Efficient Extraction of an Anthraquinone Physcion Using Response Surface Methodology (RSM) Optimized Ultrasound-Assisted Extraction Method from Aerial Parts of Senna occidentalis and Analysis by HPLC-UV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Apparatus and Reagents
2.3. Ultrasound-Assisted Extraction of Aerial Parts of S. occidentalis
2.4. BBD Experimental Design
2.4.1. Single Factor Experimental Design
2.4.2. Optimization of Extraction Variables Using the BBD Method and Method Validity Testing
2.5. HPLC-UV Analysis of Physcion in BBD Run SOMEs
2.6. Statistical Analysis
3. Results
3.1. Single-Factor Effect on Physcion Yield
3.2. BBD Method Optimization of the Extraction Conditions
3.2.1. Statistical Analysis and Model Fitting
3.2.2. Linear, Quadratic, and Interaction Effect of M1, M2, and M3 of Ultrasonic Extraction on R Yield
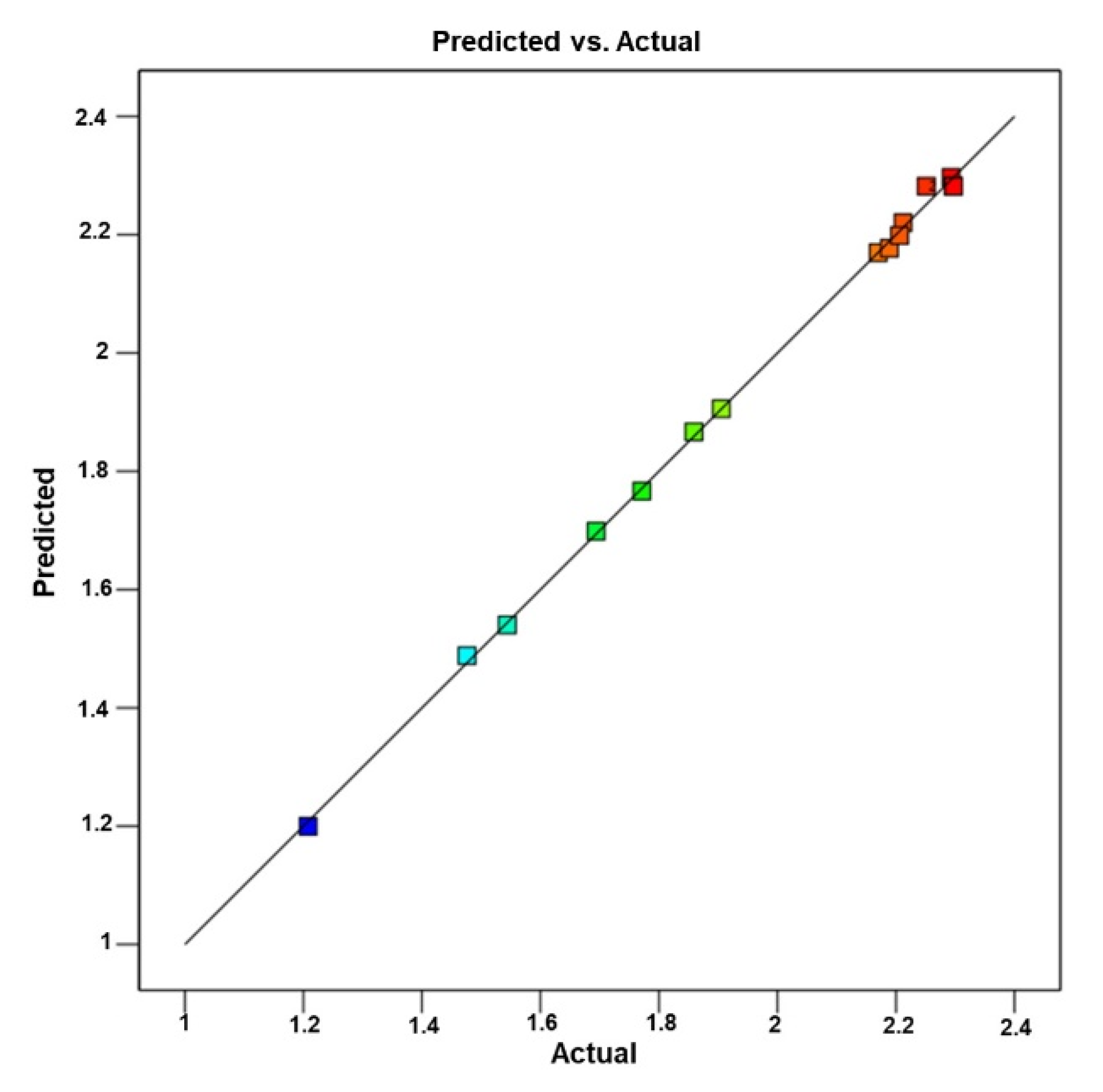
3.2.3. BBD Method Validation
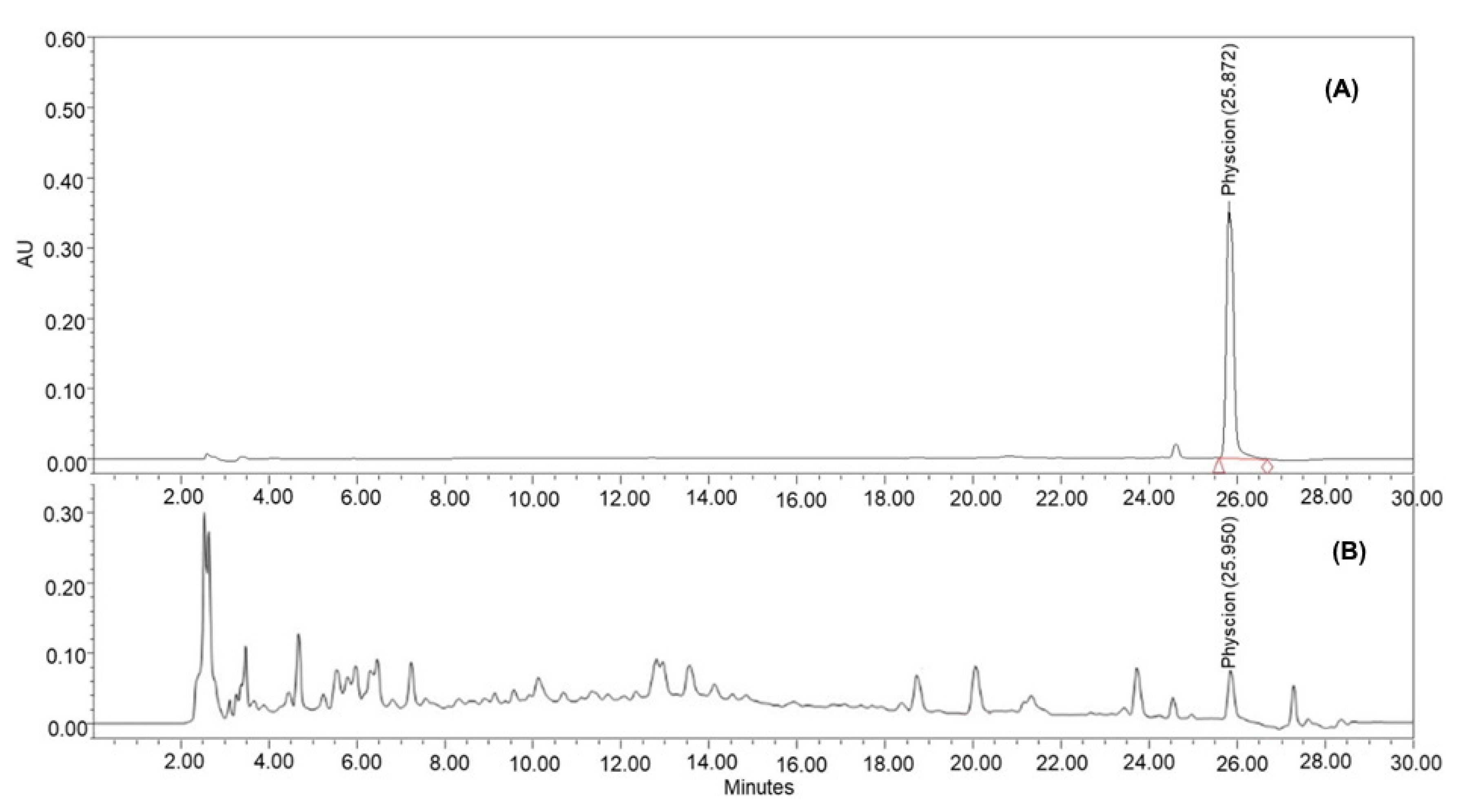
3.3. HPLC Analysis of BBD Optimized SOME
3.4. Verification of Optimized Microwave-Assisted Extraction Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liogier, H.A. Descriptive Flora of Puerto Rico and Adjacent Islands, Spermatophyla, Second Volume; Editorial de la Universidad de Puerto Rico: Rio Piedras, Puerto Rico, 1988; p. 481. [Google Scholar]
- Stevens, W.D.; Ulloa-U, C.; Pool, A.; Monitel, O.H. Flora de Nicaragua. Monographs of Systematic Botany, Volume 85; Missouri Botanical Garden Press: St. Louis, MO, USA, 2001; pp. 1–943. [Google Scholar]
- Haselwood, E.L.; Motter, G.G. Handbook of Hawaiian Weeds, Experiment Sattion; Hawaiian Sugar Planters Association: Honolulu, HI, USA, 1966; p. 479. [Google Scholar]
- Hu, S. Food Plants of China; Chinese University Press: Hong Kong China, 2005. [Google Scholar]
- Kolhapure, S.A.; Mitra, W.S. Meta-analysis of 50 phase III clinical trials in evaluation of efficacy and safety of Liv. 52 in infective hepatitis. Med. Update 2004, 12, 51–61. [Google Scholar]
- Yadav, J.P.; Arya, V.; Yadav, S.; Panghal, M.; Kumar, S.; Dhankhar, S. Cassia occidentalis L.: A review on its ethnobotany, phytochemical and pharmacological profile. Fitoterapia 2010, 81, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Smolenski, I.J.; Silinis, H.; Farnsworth, N.R. Alkaloid screening. VI. Lloydia 1975, 38, 225–255. [Google Scholar]
- Agarwal, S.K.; Singh, S.S.; Verma, S.; Kumar, S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J. Ethnopharmacol. 2000, 72, 43–46. [Google Scholar] [CrossRef]
- Ghosh, S.; Das Sarma, M.; Patra, A.; Hazra, B. Anti-inflammatory and anticancer compounds isolated from Ventilago madraspatana Gaertn, Rubia cordifolia Linn. and Lantana camara Linn. J. Pharm. Pharmacol. 2010, 62, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Tamokou, J.d.D.; Tala, M.F.; Wabo, H.K.; Kuiate, J.R.; Tane, P. Antimicrobial activities of methanol extract and compounds from stem bark of Vismia rubescens. J. Ethnopharmacol. 2009, 124, 571–575. [Google Scholar] [CrossRef]
- Hong, J.Y.; Chung, H.J.; Bae, S.Y.; Trung, T.N.; Bae, K.; Lee, S.K. Induction of cell cycle arrest and apoptosis by physcion, an anthraquinone isolated from rhubarb (Rhizomes of Rheum tanguticum), in MDA-MB-231 human breast cancer cells. J. Cancer Prev. 2014, 19, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Adnan, M.; Rasul, A.; Hussain, G.; Shah, M.A.; Sarfraz, I.; Nageen, B.; Riaz, A.; Khalid, R.; Asrar, M.; Selamoglu, Z.; et al. Physcion and Physcion 8-O-β-D-glucopyranoside: Natural Anthraquinones with Potential Anticancer Activities. Curr. Drug Targets 2021, 22, 488–504. [Google Scholar] [CrossRef]
- Cao, L.J.; Miao, J.; Liu, J.X.; Gao, W.Y.; Li, X. Research on contents of anthraquinones in Cassiae semen by principal component analysis. Zhongguo Zhong Yao Za Zhi 2015, 40, 2589–2593. [Google Scholar]
- Yan, J.; Wang, Y.; Wu, H.; Sun, Z.; Tan, S.; Wang, W.; Gong, L.; Xia, X.; Li, S. Development of a Method for Simultaneous Determination of Two Stilbenes and Four Anthraquinones from Polygonum cuspidatum by RP-HPLC. J. AOAC Int. 2019, 1, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Diao, F.; Xie, Q.; Wu, X.; Liu, C.; Li, X.; Li, Q. Determination of sennoside B, sennoside A and physcion in slimming health foods. Wei Sheng Yan Jiu 2020, 49, 809–814. [Google Scholar]
- Liu, X.; Li, H.; Wu, L.; Xing, J.; Poh, Y.; Cai, H.; Cai, B.C. Simultaneous quantification of chrysophanol and physcion in rat plasma by ultrafast liquid chromatography-tandem mass spectrometry and application of the technique to comparative pharmacokinetic studies of Radix et Rhei Rhizoma extract alone and Dahuang Fuzi Decoction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 980, 88–93. [Google Scholar]
- Alam, P.; Noman, O.M.; Herqash, R.N.; Almarfadi, O.M.; Akhtar, A.; Alqahtani, A.S. Response Surface Methodology (RSM)-Based Optimization of Ultrasound-Assisted Extraction of Sennoside A, Sennoside B, Aloe-Emodin, Emodin, and Chrysophanol from Senna alexandrina (Aerial Parts): HPLC-UV and Antioxidant Analysis. Molecules 2022, 27, 298. [Google Scholar] [CrossRef]
- Dhanani, T.; Singh, R.; Reddy, N.; Trivedi, A.; Kumar, S. Comparison on extraction yield of sennoside A and sennoside B from Senna (Cassia angustifolia) using conventional and non-conventional extraction techniques and their quantification using a validated HPLC-PDA detection method. Nat. Prod. Res. 2017, 31, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Genovese, S.; Tammaro, F.; Menghini, L.; Carlucci, G.; Epifano, F.; Locatelli, M. Comparison of three different extraction methods and HPLC determination of the anthraquinones aloe-emodine, emodine, rheine, chrysophanol and physcione in the bark of Rhamnus alpinus L. (Rhamnaceae). Phytochem. Anal. 2010, 21, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Xu, M.; Wang, Y.M.; Wu, D.M.; Chen, J.H. Optimizing ultrasonic ellagic acid extraction conditions from infructescence of Platycarya strobilacea using response surface methodology. Molecules 2010, 15, 7923–7932. [Google Scholar] [CrossRef]
- Zhong, K.; Wang, Q. Optimization of ultrasonic extraction of polysaccharides from dried longan pulp using response surface methodology. Carbohydr. Polym. 2010, 80, 19–25. [Google Scholar] [CrossRef]
- Zhao, L.C.; Liang, J.; Li, W.; Cheng, K.M.; Xia, X.; Deng, X.; Yang, G.L. The use of response surface methodology to optimize the ultrasound-assisted extraction of five anthraquinones from Rheum palmatum L. Molecules 2011, 16, 5928–5937. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.S.; Wu, Y.F.; Dai, S.L.; Chen, R.; Shao, Y. Ultrasound-assisted extraction of geniposide from Gardenia jasminoides. Ultrason. Sonochem. 2012, 19, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.J.; Paniwnyk, L.; Lorimer, J.P. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996, 3, S253–S260. [Google Scholar] [CrossRef]
- Zhao, S.; Kwok, K.C.; Liang, H. Investigation on ultrasound-assisted extraction of saikosaponins from Radix Bupleuri. Sep. Purif. Technol. 2007, 55, 307–312. [Google Scholar] [CrossRef] [PubMed]


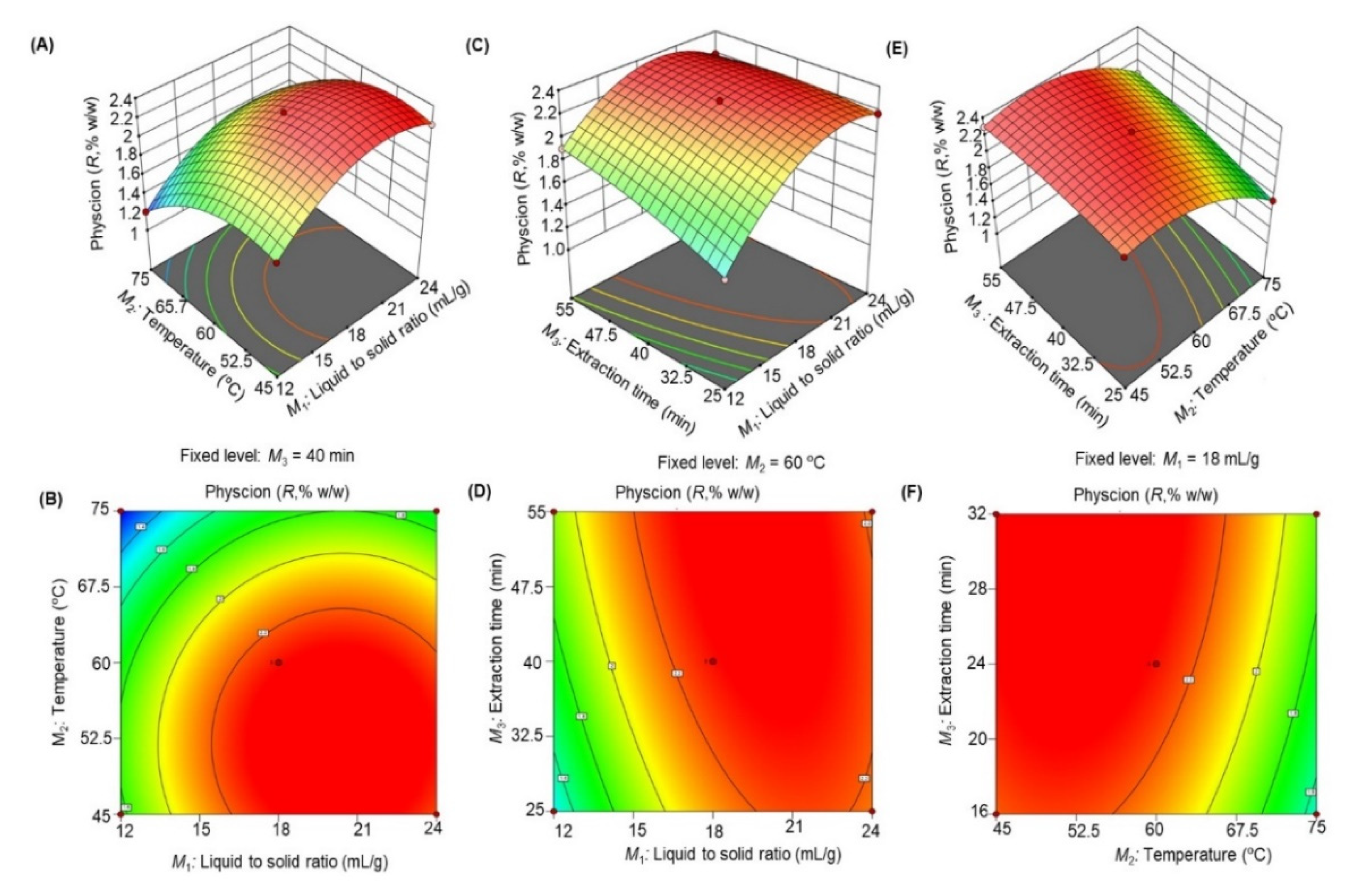
| Independent Variable | Factor level | Dependent Variable | Goal | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Liquid-to-solid ratio (M1) | 12 | 18 | 24 | Physcion yield (% w/w) (R) | Maximized |
| Extraction temperature (°C) (M2) | 45 | 60 | 75 | ||
| Extraction time (min) (M3) | 25 | 40 | 55 | ||
| Run | Factor (Coded) | Actual Variables | Physcion Yield (R) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (M1) (mL/g) | (M2) (°C) | (M3) (min) | (M1) (mL/g) | (M2) (°C) | (M3) (min) | Experimental (% w/w) | Predicted (% w/w) | Residual | |
| 1 | 0 | −1 | 1 | 18 | 45 | 55 | 2.29 ± 0.08 | 2.30 | −0.0039 |
| 2 | 1 | 0 | 1 | 24 | 60 | 55 | 2.19 ± 0.08 | 2.18 | 0.0121 |
| 3 | −1 | 0 | 1 | 12 | 60 | 55 | 1.91 ± 0.05 | 1.91 | −0.0006 |
| 4 | 0 | −1 | −1 | 18 | 45 | 25 | 2.21 ± 0.08 | 2.20 | 0.0076 |
| 5 | 1 | −1 | 0 | 24 | 45 | 40 | 2.21 ± 0.07 | 2.22 | −0.0083 |
| 6 | 0 | 0 | 0 | 18 | 60 | 40 | 2.25 ± 0.08 | 2.28 | −0.0307 |
| 7 | 0 | 0 | 0 | 18 | 60 | 40 | 2.30 ± 0.09 | 2.28 | 0.0153 |
| 8 | −1 | 0 | −1 | 12 | 60 | 25 | 1.48 ± 0.03 | 1.49 | −0.0121 |
| 9 | 0 | 1 | −1 | 18 | 75 | 25 | 1.54 ± 0.04 | 1.54 | 0.0039 |
| 10 | −1 | 1 | 0 | 12 | 75 | 40 | 1.21 ± 0.02 | 1.20 | 0.0082 |
| 11 | 1 | 0 | −1 | 24 | 60 | 25 | 2.17 ± 0.02 | 2.17 | 0.0006 |
| 12 | 0 | 0 | 0 | 18 | 60 | 40 | 2.31 ± 0.03 | 2.28 | 0.0153 |
| 13 | 1 | 1 | 0 | 24 | 75 | 40 | 1.69 ± 0.04 | 1.70 | −0.0045 |
| 14 | 0 | 1 | 1 | 18 | 75 | 55 | 1.86 ± 0.04 | 1.87 | −0.0076 |
| 15 | −1 | −1 | 0 | 12 | 45 | 40 | 1.77 ± 0.04 | 1.77 | 0.0045 |
| 16 | 0 | 0 | 0 | 18 | 60 | 40 | 2.24 ± 0.08 | 2.22 | 0.0018 |
| 17 | 0 | 0 | 0 | 18 | 60 | 40 | 2.24 ± 0.07 | 2.23 | 0.0013 |
| Dependent Variables | Source | R2 | Adjusted R2 | Predicted R2 | SD |
|---|---|---|---|---|---|
| R | Linear | 0.6547 | 0.5605 | 0.4410 | 0.2334 |
| 2FI | 0.6867 | 0.4517 | 0.0906 | 0.2607 | |
| Quadratic | 0.9988 | 0.9967 | 0.9925 | 0.0201 |
| Dependent Variables | Source | Sum of Square | Degree of Freedom | Mean Square | F-Value | p Value |
|---|---|---|---|---|---|---|
| R | Model | 1.73 | 9 | 0.1926 | 474.84 | <0.0001 |
| Residual | 0.0020 | 5 | 0.0004 | - | - | |
| Lack of fit | 0.0006 | 3 | 0.0002 | 0.2919 | 0.8319 | |
| Pure error | 0.0014 | 2 | 0.0007 | - | - |
| Dependent Variables | Independent Variables | SS a | DF b | MS c | F-Value | p-Value d |
|---|---|---|---|---|---|---|
| R | Linear effects | |||||
| M1 | 0.4536 | 1 | 0.4536 | 1118.18 | <0.0001 | |
| M2 | 0.5924 | 1 | 0.5924 | 1460.29 | <0.0001 | |
| M3 | 0.0903 | 1 | 0.0903 | 222.62 | <0.0001 | |
| Quadratic effects | ||||||
| M12 | 0.0099 | 1 | 0.3333 | 821.64 | <0.0001 | |
| M22 | 0.0014 | 1 | 0.2495 | 615.06 | <0.0001 | |
| M32 | 0.0035 | 1 | 0.0079 | 19.43 | 0.0070 | |
| Interaction effects | ||||||
| M1M2 | 0.0003 | 1 | 0.0005 | 1.25 | 0.3147 | |
| M1M3 | 0.0002 | 1 | 0.0420 | 103.59 | 0.0002 | |
| M2M3 | 0.0003 | 1 | 0.0130 | 32.03 | 0.0024 | |
| Factor | Optimal Level | ||
|---|---|---|---|
| M1(mL/g) | 20.16 | ||
| M2 (°C) | 52.2 | ||
| M3 (min) | 46.6 | ||
| Response | Predicted (%w/w) | Experimental (%w/w, n = 3) | Residual (%) |
| Physcion (% w/w) | 2.41 | 2.43 ± 0.16 | 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, P.; Noman, O.M.; Herqash, R.N.; Almarfadi, O.M.; Akhtar, A.; Alqahtani, A.S. Efficient Extraction of an Anthraquinone Physcion Using Response Surface Methodology (RSM) Optimized Ultrasound-Assisted Extraction Method from Aerial Parts of Senna occidentalis and Analysis by HPLC-UV. Separations 2022, 9, 142. https://doi.org/10.3390/separations9060142
Alam P, Noman OM, Herqash RN, Almarfadi OM, Akhtar A, Alqahtani AS. Efficient Extraction of an Anthraquinone Physcion Using Response Surface Methodology (RSM) Optimized Ultrasound-Assisted Extraction Method from Aerial Parts of Senna occidentalis and Analysis by HPLC-UV. Separations. 2022; 9(6):142. https://doi.org/10.3390/separations9060142
Chicago/Turabian StyleAlam, Perwez, Omar M. Noman, Rashed N. Herqash, Omer M. Almarfadi, Ali Akhtar, and Ali S. Alqahtani. 2022. "Efficient Extraction of an Anthraquinone Physcion Using Response Surface Methodology (RSM) Optimized Ultrasound-Assisted Extraction Method from Aerial Parts of Senna occidentalis and Analysis by HPLC-UV" Separations 9, no. 6: 142. https://doi.org/10.3390/separations9060142
APA StyleAlam, P., Noman, O. M., Herqash, R. N., Almarfadi, O. M., Akhtar, A., & Alqahtani, A. S. (2022). Efficient Extraction of an Anthraquinone Physcion Using Response Surface Methodology (RSM) Optimized Ultrasound-Assisted Extraction Method from Aerial Parts of Senna occidentalis and Analysis by HPLC-UV. Separations, 9(6), 142. https://doi.org/10.3390/separations9060142







