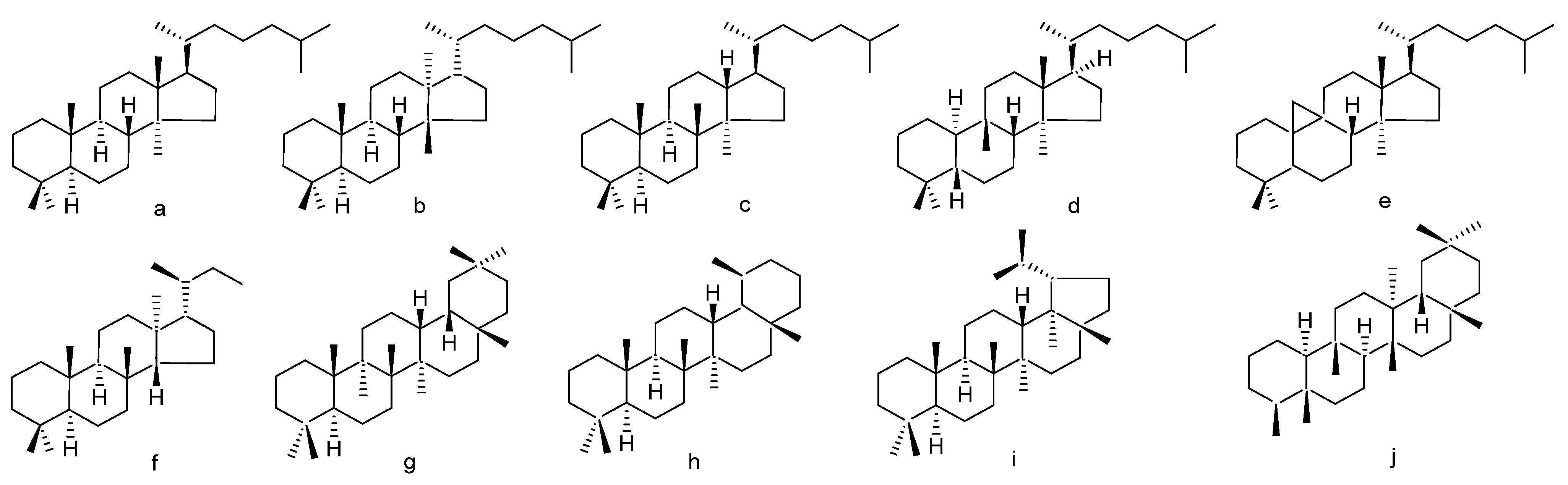
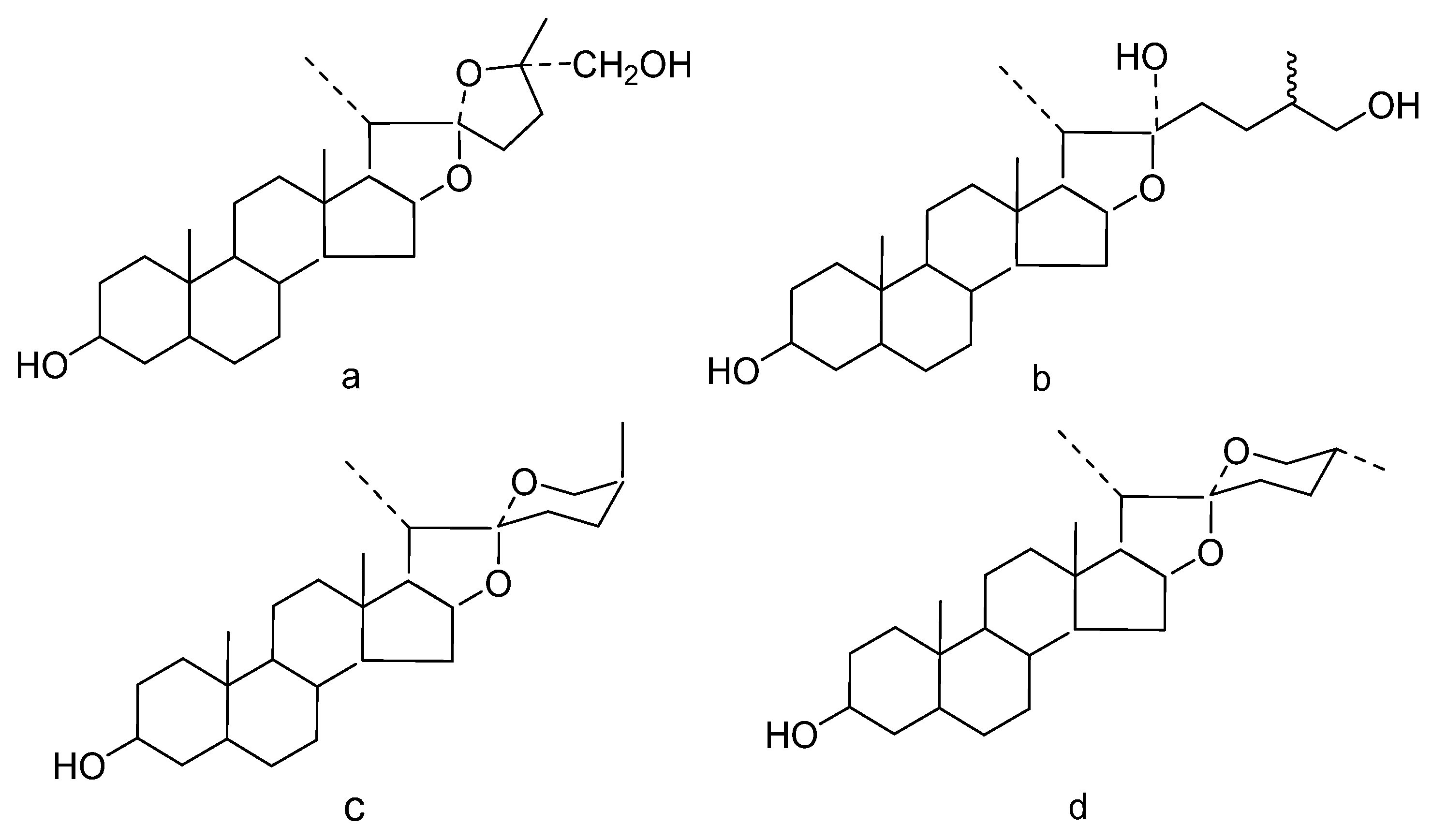
1. Introduction
2. Extraction and Separation Methods
2.1. Extraction of Saponins
2.1.1. ILs
2.1.2. UAE
2.1.3. MAE
2.2. Isolation of Saponins
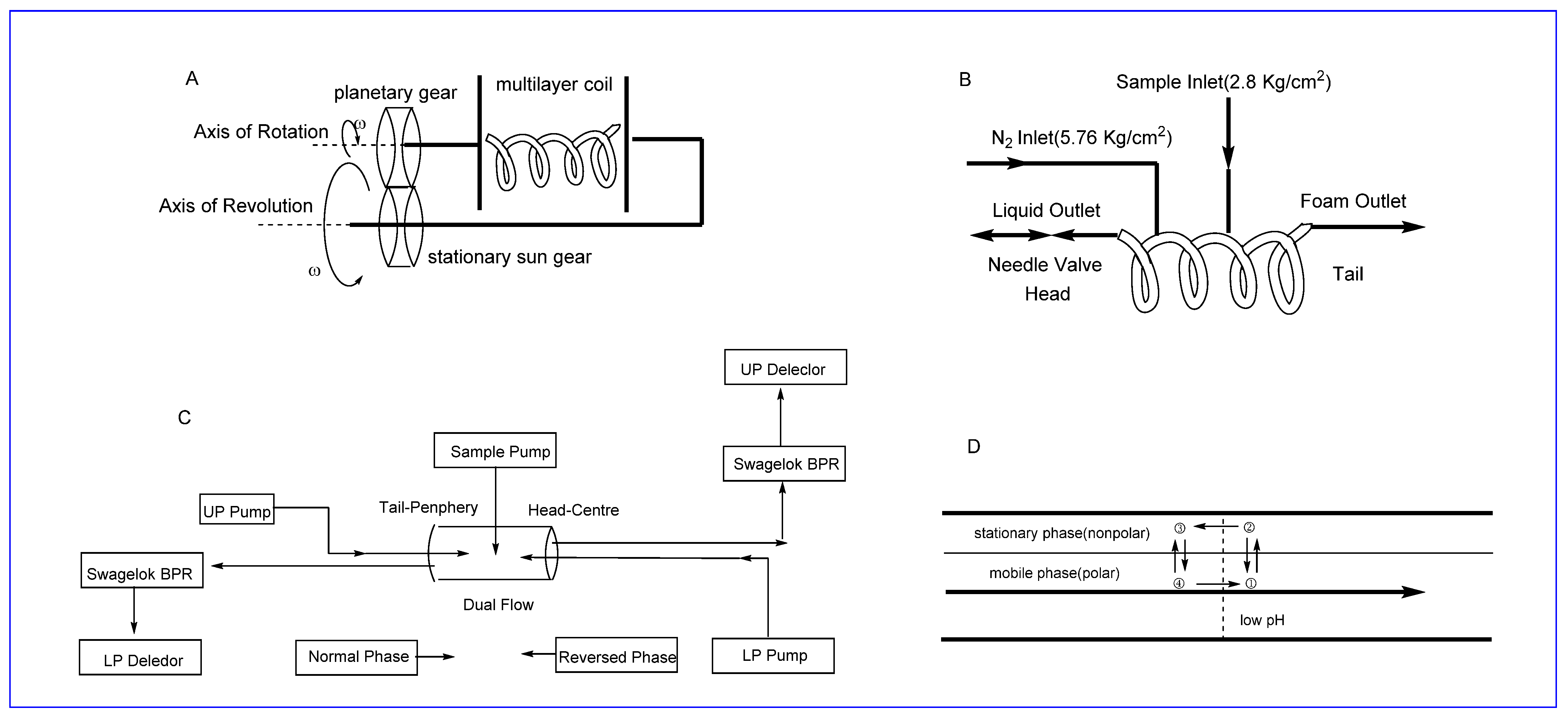
2.2.1. SFC
2.2.2. HSCCC
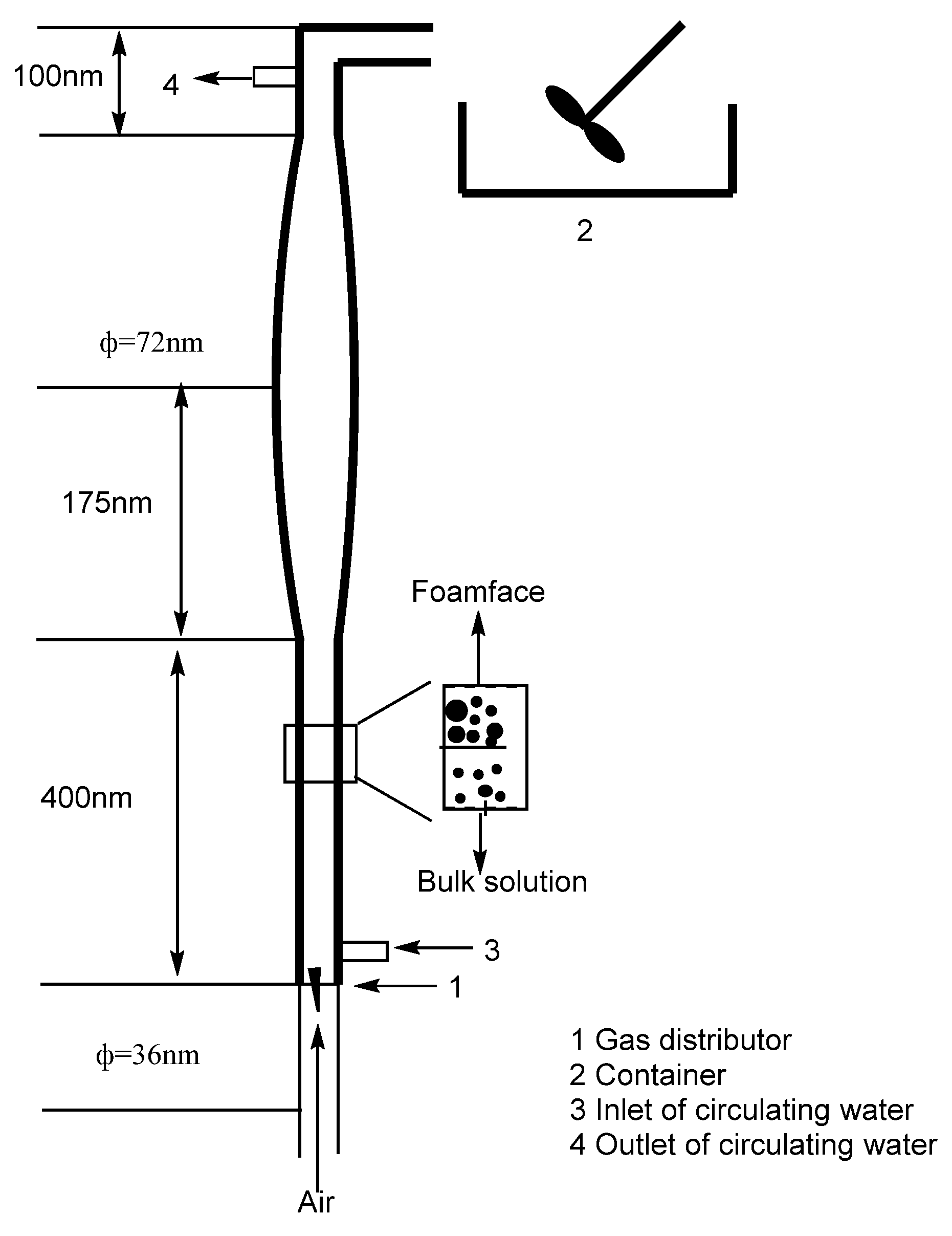
2.2.3. Foam Fractionation
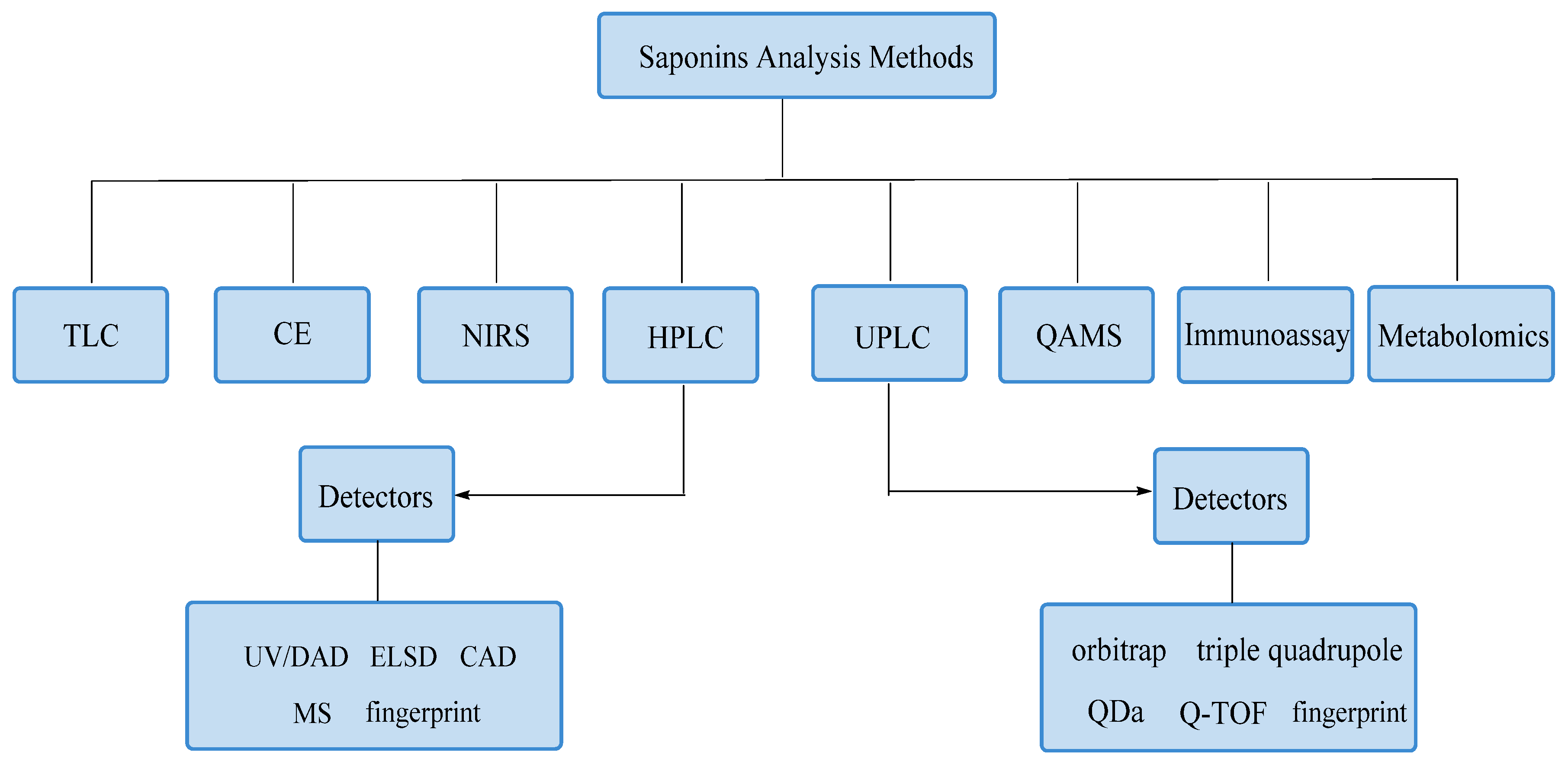
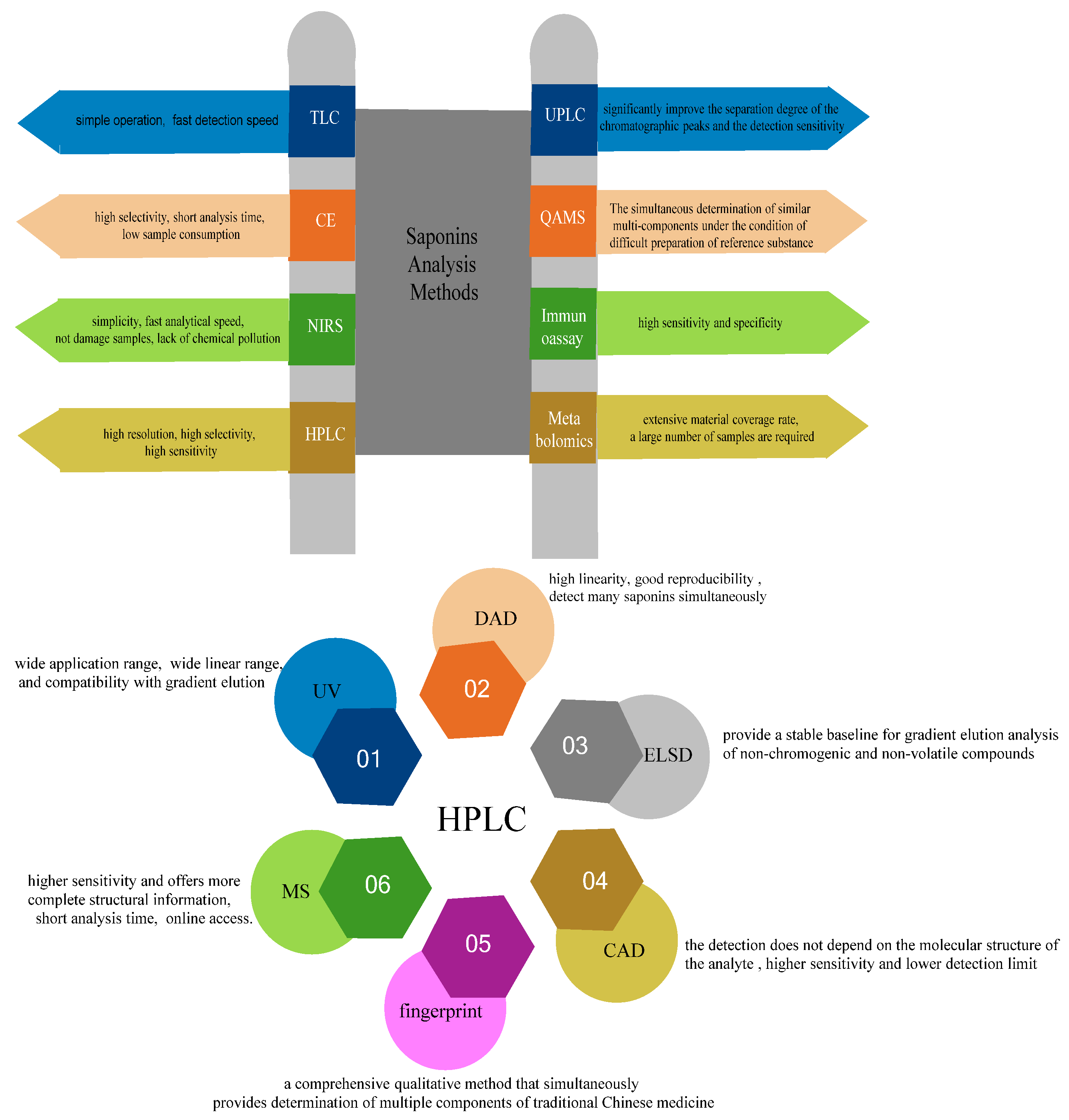
3. Analytical Methods
3.1. TLC
3.2. CE
3.3. NIRS
3.4. HPLC
3.4.1. HPLC-UV/DAD
3.4.2. HPLC-ELSD
3.4.3. HPLC-CAD
3.4.4. Chromatographic Fingerprint
3.4.5. HPLC-MS Detector
| Name | Qualitative/Quantitative | Analytical Method | Chromatographic Conditions | Test Results | Ref. |
|---|---|---|---|---|---|
| Polygonti Rhizome | Qualitative and quantitative | UHPLC-Q-Exactive Orbitrap HRMS | stationary phase: Alorich Ascentis C8 column (10 cm × 4.6 mm, 3 µm), mobile phases: water (A) and acetonitrile (B), column temperature: 25 °C | 12 diosgenin:Dioscin, Gracillin, Deltonin, Trillin, Prosapogenin A, zingiberensis New Saponin, Protodioscin, Protogracillin, Protodeltonin, Pseudoprotodioscin, Pseudoprotogracillin, Methyl protodioscin | [84] |
| Soybeans | Qualitative and quantitative | SPE-HPLC-MALDI-TOF-MS | stationary phase: Gemini C18 column (150 × 4.6 mm, 5 μm), mobile phase: water (0.25% acetic acid) (A) and methanol (0.25% acetic acid) (B) | soyasaponins I and βg | [95] |
| Achyranthes | Quantitative | LC-MS | stationary phase:Inertsil PREP-ODS column (20 × 250 mm), mobile phase: UPW (5 mM DHAA) (A) and MeCN (5 mM DHAA) (B) | chikuset-susaponins IVa and V, achyranthosides B, C, D, E and G, sulfachyranthosides B and D, and betavulgarosides II and IV | [83] |
| Paris and Trillium | Qualitative | HPLC-ESI (+/−)-MSn | stationary phase: Kromasil RP-C18 column (4.6 mm × 250 mm, 5 µm), mobile phase: water (A) and acetonitrile (B) | 12 steroidal saponins: Dichotomin, Protosaponin3Glc-Rha-Ara, Methyldichotomin, Methyl protodioscin, Methylprotosaponin2Glc-2Rha-Ara, Diosgenin 2Glc-3Rha, PolyPhyllin H, Methylprotogracillin, Diosgenin2Glc-Rha-Ara, PennogeninGlc-2Rha, Pennogenin 2Glc-Rha, Diosgenin2Ara-Rha-Glc | [69] |
| Ophiopogon japonicus | Quantitative | HPLC-MS | stationary phase: Tigerkin C18 column, mobile phase: water (0.02% formic acid ) (A) and acetonitrile (0.02% formic acid) (B) | three steroidal saponins: cixi-ophiopogon A, cixi-ophiopogon B, cixi-ophiopogon C | [81] |
| Chaihu | Quantitative | anionic adducts-based liquid chromatography tandem mass spectrometry method | stationary phase: Agilent Zorbax SB-C18 column (100 × 3.0 mm, 3.0 µm), mobile phase: water (0.06% formic acid) (A), acetonitrile (B) and methanol (C) | saikosaponin a, saikosaponin c, saikosaponin d and saikosaponin b2 | [94] |
| Chaihu-Guizhi decoction | Quantitative | HPLC-ESI- MS/MS | stationary phase: Halo® C18 column (2.1 × 100 mm, 2.7 μm), mobile phase: water (0.1% formic acid) (A) and acetonitrile (B), flow rate: 0.3 mL/min | 15 active compounds: Saikosaponin A, Baicalin, Wogonin, Glycyrrhizic acid, Glycyrrhetinic acid, Albiflorin, Paeoniflorin, Liquiritin, Isoliquiritin, Liquiritigenin, Isoliquiritigenin, Cinnamic acid, Gallic acid, Wogonoside and Oroxylin A | [98] |
| Zhimu-Baihe herb-pair | Qualitative | high-performance liquid chromatography and time-of-flight mass spectrometry | stationary phase: Zorbax XDB-C18 analytical column (2.1 × 50 mm, 1.8 µm), mobile phase: water (0.1% formic acid) (A) and acetonitrile (B), flow rate: 0.2 mL/min | 24 saponins, 3 xanthones, 1 anthraquinone and 2 alkaloids: Neomangiferin, Mangiferin, Isomangiferin, Timosaponin B-V, Timosaponin B-VI, Timosaponin H1, Timosaponin I1, Timosaponin B-IV, Timosaponin I2; Timosaponin H2, Neohyacinthoside, Timosaponin E1, Timosaponin E, Timosaponin N, Timosaponin E2, Macrostemonoside K, Timosaponin B-II, Timosaponin D, Timosaponin B-I, Timosaponin B-III, Brownioside 1, Brownioside 2, Timosaponin F, Anemarrhenasaponin I, Anemarrhenasaponin Ia, Timosaponin G, Timosaponin AIII, Timosaponin A-I, Colchicine, Emodin | [100] |
| Radix Astragali | Qualitative | HPLC-Q- TOF/MS | stationary phase: Gemini C18 column (4.6 mm × 250 mm, 5 µm), mobile phase: water (0.3% formic acid) (A) and ACN (B) | 22 types of astragaloside IV | [90] |
| Ophiopogon Japonicus Ker-Gawler | Qualitative | High-Performance Liquid Chromatography with Ion Trap Mass Spectrometry | stationary phase: Tigerkin C-18 column (4.6 × 250 mm, 5.0 µm), mobile phase: water (0.05% formic acid) (A) and acetonitrile (0.05% formic acid) (B), flow rate: 0.5 mL/min, detection wavelength: 203 nm | 8 steroidal saponins: ophiogenin 3-O-α-L-rhanose-(1→2)-β-D-xylose-(1→3)-β-D-glucose-(1→4)-β-D-glucose, ophiogenin 3-O-α-L-rhanose-(1→2)-β-D-glucose-β-D-glucose, ophiogenin 3-O-α-L-rhanose-(1→2)-β-D-xylose-β-D-glucose, pennogenin 3-O-α-L-rhanose-(1→2)-β-D-xylose-(1→3)-β-D-glucose, ruscogenin 3-O-α-L-rhanose-(1→2)-β-D-xylose-(1→3)-α-L-araβinose, ruscogenin 3-O-α-L-rhanose-(1→2)-β-D-xylose-(1→3)-β-D-fucose, pennogenin 3-O-α-L-Rha-(1→2)-O-β-D-Xyl-(1→3)-O-β-D-Xyl-(1→4)-O-β-D-Glc, pennogenin 3-O-α-L-Rha-(1→2)-O-β-D-Glc-(1→3)-O-β-D-Glc or ruscogenin 3-O-α-L-Rha-(1→2) -O-β-D-Glc-(1→3)-O-β-D-Glc | [91] |
| Glycyrrhiza uralensis | Qualitative | rapid-resolution liquid chromatography with time-of-flight mass spectrometry (RRLC/TOF-MS) | stationary phase: Agilent ZorBax SB-C18 column (4.6 × 50 mm, 1.8 µm), mobile phase: water (0.2% formic acid) (A) and acetonitrile (B) | 19 oleic acid alkanestype triterpene saponins: uralsaponin C, uralsaponin D, uralsaponin F, uralsaponin E, 24-hydroxyl-licorice E2, licorice-saponin A3, 22-acetoxyl-glycyrrhizin, licorice-saponin E2, 22-acetoxyl-Glycyrrhaldehyde, licorice-saponin G2, glycyrrhizin, 18a-glycyrrhizin and uralsaponin B | [93] |
| Shaoyao-Gancao-Decoction | Quantitative | HPLC–MS/MS | stationary phase: Zorbax XDB-C18 column (2.1 mm × 50 mm, 3.5 µm), mobile phase: water (0.1% formic acid) (A) and methanol (0.1% formic acid) (B) | Albiflorin, oxypaeoniflorin, paeoniflorin, liquiritin, isoliquiritin, liquiritigenin, isoliquiritigenin, ononin, glycyrrhizin andglycyrrhetinic acid | [101] |
| Glycyrrhiza yunnanensis | Qualitative | HPLC–MS/MS | stationary phase: YMC-Pack ODS-A column (4.6 mm × 250 mm, 5 µm), mobile phase: acetonitrile (A) and water (0.1% formic acid) (B), column temperature: 35 ℃, flow rate: 1 mL/min | glyyunnansapogenin I, yunganosides E3, L, M, N1,O, P and N2 | [92] |
| Dioscorea panthaica Prain et Burk | Quantitative | high-performance liquid chromatography-electrospray tandem mass spectrometry | stationary phase: RP-18e monolithic column (50 mm × 2 mm), mobile phase: acetonitrile (A) and water (0.1% formic acid) (B) | six steroid saponins: HSY-14, HSY-10, dioscin (DS), gracillin (GC), pseudoprotodioscin (PDD), pseudoprotogracillin (PDG) | [82] |
| Ardisia Crenata | Qualitative and quantitative | Ultra fast liquid chromatography-electrospray quadrupole mass spectrometry (UFLC-MS) | stationary phase: Zorbax Eclipse Plus C18 column (100 mm × 2.1 mm, 1.8 µm), mobile phase: water (0.1% formic acid) (A) and acetonitrile (0.1% formic acid) (B), flow rate: 0.2 mL/min | 13,28-epoxy-oleanane-type triterpenoid saponins | [96] |
| Acanthopanax henryi | Qualitative | HPLC-ESI-TOF-MS | stationary phase: Kinetex XB-C18 column (100 mm × 4.6 mm, 2.6 µm), mobile phase: acetonitrile (A) and water (B) | 15 triterpenoid saponins | [75] |
| Panax notoginseng | Qualitative | HPLC-QTOF/MS | stationary phase: agilent Eclipse XDB-C18 column (250 mm × 4.6 mm, 5 µm) mobile phase: water (0.1% formic acid) (A) and acetonitrile (0.1% formic acid) (B), flow rate: 0.8 mL/min | 234 ginsenosides | [89] |
| Triguero asparagus | Qualitative and quantitative | HPLC-MS | stationary phase: reversephase analytical column (25 cm × 4.6 mm, 5 μm), mobile phase: water (0.1% formic acid) (A) and acetonitrile (0.1% formic acid) (B) | saponins (HTSAP1 to HTSAP8) and protodioscin | [102] |
| fresh and cooked white asparagus | Qualitative and quantitative | HPLC-MS/MS | stationary phase: Zorbax Eclipse XDB-C18 column (150 × 2.1 mm, 5 μm), mobile phase: acetonitrile (0.1% formic acid) (A) and water (0.1% formic acid) (B) | the monodesmosidic saponins 5a/b, bidesmosides 1a/b and 2a/b | [97] |
| crude Glycyrrhizae radix and processed Glycyrrhizae radix | Qualitative | HPLC-ESI/MS | stationary phase: Kromasil 100-5 C18 column (4.6 × 250 mm, 5 µm), mobile phase: water (0.1% formic acid) (A) and acetonitrile (B), detection wavelength: 254 nm | eleven constituents: liquiritin apioside, liquiritin, licuraside, isoliquiritin, ononin, glycyrrhizin, liquiritigenin-7,4’-diglucoside, licorice saponin A3, 22β-acetoxylglycyrrhizic acid, licorice saponin G2, and yunganoside E2 | [99] |
3.5. UPLC
3.6. QAMS
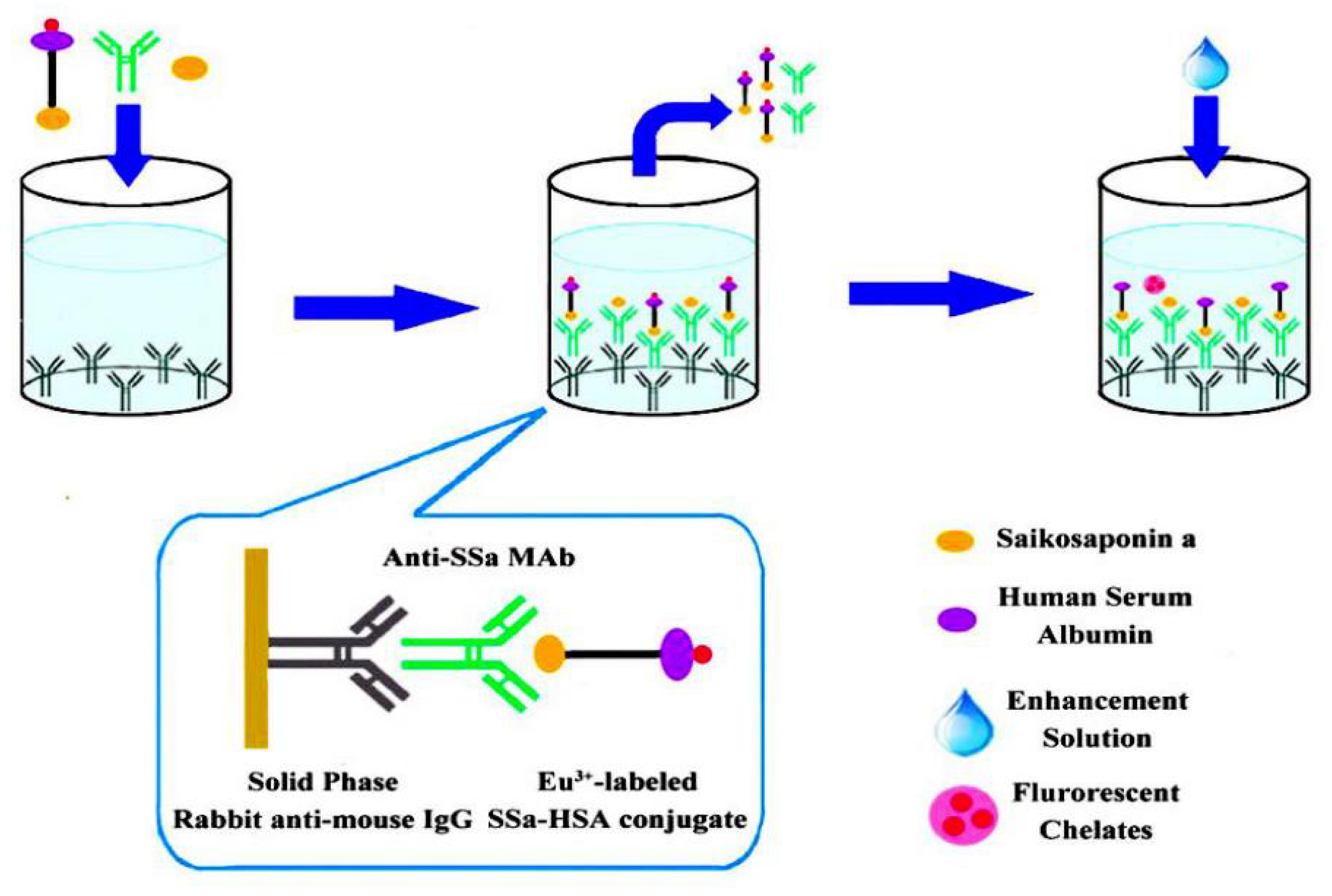
3.7. Immunoassay
3.8. Metabolomics
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ILs | ionic liquids |
| SFC | supercritical fluid chromatography |
| UAE | ultrasonic-assisted extraction |
| MAE | microwave-assisted extraction |
| HSCCC | high-speed counter-current chromatography |
| DAD | diode array detector |
| MSD | mass spectrometry detector |
| ELSD | evaporative light-scattering detector |
| CAD | charged aerosol detector |
| QAMS | quantitative analysis of multi-components by single-marker |
| NIRS | near-infrared spectroscopy |
| TLC | thin-layer chromatography |
| CE | capillary electrophoresis |
| HPLC | high-performance liquid chromatography |
| UPLC | ultra-high-performance liquid chromatography |
| UV/DAD | ultraviolet/diode array detection |
| SMD | standard method difference |
| PLS | partial least square |
| PCA | principal component analysis |
| RSD | relative standard deviation |
| CG | calycosin-7-o-β-d-glucoside |
| NMR | nuclear magnetic resonance |
| HPTLC | high-performance thin-layer chromatography |
| IT | ion-trap |
| PR | polygala radi |
References
- Abashev, M.; Stekolshchikova, E.; Stavrianidi, A. Quantitative aspects of the hydrolysis of ginseng saponins: Application in HPLC-MS analysis of herbal products. J. Ginseng Res. 2021, 45, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Ha, T.K.Q.; Yang, J.L.; Pham HT, T.; Oh, W.K. Triterpenoids from the genus Gynostemma: Chemistry and pharmacological activities. J. Ethnopharmacol. 2021, 268, 113574. [Google Scholar] [CrossRef] [PubMed]
- Kuwada, K.; Kawase, S.; Nakata, K.; Shinya, N.; Narukawa, Y.; Fuchino, H.; Kawahara, N.; Kiuchi, F. LC-MS analysis of saponins of Achyranthes root in the Japanese market. J. Nat. Med. 2020, 74, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Wang, H.; Jin, Y.; Li, Y.; Wang, Y. Isolation, identification, and quantification of triterpene saponins in the fresh fruits of Panax notoginseng. Nat. Prod. Res. 2021, 1–11. [Google Scholar] [CrossRef]
- Man, S.; Yujuan, W.; Yiming, L.; Sheng, L. Advances in pharmacology and clinical application of platycodin. J. Shanghai Univ. Tradit. Chin. Med. 2018, 32, 86–91. [Google Scholar]
- Jun, Z.; Baikun, Y.; Xiaoyang, H. Research Progress in the Chemical Constituents and Modern Pharmacology of Platycodon. J. Liaoning Univ. Tradit. Chin. Med. 2019, 21, 113–116. [Google Scholar]
- Xiongxiong, X.; Chi, Z.; Jinxiang, Z.; Chenhui, Z.; Zhu, M.; Junwei, H.; Hongling, W.; Guoyue, Z.; Shouwen, Z.; Fengyu, H. Advances in research on chemical constituents and pharmacological activity of Chinese herbal medicine Platycodon grandiflorum. Bull. Tradit. Chin. Med. 2018, 17, 13, 66–72. [Google Scholar]
- Mian, T.; Xiaofen, X. Advances in Studies on Chemical Constituents and Pharmacological Effects of Medicinal Astragalus. Her. Tradit. Chin. Med. 2018, 24, 117–122. [Google Scholar]
- Lili, W.; Li, C.; Ying, S.; Tianyang, X. Chemical compositionand pharmacological action of Rhizoma Anemarrhenae. Jilin J. Tradit. Chin. Med. 2018, 38, 90–92. [Google Scholar]
- Yashuang, C.; Shiwei, S. Advances in the research on chemical constituents and pharmacological effects of Bupleurum chinense. Heilongjiang Med. 2014, 27, 630–633. [Google Scholar]
- Meiling, Y.; Liu, Y.; Ajiao, H.; Xinyue, G.; Wenjing, M.; Xudong, X.; Hua, H. Research Progress on Chemical Composition and Pharmacological Effect of Bupleurum chinense. Inf. Tradit. Chin. Med. 2018, 35, 103–109. [Google Scholar]
- Xiaohui, S.; Liang, Z. Radix bupleuri research progress of pharmacological effects. China Med. Her. 2017, 14, 52–55. [Google Scholar]
- Dawei, L.; Liping, K.; Baiping, M. Chemical constituents and pharmacological activities of Polygala tenuifolia Willd.: Research advances. Int. J. Pharm. Res. 2012, 39, 32–36, 44. [Google Scholar]
- Yingying, S.; Yue, L.; Keji, C. Cardiovascular pharmacological effects of ginsenosides: Progress and thinking. Sci. China Life Sci. 2016, 46, 771–778. [Google Scholar]
- Juan, L.; Rufeng, W.; Li, Y.; Zhengtao, W. Structure and biological action on cardiovascularsystems of saponins from Panax notoginseng. Chin. J. Tradit. Chin. Med. 2015, 40, 3480–3487. [Google Scholar]
- Savarino, P.; Demeyer, M.; Decroo, C.; Colson, E.; Gerbaux, P. Mass spectrometry analysis of saponins. Mass Spectrom. Rev. 2021, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, H.; Wu, H.; Deng, R.; Sun, M. Deciphering the metabolic profile and pharmacological mechanisms of Achyranthes bidentata blume saponins using ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry coupled with network pharmacology-based investigation. J. Ethnopharmacol. 2021, 274, 114067. [Google Scholar] [CrossRef]
- Xinbao, Y.; Xiuwei, Y.; Jianxun, L. Study on the chemical constituents of saponins in ginseng. Mod. Chin. Med. 2013, 15, 349–358. [Google Scholar]
- Hui, L.; Liang, X.; Meijia, W.; Fanlin, Z.; Caixiang, X.; Tingguo, K. Study on the content measurement and quality evaluation of ginsenoside constituent from different habitats with UPLC analysis method. Chin. J. Tradit. Chin. Med. 2015, 30, 1963–1968. [Google Scholar]
- Chao, C. Research Progress on Determination Methods of Saponins from Sanqi(Panax Notoginseng) and its Preparations. Chin. Med. Her. 2017, 23, 49–53. [Google Scholar]
- Pengguo, X.; Shuncang, Z.; Zongsuo, L.; Zhihong, Q. Research history and overview of chemical constituents of Panax notoginseng. Chin. Herb. Med. 2014, 45, 2564–2570. [Google Scholar]
- Lingling, T.; Xiaomin, H.; Zhenghai, H. Determination of platycodin in Platycodi Radix from different habitats. Chin. Herb. Med. 2015, 46, 1682–1684. [Google Scholar]
- Qi, W.; Yumei, Z.; Zhong, D.; Jing, L.; Ruichao, L. HPLC characteristic spectrum analysis of saponins in astragali radix*. J. Pharm. Anal. 2012, 32, 1101–1104. [Google Scholar]
- Zongquan, W.; Jiming, J.; Jian, S.; Zhengjie, L.; Zuohuan, M. Determination of astragaloside Ⅰ, astragaloside Ⅱ and astragaloside Ⅳ in Radix Astragali from various habitats*. J. Pharm. Anal. 2010, 30, 1191–1194. [Google Scholar]
- Guolong, L.; Jie, Y.; Jinhao, D.; Hongbo, L.; Zhenhua, Z.; Dawei, Q.; Zhishu, T. Quality Analysis and Evaluation of Anemarrhena asphodeloides Rhizome from Different Habitats. Chin. Med. Mater. 2015, 38, 1148–1152. [Google Scholar]
- Ling, S.; Ning, W.; Jinquan, L. Determination of the content of saponins in Zhimu by one test and multiple evaluation method. Chin. Med. Mater. 2015, 38, 997–1000. [Google Scholar]
- Xing, J.; Yifan, F. Advances in studies on saponins in Anemarrhena asphodeloides. Chin. Herb. Med. 2010, 41, 680–683. [Google Scholar]
- Hanqian, H.; Xiaohan, W.; Hang, F.; Yan, W.; Shihai, Y. Research progress on medicinal plant resources of Bupleurum L. Chin. Herb. Med. 2017, 48, 2989–2996. [Google Scholar]
- Xiangyan, Z.; Changli, L. Research Overview and Development Trend of Chinese Medicine Bupleurum. Shi Zhen Guo Yao Guo Yao 2015, 26, 963–966. [Google Scholar]
- Taozhen, Z.; Weiwei, R.; Qing, L.; Kaishun, B. Research progress on Polygalae Radix. Chin. Herb. Med. 2016, 47, 2381–2389. [Google Scholar]
- Xiaojuan, G.; Dan, Z.; Jianjun, Z.; Xia, Z.; Yinghua, W.; Hanqing, W. Herbal Textural Research on Glycyrrhizae Radix et Rhizoma. Chin. J. Exp. Prescr. 2017, 23, 193–198. [Google Scholar]
- Haihua, L.; Mei, Q.; Juan, Y.; Shuaijie, L. Research progression of glycyrrhiza uralensis. J. Inn. Mong. Med. Univ. 2015, 37, 199–204. [Google Scholar]
- Yangyang, L.; Chunsheng, L.; Binfang, Z.; Bingduo, F.; Pengshou, L.; Yunhai, X.; Tonghua, L. Research progress on germplasm resources of Glycyrrhizae Radix et Rhizoma. Chin. Herb. Med. 2013, 44, 3593–3598. [Google Scholar]
- Lin, H.; Zhang, Y.; Han, M.; Yang, L. Aqueous ionic liquid based ultrasonic assisted extraction of eight ginsenosides from ginseng root. Ultrason. Sonochemistry 2013, 20, 680–684. [Google Scholar] [CrossRef]
- Li, L.J.; Jin, Y.R.; Wang, X.Z.; Liu, Y.; Wu, Q.; Shi, X.L.; Li, X.W. Ionic liquid and aqueous two-phase extraction based on salting-out coupled with high-performance liquid chromatography for the determination of seven rare ginsenosides in Xue-Sai-Tong injection. J. Sep. Sci. 2015, 38, 3055–3062. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Wang, Y.; Su, Z.; He, D.; Du, Y.; Guo, M.; Yang, D.; Tang, D. Ionic liquids-ultrasound based efficient extraction of flavonoid glycosides and triterpenoid saponins from licorice. RSC Adv. 2018, 8, 13989–13996. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Zhang, J.; Su, X.; Meng, Q.; Dou, J. Extraction and Separation of Eight Ginsenosides from Flower Buds of Panax Ginseng Using Aqueous Ionic Liquid-Based Ultrasonic-Assisted Extraction Coupled with an Aqueous Biphasic System. Molecules 2019, 24, 778. [Google Scholar] [CrossRef]
- Sun, T.; Luo, J.; Xu, Y.; Sun, X.; Yang, S.; Yang, M. Ultra-high performance supercritical fluid chromatography method for separation and quantitation of saikosaponins in herbal medicine. J. Pharm. Biomed. Anal. 2021, 199, 114039. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, T.; Zhou, H.; Feng, Y.; Fan, C.; Chen, W.; Crommen, J.; Jiang, Z. Fast separation of triterpenoid saponins using supercritical fluid chromatography coupled with single quadrupole mass spectrometry. J. Pharm. Biomed. Anal. 2016, 121, 22–29. [Google Scholar] [CrossRef]
- Zhu, L.L.; Zhao, Y.; Xu, Y.W.; Sun, Q.L.; Sun, X.G.; Kang, L.P.; Yan, R.Y.; Zhang, J.; Liu, C.; Ma, B.P. Comparison of ultra-high performance supercritical fluid chromatography and ultra-high performance liquid chromatography for the separation of spirostanol saponins. J. Pharm. Biomed. Anal. 2016, 120, 72–78. [Google Scholar] [CrossRef]
- Rho, T.; Choi, S.J.; Kil, H.W.; Ko, J.; Yoon, K.D. Separation of nine novel triterpene saponins from Camellia japonica seeds using high-performance countercurrent chromatography and reversed-phase high-performance liquid chromatography. Phytochem. Anal. PCA 2019, 30, 226–236. [Google Scholar] [CrossRef]
- Sun, H.; Ma, L.J.; Wan, J.B.; Tong, S. Preparative separation of gypenoside XVII, ginsenoside Rd2, and notoginsenosides Fe and Fd from Panax notoginseng leaves by countercurrent chromatography and orthogonality evaluation for their separation. J. Sep. Sci. 2021, 44, 2996–3003. [Google Scholar] [CrossRef] [PubMed]
- Ha, I.J.; Kang, M.; Na, Y.C.; Park, Y.; Kim, Y.S. Preparative separation of minor saponins from Platycodi Radix by high-speed counter-current chromatography. J. Sep. Sci. 2011, 34, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.D.; Chin, Y.W.; Yang, M.H.; Choi, J.; Kim, J. Application of high-speed countercurrent chromatography-evaporative light scattering detection for the separation of seven steroidal saponins from Dioscorea villosa. Phytochem. Anal. PCA 2012, 23, 462–468. [Google Scholar] [CrossRef]
- Lee, K.J.; Song, K.; Song, D.Y.; Kim, Y.S. Application of linear gradient elution in countercurrent chromatography for the separation of triterpenoid saponins from the roots of Pulsatilla koreana Nakai. J. Sep. Sci. 2017, 40, 2810–2818. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Ha, I.J.; Chun, J.; Kang, S.S.; Kim, Y.S. Separation of two cytotoxic saponins from the roots of Adenophora triphylla var. japonica by high-speed counter-current chromatography. Phytochem. Anal. PCA 2013, 24, 148–154. [Google Scholar] [CrossRef]
- Song, H.; Lin, J.; Zhu, X.; Chen, Q. Developments in high-speed countercurrent chromatography and its applications in the separation of terpenoids and saponins. J. Sep. Sci. 2016, 39, 1574–1591. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Y.; Wu, Z.; Liu, W.; Li, R.; Wang, Y. A novel technology coupling extraction and foam fractionation for separating the total saponins from Achyranthes bidentata. Prep. Biochem. Biotechnol. 2016, 46, 666–672. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, Z.; Liu, W.; Gao, Y.; Guo, S.; Kang, S. Separation of soybean saponins from soybean meal by a technology of foam fractionation and resin adsorption. Prep. Biochem. Biotechnol. 2016, 46, 346–353. [Google Scholar] [CrossRef]
- Huifen, L.; Yuan, P.; Gang, C.; Junyan, L.; Jun, H. Determination of diosgenin inRhizoma Paridis by HPLC and TLC. Chin. Herb. Med. 2003, 34, 35–37. [Google Scholar]
- Wang, L.; Wang, X.; Yuan, X.; Zhao, B. Simultaneous analysis of diosgenin and sarsasapogenin in Asparagus officinalis byproduct by thin-layer chromatography. Phytochem. Anal. PCA 2011, 22, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Yaodong, Z. Determination of Ginsenoside Rb_1 and Rg_1 in Sanqi Shangyao Capsules by TLC Scanning. J. Jiangxi Univ. Tradit. Chin. Med. 2004, 16, 53–54. [Google Scholar]
- Jing, W.; Jiajun, C.; Xueyan, C.; Hua, T. How to identify saikosaponin in Hugan tablets by thin layer chromatography. Seek. Med. Advice 2013, 11, 182–184. [Google Scholar]
- Li, Z.; Zhao, Y.; Lin, W.; Ye, M.; Ling, X. Rapid screening and identification of active ingredients in licorice extract interacting with V3 loop region of HIV-1 gp120 using ACE and CE-MS. J. Pharm. Biomed. Anal. 2015, 111, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xue, L.; Zhang, H.; Zhu, C. Determination of saikosaponins a, c, and d in Bupleurum Chinese DC from different areas by capillary zone electrophoresis. Anal. Bioanal. Chem. 2005, 382, 1610–1615. [Google Scholar] [CrossRef]
- Emara, S.; Masujima, T.; Zarad, W.; Mohamed, K.; Kamal, M.; Fouad, M.; El-Bagary, R. Field-amplified sample stacking beta-cyclodextrin modified capillary electrophoresis for quantitative determination of diastereomeric saponins. J. Chromatogr. Sci. 2014, 52, 1308–1316. [Google Scholar] [CrossRef]
- Wu, L.; Su, Y.; Yu, H.; Qian, X.; Zhang, X.; Wang, Q.; Kuang, H.; Cheng, G. Rapid Determination of Saponins in the Honey-Fried Processing of Rhizoma Cimicifugae by Near Infrared Diffuse Reflectance Spectroscopy. Molecules 2018, 23, 1617. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, H.; Zhang, J.; Wang, Y. Determination of Total Steroid Saponins in Different Species of Paris Using FTIR Combined with Chemometrics. J. AOAC Int. 2018, 101, 732–738. [Google Scholar] [CrossRef]
- Kewei, Y.; Meijun, C.; Guorong, M.; Fu, W.; Junyu, L.; Hongping, C.; Youping, L.; Lin, C. Determination of three saponins in Panax notoginseng by NIR*. J. Pharm. Anal. 2016, 36, 691–696. [Google Scholar]
- Lu, X.; Qiu, F.; Pan, X.; Li, J.; Wang, M.; Gong, M. Simultaneous quantitative analysis of nine triterpenoid saponins for the quality control of Stauntonia obovatifoliola Hayata subsp. intermedia stems. J. Sep. Sci. 2014, 37, 3632–3640. [Google Scholar] [CrossRef]
- Yu, M.; Shin, Y.J.; Kim, N.; Yoo, G.; Park, S.; Kim, S.H. Determination of saponins and flavonoids in ivy leaf extracts using HPLC-DAD. J. Chromatogr. Sci. 2015, 53, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, M.P.; Kaiser, S.; Verza, S.G.; de Resende, P.E.; Treter, J.; Pavei, C.; Borre, G.L.; Ortega, G.G. LC-UV assay method and UPLC/Q-TOF-MS characterisation of saponins from Ilex paraguariensis A. St. Hil. (mate) unripe fruits. Phytochem. Anal. 2012, 23, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Park, Y.D. Determination of astragalin and astragaloside content in Radix Astragali using high-performance liquid chromatography coupled with pulsed amperometric detection. J. Chromatogr. A 2012, 1232, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Jeong, J.S.; Kwon, H.J.; Hong, S.P. Quantification of isoflavonoids and triterpene saponins in Astragali Radix, the root of Astragalus membranaceus, via reverse-phase high-performance liquid chromatography coupled with integrated pulsed amperometric detection. J. Chromatogr. B 2017, 1070, 76–81. [Google Scholar] [CrossRef]
- Li, B.Q.; Chen, J.; Wu, T.X.; Zhai, H.L.; Zhang, X.Y. Fast determination of four active compounds in Sanqi Panax Notoginseng Injection samples by high-performance liquid chromatography with a chemometric method. J. Sep. Sci. 2015, 38, 1449–1457. [Google Scholar] [CrossRef]
- Avula, B.; Wang, Y.H.; Ali, Z.; Smillie, T.J.; Khan, I.A. Quantitative determination of triperpene saponins and alkenated-phenolics from Labisia pumila using an LC-UV/ELSD method and confirmation by LC-ESI-TOF. Planta Med. 2011, 77, 1742–1748. [Google Scholar] [CrossRef]
- Sun, Y.; Li, B.; Lin, X.; Xue, J.; Wang, Z.; Zhang, H.; Jiang, H.; Wang, Q.; Kuang, H. Simultaneous Determination of Four Triterpenoid Saponins in Aralia elata Leaves by HPLC-ELSD Combined with Hierarchical Clustering Analysis. Phytochem. Anal. 2017, 28, 202–209. [Google Scholar] [CrossRef]
- Lee, K.Y.; Cho, Y.W.; Park, J.; Lee, D.Y.; Kim, S.H.; Kim, Y.C.; Sung, S.H. Quality control of Pulsatilla koreana based on the simultaneous determination of triterpenoidal saponins by HPLC-ELSD and principal component analysis. Phytochem. Anal. 2010, 21, 314–321. [Google Scholar] [CrossRef]
- Man, S.; Gao, W.; Zhang, Y.; Wang, J.; Zhao, W.; Huang, L.; Liu, C. Qualitative and quantitative determination of major saponins in Paris and Trillium by HPLC-ELSD and HPLC-MS/MS. J. Chromatogr. B 2010, 878, 2943–2948. [Google Scholar] [CrossRef]
- Lu, H.; Ju, M.; Chu, S.; Xu, T.; Huang, Y.; Chan, Q.; Peng, H.; Gui, S. Quantitative and Chemical Fingerprint Analysis for the Quality Evaluation of Platycodi Radix Collected from Various Regions in China by HPLC Coupled with Chemometrics. Molecules 2018, 23, 1823. [Google Scholar] [CrossRef]
- Li, X.E.; Wang, Y.X.; Sun, P.; Liao, D.Q. Determination of Saponin Content in Hang Maidong and Chuan Maidong via HPLC-ELSD Analysis. J. Anal. Methods Chem. 2016, 2016, 7214607. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Yang, M.; Chu, S.; Li, R.; Zhao, Y.; Peng, H.; Zhan, Z.; Sun, H.F. Quality Analysis of Different Specification Grades of Astragalus membranaceus var. mongholicus (Huangqi) from Hunyuan, Shanxi. J. AOAC Int. 2019, 102, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Yujia, M.; Yan, J. The application progress of electrospray detector. Int. J. Pharm. Res. 2020, 47, 514–521. [Google Scholar]
- Liyang, L.; Xiao, L. A new type of universal detector-electrospray detector. Mod. Sci. Instrum. 2011, 2011, 141–145. [Google Scholar]
- Zhang, X.D.; Li, Z.; Liu, G.Z.; Wang, X.; Kwon, O.K.; Lee, H.K.; Whang, W.K.; Liu, X.Q. Quantitative determination of 15 bioactive triterpenoid saponins in different parts of Acanthopanax henryi by HPLC with charged aerosol detection and confirmation by LC-ESI-TOF-MS. J. Sep. Sci. 2016, 39, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Yang, S.L.; Han, Y.Y.; Zhao, L.; Lu, G.L.; Xia, T.; Gao, L.P. Qualitative and quantitative analysis of triterpene saponins from tea seed pomace (Camellia oleifera Abel) and their activities against bacteria and fungi. Molecules 2014, 19, 7568–7580. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, Q.; Sun, L.N.; Gao, S.H.; Tao, X.; Chen, W.S. Fingerprint analysis of Zhimu-Huangbai herb pair and simultaneous determination of its alkaloids, xanthone glycosides and steroidal saponins by HPLC-DAD-ELSD. Chin. J. Nat. Med. 2014, 12, 525–534. [Google Scholar] [CrossRef]
- Tian, R.T.; Xie, P.S.; Liu, H.P. Evaluation of traditional Chinese herbal medicine: Chaihu (Bupleuri Radix) by both high-performance liquid chromatographic and high-performance thin-layer chromatographic fingerprint and chemometric analysis. J. Chromatogr. A 2009, 1216, 2150–2155. [Google Scholar] [CrossRef]
- Yao, H.; Shi, P.; Shao, Q.; Fan, X. Chemical fingerprinting and quantitative analysis of a Panax notoginseng preparation using HPLC-UV and HPLC-MS. Chin. Med. 2011, 6, 9. [Google Scholar] [CrossRef]
- Shakeri, A.; Masullo, M.; D’Urso, G.; Iranshahi, M.; Montoro, P.; Pizza, C.; Piacente, S. In depth chemical investigation of Glycyrrhiza triphylla Fisch roots guided by a preliminary HPLC-ESIMS(n) profiling. Food Chem. 2018, 248, 128–136. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Qu, H. Determination of three steroidal saponins from Ophiopogon japonicus (Liliaceae) via high-performance liquid chromatography with mass spectrometry. Nat. Prod. Res. 2013, 27, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, P.; Wang, X.; Jing, W.; Chen, L.; Liu, A. Quantification of saponins in Dioscorea panthaica Prain et Burk rhizomes with monolithic column using rapid resolution liquid chromatography coupled with a triple quadruple electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 71, 152–156. [Google Scholar] [CrossRef]
- Kawahara, Y.; Hoshino, T.; Morimoto, H.; Shinizu, T.; Narukawa, Y.; Fuchino, H.; Kawahara, N.; Kiuchi, F. LC-MS-based quantification method for Achyranthes root saponins. J. Nat. Med. 2016, 70, 102–106. [Google Scholar] [CrossRef]
- Qi, H.; Feng, F.; Zhai, J.; Chen, F.; Liu, T.; Zhang, F.; Zhang, F. Development of an analytical method for twelve dioscorea saponins using liquid chromatography coupled to Q-Exactive high resolution mass spectrometry. Talanta 2019, 191, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Miyake, S.; Miki, Y.; Ninomiya, K.; Yoshikawa, M.; Muraoka, O. Quantitative analysis of acylated oleanane-type triterpene saponins, chakasaponins I-III and floratheasaponins A-F, in the flower buds of Camellia sinensis from different regional origins. J. Nat. Med. 2012, 66, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, J.; Xu, W.; Sun, J. Quantification of polygalasaponin F in rat plasma using liquid chromatography-tandem mass spectrometry and its pharmacokinetics application. Biomed. Chromatogr. BMC 2015, 29, 1388–1392. [Google Scholar] [CrossRef]
- Li, S.P.; Qiao, C.F.; Chen, Y.W.; Zhao, J.; Cui, X.M.; Zhang, Q.W.; Liu, X.M.; Hu, D.J. A novel strategy with standardized reference extract qualification and single compound quantitative evaluation for quality control of Panax notoginseng used as a functional food. J. Chromatogr. A 2013, 1313, 302–307. [Google Scholar] [CrossRef]
- Liu, F.; Ma, N.; He, C.; Hu, Y.; Li, P.; Chen, M.; Su, H.; Wan, J.B. Qualitative and quantitative analysis of the saponins in Panax notoginseng leaves using ultra-performance liquid chromatography coupled with time-of-flight tandem mass spectrometry and high performance liquid chromatography coupled with UV detector. J. Ginseng. Res. 2018, 42, 149–157. [Google Scholar] [CrossRef]
- Lai, C.J.; Tan, T.; Zeng, S.L.; Qi, L.W.; Liu, X.G.; Dong, X.; Li, P.; Liu, E.H. An integrated high resolution mass spectrometric data acquisition method for rapid screening of saponins in Panax notoginseng (Sanqi). J. Pharm. Biomed. Anal. 2015, 109, 184–191. [Google Scholar] [CrossRef]
- Chu, C.; Cai, H.X.; Ren, M.T.; Liu, E.H.; Li, B.; Qi, L.W.; Li, P. Characterization of novel astragaloside malonates from Radix Astragali by HPLC with ESI quadrupole TOF MS. J. Sep. Sci. 2010, 33, 570–581. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Qu, H. Structure characterization and identification steroidal saponins from Ophiopogon japonicus Ker-Gawler (Liliaceae) by high-performance liquid chromatography with ion trap mass spectrometry. Phytochem. Anal. PCA 2011, 22, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Wang, Q.; Qiao, X.; Guo, H.C.; Yang, Y.F.; Bo, T.; Xiang, C.; Guo, D.A.; Ye, M. New triterpene saponins from the roots of Glycyrrhiza yunnanensis and their rapid screening by LC/MS/MS. J. Pharm. Biomed. Anal. 2014, 90, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.F.; Qi, L.W.; Zhou, J.L.; Li, P. Structural characterization and identification of oleanane-type triterpene saponins in Glycyrrhiza uralensis Fischer by rapid-resolution liquid chromatography coupled with time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. RCM 2010, 24, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Song, R.; Tian, J.X.; Tian, Y.; Liu, G.Q.; Zhang, Z.J. Analysis of saikosaponins in rat plasma by anionic adducts-based liquid chromatography tandem mass spectrometry method. Biomed. Chromatogr. BMC 2012, 26, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Sagratini, G.; Zuo, Y.; Caprioli, G.; Cristalli, G.; Giardina, D.; Maggi, F.; Molin, L.; Ricciutelli, M.; Traldi, P.; Vittori, S. Quantification of soyasaponins I and betag in Italian lentil seeds by solid-phase extraction (SPE) and high-performance liquid chromatography-mass spectrometry (HPLC-MS). J. Agric. Food Chem. 2009, 57, 11226–11233. [Google Scholar] [CrossRef]
- Ma, L.; Li, W.; Wang, H.; Kuang, X.; Li, Q.; Wang, Y.; Xie, P.; Koike, K. A simple and rapid method to identify and quantitatively analyze triterpenoid saponins in Ardisia crenata using ultrafast liquid chromatography coupled with electrospray ionization quadrupole mass spectrometry. J. Pharm. Biomed. Anal. 2015, 102, 400–408. [Google Scholar] [CrossRef]
- Dawid, C.; Hofmann, T. Quantitation and bitter taste contribution of saponins in fresh and cooked white asparagus (Asparagus officinalis L.). Food Chem. 2014, 145, 427–436. [Google Scholar] [CrossRef]
- Li, Z.; Wen, R.; Du, Y.; Zhao, S.; Zhao, P.; Jiang, H.; Rong, R.; Lv, Q. Simultaneous quantification of fifteen compounds in rat plasma by LC-MS/MS and its application to a pharmacokinetic study of Chaihu-Guizhi decoction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1105, 15–25. [Google Scholar] [CrossRef]
- Huang, W.W.; Wang, M.Y.; Shi, H.M.; Peng, Y.; Peng, C.S.; Zhang, M.; Li, Y.; Lu, J.; Li, X.B. Comparative study of bioactive constituents in crude and processed Glycyrrhizae radix and their respective metabolic profiles in gastrointestinal tract in vitro by HPLC-DAD and HPLC-ESI/MS analyses. Arch. Pharmacal Res. 2012, 35, 1945–1952. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, Z.; Yang, L.; Gao, Y.; Liu, W.; Zhang, H.; Chai, Y. Detection, characterization and identification of major constituents in Zhimu-Baihe herb-pair extract by fast high-performance liquid chromatography and time-of-flight mass spectrometry through dynamic adjustment of fragmentor voltage. Rapid Commun. Mass Spectrom. RCM 2011, 25, 9–19. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Wang, P.; Lin, X.; Yang, Y.; Li, D.; Li, H.; Wu, X.; Liu, H. Pharmacokinetic comparisons of different combinations of Shaoyao-Gancao-Decoction in rats: Simultaneous determination of ten active constituents by HPLC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 932, 76–87. [Google Scholar] [CrossRef]
- Vazquez-Castilla, S.; Jaramillo-Carmona, S.; Fuentes-Alventosa, J.M.; Jimenez-Araujo, A.; Rodriguez-Arcos, R.; Cermeno-Sacristan, P.; Espejo-Calvo, J.A.; Guillen-Bejarano, R. Optimization of a method for the profiling and quantification of saponins in different green asparagus genotypes. J. Agric. Food Chem. 2013, 61, 6250–6258. [Google Scholar] [CrossRef] [PubMed]
- Shuai, Z.; Hailin, C.; Bing, Y. Development of ultra—high performan ce liquid chromatography and its application in the field of analysis. Anal. Instrum. 2017, 2017, 16–27. [Google Scholar]
- Avula, B.; Wang, Y.H.; Ali, Z.; Smillie, T.J.; Khan, I.A. Chemical fingerprint analysis and quantitative determination of steroidal compounds from Dioscorea villosa, Dioscorea species and dietary supplements using UHPLC-ELSD. Biomed. Chromatogr. 2014, 28, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Ouyang, P.Y.; Gao, W.; Yi, T.; Zhang, X.T.; Zhao, Z.D.; Yang, H. Rapid and Sensitive Determination of the Major Steroidal Saponins of Ypsilandra thibetica Franch by Ultra High-Performance Liquid Chromatography Coupled with Triple Quadrupole Mass Spectrometry. J. Chromatogr. Sci. 2016, 54, 1010–1015. [Google Scholar] [CrossRef][Green Version]
- Huang, J.; Yin, L.; Dong, L.; Quan, H.; Chen, R.; Hua, S.; Ma, J.; Guo, D.; Fu, X. Quality evaluation for Radix Astragali based on fingerprint, indicative components selection and QAMS. Biomed. Chromatogr. BMC 2018, 32, e4343. [Google Scholar] [CrossRef]
- Verza, S.G.; Silveira, F.; Cibulski, S.; Kaiser, S.; Ferreira, F.; Gosmann, G.; Roehe, P.M.; Ortega, G.G. Immunoadjuvant activity, toxicity assays, and determination by UPLC/Q-TOF-MS of triterpenic saponins from Chenopodium quinoa seeds. J. Agric. Food. Chem. 2012, 60, 3113–3118. [Google Scholar] [CrossRef]
- Yang, L.; Li, C.L.; Cheng, Y.Y.; Tsai, T.H. Development of a Validated UPLC-MS/MS Method for Analyzing Major Ginseng Saponins from Various Ginseng Species. Molecules 2019, 24, 4065. [Google Scholar] [CrossRef]
- Tang, Y.; Yi, T.; Chen, H.; Zhao, Z.; Liang, Z.; Chen, H. Quantitative comparison of multiple components in Dioscorea nipponica and D. panthaica by ultra-high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Phytochem.Anal. 2013, 24, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liang, Z.; Brand, E.; Chen, H.; Zhao, Z. Distributive and Quantitative Analysis of the Main Active Saponins in Panax notoginseng by UHPLC-QTOF/MS Combining with Fluorescence Microscopy and Laser Microdissection. Planta Med. 2016, 82, 263–272. [Google Scholar] [CrossRef]
- Zhao, M.; Dai, Y.; Li, Q.; Li, P.; Qin, X.M.; Chen, S. A Practical Quality Control Method for Saponins Without UV Absorption by UPLC-QDA. Front. Pharm. 2018, 9, 1377. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, S.; Zhu, Y.; Yan, H.; Qian, D.W.; Wang, H.Q.; Yu, J.Q.; Duan, J.A. Comparative analysis of twenty-five compounds in different parts of Astragalus membranaceus var. mongholicus and Astragalus membranaceus by UPLC-MS/MS. J. Pharm. Anal. 2019, 9, 392–399. [Google Scholar] [CrossRef]
- Wang, C.J.; He, F.; Huang, Y.F.; Ma, H.L.; Wang, Y.P.; Cheng, C.S.; Cheng, J.L.; Lao, C.C.; Chen, D.A.; Zhang, Z.F.; et al. Discovery of chemical markers for identifying species, growth mode and production area of Astragali Radix by using ultra-high-performance liquid chromatography coupled to triple quadrupole mass spectrometry. Phytomedicine Int. J. Phytother. Phytopharm. 2020, 67, 153155. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Yang, N.; Zhao, Y.; Sheng, X.; Yang, S.; Li, Y. A rapid classification and identification method applied to the analysis of glycosides in Bupleuri radix and liquorice by ultra high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2018, 41, 3791–3805. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, Y.; Zheng, Y.; Yang, J.; Zhang, L. Ultra high performance liquid chromatography coupled with triple quadrupole mass spectrometry and chemometric analysis of licorice based on the simultaneous determination of saponins and flavonoids. J. Sep. Sci. 2016, 39, 2928–2940. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Cao, J.; Qiu, F.; Kong, W.; Yang, S.; Yang, M. Simultaneous determination of five bioactive components in radix glycyrrhizae by pressurised liquid extraction combined with UPLC-PDA and UPLC/ESI-QTOF-MS confirmation. Phytochem. Anal. PCA 2013, 24, 527–533. [Google Scholar] [CrossRef]
- Tao, W.; Duan, J.; Zhao, R.; Li, X.; Yan, H.; Li, J.; Guo, S.; Yang, N.; Tang, Y. Comparison of three officinal Chinese pharmacopoeia species of Glycyrrhiza based on separation and quantification of triterpene saponins and chemometrics analysis. Food Chem 2013, 141, 1681–1689. [Google Scholar] [CrossRef]
- Tao, W.; Duan, J.; Guo, J.; Li, J.; Tang, Y.; Liu, P.; Yang, N. Simultaneous determination of triterpenoid saponins in dog plasma by a validated UPLC-MS/MS and its application to a pharmacokinetic study after administration of total saponin of licorice. J. Phamaceutical Biomed. Anal. 2013, 75, 248–255. [Google Scholar] [CrossRef]
- Qi, Y.; Li, S.; Pi, Z.; Song, F.; Lin, N.; Liu, S.; Liu, Z. Chemical profiling of Wu-tou decoction by UPLC-Q-TOF-MS. Talanta 2014, 118, 21–29. [Google Scholar] [CrossRef]
- Li, G.; Nikolic, D.; van Breemen, R.B. Identification and Chemical Standardization of Licorice Raw Materials and Dietary Supplements Using UHPLC-MS/MS. J. Agric. Food Chem. 2016, 64, 8062–8070. [Google Scholar] [CrossRef]
- Zhimin, W.; Huimin, G.; Xuetao, F.; Weihao, W. Multi- components quantitation by onemarker new method for quality evaluation of Chinese herbalmedicine. China J. Chin. Mater. Med. 2006, 2006, 1925–1928. [Google Scholar]
- Meng, F.C.; Wu, Q.S.; Wang, R.; Li, S.P.; Lin, L.G.; Chen, P.; Zhang, Q.W. A Novel Strategy for Quantitative Analysis of Major Ginsenosides in Panacis Japonici Rhizoma with a Standardized Reference Fraction. Molecules 2017, 22, 2067. [Google Scholar] [CrossRef]
- Lingzhou, J.; Zufang, G. Determination of Three Ingredients in Platycodonis Radix by Quantitative Analysis of Multi-components by Single Marker. Mod. Chin. Appl. Pharm. 2017, 34, 729–732. [Google Scholar]
- Yang, X.; Zhang, X.; Yang, S.P.; Le, T.; Fan, X.D.; Guo, X.; Chen, B. Simultaneous quantitative analysis of multi-compounds by a single marker in Radix Astragali by using serum HPLC-MS feature. Pak. J. Pharm. Sci. 2016, 29, 1243–1249. [Google Scholar] [PubMed]
- Stavrianidi, A.; Stekolshchikova, E.; Porotova, A.; Rodin, I.; Shpigun, O. Combination of HPLC-MS and QAMS as a new analytical approach for determination of saponins in ginseng containing products. J. Pharm. Biomed. Anal. 2017, 132, 87–92. [Google Scholar] [CrossRef]
- Stekolshchikova, E.; Turova, P.; Shpigun, O.; Rodin, I.; Stavrianidi, A. Application of quantitative analysis of multi-component system approach for determination of ginsenosides in different mass-spectrometric conditions. J. Chromatogr. A 2018, 1574, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q.; Jia, X.H.; Zhu, S.; Komatsu, K.; Wang, X.; Cai, S.Q. A systematic study on the influencing parameters and improvement of quantitative analysis of multi-component with single marker method using notoginseng as research subject. Talanta 2015, 134, 587–595. [Google Scholar] [CrossRef]
- Chao, Z.; Cui, Q.; Tian, E.; Zeng, W.; Cai, X.; Li, X.; Tanaka, H.; Shoyama, Y.; Wu, Y. Ultrasensitive Time-Resolved Fluoroimmunoassay for Saikosaponin a in Chaihu (Bupleuri Radix). PLoS ONE 2016, 11, e0151032. [Google Scholar] [CrossRef]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Metabolomics: The apogee of the omic triology. Nat. Rev. Mol. Cell Biol. 2013, 13, 263. [Google Scholar] [CrossRef]
- Farag, M.A.; Porzel, A.; Wessjohann, L.A. Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC-MS, LC-MS and 1D NMR techniques. Phytochemistry 2012, 76, 60–72. [Google Scholar] [CrossRef]
- Ha, Y.W.; Na, Y.C.; Ha, I.J.; Kim, D.H.; Kim, Y.S. Liquid chromatography/mass spectrometry-based structural analysis of new platycoside metabolites transformed by human intestinal bacteria. J. Pharm. Biomed. Anal. 2010, 51, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Chen, X.; Godevac, D.; Bueno, P.C.P.; Salome Abarca, L.F.; Jang, Y.P.; Wang, M.; Choi, Y.H. Metabolic Profiling of Saponin-Rich Ophiopogon japonicus Roots Based on 1H NMR and HPTLC Platforms. Planta Med. 2019, 85, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R.; Yau, L.F.; Gao, W.N.; Liu, Y.; Yick, P.W.; Liu, L.; Jiang, Z.H. Quantitative comparison and metabolite profiling of saponins in different parts of the root of Panax notoginseng. J. Agric. Food Chem. 2014, 62, 9024–9034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, X.; Li, Z.; Xu, X.; Peng, B.; Qin, X.; Du, G. UPLC/Q-TOF MS-based metabolomics and qRT-PCR in enzyme gene screening with key role in triterpenoid saponin biosynthesis of Polygala tenuifolia. PLoS ONE 2014, 9, e105765. [Google Scholar] [CrossRef] [PubMed]

| No. | Drug Name | Medication Site | Main Ingredient | Efficacy | Ref. |
|---|---|---|---|---|---|
| 1 | Panax ginseng C. A. Mey. | Dry roots and rhizomes | Triterpenoid saponins, ginseng polysaccharides, ginseng alkynols, amino acid proteins, sugars, vitamins, organic acids, trace elements, flavonoids and peptides | It can strengthen the vitality, strengthen the body, nourish blood and blood, nourish the spleen and benefit the lungs, calm the heart, calm the mind and promote wisdom | [14,18,19] |
| 2 | Panax pseudoginseng Wall. Var. notoginseng(Burkill) Hoo et Tseng | Roots and rhizomes, leaves, flowers | Dammar type tetracyclic triterpene saponins, flavonoids, notoginsenosides (amino acids), proteins, volatile oils, acetylenes, alcohols, polysaccharides, polyols, polyacetylene alcohols, organic acids, trace elements, etc. | Diffuse stasis to stop bleeding, reduce swelling and relieve pain | [15,20,21] |
| 3 | Platycodon grandiflorus (Jacq.) A. DC. | Dry root | Oleanane-type pentacyclic triterpene saponins, flavonoids, phenols, sterols, polysaccharides, polyacetylenes, steroids, phenolic acids, polysaccharides, fatty acids, fatty acids and trace elements, inorganic elements, etc. | Promoting lung, relieving asthma, dispelling cold, benefiting pharynx, expectorating phlegm, discharging pus, inducing drugs to increase | [5,6,7,22] |
| 4 | Astragali Radix | Dry root | Triterpene saponins, polysaccharides, flavonoids, amino acids | Fill the air to raise Yang, fixed surface antiperspirant, diuretic to poison, discharge pus, collect sore muscle, benefit water to reduce swelling | [8,23,24] |
| 5 | Anemarrhena asphodeloides Bunge | Dry rhizome | Steroid saponins diphenpyrone, flavonoids, lignin, polysaccharides, alkaloids, amino acids, volatile oils, organic acids and trace elements, inorganic elements, etc. | Clearing heat and purging fire, nourishing Yin and moistening dryness, quenching thirst and eliminating annoyance | [9,25,26,27] |
| 6 | Bupleuri Radix | Dried roots, whole grasses | Bupleurum saponins, flavonoids, volatile oils, polysaccharides, sterols, polyols, coumarins, lignans, fatty acids (oleic acid, linolenic acid, palmitic acid, stearic acid, etc.), tryptophan, wood sugar alcohol, uridine, adenosine and trace elements, etc. | Antipyretic, anti-inflammatory, lowering blood cholesterol, reconciling the inside and outside, soothing the liver and stagnating depression, raising Yang, lifting depression, protecting liver and boldness, cooling down, relieving the stasis of the liver qi, relieving qi, relieving pain and reducing inflammation, anticancer, resisting liver fibrosis, evacuating fever, soothing liver and relieving depression, raising Yang, lifting qi | [10,11,12,28,29] |
| 7 | Polygala tenuifolia Willd | Dry roots | Triterpene saponins, ketones, oligosaccharides, alkaloids, phenylpropanoid flavonoids, lactones, coumarins, lignin, etc. | Dispelling phlegm, reducing swelling, calming the mind and improving intelligence | [13,30] |
| 8 | Glycyrrhiza ur alensis Fisch. | Dried roots and rhizomes | Triterpenoid saponins flavonoids and polysaccharides | Replenishing spleen and qi, clearing away heat and detoxifies, expelling phlegm and cough, relieving pain, reconciling all drugs | [31,32,33] |
| No. | Drug Name | Content Determination |
|---|---|---|
| 1 | Panax ginseng C. A. Mey. | Total amount of ginsenoside Rg1 (C42H72O14) and ginsenoside Re (C48H82O18) should not be less than 0.27%, and ginsenoside Rb1 (C54H92O23) should not be less than 0.18% |
| 2 | Panax pseudoginseng Wall. var. notoginseng (Burkill) Hoo et Tseng | Total amount of ginsenoside Rg1 (C42H72O14), ginsenoside Rb1 (C54H92O23) and notoginseng R1 (C47H80O18) should not be less than 5.0% |
| 3 | Platycodon grandifloras (Jacq.) A. DC. | Platycodon grandiflorum saponin D (C57H92O28) shall not be less than 0.10% |
| 4 | Astragali Radix | Astragaloside IV (C41H68O14) shall not be less than 0.080%, and calycoflavone glucoside (C22H22O10) shall not be less than 0.020% |
| 5 | Anemarrhena asphodeloides Bunge | Mangiferin (C19H18O11) shall not be less than 0.70%, and Anemarrhena saponin BII (C45H76O19) shall not be less than 3.0% |
| 6 | Bupleuri Radix | Total content of saponin a (C42H68O13) and saponin d (C42H68O13) should not be less than 0.30% |
| 7 | Polygala tenuifolia Willd. | Polygala tenuifolia saponins (C36H56O12), not less than 2.0%, Polygala ketone III (C25H28O15) not less than 0.15%, containing 3,6’-dierucyl sucrose (C36H46O17) not less than 0.50% |
| 8 | Glycyrrhiza uralensis Fisch. | Glycyrrhizin (C21H22O9) shall not be less than 0.50%, glycyrrhizic acid (C42H62O16) shall not be less than 2.0% |
| Detection Compound | Stationary Phase | Mobile Phase | The Detector | Ref. |
|---|---|---|---|---|
| 4 triterpenoid saponins from Sophora flavescens | Luna C18 (2) column (150 × 4.6 mm; 5 μ particle size) | water (0.1% acetic acid) (A) and acetonitrile (0.1% acetic acid) (B) | 996 Photodiode array detector (Waters Corp.), 75 ELS detector Sedex (SEDERE) And MS: ESI: TOF | [66] |
| 3 steroidal saponins | Tigerkin C18 column | water (0.02% formic) (A) and acetonitrile (0.02% formic) (B) | Mass spectrometry | [81] |
| 6 steroidal saponins | RP-18e monolithic column (50 × 2 mm) | acetonitrile (A) and formic acid aqueous solution (0.1%, v/v) (B) | Mass spectrometry | [82] |
| 11 saponins of Achyranthes bidentate | Inertsil PREP-ODS column (20 × 250 mm) | volatile ion pair reagent (dihexyl ammonium acetate) | SPDM10AVP Photodiode Array Detector and Shimadzu LC-MS-2020 Mass spectrometry | [83] |
| 12 diosgenin in six batches of polygala samples | Rich Alorich Ascentis C8 column (100 mm × 4.6 mm, 3 μm) | diosgenin methyl: water (A) and methanol (B) 11 other saponins: water (A) and acetonitrile (B) | High Resolution Mass Spectrometry: (-):HESI(+/−) | [84] |
| 4 triterpene saponins in the Asparagus leaves | Dikma Diamonsil C18 column (4.6 mm × 250 mm, 5 μm) | acetonitrile (A) and water (B) | 2000ES Diode array detector | [67] |
| 9 oleic acid saponins | Kromasil 100-5 C18 column (250 mm × 4.6 mm, 5 μm) | water (0.1% formic acid) (A) and acetonitrile (0.1% formic acid) (B) | SPD-M20A DAD detector and LTQ Orbitrap Velos Pro mass spectrometer: (-): electrospray | [60] |
| 5 triterpenoidal saponins In Pulsatilla koreana | Shiseido CapCell PAK C18 analytical column (4.6 mm × 150 mm, 5 μm) | water (A) and acetonitrile (B) | MS: (-): ESI | [68] |
| 15 triterpenoid saponins from the leaves, stems, root skins and fruits of Acanthopanax quiculata | Kinetex XB-C18 column (100 mm × 4.6 mm, 2.6 μm) | acetonitril (A) and water (B) | Charged Aerosol Detection and Agilent 6530q-TOF mass spectrometry: (+) | [75] |
| 4 steroidal saponins | Diamonsil C18 column (4.6 mm × 250 mm, 5 μm) | acetonitrile (A) and water (B) | UV detector, Sedex75 ELSD system and DAD | [77] |
| 6 components in extract of ivy leaf | YMC Hydrosphere C18 analytical column (150 × 4.6 mm, 5 μm) | acetonitrile (A) and 0.1% phosphoric acid (B) | G4212A UV-Visible Diode Array Detector | [61] |
| triterpene saponins in Camellia plants | Inertsil ODS-3 column (2.1 mm × 100 mm) | methanol (A) and 5 mM trifluoroacetic acid (B) | UV-visible light detector | [85] |
| Arachnoside F in rat plasma | Reverse phase Zorbax SB-C18 column (150 × 4.6 mm, 5 μm) | ammonium acetate (A) and acetonitrile (B) | Agilent 6460 Triple Quadrupole Mass Spectrometer: Electrospray (+) | [86] |
| 5 saponins in 10 batches of Panax notoginseng | Agilent Zorbax SB-AQ analytical column (4.6 mm × 50 mm, 3.5 μm) | deionized water (A) and acetonitrile (B) | Diode array detector | [87] |
| Panax notoginseng saponin Fc and Ginsenoside Rc in Notoginseng leaf | Zorbax ODS C8 column (250 mm × 4.6 mm, 5 µm) | water (A) and acetonitrile (B) | Waters 2996 photodiode array detector | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ma, Y.; Tao, L.; Zhang, X.; Hao, F.; Zhao, S.; Han, L.; Bai, C. Recent Advances in Separation and Analysis of Saponins in Natural Products. Separations 2022, 9, 163. https://doi.org/10.3390/separations9070163
Wang Y, Ma Y, Tao L, Zhang X, Hao F, Zhao S, Han L, Bai C. Recent Advances in Separation and Analysis of Saponins in Natural Products. Separations. 2022; 9(7):163. https://doi.org/10.3390/separations9070163
Chicago/Turabian StyleWang, Yi, Yan Ma, Li Tao, Xiaoyan Zhang, Fusheng Hao, Shipeng Zhao, Lu Han, and Changcai Bai. 2022. "Recent Advances in Separation and Analysis of Saponins in Natural Products" Separations 9, no. 7: 163. https://doi.org/10.3390/separations9070163
APA StyleWang, Y., Ma, Y., Tao, L., Zhang, X., Hao, F., Zhao, S., Han, L., & Bai, C. (2022). Recent Advances in Separation and Analysis of Saponins in Natural Products. Separations, 9(7), 163. https://doi.org/10.3390/separations9070163






