Optimization of Steam Distillation Process and Chemical Constituents of Volatile Oil from Angelicaesinensis Radix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Reagents, and Instruments
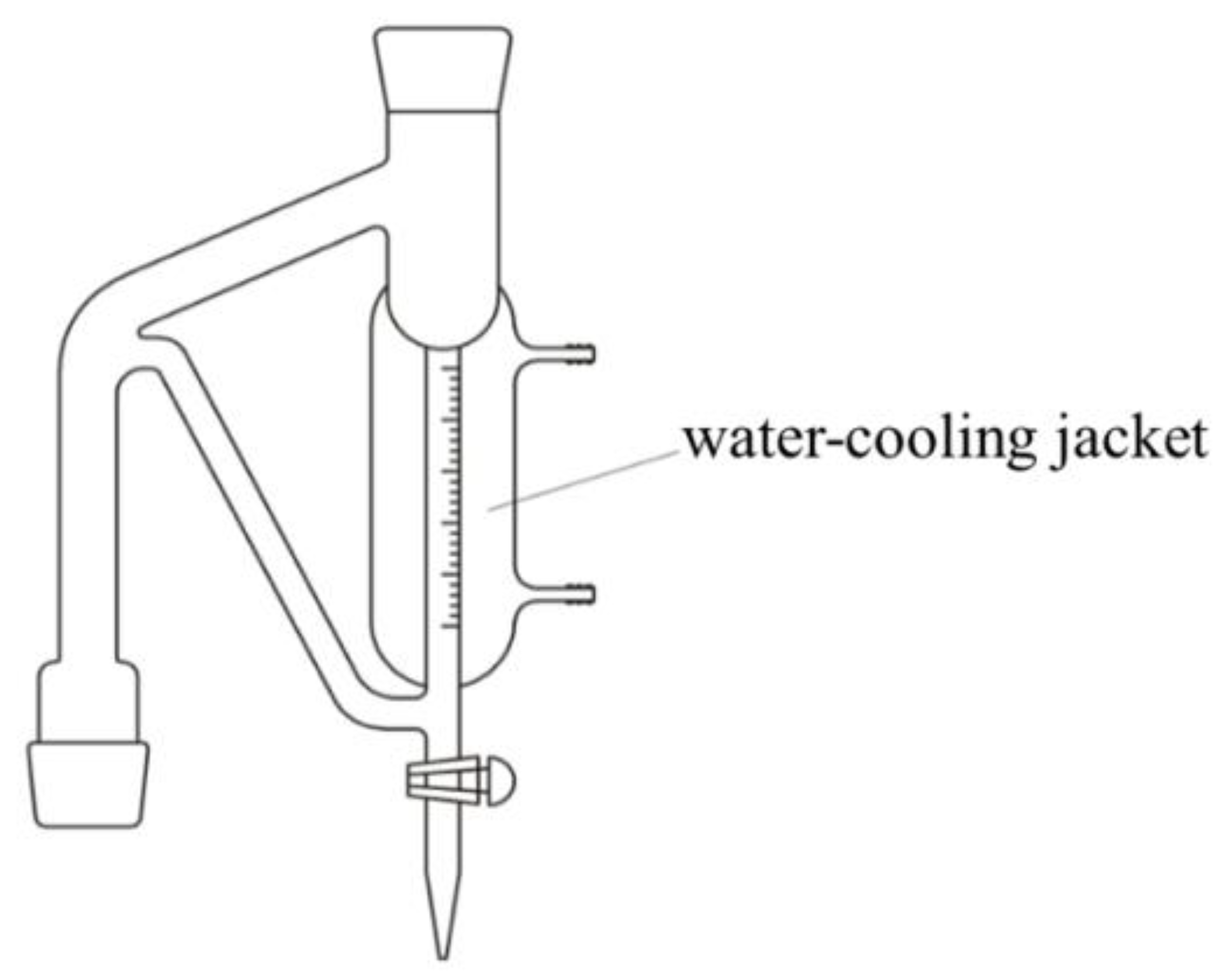
2.2. Distillation Method
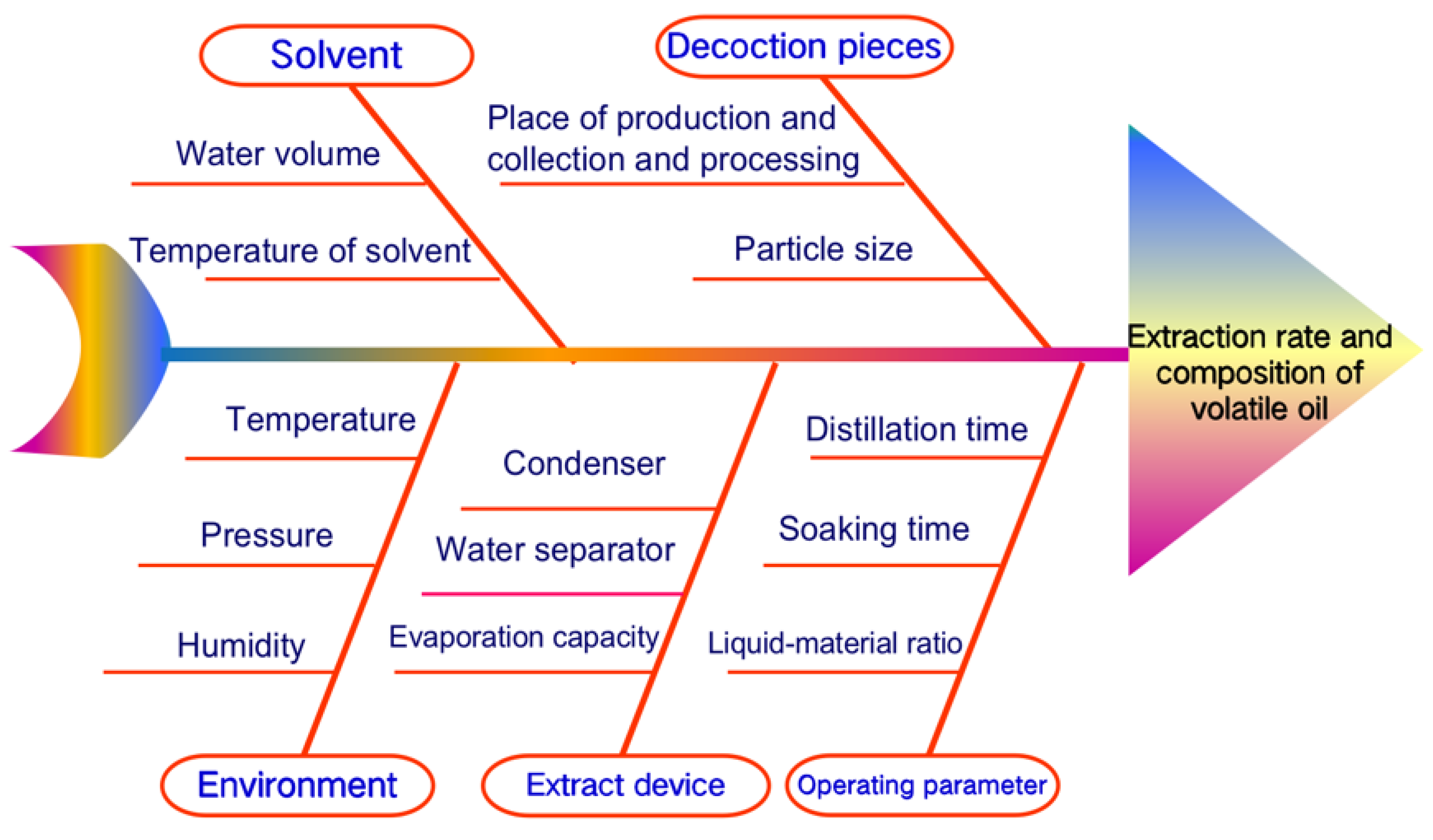
2.3. Optimization of Distillation Process Parameters
2.4. Analysis of Chemical Constituents of Volatile Oil from Angelicae sinensis Radix
2.5. Data Processing Method
2.5.1. Kinetic Models
2.5.2. Data Processing of Volatile Oil Distillation Rate Obtained from Experimental Design
2.5.3. Optimization of Distillation Parameters of Volatile Oil by the Monte Carlo Method
3. Results and Discussion
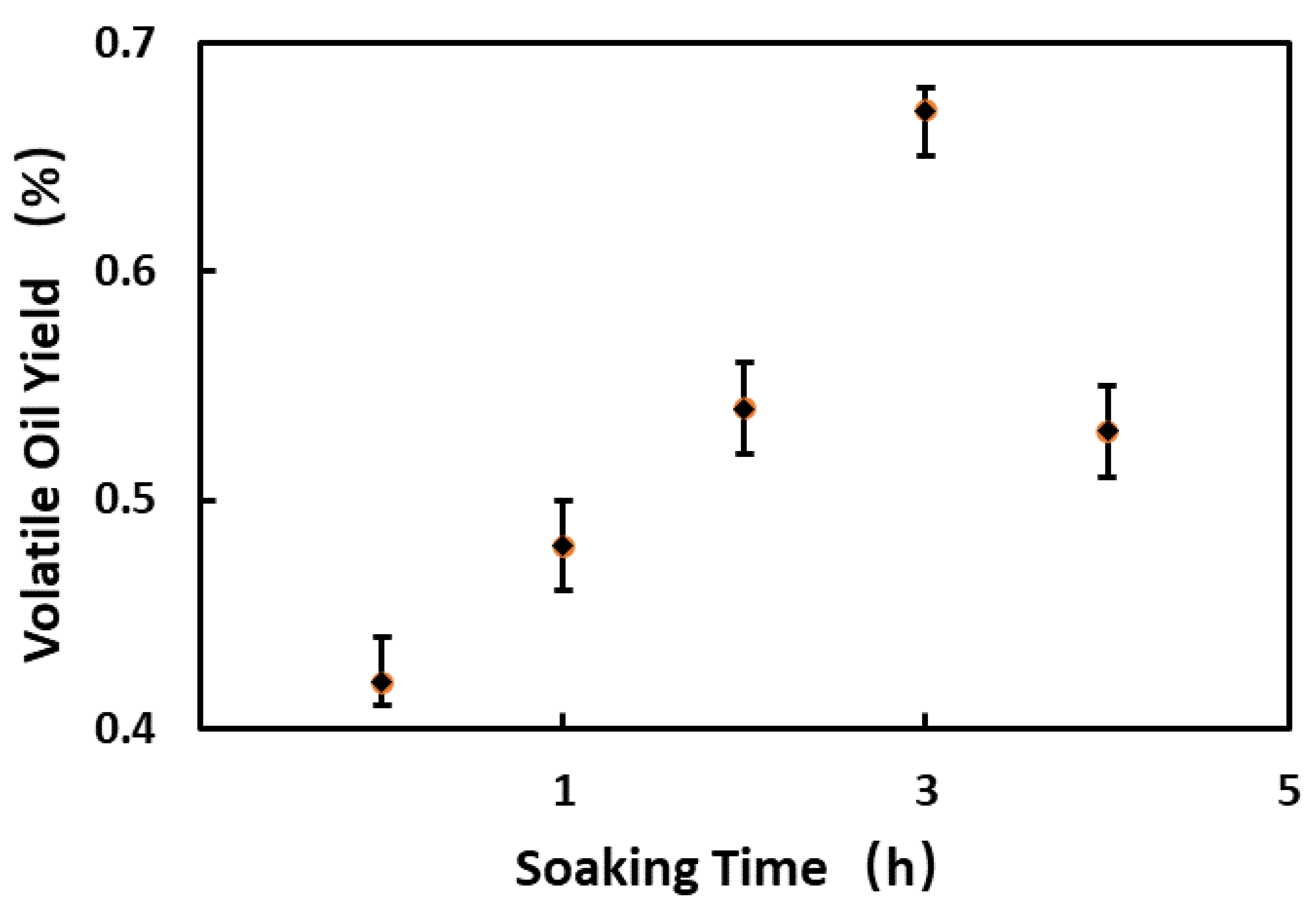
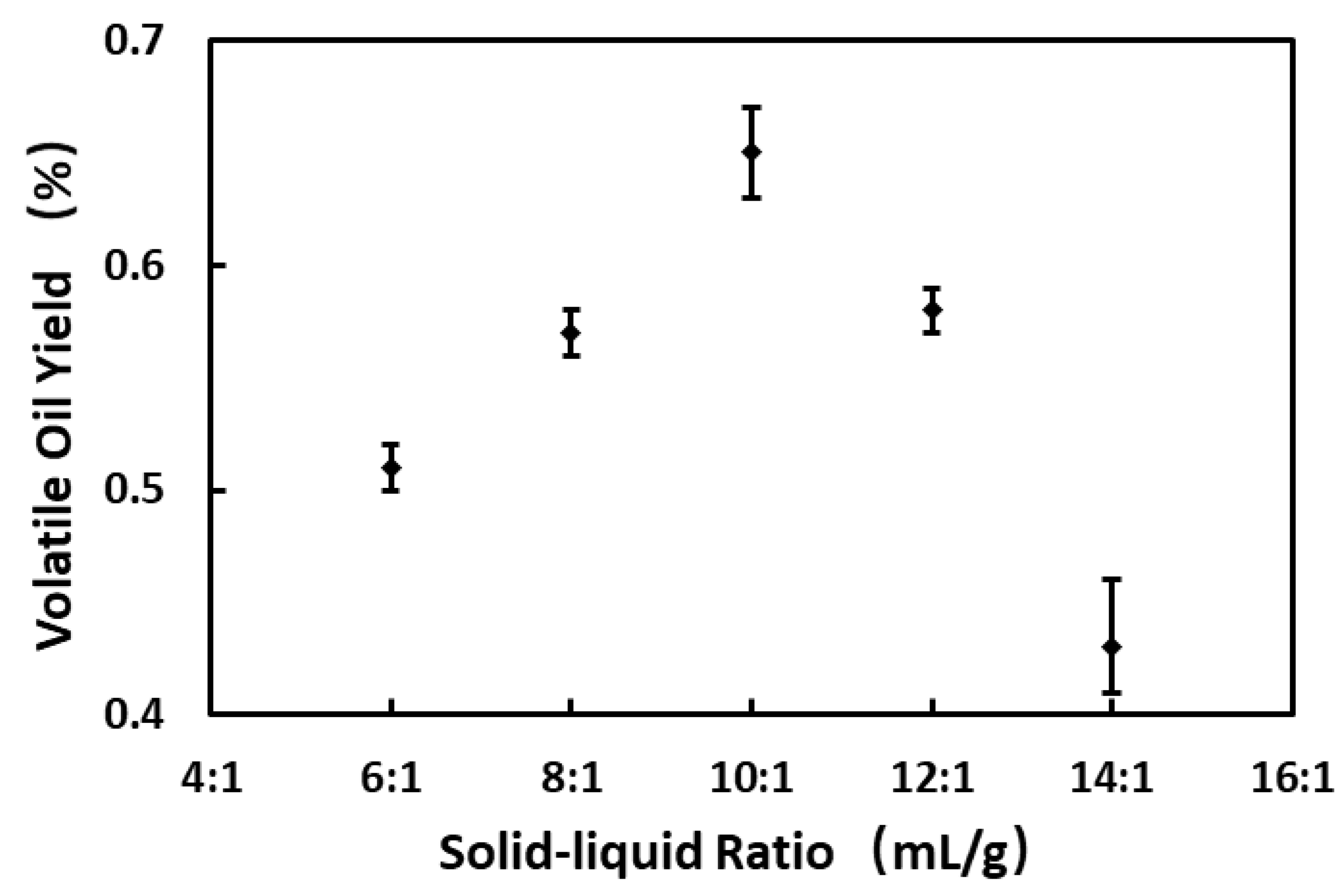
3.1. Critical Process Parameters of Volatile Oil Distillation
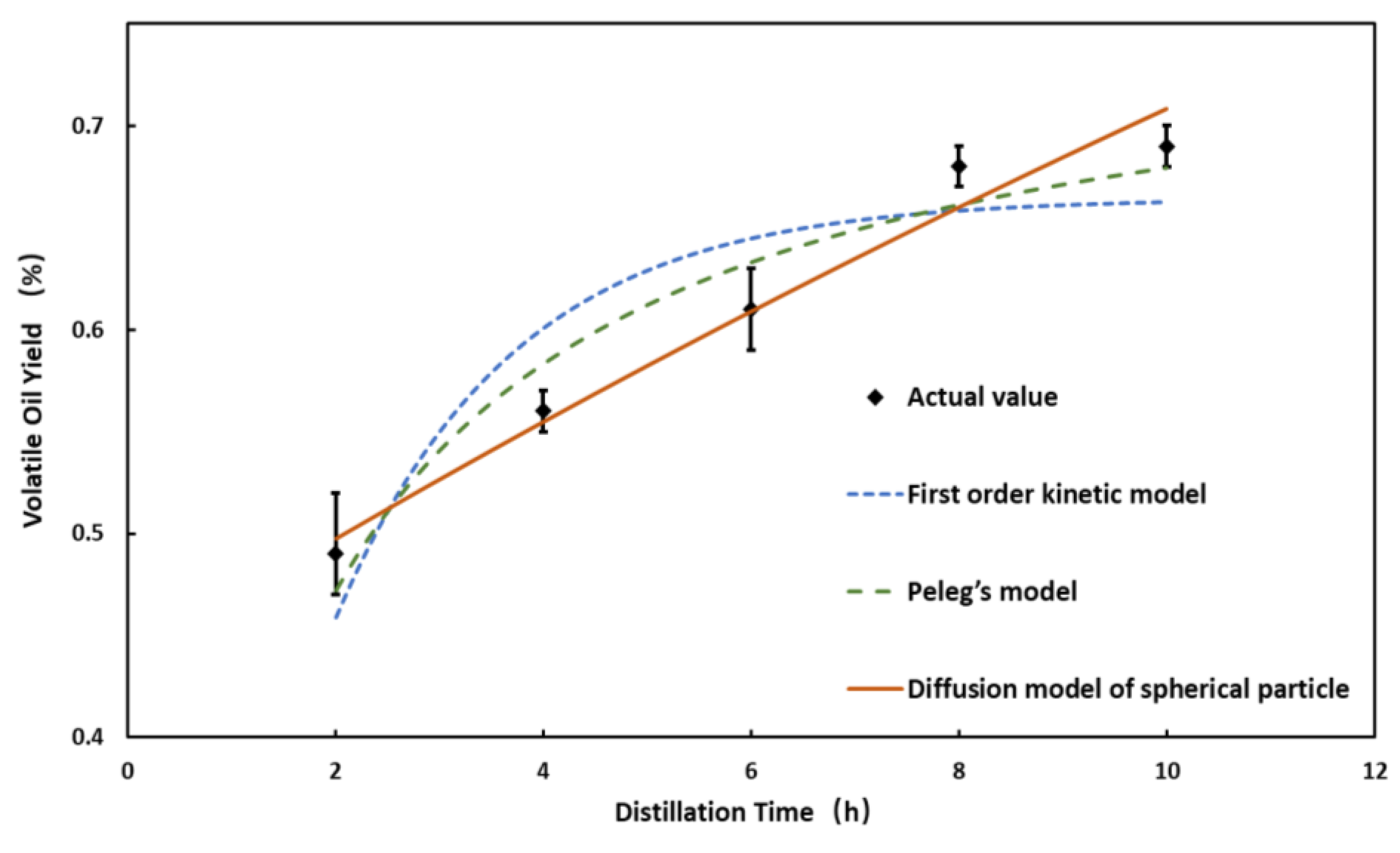
3.2. Study on Kinetics of Volatile Oil Distillation
3.3. Optimization of Distillation Parameters of Volatile Oil
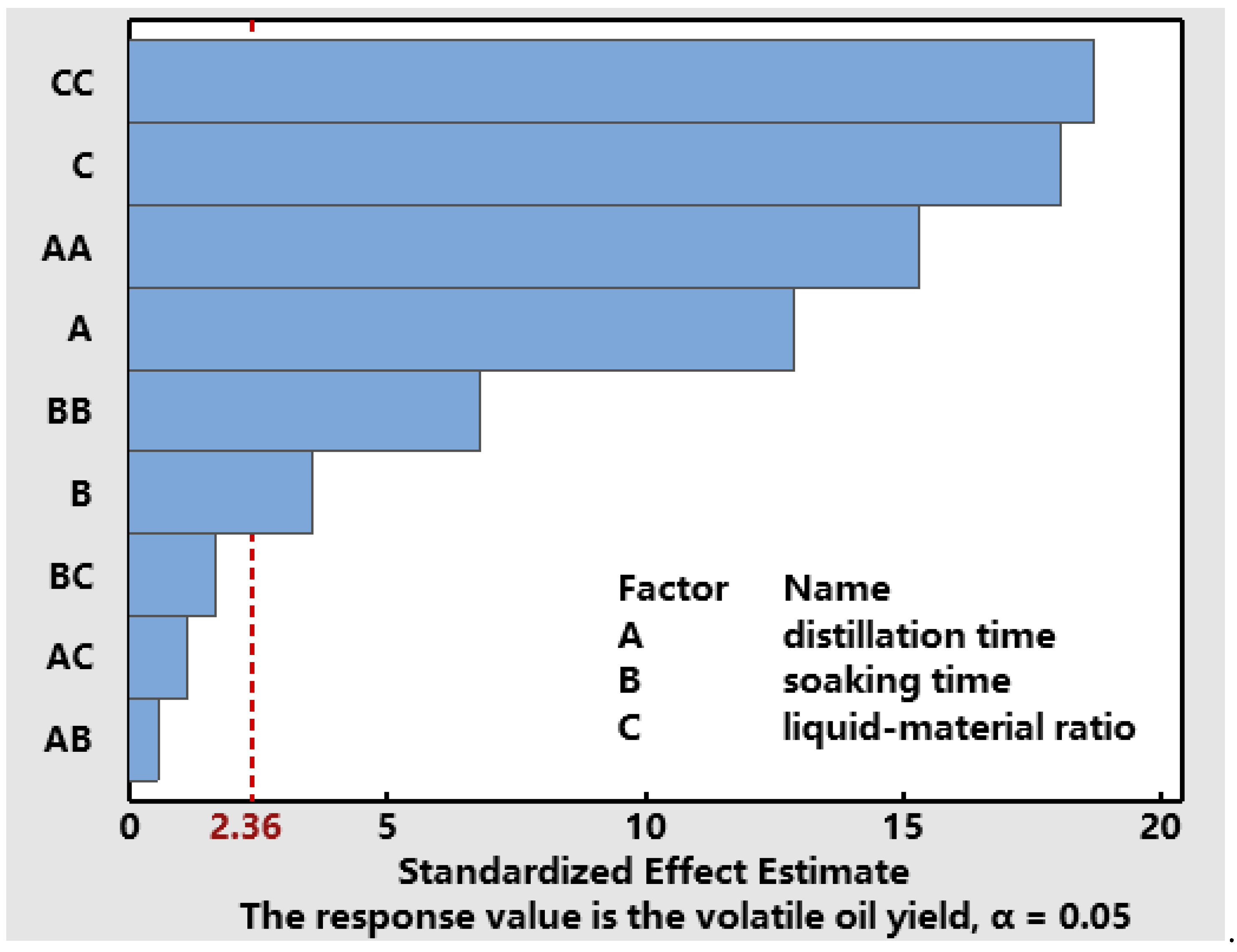
3.3.1. Data Processing and Model Fitting
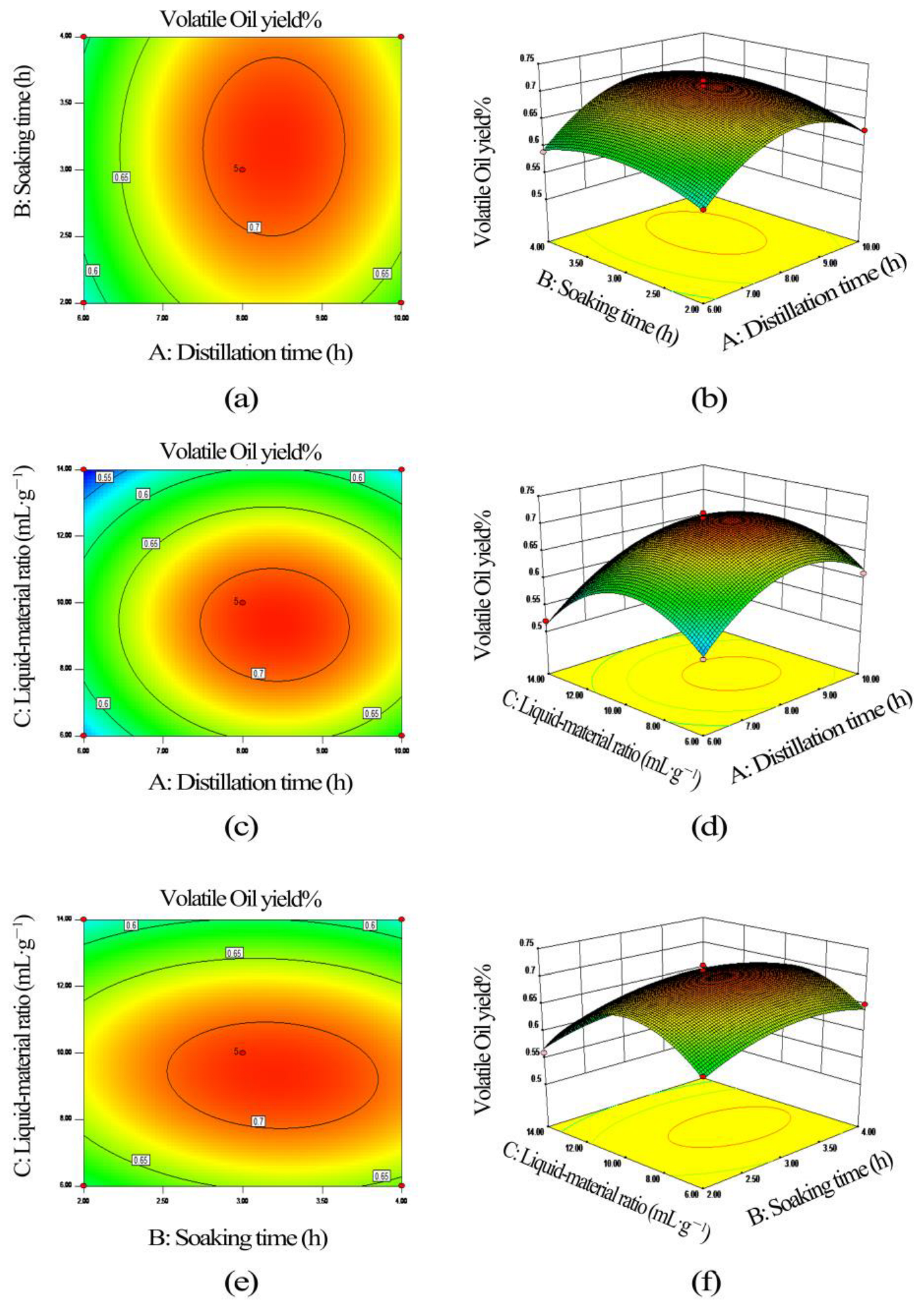
3.3.2. Response Surface Diagram and Contour Diagram
3.3.3. Design Space Calculation and Verification
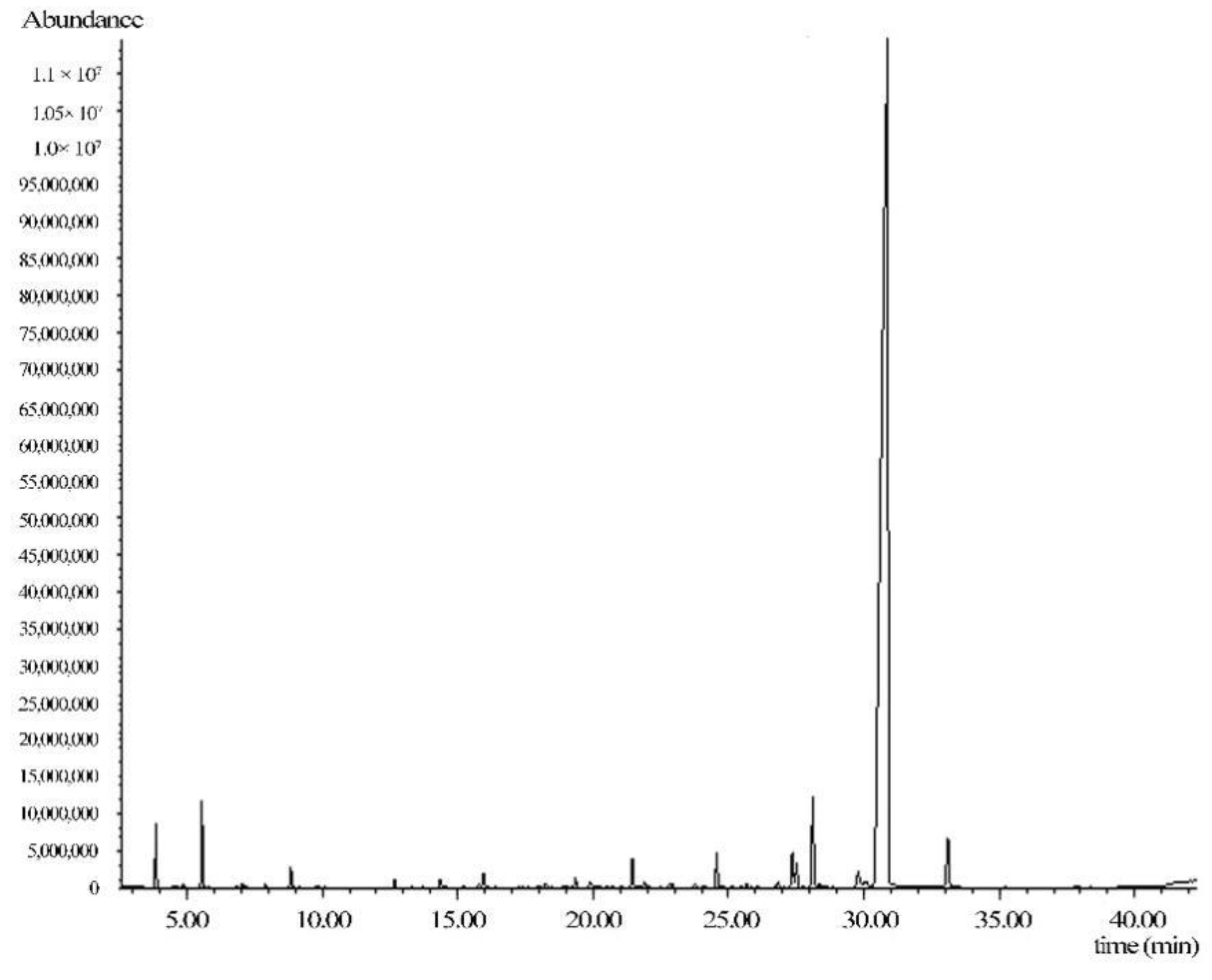
3.4. Qualitative Analysis of Chemical Constituents of Volatile Oil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Wu, G.; Sun, M.; Du, L.; Ren, Y. Research progress of ligustilide in Angelica sinensis. J. Gansu Univ. Chin. Med. 2018, 35, 102–105. [Google Scholar]
- Zhao, X.; Wang, D.; Zhao, D.; Ji, H.; Pei, Y.; Bai, J. Isolation and identification of the chemical constituents from roots of Angelica sinenses (Oliv.) Diels. J. Shenyang Pharm. Univ. 2013, 30, 182–185. [Google Scholar]
- Wu, H.; Hua, Y.; Guo, Y.; Wei, Y. Extraction and Analysis of Volatile Oil in Different Parts of Radix Angelicae Sinensis from Min County in Gansu. Nat. Prod. Res. Dev. 2012, 24, 1225–1229. [Google Scholar]
- Sun, M.; Ma, Q.; Liu, F.; Ren, Y. New Progress in the Study of Volatile Oil from Angelica sinensis. Abstr. World Curr. Med. Inf. 2019, 19, 56–58. [Google Scholar]
- Cui, F.; Feng, L.; Hu, J. Factors affecting stability of z-ligustilide in the volatile oil of radix angelicae sinensis and ligusticum chuanxiong and its stability prediction. Drug Dev Ind Pharm. 2006, 32, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Zou, J.; Jia, Y.; Wang, C.; Shi, Y.; Guo, D. Network analysis of the pharmacological mechanism of Angelica sinensis volatile oil in the treatment of hypertension. Nat. Prod. Res. Dev. 2021, 33, 657–666. [Google Scholar]
- Liu, X.; Huang, X.; Zhang, X.; Wang, L.; Zhu, L.; Xu, J.; Chen, Q.; Yang, M.; Wang, F. Q-Marker prediction of volatile oil of Angelicae Sinensis Radix based on GC-MS analysis combined with network pharmacology. Chin. Tradit. Herb. Drugs 2021, 52, 2696–2706. [Google Scholar]
- Zhu, L.; Luo, J.; Song, R.; Wang, L.; Zhang, A.; Zang, K. Effect and mechanism of volatile oil of angelica on apoptosis and autophagy in human colorectal cancer SW480 cells. Chin. J. Clin. Pharmacol. 2021, 37, 3253–3256. [Google Scholar]
- Kusuma, H.S.; Mahfud, M. Chemical composition of essential oil of Indonesia sandalwood extracted by microwave-assisted hydrodistillation. AIP Conf. Proc. 2016, 1755, 50001. [Google Scholar]
- Kusuma, H.S.; Mahfud, M. Box-Behnken design for investigation of microwave-assisted extraction of patchouli oil. AIP Conf. Proc. 2015, 1699, 50014. [Google Scholar]
- Priyadarshi, S.; Kashyap, P.; Gadhave, R.K.; Jindal, N. Effect of ultrasound-assisted hydrodistillation on extraction kinetics, chemical composition, and antimicrobial activity of Citrus jambhiri peel essential oil. J. Food Process Eng. 2021, 44, e13904. [Google Scholar] [CrossRef]
- Lan, Z.; Wang, L.; Li, Q.; Wang, S.; Meng, J. Analysis of volatile oil components of different species of Curcumae rhizoma based on GC-MS and chemometrics. China J. Chin. Mater. Med. 2021, 46, 3614–3624. [Google Scholar]
- Zou, J.; Zhang, X.; Shi, Y.; Guo, D.; Cheng, J.; Cui, C.; Tai, J.; Liang, Y.; Wang, Y.; Wang, M. Kinetic study of extraction of volatile components from turmeric by steam distillation. China J. Tradit. Chin. Med. Pharm. 2020, 35, 1175–1180. [Google Scholar]
- Wu, Z.; Xie, L.; Li, Y.; Wang, Y.; Wang, X.; Wan, N.; Huang, X.; Zhang, X.; Yang, M. A novel application of the vacuum distillation technology in extracting Origanum vulgare L. essential oils. Ind. Crop. Prod. 2019, 139, 111516. [Google Scholar] [CrossRef]
- Gong, X.; Chen, T.; Qu, H. Research advances in secondary development of Chinese patentmedicines based on quality by design concept. China J. Chin. Mater. Med. 2017, 42, 1031–1036. [Google Scholar]
- Tai, Y.; Qu, H.; Gong, X. Design Space Calculation and Continuous Improvement Considering a Noise Parameter: A Case Study of Ethanol Precipitation Process Optimization for Carthami Flos Extract. Separations 2021, 8, 74. [Google Scholar] [CrossRef]
- El-Shamy, A.M.; El-Hadek, M.A.; Nassef, A.E.; El-Bindary, R.A. Box-Behnken design to enhance the corrosion resistance of high strength steel alloy in 3.5 wt.% NaCl solution. Moroc. J. Chem. 2020, 8, 788–800. [Google Scholar]
- Kusuma, H.S.; Sudrajat, R.G.M.; Febrilliant, D.; Susanto, D.F.; Gala, S.; Mahfud, M. Response Surface Methodology (RSM) Modeling of Microwave-Assisted Extraction of Natural Dye from Swietenia mahagony: A comparation Between Box-Behnken and Central Composite Design Method. AIP Conf. Proc. 2015, 1699, 50009. [Google Scholar]
- Zhao, M.; Yang, S.; Sun, Y.; Li, F.; Zhang, S.; Jiao, J. Gas Chromatography-mass Spectrometry Analysis of Volatile Oil of Angelica sinensis Root Dry Chemical Composition. Chem. World 2018, 59, 231–234. [Google Scholar]
- Shao, J.; Qu, H.; Gong, X. Comparison of two algorithms for development of design space-overlapping method and probability-based method. China J. Chin. Mater. Med. 2018, 43, 2074–2080. [Google Scholar]
- Zhang, Y.; Zhang, Y.; Han, Y.; Tian, Y.; Wu, P.; Xin, A.; Wei, X.; Shi, Y.; Zhang, Z.; Su, G.; et al. Pharmacokinetics, tissue distribution, and safety evaluation of a ligustilide derivative (LIGc). J. Pharm. Biomed. Anal. 2020, 182, 113140. [Google Scholar] [CrossRef]
- Li, T.; He, X. Analysis of essential oil from the roots of Angelica sinensis by GC-MS. West China J. Pharm. Sci. 2015, 30, 249–250. [Google Scholar]
- Yan, H.; Zhang, X.-B.; Zhu, S.-D.; Qian, D.-W.; Guo, L.-P.; Huang, L.-Q.; Duan, J.-A. Production regionalization study of Chinese angelica based on MaxEnt model. China J. Chin. Mater. Med. 2016, 41, 3139–3147. [Google Scholar]
- Liu, P.; Chen, J.; Zhou, B.; Xu, Y.; Qian, D.W.; Duan, J.A. Analysis of variation of coumarin and volatile compounds in Angelica Dahuricae radix in different drying methods and conditions. Zhongguo Zhong Yao Za Zhi 2014, 39, 2653–2659. [Google Scholar]
- Ji, P.; Hua, Y.; Xue, W.; Wu, H.; Guo, Y.; Wei, Y. Extraction and Composition Analysis of Essential Oil from Raw Radix Angelicae Sinensis and Its Different Processed Products. Nat. Prod. R D. 2012, 24, 1230–1234, 1238. [Google Scholar]
- Li, R. Analysis of Volatile Oil Ingredients in Wild Angelica sinensis from Different Origin. Chin. J. Ethnomed. Ethnopharm. 2019, 28, 41–44. [Google Scholar]
- Li, Z.; Cui, J.; Fu, Z.; Mu, J. Supercritical CO2 Fluid Extraction and Chemical Composition Analysis of Volatile Oil from Atractylodis macrocephalae rhizoma. Food Drug 2019, 21, 269–273. [Google Scholar]
- Wang, T.; Cheng, Z. Research on Angelica Naphtha Components form Different Regions. Pharm. Biotechnol. 2013, 20, 535–537. [Google Scholar]
- Wang, X.; Zhao, Z.; Zhang, Y.; Shi, X.; Cheng, F.; Guo, M.; Wen, X. Comparative analysis of volatile compounds in Angelica Sinensis by different acquisition methods with GC-MS technique. J. Tradit. Chin. Vet. Med. 2018, 37, 61–65. [Google Scholar]
- Li, D.; Ma, X.; Song, P.; Zhao, J.; Ding, Y. Analysis of Three Methods for Extracting Volatile Constituent from DangGui by Combination of Gas Chromatography and Mass Spectrometry. West. J. Tradit. Chin. Med. 2013, 26, 15–18. [Google Scholar]
- Yue, H.; Jia, H.; Wang, J. Study on the Extraction Technology of the Volatile Oil in Angelica sinensis (Oliv.) Diels Based on Supercritical CO2 Extraction. J. Anhui Agric. Sci. 2010, 1, 47–50. [Google Scholar]

| Factor | Level | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (1) | |
| A: Distillation time (h) | 6 | 8 | 10 |
| B: Soaking time (h) | 2 | 3 | 4 |
| C: Liquid–material ratio (mL·g−1) | 6:1 | 10:1 | 14:1 |
| Serial Number | A (Distillation Time/h) | B (Soaking Time/h) | C [Liquid-Material Ratio (mL·g−1)] | Y (Volatile Oil Yield/%) |
|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 0.58 |
| 2 | 1 | −1 | 0 | 0.63 |
| 3 | −1 | 1 | 0 | 0.59 |
| 4 | 1 | 1 | 0 | 0.65 |
| 5 | −1 | 0 | −1 | 0.55 |
| 6 | 1 | 0 | −1 | 0.61 |
| 7 | −1 | 0 | 1 | 0.52 |
| 8 | 1 | 0 | 1 | 0.56 |
| 9 | 0 | −1 | −1 | 0.61 |
| 10 | 0 | 1 | −1 | 0.65 |
| 11 | 0 | −1 | 1 | 0.56 |
| 12 | 0 | 1 | 1 | 0.57 |
| 13 | 0 (8 h) | 0 (3 h) | 0 (10:1) | 0.70 |
| 14 | 0 (8 h) | 0 (3 h) | 0 (10:1) | 0.71 |
| 15 | 0 (8 h) | 0 (3 h) | 0 (10:1) | 0.71 |
| 16 | 0 (8 h) | 0 (3 h) | 0 (10:1) | 0.72 |
| 17 | 0 (8 h) | 0 (3 h) | 0 (10:1) | 0.71 |
| Model | RMSE (10−2 mL/g) | SSE (10−4 mL2/g2) | MSE (10−4 mL2/g2) | MAE (10−2 mL/g) |
|---|---|---|---|---|
| First-order kinetic model | 0.032 | 0.0051 | 0.0010 | 0.031 |
| Peleg’s model | 0.019 | 0.0019 | 0.00037 | 0.019 |
| Diffusion model of spherical particle | 0.013 | 0.00082 | 0.00016 | 0.010 |
| Variance Source | Sum of Square | Degree of Freedom | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Model | 0.069 | 9 | 7.65 × 10−3 | 93.13 | <0.0001 |
| A | 5.513 × 10−3 | 1 | 5.513 × 10−3 | 67.11 | <0.0001 |
| B | 8 × 10−4 | 1 | 8 × 10−4 | 9.74 | 0.0168 |
| C | 5.512 × 10−3 | 1 | 5.512 × 10−3 | 67.11 | <0.0001 |
| AB | 2.5 × 10−5 | 1 | 2.5 × 10−5 | 0.3 | 0.5983 |
| AC | 1 × 10−4 | 1 | 1 × 10−4 | 1.22 | 0.3064 |
| BC | 2.25 × 10−4 | 1 | 2.25 × 10−4 | 2.74 | 0.1419 |
| A22 | 0.019 | 1 | 0.019 | 233.55 | <0.0001 |
| B22 | 3.789 × 10−3 | 1 | 3.789 × 10−3 | 46.13 | 0.0003 |
| C22 | 0.029 | 1 | 0.029 | 348.88 | <0.0001 |
| Residual error | 5.75 × 10−4 | 7 | 8.214 × 10−5 | ||
| Misfit term | 3.75 × 10−4 | 3 | 1.25 × 10−4 | 2.5 | 0.1985 |
| Pure error | 2 × 10−4 | 4 | 5 × 10−5 | ||
| Total error | 0.069 | 16 |
| Serial Number | T/min | Compound | Chemical Formula | Relative Content/% | m/z Value of the Fragment with Maximum Abundance | Abundance |
|---|---|---|---|---|---|---|
| 1 | 3.85 | α-Pinene 2,6,6-trimethylbicyclo [3.1.1]hept-2-ene | C10H16 | 1.21 | 93.1 | 239,680 |
| 2 | 5.55 | β-Ocimene 3,7-dimethylocta−1,3,6-triene | C10H16 | 1.67 | 93.1 | 298,432 |
| 3 | 8.84 | 6-butylcyclohepta−1,4-diene | C11H18 | 0.53 | 79.1 | 61,936 |
| 4 | 12.68 | Diamyl Ketone undecan-6-one | C11H22O | 0.21 | 71.1 | 22,096 |
| 5 | 14.33 | 4-ethenyl-2-methoxyphenol | C9H10O2 | 0.30 | 150.1 | 32,720 |
| 6 | 15.95 | Duraldehyde 2,4,5-trimethylbenzaldehyde | C10H12O | 0.46 | 147.1 | 34,272 |
| 7 | 18.24 | β-Cedrene (1S,2R,5S,7S)-2,6,6-trimethyl-8-methylidenetricyclo [5.3.1.01,5]undecane | C15H24 | 0.27 | 161.2 | 7463 |
| 8 | 19.36 | 4-(1,2-dimethylcyclopent-2-en−1-yl)butan-2-one | C11H18O | 0.25 | 93.1 | 12,845 |
| 9 | 19.88 | Sesquichamene 2,4a,8,8-tetramethyl−1,1a,4,5,6,7-hexahydrocyclopropa[j]naphthalene | C15H24 | 0.02 | 93.1 | 4696 |
| 10 | 21.45 | (1R,2R)−1-ethenyl−1-methyl−4-propan-2-ylidene-2-prop−1-en-2-ylcyclohexane | C15H24 | 0.75 | 121.1 | 50,288 |
| 11 | 21.90 | β-Bisabolene (4S)−1-methyl−4-(6-methylhepta−1,5-dien-2-yl)cyclohexene | C15H24 | 0.17 | 69.1 | 9638 |
| 12 | 23.77 | Alloaromadendrene 1,1,7-trimethyl−4-methylidene-2,3,4a,5,6,7,7a,7b-octahydro−1aH-cyclopropa[e]azulene | C15H24 | 0.11 | 121.1 | 4412 |
| 13 | 24.57 | Spathulenol (7S)−1,1,7-trimethyl−4-methylidene−1a,2,3,4a,5,6,7a,7b-octahydrocyclopropa[h]azulen-7-ol | C15H24O | 0.96 | 91.1 | 22,384 |
| 14 | 27.36 | 2-methyl−1,3-benzoxazole | C8H7NO | 1.06 | 133.0 | 240,832 |
| 15 | 27.52 | 1-oxido−4-[2-(1-oxidopyridin−1-ium−4-yl)ethyl]pyridin−1-ium | C12H12N2O2 | 0.71 | 108.1 | 70,000 |
| 16 | 27.75 | Cyclopentadiene 2,5,5-trimethylcyclopenta−1,3-diene | C8H12 | 0.09 | 159.0 | 373,632 |
| 17 | 28.12 | Butylidenephthalide (3E)-3-butylidene-2-benzofuran−1-one | C12H12O2 | 2.79 | 159.0 | 56,168 |
| 18 | 30.06 | Senkyunolide A (3S)-3-butyl−4,5-dihydro-3H-2-benzofuran−1-one | C12H16O2 | 0.36 | 107.1 | 27,088 |
| 19 | 30.88 | Z-ligustilide (3Z)-3-butylidene−4,5-dihydro-2-benzofuran−1-one | C12H14O2 | 85.4 | 161.1 | 1,526,784 |
| 20 | 32.34 | E-ligustilide (3E)-3-butylidene−4,5-dihydro-2-benzofuran−1-one | C12H14O2 | 2.04 | 161.1 | 86,504 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, N.; Lan, J.; Wu, Z.; Chen, X.; Zheng, Q.; Gong, X. Optimization of Steam Distillation Process and Chemical Constituents of Volatile Oil from Angelicaesinensis Radix. Separations 2022, 9, 137. https://doi.org/10.3390/separations9060137
Wan N, Lan J, Wu Z, Chen X, Zheng Q, Gong X. Optimization of Steam Distillation Process and Chemical Constituents of Volatile Oil from Angelicaesinensis Radix. Separations. 2022; 9(6):137. https://doi.org/10.3390/separations9060137
Chicago/Turabian StyleWan, Na, Jing Lan, Zhenfeng Wu, Xinying Chen, Qin Zheng, and Xingchu Gong. 2022. "Optimization of Steam Distillation Process and Chemical Constituents of Volatile Oil from Angelicaesinensis Radix" Separations 9, no. 6: 137. https://doi.org/10.3390/separations9060137
APA StyleWan, N., Lan, J., Wu, Z., Chen, X., Zheng, Q., & Gong, X. (2022). Optimization of Steam Distillation Process and Chemical Constituents of Volatile Oil from Angelicaesinensis Radix. Separations, 9(6), 137. https://doi.org/10.3390/separations9060137







