Phytochemical Characterization and Heavy Metal and Thermal Analyses of Saussurea hypoleuca Root and Evaluation of Its Anthelmintic and Antioxidant Activity In Vitro and In Silico
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Extraction and Isolation
2.4. Compound Characterization
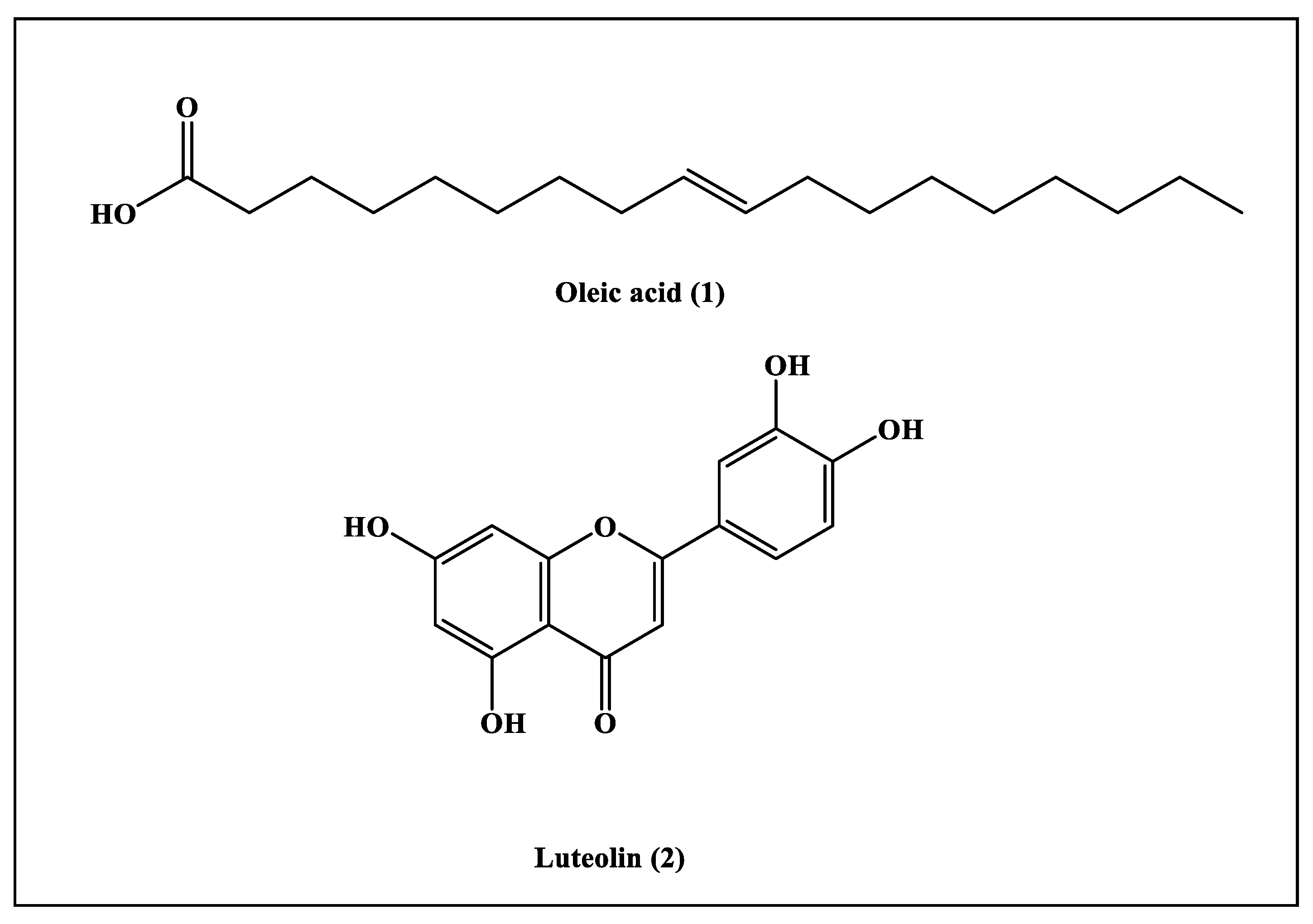
2.4.1. Characterization of Compound (1)
2.4.2. Characterization of Compound (2)
2.5. Heavy Metal Analysis
2.6. Thermal Analysis
2.7. HPLC Standardization of Saussurea Hypoleuca Root
2.7.1. Sample Preparation
2.7.2. Reversed-Phase HPLC Analysis
2.7.3. Standard Curve Construction
2.8. Determination of the Anthelmintic Activity
2.9. Determination of the Antioxidant Activity
2.10. Computational Studies
2.10.1. Molecular Docking Studies
2.10.2. ADME and TOPKAT Prediction
3. Results and Discussion
3.1. Phytochemical Characterization
3.2. Heavy Metal Analysis
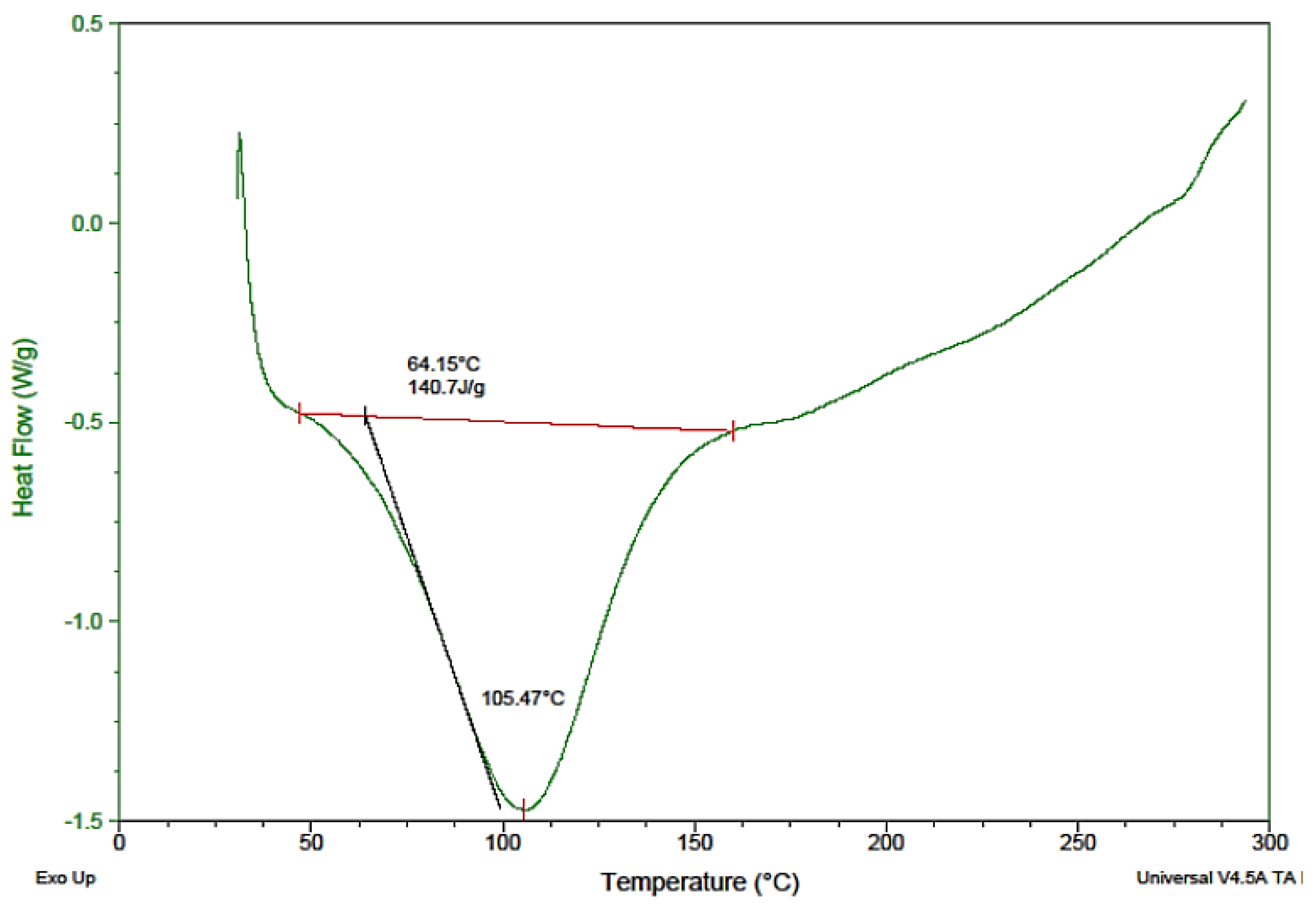
3.3. Thermal Analysis
3.4. HPLC Standardization of Saussurea Hypoleuca Root
3.5. Determination of the Anthelmintic Activity
3.6. Determination of the Antioxidant Activity
3.7. Computational Studies
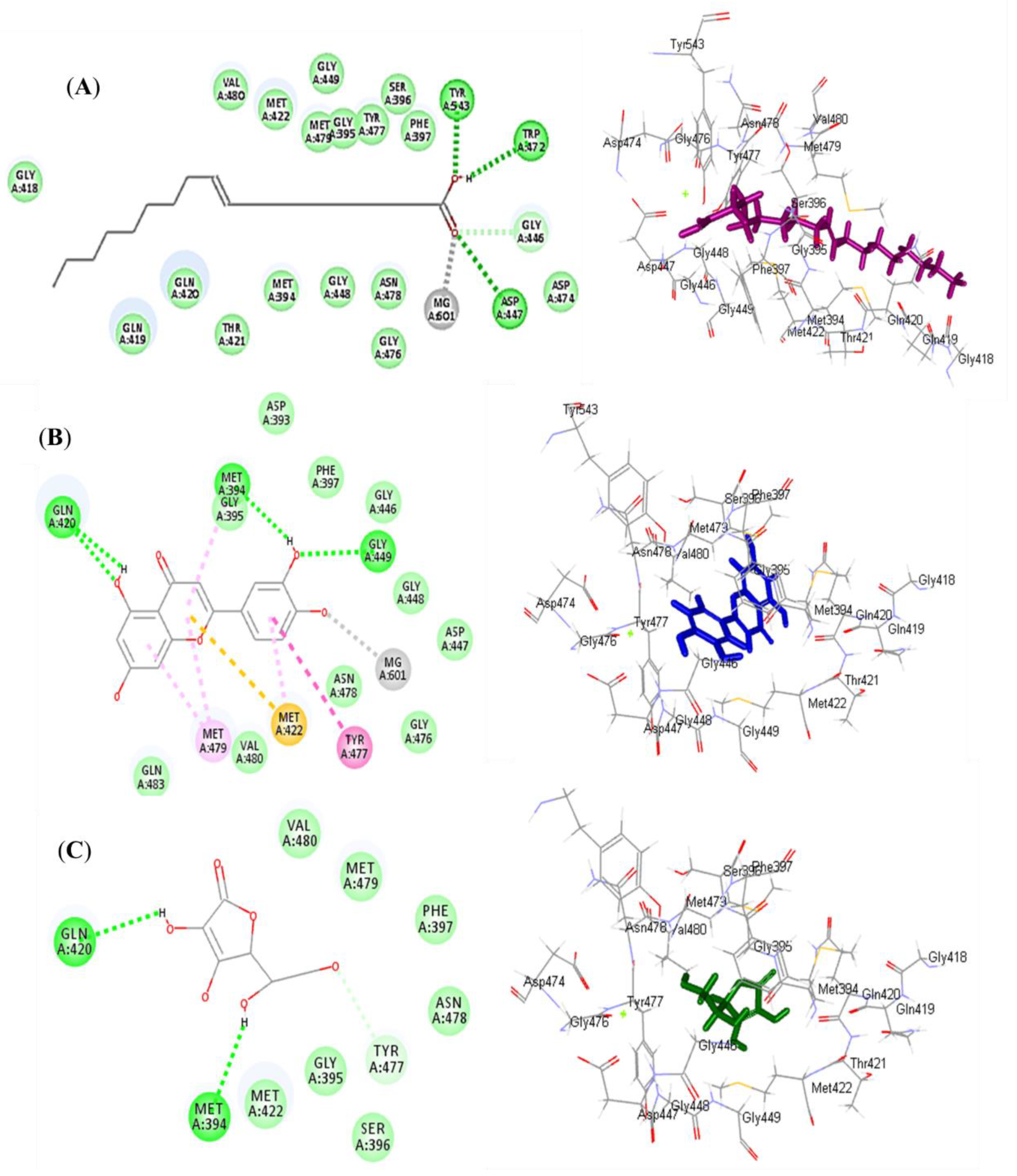
3.7.1. Molecular Docking Studies
3.7.2. ADME and TOPKAT Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fathy, S.; Emam, M.; Agwa, S.A.; Zahra, F.A.; Youssef, F.; Sami, R. The antiproliferative effect of Origanum majorana on human hepatocarcinoma cell line: Suppression of NF-kB. Cell. Mol. Biol. 2016, 62, 80–84. [Google Scholar] [PubMed]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obs. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, S.-J.; Zhang, Z.-X.; Zhang, M.-L.; Shi, Q.-W.; Gu, Y.-C.; Dong, M.; Kiyota, H. Chemical constituents from the genus Saussurea and their biological activities. Heterocycl. Commun. 2017, 23, 331–358. [Google Scholar] [CrossRef]
- Pandey, M.M.; Rastogi, S.; Rawat, A.K.S. Saussurea costus: Botanical, chemical and pharmacological review of an ayurvedic medicinal plant. J. Eethnopharmacol. 2007, 110, 379–390. [Google Scholar] [CrossRef]
- Ghimire, B.; Jeong, M.J.; Lee, K.M.; Heo, K.; Lee, C.H.; Suh, G.U. Achene morphology of Saussurea species (Asteraceae, Cardueae) in Korea and its systematic implications. Bot. J. Linnean Soci. 2016, 181, 692–710. [Google Scholar] [CrossRef][Green Version]
- Singh, G.; Rai, I.D.; Rawat, G.S.; Goraya, G.S.; Jalal, J.S. Additions to the flora of Great Himalayan National Park, Western Himalaya. Ind. J. Forest. 2015, 38, 375–381. [Google Scholar] [CrossRef]
- Arshad, N.; Ishtiaq, S.; Khan, F.Z. HPLC, GC-MS Analysis, hepatoprotective and antioxidant activities of Saussurea hypoleuca spreng. root. Egypt. J. Chem. 2021, 64, 4343–4349. [Google Scholar] [CrossRef]
- Arshad, N.; Ishtiaq, S. Proximate analysis and in vitro biological assays of Saussurea hypoleuca spreng. Root. Pak. J. Pharm. Sci. 2019, 32, 1235–1243. [Google Scholar]
- Arshad, N.; Ishtiaq, S.; Khan, F.Z.; Danish, Z.; Rashid, A.J.; Ijaz, B.; Tariq, S. GC-MS analysis, anticancer and anti-inflammatory activities of Saussurea hypoleuca spreng. Root. Pak. J. Pharm. Sci. 2021, 34, 291–300. [Google Scholar]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Rai, V.; Kakkar, P.; Khatoon, S.; Rawat, A.; Mehrotra, S. Heavy metal accumulation in some herbal drugs. Pharm. Biol. 2001, 39, 384–387. [Google Scholar] [CrossRef]
- Pai, S.C.; Joshi, M.; Mohan, S.R.; Deshpande, U.; Dhami, T.; Khatei, J.; Rao, K.K.; Sanjeev, G. Electron irradiation effects on TGA-capped CdTe quantum dots. J. Phys. D Appl.Phys. 2013, 46, 175304. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Salama, M.M.; ElMeshad, A.N.; Teaima, M.H.; Rashad, L.A. HPLC–DAD–MS/MS profiling of standardized rosemary extract and enhancement of its anti-wrinkle activity by encapsulation in elastic nanovesicles. Arch. Pharm. Res. 2016, 39, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Ajaiyeoba, E.; Onocha, P.; Olarenwaju, O. In vitro anthelmintic properties of Buchholzia coriaceae and Gynandropsis gynandra extracts. Pharm. Biol. 2001, 39, 217–220. [Google Scholar] [CrossRef]
- Veeru, P.; Kishor, M.P.; Meenakshi, M. Screening of medicinal plant extracts for antioxidant activity. J. Med. Plants Res. 2009, 3, 608–612. [Google Scholar]
- Youssef, F.S.; Ashour, M.L.; El-Beshbishy, H.A.; Singab, A.N.B.; Wink, M. Metabolic profiling of Buddleia indica leaves using LC/MS and evidence of their antioxidant and hepatoprotective activity using different in vitro and in vivo experimental models. Antioxidants 2019, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Janibekov, A.A.; Youssef, F.S.; Ashour, M.L.; Mamadalieva, N.Z. New flavonoid glycosides from two Astragalus species (Fabaceae) and validation of their antihyperglycaemic activity using molecular modelling and in vitro studies. Ind. Crops Prod. 2018, 118, 142–148. [Google Scholar] [CrossRef]
- Altyar, A.E.; Ashour, M.L.; Youssef, F.S. Premna odorata: Seasonal metabolic variation in the essential oil composition of its leaf and verification of its anti-ageing potential via in vitro assays and molecular modelling. Biomolecules 2020, 10, 879. [Google Scholar] [CrossRef]
- Youssef, F.S.; Ovidi, E.; Musayeib, N.M.A.; Ashour, M.L. Morphology, anatomy and secondary metabolites investigations of Premna odorata Blanco and evaluation of its anti-Tuberculosis activity using in vitro and in silico studies. Plants 2021, 10, 1953. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Youssef, F.S.; Hussain, H.; Zengin, G.; Mollica, A.; Al Musayeib, N.M.; Ashour, M.L.; Westermann, B.; Wessjohann, L.A. Validation of the antioxidant and enzyme inhibitory potential of selected triterpenes using in vitro and in silico studies, and the evaluation of their ADMET properties. Molecules 2021, 26, 6331. [Google Scholar] [CrossRef]
- Jian-Jan, L.; Xi-Kui, L. Chemical constituents from Yannanopilia longistaminata. Nat. Prod. Res. 2008, 20, 8–13. [Google Scholar]
- Lin, L.-C.; Pai, Y.-F.; Tsai, T.-H. Isolation of luteolin and luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and their pharmacokinetics in rats. J. Agric. Food Chem. 2015, 63, 7700–7706. [Google Scholar] [CrossRef] [PubMed]
- Nibret, E.; Wink, M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Phytomedicine 2010, 17, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-C.; Chou, C.-J. Chemical constituents from Pterocypsela formosana. Chin. Pharm. J. 2002, 54, 181–185. [Google Scholar]
- World Health Organization. WHO Guidelines on Good Manufacturing Practices (GMP) for Herbal Medicines; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Lee, W.-H.; Loo, C.-Y.; Nomura, C.T.; Sudesh, K. Biosynthesis of polyhydroxyalkanoate copolymers from mixtures of plant oils and 3-hydroxyvalerate precursors. Bioresour. Technol. 2008, 99, 6844–6851. [Google Scholar] [CrossRef]
- Crompton, D.W.; Nesheim, M.C. Nutritional impact of intestinal helminthiasis during the human life cycle. Ann. Rev. Nutr. 2002, 22, 35–59. [Google Scholar] [CrossRef]
- Spiegler, V.; Liebau, E.; Hensel, A. Medicinal plant extracts and plant-derived polyphenols with anthelmintic activity against intestinal nematodes. Nat. Prod. Rep. 2017, 34, 627–643. [Google Scholar] [CrossRef]
- Jayaprakasha, G.; Negi, P.; Jena, B.; Rao, L.J.M. Antioxidant and antimutagenic activities of Cinnamomum zeylanicum fruit extracts. J. Food Comp. Anal. 2007, 20, 330–336. [Google Scholar] [CrossRef]
- Lee, Y.; Howard, L.; Villalon, B. Flavonoids and antioxidant activity of fresh pepper (Capsicum annuum) cultivars. J. Food Sci. 1995, 60, 473–476. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-antioxidant activity relationships of luteolin and catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef]
- Blasi, F.; Cossignani, L. An Overview of Natural Extracts with Antioxidant Activity for the Improvement of the Oxidative Stability and Shelf Life of Edible Oils. Processes 2020, 8, 956. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Matarese, L.E.; Mullin, G.E. Vitamins and minerals. Yamada’s Textb. Gastroenterol. 2022, 426–456. [Google Scholar] [CrossRef]
- Shimoi, K.; Okada, H.; Furugori, M.; Goda, T.; Takase, S.; Suzuki, M.; Hara, Y.; Yamamoto, H.; Kinae, N. Intestinal absorption of luteolin and luteolin 7-O-β-glucoside in rats and humans. FEBS Lett. 1998, 438, 220–224. [Google Scholar] [CrossRef]
- Kure, A.; Nakagawa, K.; Kondo, M.; Kato, S.; Kimura, F.; Watanabe, A.; Shoji, N.; Hatanaka, S.; Tsushida, T.; Miyazawa, T. Metabolic fate of luteolin in rats: Its relationship to anti-inflammatory effect. J. Agric. Food Chem. 2016, 64, 4246–4254. [Google Scholar] [CrossRef] [PubMed]
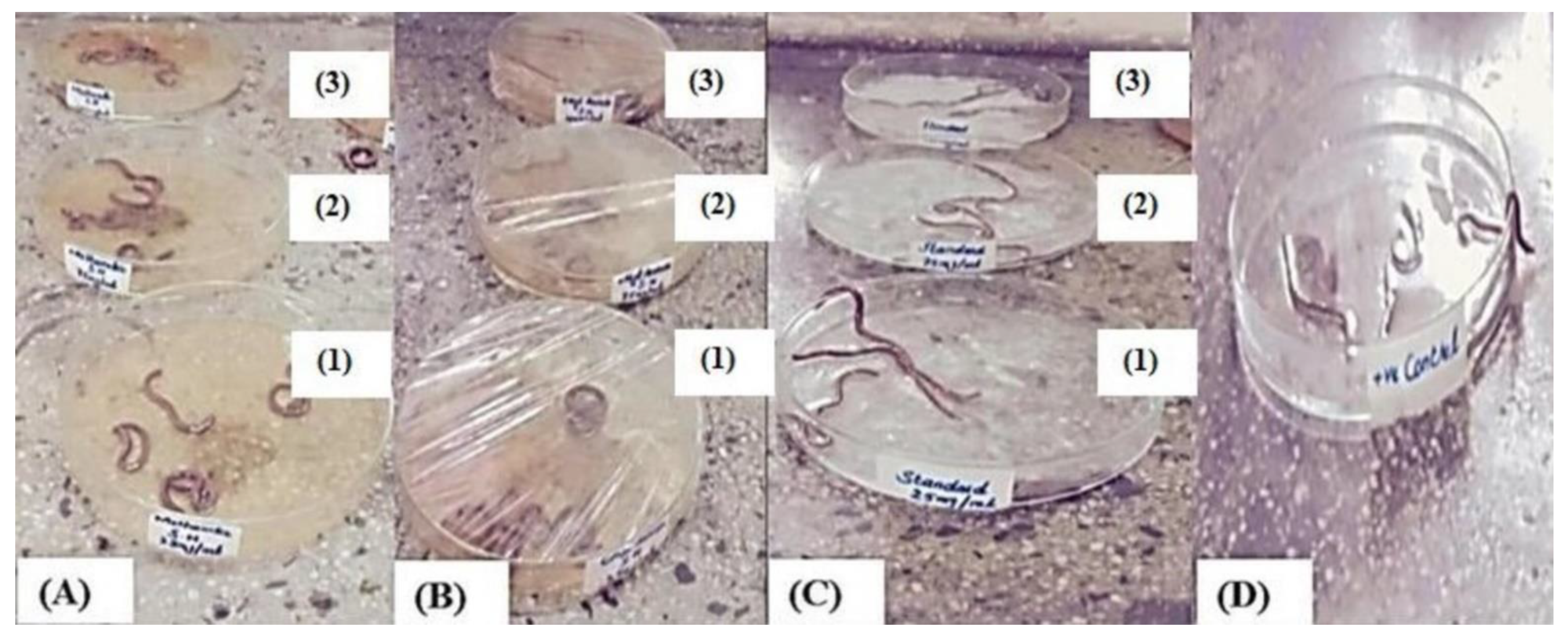


| Treatment Groups | Concentrations (mg/mL) | Paralysis Time (min) | Death Time (min) |
|---|---|---|---|
| Total methanol extract | 25 | 66.4 ± 2.41 | 82.7 ± 1.92 |
| 75 | 36.6 ± 1.14 | 54.4 ± 2.41 | |
| 100 | 24.0 ± 1.58 *** | 43.1 ± 1.52 *** | |
| Ethyl acetate fraction | 25 | 85.3 ± 1.20 | 104.4 ± 2.07 |
| 75 | 53.0 ±1.58 | 65.4 ± 2.88 * | |
| 100 | 42.2 ± 1.92 * | 62.6 ± 2.07 * | |
| Albendazole | 25 | 108.4 ± 3.05 | 132.9 ± 1.24 |
| 75 | 65.6 ± 2.07 | 87.4 ± 1.14 * | |
| 100 | 53.0 ± 1.58 * | 74.3 ± 2.64 ** | |
| Control | - | - | - |
| Compounds | NADPH Oxidase (Nox) | Number of Formed Hydrogen Bonds with the Amino Acid Residues | Myeloperoxidase (MP) | Number of Formed Hydrogen Bonds with the Amino Acid Residues |
|---|---|---|---|---|
| Oleic acid (1) | −35.06 | 3; Asp179, Tyr186, Lys187 | −46.80 | 3; Asp447, Trp472, Tyr543 |
| Luteolin (2) | −37.63 | 2; Val214, Asp179 | −37.71 | 4; Gln420, Met394, Gly449 |
| Ascorbic acid | −11.06 | 4;Gly158, Ser157, Lys187 | −22.71 | 2; Gln420, Met394 |
| Compounds | Oleic Acid (1) | Luteolin (2) | Ascorbic Acid |
|---|---|---|---|
| ADMET parameters | |||
| Absorption Level | 2 | 0 | 1 |
| Solubility Level | 2 | 3 | 5 |
| BBB Level | 4 | 4 | 4 |
| PPB Level | True | False | False |
| CPY2D6 | NI | I | NI |
| Hepatotoxic | Non-toxic | Toxic | Non-toxic |
| PSA-2D | 38.116 | 109.492 | 109.492 |
| Alog p98 | 6.86 | 2.168 | −1.709 |
| TOPKAT parameters | |||
| Ames prediction | Non-mutagen | Non-mutagen | Non-mutagen |
| Rat chronic LOAEL(g/kg·bw) | 0.35 | 0.11 | 0.02 |
| Rat oral LD50 (g/kg·bw) | 6.73 | 0.77 | 1.75 |
| Rat inhalational LD50 (mg/m3/h) | 9501.28 | 1387.9 | 1919.89 |
| Rat female NPT | Non-carcinogen | Non-carcinogen | Non-carcinogen |
| Rat male NPT | Non-carcinogen | Carcinogen | Non-carcinogen |
| Skin irritancy | Moderate | None | None |
| Ocular irritancy | None | Mild | Moderate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhady, S.S.; Arshad, N.; Ishtiaq, S.; Bayram, R.; Amin, A.; Bogari, H.A.; Abdelhameed, R.F.A.; Youssef, F.S.; Ashour, M.L. Phytochemical Characterization and Heavy Metal and Thermal Analyses of Saussurea hypoleuca Root and Evaluation of Its Anthelmintic and Antioxidant Activity In Vitro and In Silico. Separations 2022, 9, 138. https://doi.org/10.3390/separations9060138
Elhady SS, Arshad N, Ishtiaq S, Bayram R, Amin A, Bogari HA, Abdelhameed RFA, Youssef FS, Ashour ML. Phytochemical Characterization and Heavy Metal and Thermal Analyses of Saussurea hypoleuca Root and Evaluation of Its Anthelmintic and Antioxidant Activity In Vitro and In Silico. Separations. 2022; 9(6):138. https://doi.org/10.3390/separations9060138
Chicago/Turabian StyleElhady, Sameh S., Numera Arshad, Saiqa Ishtiaq, Roula Bayram, Adnan Amin, Hanin A. Bogari, Reda F. A. Abdelhameed, Fadia S. Youssef, and Mohamed L. Ashour. 2022. "Phytochemical Characterization and Heavy Metal and Thermal Analyses of Saussurea hypoleuca Root and Evaluation of Its Anthelmintic and Antioxidant Activity In Vitro and In Silico" Separations 9, no. 6: 138. https://doi.org/10.3390/separations9060138
APA StyleElhady, S. S., Arshad, N., Ishtiaq, S., Bayram, R., Amin, A., Bogari, H. A., Abdelhameed, R. F. A., Youssef, F. S., & Ashour, M. L. (2022). Phytochemical Characterization and Heavy Metal and Thermal Analyses of Saussurea hypoleuca Root and Evaluation of Its Anthelmintic and Antioxidant Activity In Vitro and In Silico. Separations, 9(6), 138. https://doi.org/10.3390/separations9060138









