Abstract
Phytochemical characterization of the ethyl acetate fraction of Saussurea hypoleuca root extract resulted in the isolation of oleic acid (1) and luteolin (2), which were isolated for the first time from Saussurea hypoleuca root. A heavy metal analysis of the root powder performed using atomic absorption spectroscopy showed that the contents of iron, cadmium, lead, zinc, nickel, and copper were within the certified limits according to the WHO guidelines. Differential scanning calorimetry (DSC) revealed its crystalline and amorphous nature; meanwhile, standardization of the root with UHPLC revealed the presence of 14.79 ± 0.015 µg/mL of luteolin. Both the total methanol extract and the ethyl acetate fraction of the plant root held significant anthelmintic activity. Oleic acid and luteolin exhibited potent antioxidant activity, evidenced by their IC50 values, which were equal to 47.0 and 119.8 µg/mL, respectively, in a 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging assay. In silico studies showed that luteolin exerted the highest fitting within the binding sites of NADPH oxidase (Nox). For myeloperoxidase (MP), oleic acid revealed the best fitting in its active sites. The results of ADMET (absorption, distribution, metabolism, excretion, and toxicity) and TOPKAT (toxicity prediction) protocols revealed acceptable pharmacodynamic and pharmacokinetic characteristics, in addition to reasonable toxicity characteristics for both compounds. Thus, they can be incorporated into pharmaceutical dosage forms to combat oxidative stress.
1. Introduction
Oxidative stress appears when the generation of reactive oxygen species (ROS) predominates the antioxidant defenses that exist intrinsically within the body of a living organism. When ROS exceed the permitted limits, they exert drastic changes with concomitant indiscriminate destruction to biological molecules, causing deterioration in their functions and, ultimately, death. [1]. The concept of a pro-oxidant–antioxidant balance is basically used to highlight oxidative stress for many reasons. At first, it confirms that alteration on either side of the equilibrium, comprising abnormal great production of ROS or insufficient antioxidant defenses, may result in its disturbance. Secondly, it reflects ROS homeostatic concentrations. Finally, the term “balance” attracts concern to the concept of the presence of a graded response to oxidative stress. Thus, small disturbances in the balance result in homeostatic adaptations with respect to variations in the immediate environment. However, a major disturbance may cause permanent damage with concomitant cell death. The limit existing between normal physiological changes and pathological insults is, therefore, inevitably indistinct [2]. Saussurea is a plant genus belonging to the Asteraceae family (Compositae) and includes nearly 400 species spread throughout Europe and Asia and about 264 species in China [3]. Saussurea species vary in height, between dwarf to tall, thistle-like species. The leaves are arranged in a dense basal rosette that spirals up along the stem. They form a dense head of small capitula with surrounding dense, white to purple woolly hairs with additional white to purple florets. Traditionally, many members of the genus Saussurea have been used to alleviate fevers, colds, colic, headaches, lumbar pain, menorrhagia, and renal pain, in addition to their utilization in the treatment of asthma, dysentery, rheumatism, stomachaches, ulcers, and toothaches [4,5].
Saussurea hypoleuca (synonym: Saussurea auriculata) is locally named Qust and is found in Quetta, Balochistan, in the Pakistan mountains. In addition, S. hypoleuca exists in Himachal Pradesh in the Himalayan region of India [6]. A comprehensive literature review on Saussurea hypoleuca roots revealed that only some chemical constituents have been previously identified by authors, represented by phenolic compounds such as caffeic acid and sinapic acid from methanol extract. Additionally, the detected flavonoids in methanol were quercetin, myricetin, and kaempferol [7,8]. A proximate analysis and in vitro biological assessments highlighted that the roots contain many phytoconstituents worthy of being isolated [9]. Meanwhile, a gas chromatography analysis accompanied by mass spectrometry (GC-MS) displayed the presence of hexadecanoic acid, isopropyl myristate, decanedioic acid, tetracosapentaene, 3,4-hexanedione, and tetracosapentaene as major compounds identified from the n-hexane and chloroform fractions of the roots [7]. Furthermore, the anticancer, anti-inflammatory, hepatoprotective, and antioxidant activities of the crude extract were investigated in vitro and in vivo and strongly supported that the roots possess considerable pharmacological potential that is worthy of being deeply investigated [7].
In this study, we aim to perform for the first time a phytochemical characterization of the major compounds existing in the Saussurea hypoleuca root ethyl acetate fraction after their isolation using different chromatographic methods. The identification and structural elucidation of these compounds are performed employing nuclear magnetic resonance (NMR) analyses, where oleic acid (1) and luteolin (2) are the first to be isolated from the roots. Furthermore, heavy metal and thermal analyses of the plant root powder are carried out for the first time. The standardization of the S. hypoleuca root is also performed using ultra-high-performance liquid chromatography (UHPLC). In addition, the determination of anthelmintic activity is first performed on the total methanol extract and then the ethyl acetate fraction. The isolated compounds are evaluated for their free radical scavenging properties using 2,2-diphenyl-1-picrylhydrazyl scavenging capacity (DPPH). Molecular docking is also carried out for the isolated compounds within the active core of enzymes implicated in generating free radicals that further aggravate oxidative stress, aiming to ascertain the in vitro studies. Finally, a computational assessment of ADMET (absorption, distribution, metabolism, excretion, and toxicity) behavior and TOPKAT (toxicity prediction) of the obtained constituents is carried out to evaluate their toxicity, as well as their pharmacokinetic and pharmacodynamic characteristics.
2. Materials and Methods
2.1. Chemicals and Reagents
Analytical-grade solvents comprising methanol (CAS NO, 67-56-1), n-hexane (CAS NO, 110-54-3), and ethyl acetate (CAS NO, 141-78-6) were supplied by Merck Millipore, Darmstadt, Germany. n-butanol (CAS NO, 71-36-3), chloroform (CAS NO, 67-66-3), and standard DPPH (CAS NO, 1898-66-4) were supplied by Sigma Aldrich, USA. Silica gel (CAS 7631-86-9), acetonitrile (CAS NO, 75-05-8), and TLC Silica gel 60 F254 (CAS NO, 105554) were supplied by Merck Millipore, Germany.
2.2. Plant Material
Saussurea hypoleuca Spreng. ex DC (Asteraceae) roots were gathered from the mountains of Baluchistan, Pakistan, in September 2016 and were kindly identified by Prof Dr. Zaheer-ul-deen from the Department of Botany at Government College University of Lahore, Pakistan. A specimen was maintained under voucher number (GC. Herb. Bot. 3453) at the Government College University herbarium museum in Lahore, Pakistan.
2.3. Extraction and Isolation
The extraction of 7 kg of S. hypoleuca powdered, air-dried roots was performed using methanol (21 L), and the roots were consequently extracted three times by employing a cold maceration technique at room temperature. The methanol extract was successively fractionated using different solvents of increasing polarity, comprising n-hexane, chloroform, ethyl acetate, and n-butanol, to give the respective fractions [10]. The ethyl acetate fraction was chosen for its compound isolation in a pure form, highlighted by its promising thin-layer chromatography (TLC), which showed many promising compounds. An amount of 10 g of the ethyl acetate fraction sample was loaded on a silica gel column for chromatography and successively eluted with mobile phase, starting with n-hexane and followed by a gradual increase in polarity adopting the following order (n-hexane: chloroform, chloroform: ethyl acetate, and ethyl acetate: methanol). Finally, seventy fractions (20 mL each) were collected in glass vials, and an examination was performed using TLC that was exposed to a UV lamp. Similar fractions were collected together, resulting in ten main fractions. Based on the examined TLC, the mobile phases were adjusted for these fractions, and they were exposed to purification using subcolumns containing silica gel as a stationary phase for further purification. Two compounds were successfully isolated. Compound (1) (25 mg) was eluted using a solvent system of n-hexane: chloroform (95:05); meanwhile, compound (2) (38 mg) was obtained with ethyl acetate: methanol (70:30) as a solvent system.
2.4. Compound Characterization
2.4.1. Characterization of Compound (1)
Compound (1) was obtained as a pale, yellow, oily liquid. 1H-NMR (δ ppm); (CDCl3-400 MHz); J in Hz: 0.89 (3H, m, H-18), 1.30 (8H, m, H-4, H-7, H-12, H-17), 1.64 (2H, m, H-3), 2.03 (4H, m, H-8, H-11), 2.36 (2H, m, H-2), and 5.36 (4H, m, H-9, H-10); 13C-NMR (δ ppm); (CDCl3-135 MHz); 180.6 (COOH), 34.1 (C-2), 24.68 (C-3), 29.10 (C-4), 29.10 (C-5), 29.44 (C-6), 29.60 (C-7), 27.10 (C-8), 128.81 (C-9), 130.87 (C-10), 27.20 (C-11), 29.70 (C-12), 29.34 (C-13), 29.53 (C-14), 29.25 (C-15), 31.90 (C-16), 22.7 (C-17), and 14.1(C-18). The 1H-NMR and 13C-NMR spectra of this compound (1) are illustrated in Figure S1 in the Supplementary Data.
2.4.2. Characterization of Compound (2)
Compound (2) was obtained as a light yellow powder. 1H-NMR (δ ppm); (CDCl3-400 MHz); J in Hz: 12.98 (s, 1H, 5-OH), 7.42 (m, 2H, H-2′, 6′), 6.90 (d, J = 8.0 Hz, 1H, H-5′), 6.67 (s, 1H, H-3), 6.45 (d, J = 2.0 Hz, 1H, H-8), and 6.19 (d, J = 2.0 Hz, 1H, H-6); 13C-NMR (δ ppm); (CDCl3-135 MHz); 163.92 (C-2), 102.91 (C-3), 183.86 (C-4), 161.52 (C-5), 100.12 (C-6), 164.16 (C-7), 95.00 (C-8), 157.32 (C-9), 103.74 (C-10), 121.56 (C-1′), 113.40 (C-2′), 145.77 (C-3′), 149.73 (C-4′), 116.05 (C-5′), and 119.02 (C-6′). The 1H-NMR and 13C-NMR spectra of this compound are displayed in Figure S2 in the Supplementary Data.
2.5. Heavy Metal Analysis
Atomic absorption spectroscopy was used to identify and quantify the plant root powder for heavy metal analysis. The analysis was conducted in accordance to a wet digestion method. A total of 1 g of the sample was subjected in a round-bottom flask to concentrated HNO3 for sixteen hours and then heated at 80 °C on a hot plate. An amount of 2 mL of 60% perchloric acid was added until a clear solution was formed, as previously described by Rai et al. (2001) [11]. Then, 10 mL of distilled water was added gradually to the solution and filtered, and the final volume was made with 25 mL of distilled water. Various metals were analyzed in the powder by comparison with the standard.
2.6. Thermal Analysis
The physiochemical nature of the plant root powder was determined with the procedure previously reported by Pai et al. [12]. The tested sample was screened at a predefined rate, which was 10 °C/min, over temperatures ranging from 40–300 °C in an inert environment with nitrogen gas (N2) at a rate of 10 mL/min. A thermogram was obtained regarding the thermal stability of the root powder.
2.7. HPLC Standardization of Saussurea Hypoleuca Root
2.7.1. Sample Preparation
Saussurea hypoleuca root extract was prepared via the sonication of 2 g of powdered root with 70% ethanol (3 × 5 mL) in a stoppered conical flask. Consequently, the extract was filtered and transferred to a 25 mL volumetric flask where the final volume was adjusted using 70% ethanol until reaching the mark.
2.7.2. Reversed-Phase HPLC Analysis
The HPLC analysis was performed employing the method previously described by Ezzat et al. [13] using a Flexar FX-15 UHPLC pump (Perkin Elmer, Waltham, MA, USA) furnished with a C18 column (250 × 4.6 mm, 2.6 μm) (Supelco, Kromacil®, Sigma-Aldrich, Gillingham, Dorset, UK) and supplemented with a UV–visible (UV–vis) detector operating at a flow rate of 1 mL/min. Isocratic elution was used with a mobile phase composed of 70% acetonitrile and 30% water. The samples were filtered with a syringe filter (0.22 µm), and 10 μL of the prepared sample was injected. The wavelength at 320 nm was the used UV–vis detector wavelength for detection of the eluted compounds. Luteolin was eluted at a retention time of 2.965 min.
2.7.3. Standard Curve Construction
A stock solution of luteolin was prepared in a 25 mL volumetric flask via dissolving an accurate weight in methanol (HPLC-grade) to obtain a final concentration of 3 mg/mL. A calibration curve was then plotted from the stock solution by the serial dilution of different aliquots in the range of 9.37–300 μg/mL.
2.8. Determination of the Anthelmintic Activity
Earthworms were collected from moist soil and washed with 0.9% NaCl solution to remove soil and fecal matter to investigate the anthelmintic activity. The study was designed in Pheretima posthuma due to its similarity anatomically and physiologically to human intestinal roundworms. Earthworms with 6–8 cm lengths and 0.2–0.3 cm widths were used in all the experimental protocols. The earthworms were allocated into ten groups, each having five worms. The total methanol extract and the ethyl acetate fraction, as well as albendazole, at concentrations of 25, 75, and 100 mg/mL were prepared in normal saline. Albendazole was prepared in similar concentrations to the samples and served as the standard drug. The control group received normal saline only. Each concentration was made in 10 mL petri dishs. Each petri dish contained 10 mL of each concentration. All the earthworms were released into these petri dishes. Paralysis and death of the worms were noticed. The time of paralysis was recorded when there was no kind of movement, even if they were shaken vigorously, and death was detected when there was no motility after dipping them in warm water, followed by fading of their body color. This assay was performed by Ajaiyeoba et al. (2001) with slight modifications [14].
2.9. Determination of the Antioxidant Activity
The isolated compounds were measured for their free radical scavenging properties using 2,2-diphenyl-1-picrylhydrazyl scavenging capacity (DPPH) as an indicator for their antioxidant activity [15,16]. The preparation of stock solutions (1 mg/mL) of the isolated compounds was performed using methanol, which acted as the control. Various concentrations (5–250 µg/mL) of the tested compounds and the standard compound, ascorbic acid, were assessed. The compounds and standard were kept at 25° for 30 min. Absorbance was measured using a UV spectrophotometer at 517 nm. Repetition of the assay was performed three times, and the decline in absorbance was recorded. The percentage of inhibition was calculated using the following equation:
The concentration that caused 50% inhibition (IC50) was also determined; the results are presented as mean ± SD.
2.10. Computational Studies
2.10.1. Molecular Docking Studies
In silico molecular modelling studies were conducted for the compounds isolated from Saussurea hypoleuca ethyl acetate root extract, namely compounds (1) and (2), to examine their inhibitory potential on certain enzymes implicated in the production of ROS. These enzymes included NADPH oxidase (Nox) (PDB ID: 2CDU; 1.80 Aº) and myeloperoxidase (MP) (PDB ID: 5WDG; 2.40 Aº), which were obtained from the Protein Data Bank (PDB). This was performed in an effort to confirm the results of the in vitro analysis. This was conducted using Discovery Studio 4.5 (Accelrys Inc., San Diego, CA, USA) with a C-docker protocol, following what has been previously reported [17,18]. Binding energies (∆G) were calculated using the following equation:
where:
ΔGbinding = Ecomplex − (Eprotein + E ligand)
ΔGbinding: The ligand–protein interaction binding energy;
Ecomplex: The potential energy for the complex of protein bound with the ligand;
Eprotein: The potential energy of protein alone;
Eligand: The potential energy for the ligand alone.
2.10.2. ADME and TOPKAT Prediction
The pharmacokinetic, pharmacodynamic, and toxicity characteristics of compounds (1) and (2) isolated from Saussurea hypoleuca ethyl acetate root extract were predicted with ADMET (absorption, distribution, metabolism, excretion, and toxicity) and TOPKAT (toxicity prediction) protocols using Discovery Studio 4.5 (Accelrys Inc., San Diego, CA, USA). Human intestinal absorption (HIA), hepatotoxicity level, cytochrome P450 2D6, plasma–protein binding prediction (PPB), aqueous solubility, and blood–brain barrier penetration (BBB) were taken as ADMET descriptors. Meanwhile, rat oral LD50, rat inhalational LD50, rat chronic LOAEL, Ames mutagenicity, and the carcinogenic impact on both female and male rat NPTs (National Toxicology Programs), in addition to skin and ocular irritant effects, were selected as TOPKAT criteria [19,20].
3. Results and Discussion
3.1. Phytochemical Characterization
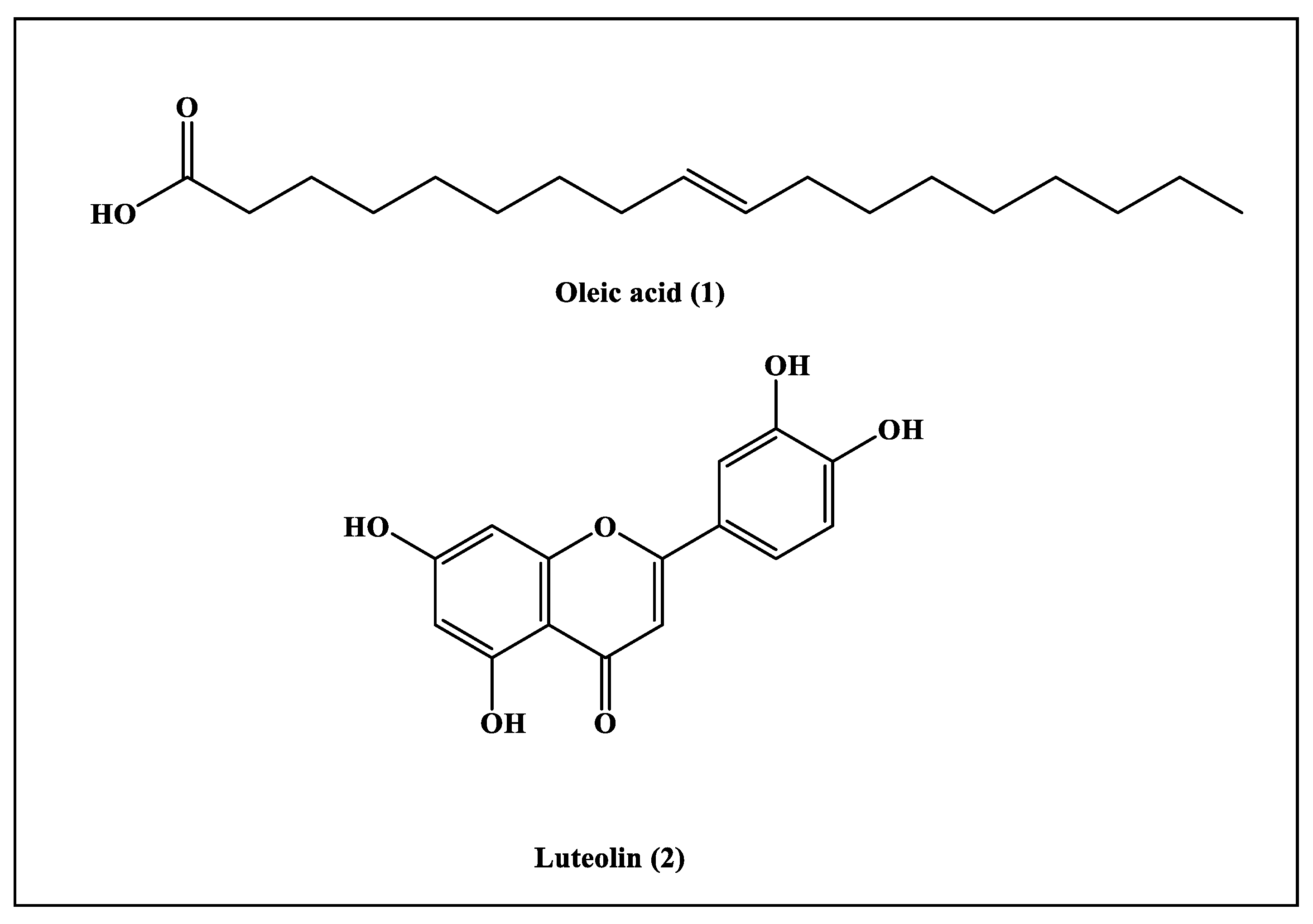
The phytochemical investigation performed on the ethyl acetate fraction prepared from Saussurea hypoleuca revealed the isolation of two compounds. Their structures were determined by 1H-NMR and 13C-NMR data and by comparing the obtained data with those previously documented. They were identified as oleic acid (1) and luteolin (2) [21,22], which were isolated for the first time from Saussurea hypoleuca roots (Figure 1).
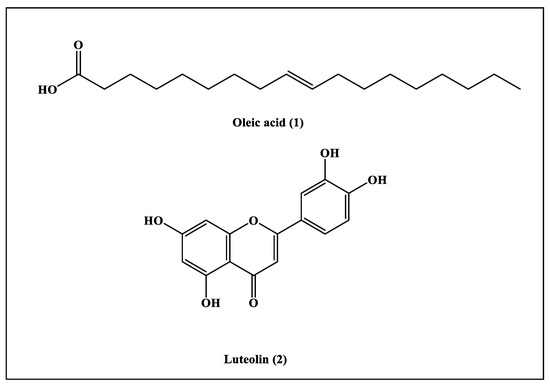
Figure 1.
Ethyl acetate fractions of compounds isolated from Saussurea hypoleuca roots.
Compound (1) was obtained as a pale, yellow, oily liquid. The 1H-NMR spectrum revealed the presence of protons of -CH3 groups, which was indicated by the triplet peaks at δH 0.89 (3H; m; H-18). Peaks at the δH of 1.30 (8H; m; H-4, H-7, H-12, and H-17) and 1.64 (2H; m; H-3) were due to the protons of the aliphatic methylene group (-CH2). In the 1H-NMR spectrum, there was a peak at the δH of 2.03 (4H; m; H-8 and H-11) that highlighted the existence of protons of methylene groups that were bonded by C=C bonds; meanwhile, the peak at the δH of 2.36 (2H; m; H-2) indicated the existence of protons of methylene groups that were attached to the carbonyl group. The presence of olefinic protons was detected by the presence of a peak at the δH of 5.36 (4H; m; H-9 and H-10). In the 13C-NMR spectrum, peaks at the δc of 130.1 (C-9 and C-10) specified the C=C bond in the compound, and a peak at the δc of 180.6 (C-1) confirmed the presence of carboxylic acid. Peaks from the δc of 14.08 to 33.96 revealed the presence of methyl and methylene groups. Thus, by comparing the obtained data with those previously documented in the literature, compound (1) was characterized as oleic acid [21,23].
Compound (2) was isolated as light yellow powder. The 1H-NMR spectrum revealed the presence of aromatic hydroxyl groups at the δH of 12.98 (s; 1H; 5-OH); an aromatic singlet at the δH of 6.67 (s; 1H; H-3); and ABC-coupled aromatic protons at 6.90, 7.42 and 7.36. There was also a pair of meta coupled aromatic protons at the δH of 6.45 (d; J = 2.0 Hz; 1H and H-8) and 6.19 (d; J = 2.0 Hz; 1H and H-6). In the 13C-NMR spectrum, there was a peak at the δc of 183.86 (C-4) for a carbonyl group; six oxygenated quaternary carbons at 149.73, 164.16, 163.92, 161.52, 157.32 and 145.7; and two sp2 quaternary carbons at 121.56 and 103.74. There were also peaks at 119.02, 116.05, 113.40, 102.91, 100.12 and 95.00, which represented six sp2 tertiary carbons. Thus, by comparing the obtained data with those previously documented in the literature, compound (2) was characterized as luteolin [22,24].
3.2. Heavy Metal Analysis
The plant powder was treated with a heavy metal analysis to estimate the quality and quantity of toxic metals using atomic absorption spectroscopy. The concentrations of iron, zinc, copper, nickel, cadmium, and lead were 386, 15.93, 3.19, 0.12, 0.073 and 0.015 mg/kg respectively. During cultivation, drying, and even in collection, plant materials can become adulterated with heavy metals. Natural products formulated from these contaminated heavy metals can, ultimately, reach the human body, where they cause disruption in the functioning of several organs and can lead to the development of skin eruption, hypertension, intestinal colitis, and different forms of tumors. Therefore, the amounts of these heavy metals in medicinal plants should remain within the permitted certified limits, as described by the WHO. The results showed that the extents of iron, cadmium, lead, zinc, nickel, and copper were within the certified limits according to the WHO guidelines (WHO 2007) [25]. Therefore, it was perceived that Saussurea is nontoxic and can be used safely for therapeutic purposes.
3.3. Thermal Analysis
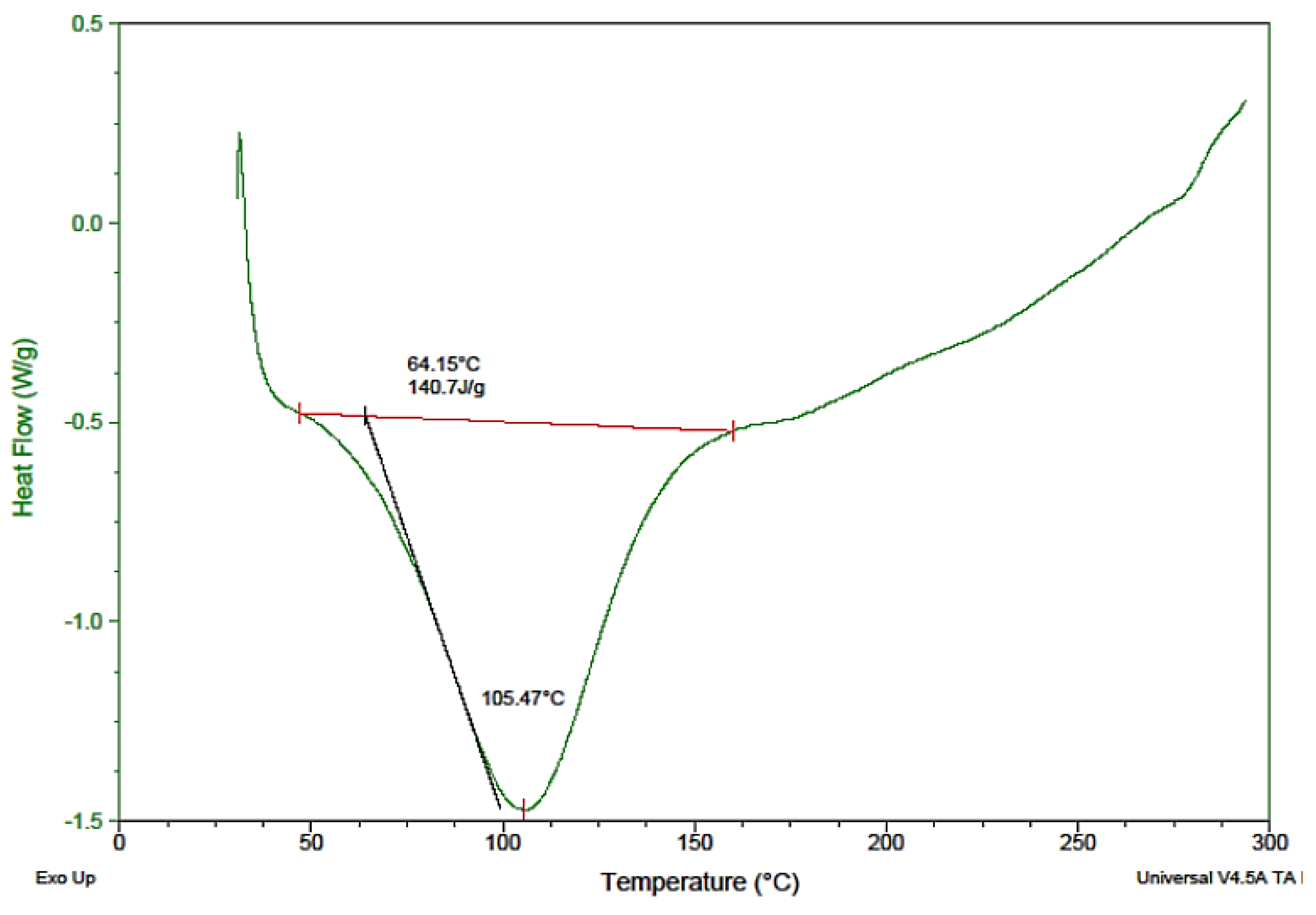
Thermal analysis is one the effective tools for the characterization of herbal materials. The determination of variation in both isothermal and non-isothermal conditions contributes to the stability of aspects of the substances under thermal analysis. DSC is used as an evaluating tool for polymer and drug compatibility as a pre-formulation study of new formulations to be established. DSC was implemented to determine incompatibility among the components of the mixture, as well as the nature of the powder, i.e., whether it was amorphous, crystalline, or polymeric, and to study the physicochemical nature of Saussurea hypoleuca powdered roots at a predefined heat and temperature. A thermograph is represented in Figure 2. For the DSC, the thermograph regarding heat flow at temperatures of 50–300 °C is illustrated in Figure 2. The peak at 64.15 °C revealed the onset temperature of the melting procedure, corresponding to the peak at 105.47 °C, which was the melting point of some components present in the mixture [26].
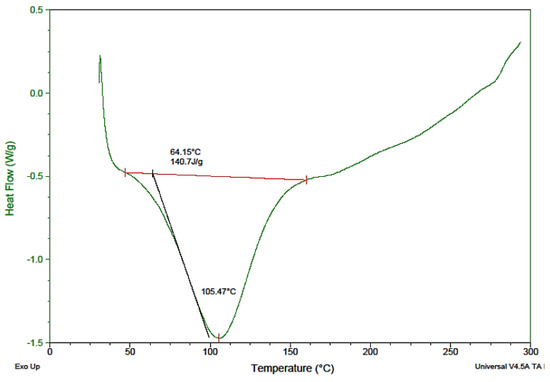
Figure 2.
Differential scanning calorimetry (DSC) thermogram of Saussurea hypoleuca powdered roots.
3.4. HPLC Standardization of Saussurea Hypoleuca Root
The standard calibration curve constructed by luteolin revealed a linear relationship (r = 0.9986) between the peak area and the concentration of luteolin. From the standard calibration curve plotted, Saussurea hypoleuca root was standardized to contain 14.79 ± 0.015 µg/mL of luteolin, where 38 mg of luteolin was obtained from 10 g of ethyl acetate root extract.
3.5. Determination of the Anthelmintic Activity
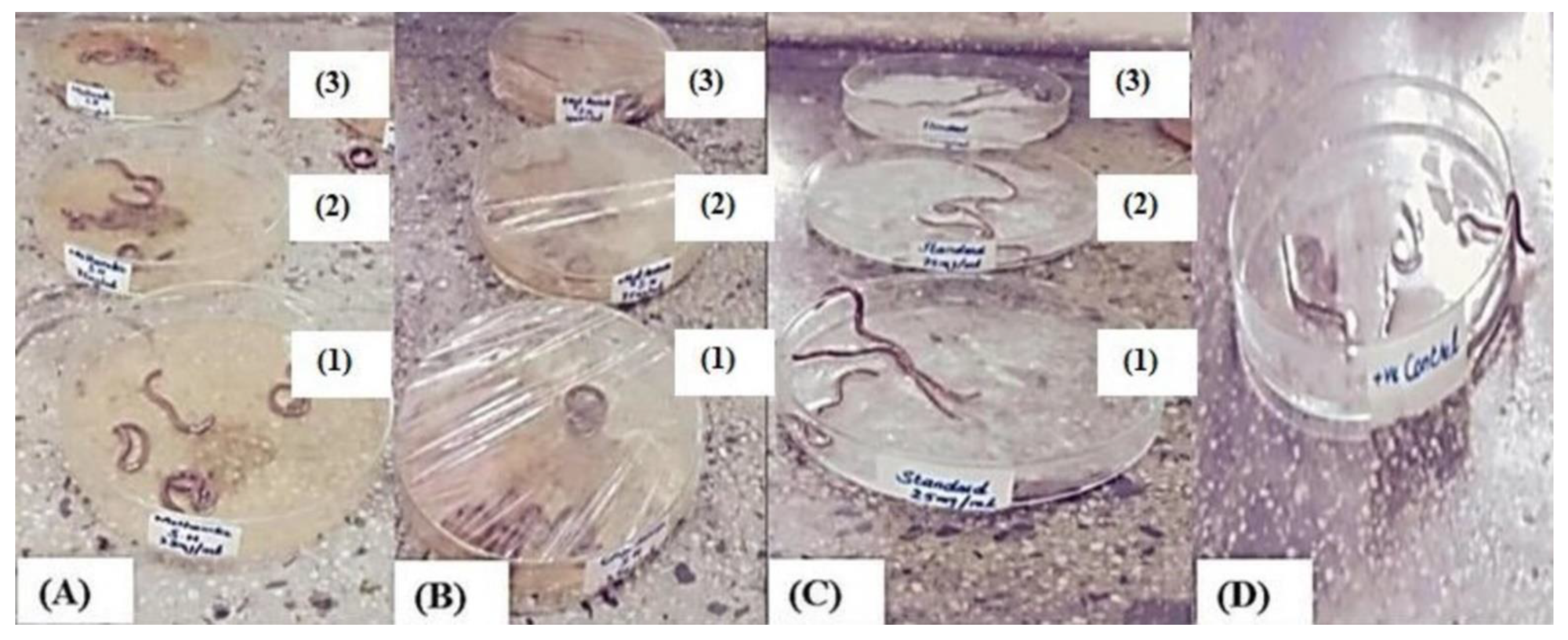
Helminthiasis is an infectious disease that occurs in all ages, especially in developing countries. Helminth infections are among the most common infections in humans, affecting a large population and posing huge peril to public well-being, promoting the prevalence of malnourishment, eosinophilia, anemia, and pneumonia. Although the infection is mostly limited to tropical areas, it can occur in travellers, and some of them can occur in temperate climates. In helminthiasis, a body part is infested with worms such as pinworms, roundworms, or tapeworms. Typically, worms exist in the GIT, but some of them can transfer to the liver, in addition to many other organs. An infected person produces eggs in their feces, which contaminate the soil and water. People become infected by ingesting food or water contaminated with eggs or larvae. According to the WHO, a large number of synthetic drugs are used against helminth infections, but they have huge side effects and cost values [27]. Keeping with these observations, our research was conducted to investigate the anthelmintic activity of the total methanol extract and the ethyl acetate fraction at concentrations of 25, 75, and 100 mg/mL in adult Indian earthworms with a comparison to albendazole (standard drug). The total methanol extract revealed paralysis and death times at 24 and 43 min, respectively, at the highest concentration. Meanwhile, the ethyl acetate fraction displayed paralysis and death times at 42.2 and 62.6 min, respectively, whereas albendazole displayed 53 and 74.3 min for paralysis and death times, respectively (Table 1). Anthelmintic agents may act locally to expel the parasites from the GIT or systematically to eradicate the adult form of the parasite that invaded the organs and tissues. The study concluded that both the total methanol extract and the ethyl acetate fraction of the plant root held significant anthelmintic activity in a dose-dependent manner when compared with albendazole (Figure 3). The exact mechanism of the anthelmintic activity could be attributed to the presence of flavonoids and other polyphenolic compounds that can bind to free proteins in the gastrointestinal tract of the host animal or to glycoprotein on the cuticle of the parasite, causing its death [28].

Table 1.
Anthelmintic effect of the total methanol extract and the ethyl acetate fraction of S. hypoleuca in earthworms.
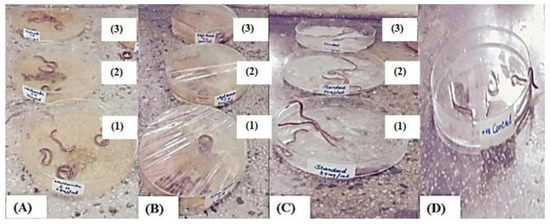
Figure 3.
Treatment of earthworms with different concentrations of total methanol extract (A) and ethyl acetate fraction (B) of S. hypoleuca, albendazole (C), and the control (D). where 1, 2 and 3 represent a concentration of 25 mg/mL, 75 mg/mL and 100 mg/mL, respectively.
3.6. Determination of the Antioxidant Activity
DPPH is a quick, simple, and well-known method for the in vitro determination of antioxidant potential that is characterized by its stability at room temperature. Moreover, it reacts with the entire sample, and sufficient time is permitted for all compounds that reveal a certain antioxidant potential to react with DPPH. The antioxidant activity in the examined sample is correlated to the discoloration of the DPPH solution. Isolated compounds that change the purple color of DPPH to yellow indicate antioxidant potential for the examined compounds [29]. The results showed that the compounds of oleic acid and luteolin exhibited potent antioxidant activity, evidenced by their IC50 values, which were equal to 47.0 and 11.98 µg/mL, respectively (IC50 for ascorbic acid was estimated with 12.60 µg/mL). It is worthy to highlight that luteolin was previously reported to possess significant antioxidant potential [30]. Luteolin is among the flavones that show remarkable therapeutic and antioxidant potential that is mainly attributed to structural features manifested by the presence of a catechol group, 3′,4′-dihydroxy moieties in the phenolic B ring, and the conjugation of a 2,3-double bond with a 1,4-pyrone moiety, in addition to 4-oxo function on luteolin’s pyranyl C ring that permits the delocalization of unpaired electrons between the A, B, and C rings, resulting in the production of a stabilized phenoxyl radical [31]. However, only a few reports have dealt with the antioxidant activity of the fatty acids due the nature of these compounds, which might show promising in vitro activity but very poor in vivo activity [32].
3.7. Computational Studies
3.7.1. Molecular Docking Studies
In silico molecular modelling studies were carried on compounds obtained from Saussurea hypoleuca ethyl acetate root extract, namely oleic acid (1) and luteolin (2), in addition to ascorbic acid, which acted as a standard antioxidant. This was performed to examine their inhibitory potentials on NADPH oxidase (Nox), as well as myeloperoxidase (MP), which are implicated in the production of reactive oxygen species that worsen oxidative stress, by Discovery Studio 4.5 (Accelrys Inc., San Diego, CA, USA) with a C-docker protocol. Oleic acid (1) and luteolin (2) showed significant activity in the inhibition of NADPH oxidase (Nox) and myeloperoxidase (MP), exceeding that of ascorbic acid, as illustrated in Table 2.

Table 2.
Binding energies (kcal/mol) of oleic acid (1) and luteolin (2) isolated from Saussurea hypoleuca root ethyl acetate fractions and ascorbic acid used as standard within the active sites of NADPH oxidase (Nox) and myeloperoxidase (MP).
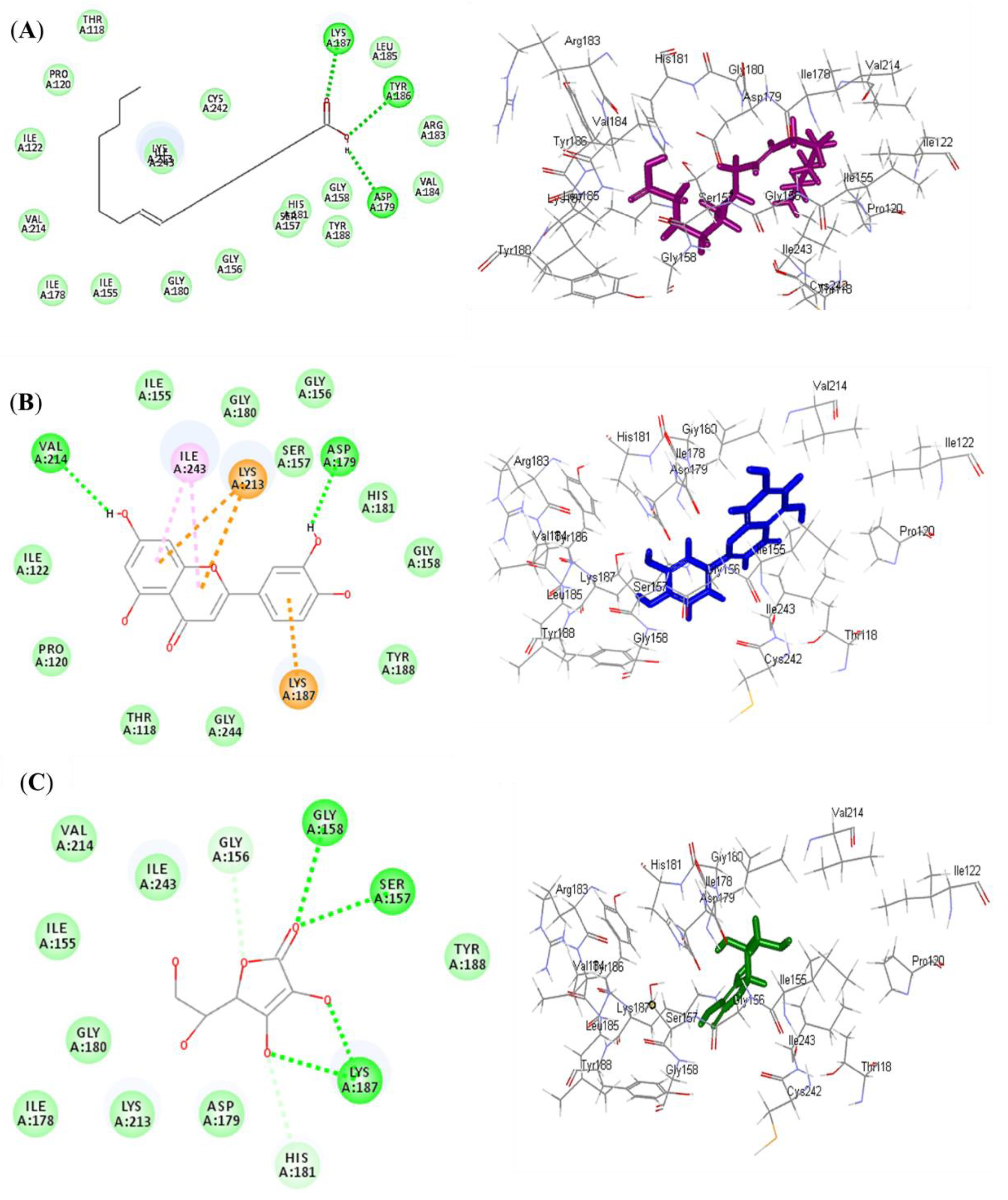
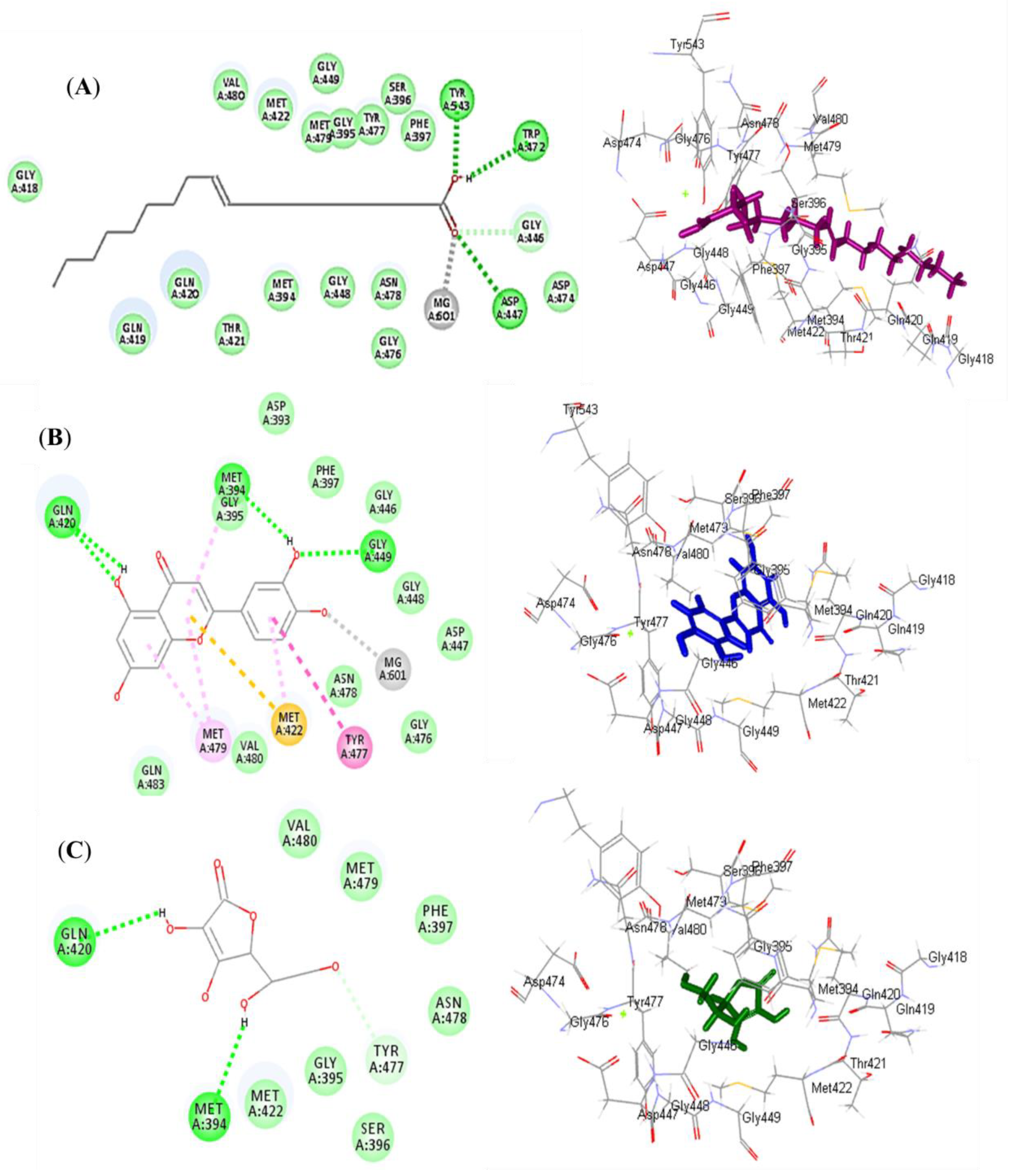
Luteolin (2) exerted the highest fitting within the binding sites of NADPH oxidase (Nox) followed by oleic acid (1), displaying ∆G of −37.63 and −35.06 kcal/mol, respectively. Meanwhile, ascorbic acid exhibited a ∆G of −11.06 kcal/mol. This was mainly attributed to the presence of many bonds with the amino acid groups existing at the binding sites. Oleic acid formed three conventional H-bonds with Asp179, Tyr186, and Lys187, in addition to many Van der Waals interactions (Figure 4A). Luteolin formed two H-bonds with Val214 and Asp179; two π-alkyl bonds with Ile243; three π-cation bonds with Lys213 and Lys187; and various Van der Waals interactions (Figure 4B). However, ascorbic acid formed four H-bonds with Gly158, Ser157, and Lys187; two C-H bonds with Gly156 and His181; and many Van der Waals interactions (Figure 4C). Regarding myeloperoxidase (MP), oleic acid revealed the best fitting in its active sites followed by luteolin, showing ∆G of −46.80 and −37.71 kcal/mol, respectively. Meanwhile, ascorbic acid exhibited a ∆G of −22.71 kcal/mol. Oleic acid formed three conventional H-bonds with Asp447, Trp472, and Tyr543; one C-H bond with Gly446; a metal acceptor bond with MG601; and many Van der Waals interactions (Figure 5A). Meanwhile, luteolin formed four H-bonds with Gln420, Met394, and Gly449; four π-alkyl bonds with Met479, Met422, and Met394; one π-π T-shaped bond with Tyr477; one π-sulphur bond with Met422; one metal acceptor bond with MG601; and multiple Van der Waals interactions (Figure 5B). However, ascorbic acid formed two H-bonds with Gln420 and Met394; one C-H bond with Tyr477; and many Van der Waals interactions (Figure 5C).
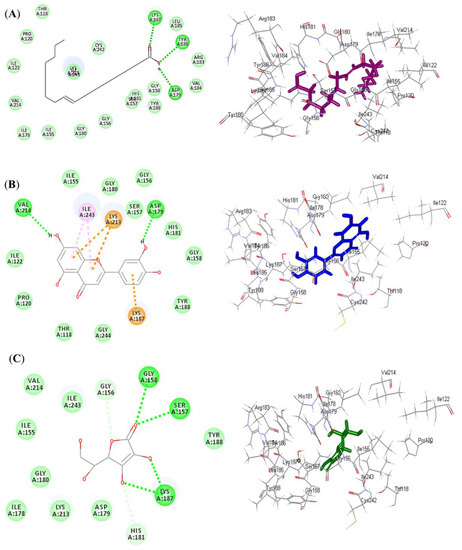
Figure 4.
2D and 3D binding modes of oleic acid (A) and luteolin (B) isolated from Saussurea hypoleuca root ethyl acetate fractions and ascorbic acid (C) used as standard in the binding site of NADPH oxidase (Nox). H-bonds: dotted green; C-H bonds: dotted light green; π-alkyl: dotted light purple; π-cation: dotted orange.
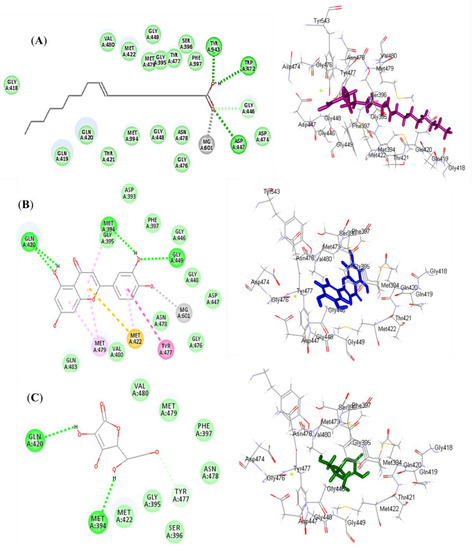
Figure 5.
2D and 3D binding mode of oleic acid (A) and luteolin (B) isolated from Saussurea hypoleuca root ethyl acetate fractions and ascorbic acid (C) used as standard in the binding site of myeloperoxidase (MP). H-bonds: dotted green; C-H bonds: dotted light green; π-alkyl: dotted light purple; π-π: dotted dark purple; π-sulphur: dotted light orange.
3.7.2. ADME and TOPKAT Prediction
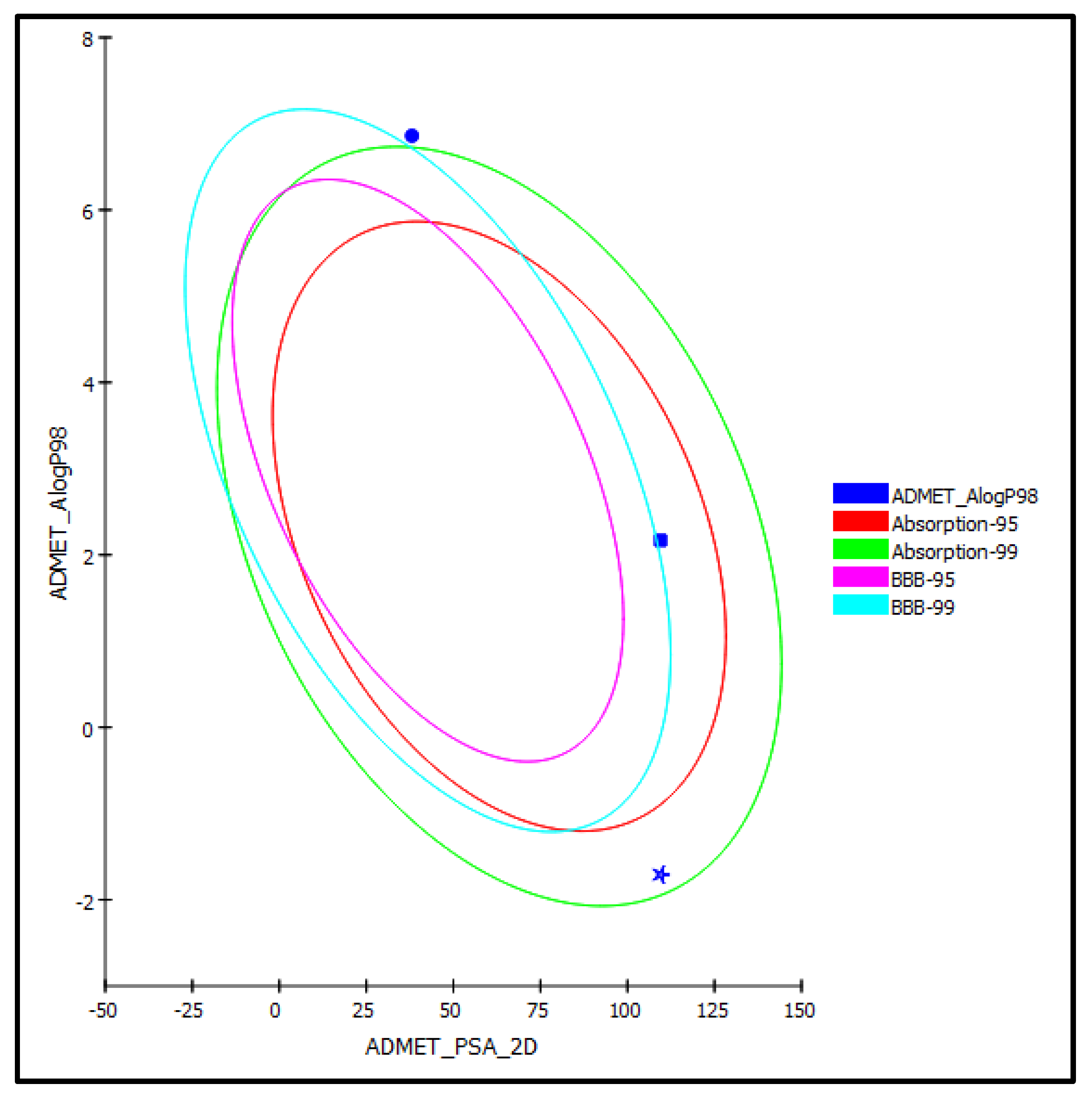
Oleic acid (1) and luteolin (2) identified from Saussurea hypoleuca ethyl acetate root extract, in addition to ascorbic acid that acted as a standard antioxidant, were exposed to ADME and TOPKAT predictions in order to explore their pharmacodynamic, pharmacokinetic, and toxicity criteria. Concerning ADME prediction, oleic acid (1) showed low human intestinal absorption, less than 90% plasma–protein binding, low solubility, and an undefined BBB penetration level and, hence, was located outside the 99% absorption ellipse, as revealed in the ADMET plot (Figure 6). Additionally, oleic acid showed no hepatotoxic effect with no inhibitory potential observed with respect to cytochrome P450 2D6 (Table 3). In contrast, luteolin (2) revealed more than 90% plasma–protein binding, in addition to good human intestinal absorption with good solubility and an undefined BBB penetration level, and hence, was located within the 95% absorption ellipse, but outside the 99% confidence limit ellipse corresponding to the blood–brain barrier (BBB), as revealed in the ADMET plot (Figure 6). However, it showed certain inhibitory potential towards cytochrome P450 2D6 with a certain hepatotoxic effect. Meanwhile, ascorbic acid showed moderate human intestinal absorption and was too soluble, showing an undefined BBB penetration level and, thus, lay within the 99% absorption ellipse, but outside the 99% confidence limit ellipse corresponding to the blood–brain barrier (BBB). Moreover, it showed no hepatotoxic effect with no inhibition to cytochrome P450 2D6. Additionally, ascorbic acid was actively absorbed by the sodium vitamin C co-transporter (SVCT) at the distal intestine, showing that the usual dietary doses (up to 100 mg/day) were almost completely absorbed. Thus, it had a high absorption rate, physiologically [33]. Regarding luteolin, its considerable absorption has previously been investigated physiologically in many studies [34,35]. Regarding TOPKAT determination, all of the examined compounds revealed no mutagenicity in the chemical Ames mutagenicity test and were non-carcinogenic for male versus female NTP rats, except for luteolin, which revealed certain carcinogenic effects against female NTP rats. Rat oral LD50 equaled 6.73, 0.77 and 1.75 g/kg bw for oleic acid, luteolin, and ascorbic acid, respectively, with rat inhalational LD50 equaling 9501.28, 1387.9 and 1919.89 mg/m3/h, respectively. Chronic rat LOAEL was found to be equal to 0.35, 0.11 and 0.02 for g/kg bw for oleic acid, luteolin, and ascorbic acid, respectively. Both luteolin and ascorbic acid showed no skin irritancy, whereas oleic acid revealed moderate dermal irritation but no ocular irritation. In contrast, luteolin and ascorbic acid exerted mild and moderate eye irritation, respectively. From results illustrated by the ADME and TOPKAT evaluations, it was clearly obvious that all the tested compounds revealed acceptable pharmacokinetic, pharmacodynamic, and toxicity properties. However, luteolin showed better pharmacokinetic and pharmacodynamic characteristics, while oleic acid showed a better safety profile. Thus, they can be incorporated in pharmaceutical preparations to combat oxidative stress.
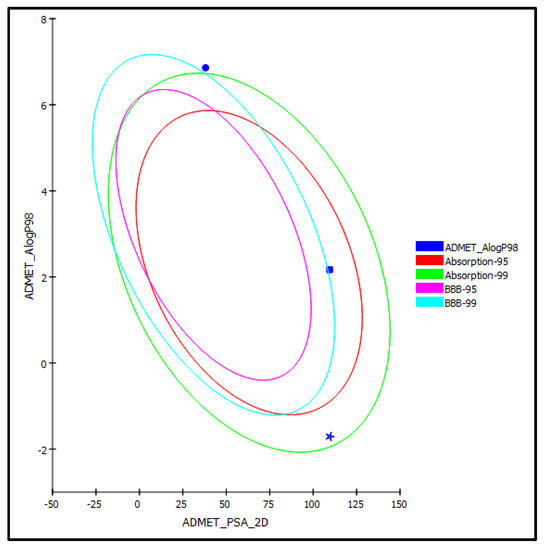
Figure 6.
ADMET Plot for oleic acid (1) (circle) and luteolin (2) (square) isolated from Saussurea hypoleuca root ethyl acetate fractions and ascorbic acid (star) as standard showing 95% and 99% confidence limit ellipse corresponding to the blood–brain barrier (BBB) and the human intestinal absorption models in ADMET_AlogP98.

Table 3.
ADME and TOPKAT (absorption, distribution, metabolism, excretion, and toxicity) predictions of oleic acid (1) and luteolin (2) isolated from Saussurea hypoleuca root ethyl acetate fractions and ascorbic acid used as standard.
4. Conclusions
Herein, isolation of oleic acid and luteolin was achieved for the first time from Saussurea hypoleuca root and they were characterized and assessed for their antioxidant potential. Saussurea hypoleuca root showed promising anthelmintic activity approaching that of albendazole. Its contents of heavy metals are within the certified limits according to the WHO guidelines. Both isolated compounds exhibited higher in vitro antioxidant potential compared to ascorbic acid. Furthermore, molecular docking studies showed that both oleic acid and luteolin revealed significant activity in the inhibition of NADPH oxidase (Nox), in addition to myeloperoxidase (MP), exceeding that of ascorbic acid, and thus, confirmed the results of the in vitro experiment. This suggested that Saussurea hypoleuca root can serve as a reservoir for lead compounds that can help in the discovery of therapeutically effective and safe drugs. ADME and TOPKAT evaluations revealed acceptable pharmacokinetic, pharmacodynamic, and toxicity properties for both luteolin and oleic. Thus, they can be incorporated in pharmaceutical preparations to combat oxidative stress and worms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations9060138/s1, Figure S1: 1H-NMR and 13C-NMR spectral data of compound (1); Figure S2: 1H-NMR and 13C-NMR spectral data of compound (2).
Author Contributions
Designed the experiments, N.A., S.I. and A.A; performed the experiments and purified the compounds, N.A., S.I. and A.A.; interpreted the NMR data, N.A., S.I., R.B., A.A. and M.L.A.; molecular docking experiment, F.S.Y. and M.L.A.; resources, H.A.B., R.F.A.A. and S.S.E.; funding acquisition, S.S.E., H.A.B. and R.F.A.A.; wrote and revised the manuscript, N.A., S.I., A.A., M.L.A., R.B. and F.S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia, funded this project, under grant No. G-1492-166-1440. The authors, therefore, acknowledge with thanks the DSR for technical and financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this manuscript.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia, under grant No. G-1492-166-1440. The authors, therefore, acknowledge with thanks the DSR for technical and financial support.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Fathy, S.; Emam, M.; Agwa, S.A.; Zahra, F.A.; Youssef, F.; Sami, R. The antiproliferative effect of Origanum majorana on human hepatocarcinoma cell line: Suppression of NF-kB. Cell. Mol. Biol. 2016, 62, 80–84. [Google Scholar] [PubMed]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obs. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, S.-J.; Zhang, Z.-X.; Zhang, M.-L.; Shi, Q.-W.; Gu, Y.-C.; Dong, M.; Kiyota, H. Chemical constituents from the genus Saussurea and their biological activities. Heterocycl. Commun. 2017, 23, 331–358. [Google Scholar] [CrossRef]
- Pandey, M.M.; Rastogi, S.; Rawat, A.K.S. Saussurea costus: Botanical, chemical and pharmacological review of an ayurvedic medicinal plant. J. Eethnopharmacol. 2007, 110, 379–390. [Google Scholar] [CrossRef]
- Ghimire, B.; Jeong, M.J.; Lee, K.M.; Heo, K.; Lee, C.H.; Suh, G.U. Achene morphology of Saussurea species (Asteraceae, Cardueae) in Korea and its systematic implications. Bot. J. Linnean Soci. 2016, 181, 692–710. [Google Scholar] [CrossRef][Green Version]
- Singh, G.; Rai, I.D.; Rawat, G.S.; Goraya, G.S.; Jalal, J.S. Additions to the flora of Great Himalayan National Park, Western Himalaya. Ind. J. Forest. 2015, 38, 375–381. [Google Scholar] [CrossRef]
- Arshad, N.; Ishtiaq, S.; Khan, F.Z. HPLC, GC-MS Analysis, hepatoprotective and antioxidant activities of Saussurea hypoleuca spreng. root. Egypt. J. Chem. 2021, 64, 4343–4349. [Google Scholar] [CrossRef]
- Arshad, N.; Ishtiaq, S. Proximate analysis and in vitro biological assays of Saussurea hypoleuca spreng. Root. Pak. J. Pharm. Sci. 2019, 32, 1235–1243. [Google Scholar]
- Arshad, N.; Ishtiaq, S.; Khan, F.Z.; Danish, Z.; Rashid, A.J.; Ijaz, B.; Tariq, S. GC-MS analysis, anticancer and anti-inflammatory activities of Saussurea hypoleuca spreng. Root. Pak. J. Pharm. Sci. 2021, 34, 291–300. [Google Scholar]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Rai, V.; Kakkar, P.; Khatoon, S.; Rawat, A.; Mehrotra, S. Heavy metal accumulation in some herbal drugs. Pharm. Biol. 2001, 39, 384–387. [Google Scholar] [CrossRef]
- Pai, S.C.; Joshi, M.; Mohan, S.R.; Deshpande, U.; Dhami, T.; Khatei, J.; Rao, K.K.; Sanjeev, G. Electron irradiation effects on TGA-capped CdTe quantum dots. J. Phys. D Appl.Phys. 2013, 46, 175304. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Salama, M.M.; ElMeshad, A.N.; Teaima, M.H.; Rashad, L.A. HPLC–DAD–MS/MS profiling of standardized rosemary extract and enhancement of its anti-wrinkle activity by encapsulation in elastic nanovesicles. Arch. Pharm. Res. 2016, 39, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Ajaiyeoba, E.; Onocha, P.; Olarenwaju, O. In vitro anthelmintic properties of Buchholzia coriaceae and Gynandropsis gynandra extracts. Pharm. Biol. 2001, 39, 217–220. [Google Scholar] [CrossRef]
- Veeru, P.; Kishor, M.P.; Meenakshi, M. Screening of medicinal plant extracts for antioxidant activity. J. Med. Plants Res. 2009, 3, 608–612. [Google Scholar]
- Youssef, F.S.; Ashour, M.L.; El-Beshbishy, H.A.; Singab, A.N.B.; Wink, M. Metabolic profiling of Buddleia indica leaves using LC/MS and evidence of their antioxidant and hepatoprotective activity using different in vitro and in vivo experimental models. Antioxidants 2019, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Janibekov, A.A.; Youssef, F.S.; Ashour, M.L.; Mamadalieva, N.Z. New flavonoid glycosides from two Astragalus species (Fabaceae) and validation of their antihyperglycaemic activity using molecular modelling and in vitro studies. Ind. Crops Prod. 2018, 118, 142–148. [Google Scholar] [CrossRef]
- Altyar, A.E.; Ashour, M.L.; Youssef, F.S. Premna odorata: Seasonal metabolic variation in the essential oil composition of its leaf and verification of its anti-ageing potential via in vitro assays and molecular modelling. Biomolecules 2020, 10, 879. [Google Scholar] [CrossRef]
- Youssef, F.S.; Ovidi, E.; Musayeib, N.M.A.; Ashour, M.L. Morphology, anatomy and secondary metabolites investigations of Premna odorata Blanco and evaluation of its anti-Tuberculosis activity using in vitro and in silico studies. Plants 2021, 10, 1953. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Youssef, F.S.; Hussain, H.; Zengin, G.; Mollica, A.; Al Musayeib, N.M.; Ashour, M.L.; Westermann, B.; Wessjohann, L.A. Validation of the antioxidant and enzyme inhibitory potential of selected triterpenes using in vitro and in silico studies, and the evaluation of their ADMET properties. Molecules 2021, 26, 6331. [Google Scholar] [CrossRef]
- Jian-Jan, L.; Xi-Kui, L. Chemical constituents from Yannanopilia longistaminata. Nat. Prod. Res. 2008, 20, 8–13. [Google Scholar]
- Lin, L.-C.; Pai, Y.-F.; Tsai, T.-H. Isolation of luteolin and luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and their pharmacokinetics in rats. J. Agric. Food Chem. 2015, 63, 7700–7706. [Google Scholar] [CrossRef] [PubMed]
- Nibret, E.; Wink, M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Phytomedicine 2010, 17, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-C.; Chou, C.-J. Chemical constituents from Pterocypsela formosana. Chin. Pharm. J. 2002, 54, 181–185. [Google Scholar]
- World Health Organization. WHO Guidelines on Good Manufacturing Practices (GMP) for Herbal Medicines; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Lee, W.-H.; Loo, C.-Y.; Nomura, C.T.; Sudesh, K. Biosynthesis of polyhydroxyalkanoate copolymers from mixtures of plant oils and 3-hydroxyvalerate precursors. Bioresour. Technol. 2008, 99, 6844–6851. [Google Scholar] [CrossRef]
- Crompton, D.W.; Nesheim, M.C. Nutritional impact of intestinal helminthiasis during the human life cycle. Ann. Rev. Nutr. 2002, 22, 35–59. [Google Scholar] [CrossRef]
- Spiegler, V.; Liebau, E.; Hensel, A. Medicinal plant extracts and plant-derived polyphenols with anthelmintic activity against intestinal nematodes. Nat. Prod. Rep. 2017, 34, 627–643. [Google Scholar] [CrossRef]
- Jayaprakasha, G.; Negi, P.; Jena, B.; Rao, L.J.M. Antioxidant and antimutagenic activities of Cinnamomum zeylanicum fruit extracts. J. Food Comp. Anal. 2007, 20, 330–336. [Google Scholar] [CrossRef]
- Lee, Y.; Howard, L.; Villalon, B. Flavonoids and antioxidant activity of fresh pepper (Capsicum annuum) cultivars. J. Food Sci. 1995, 60, 473–476. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-antioxidant activity relationships of luteolin and catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef]
- Blasi, F.; Cossignani, L. An Overview of Natural Extracts with Antioxidant Activity for the Improvement of the Oxidative Stability and Shelf Life of Edible Oils. Processes 2020, 8, 956. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Matarese, L.E.; Mullin, G.E. Vitamins and minerals. Yamada’s Textb. Gastroenterol. 2022, 426–456. [Google Scholar] [CrossRef]
- Shimoi, K.; Okada, H.; Furugori, M.; Goda, T.; Takase, S.; Suzuki, M.; Hara, Y.; Yamamoto, H.; Kinae, N. Intestinal absorption of luteolin and luteolin 7-O-β-glucoside in rats and humans. FEBS Lett. 1998, 438, 220–224. [Google Scholar] [CrossRef]
- Kure, A.; Nakagawa, K.; Kondo, M.; Kato, S.; Kimura, F.; Watanabe, A.; Shoji, N.; Hatanaka, S.; Tsushida, T.; Miyazawa, T. Metabolic fate of luteolin in rats: Its relationship to anti-inflammatory effect. J. Agric. Food Chem. 2016, 64, 4246–4254. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).