Antiallergic Properties of Biflavonoids Isolated from the Flowers of Mesua ferrea Linn.
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Plant Material
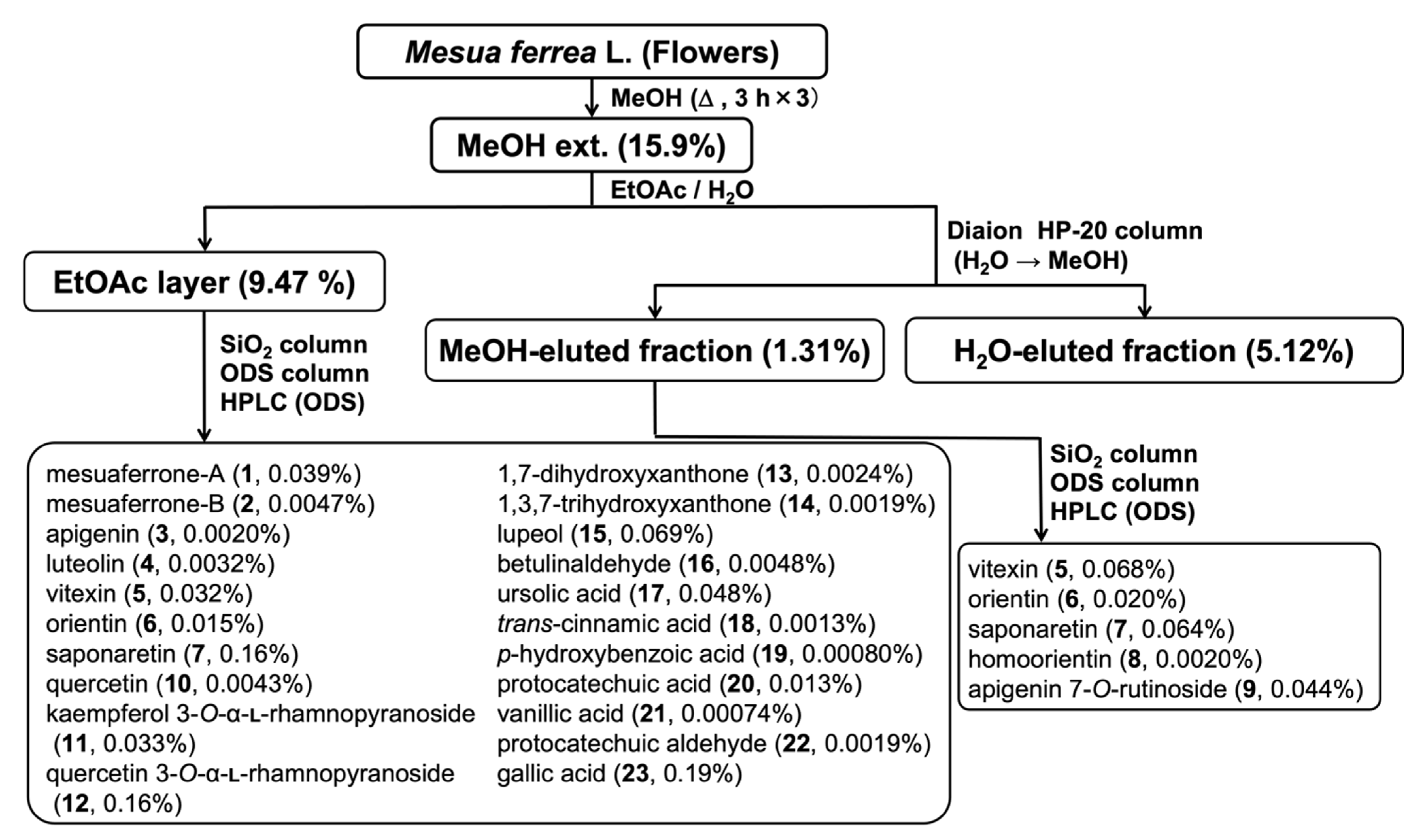
2.3. Extraction and Isolation
2.4. Hyaluronidase Inhibitory Activity
2.5. Inhibitory Effects on the Release of β-Hexosaminidase from RBL-2H3 Cells
2.6. Statistics
3. Results and Discussion
3.1. Inhibitory Effects of the M. ferrea Flower Methanolic Extract and Its Fractions on Hyaluronidase
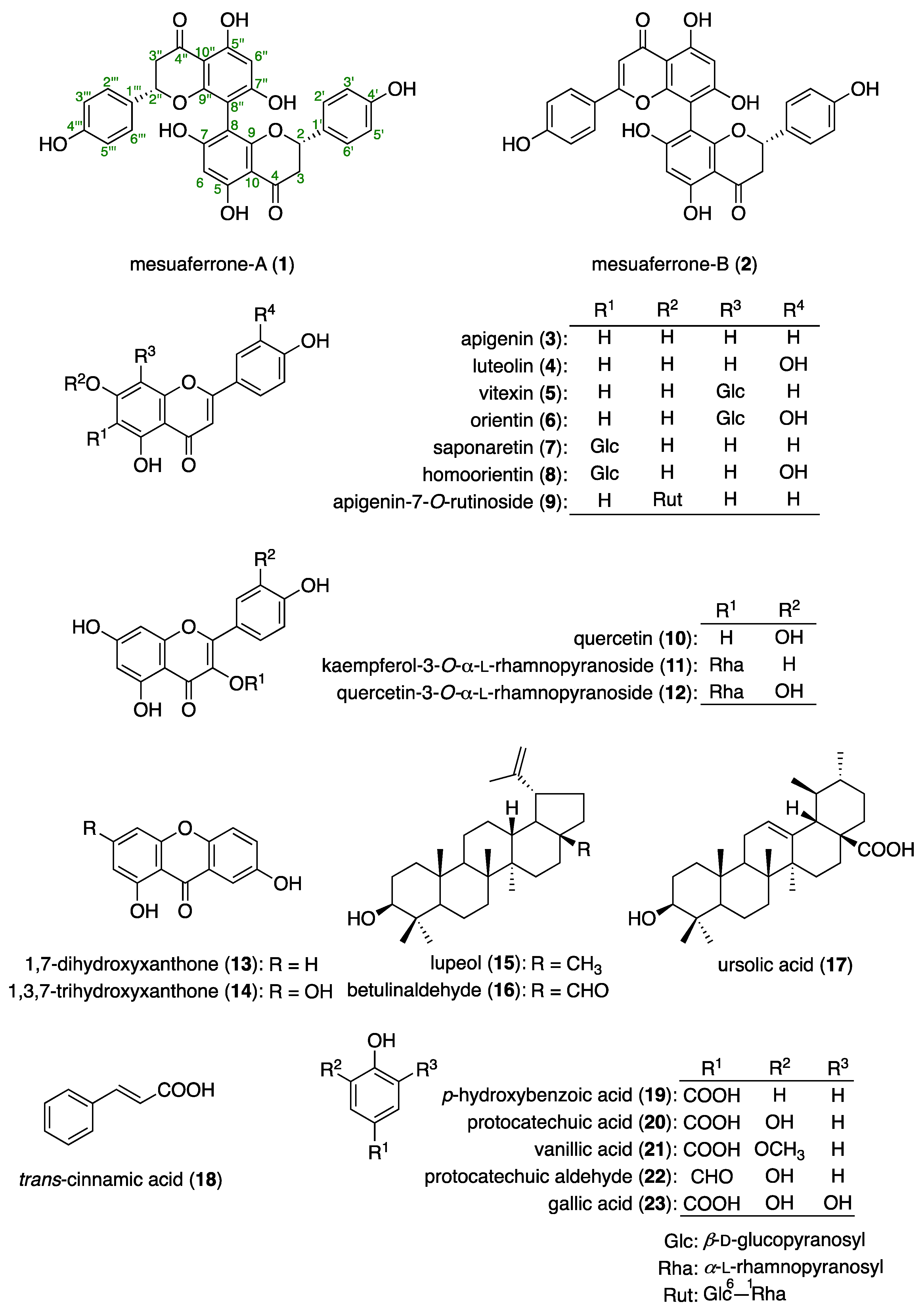
3.2. Chemical Constituents of the M. ferrea Flower
3.3. Inhibitory Effects of M. ferrea Flower Isolates (1–23) on Hyaluronidase
3.4. Inhibitory Effects of 1 and 2 on the Release of β-Hexosaminidase in RBL-2H3 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, A.B.; Shinde, S.S.; Raghavendra, S.; Satish, B.N.; Kushalappa, C.G.; Vijay, N. The genome sequence of Mesua ferrea and comparative demographic histories of forest trees. Gene 2021, 15, 145214. [Google Scholar] [CrossRef] [PubMed]
- Chukaew, A.; Saithong, S.; Chusri, S.; Limsuwan, S.; Watanapokasin, R.; Voravuthikunchai, S.P.; Chakthong, S. Cytotoxic xanthones from the roots of Mesua ferrea L. Phytochemistry 2019, 157, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Shirsat, P.; Ziyaurrahman, A.R.; Kashikar, R.; Athavale, M.; Athavale, T.; Taware, P.; Saldanha, T.; Kolhe, S.; Tembhurne, S. Subacute toxicity study of the ethanolic extract of Mesua ferrea (L.) flowers in rats. Drug Chem. Toxicol. 2020, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Manoj, K.C.; Sanjaya, K.D.S.; Geetha, L.; Lokesh, T.; Manohara, K.P. Mesua ferrea L.: A review of the medical evidence for its phytochemistry and pharmacological actions. Afr. J. Pharm. Pharmacol. 2013, 7, 211–219. [Google Scholar] [CrossRef]
- Sruthikrishna, P.K. Review on Ethnobotany and Phytopharmacology on Mesua ferrea Linn. Res. J. Pharmacogn. Phytochem. 2021, 13, 195–199. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, R.; Liu, Y.; Cong, Y.; Zhang, D.; Zhang, Y.; Yang, X.; Lu, C.; Shen, Y. Anti-virulence activities of biflavonoids from Mesua ferrea L. flower. Drug. Discov. Ther. 2019, 13, 222–227. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Y.; Yao, D.; Liu, L.; Duan, H.; Meng, L.; Yang, H.; Zhang, K.; Huang, J.; Li, Q.; et al. 4-Alkyl-5,7-dihydroxycoumarins from the flowering buds of Mesua ferrea. Fitoterapia 2019, 138, 104192. [Google Scholar] [CrossRef]
- Alam, M.S.; Jain, N.; Kamil, M. Mesuein: A novel flavanone glycoside from Mesua ferrea. Chem. Ind. Lond. 1987, 16, 565–566. [Google Scholar]
- Dennis, T.J.; Akshay, K.K.; Srimannarayana, G. A new cyclo hexadione from Mesua ferrea. Phytochemistry 1988, 27, 2325–2327. [Google Scholar] [CrossRef]
- Kamarusalihin, N.; Faujan, B.H.A.; Taufiq-Yap, Y.H.; Ali, A.M. Volatile components of methanol extract from the flower of Malaysian Mesua ferrea Linn. Orient. J. Chem. 2004, 20, 69–72. [Google Scholar]
- Chahar, M.K.; Sanjaya-Kumar, D.S.; Lokesh, T.; Manohara, K.P. In-vivo antioxidant and immunomodulatory activity of mesuol isolated from Mesua ferrea L. seed oil. Int. Immunopharmacol. 2012, 13, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Shafaei, A.; Jafari, S.F.; Mohamed, S.K.; Ezzat, M.O.; Abdul-Majid, A.S.; Oon, C.E.; Petersen, S.H.; Kono, K.; Abdul-Majid, A.M. Isoledene from Mesua ferrea oleo-gum resin induces apoptosis in HCT 116 cells through ROS-mediated modulation of multiple proteins in the apoptotic pathways: A mechanistic study. Toxicol. Lett. 2016, 22, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Akaki, J.; Ninomiya, K.; Kinouchi, E.; Tanabe, G.; Pongpiriyadacha, Y.; Yoshikawa, M.; Muraoka, O. Salacinol and related analogs: New leads for type 2 diabetes therapeutic candidates from the Thai traditional natural medicine Salacia chinensis. Nutrients 2015, 7, 1480–1493. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Matsumoto, T.; Chaipech, S.; Miyake, S.; Katsuyama, Y.; Tsuboyama, A.; Pongpiriyadacha, Y.; Hayakawa, T.; Muraoka, O.; Morikawa, T. Simultaneous quantitative analysis of 12 methoxyflavones with melanogenesis inhibitory activity from the rhizomes of Kaempferia parviflora. J. Nat. Med. 2016, 70, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Shibatani, K.; Sueyoshi, M.; Chaipech, S.; Pongpiriyadacha, Y.; Hayakawa, T.; Muraoka, O.; Morikawa, T. Aromatase inhibitory activity of geranylated coumarins, mammeasins C and D, isolated from the flowers of Mammea siamensis. Chem. Pharm. Bull. 2016, 64, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Manse, Y.; Ninomiya, K.; Nishi, R.; Kamei, I.; Katsuyama, Y.; Imagawa, T.; Chaipech, S.; Muraoka, O.; Morikawa, T. Melanogenesis inhibitory activity of a 7-O-9′-linked neolignan from Alpinia galanga fruit. Bioorg. Med. Chem. 2016, 24, 6215–6224. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Chaipech, S.; Kunikata, Y.; Yagi, R.; Pongpiriyadacha, Y.; Muraoka, O.; Morikawa, T. Quantitative determination of stilbenoids and dihydroisocoumarins in Shorea rexburghii and evaluation of their hepatoprotective activity. Int. J. Mol. Sci. 2017, 18, 451. [Google Scholar] [CrossRef]
- Tanabe, G.; Tsutsui, N.; Shibatani, K.; Marumoto, S.; Ishikawa, F.; Ninomiya, K.; Morikawa, T. Total syntheses of the aromatase inhiitors, mammeasins C and D, from Thai medicinal plant Mammea siamensis. Tetrahedron 2017, 73, 4481–4486. [Google Scholar] [CrossRef]
- Manse, Y.; Ninomiya, K.; Nishi, R.; Hashimoto, Y.; Chaipech, S.; Muraoka, O.; Morikawa, T. Labdane-type diterpenes, galangalditerpenes A-C, with melanogenesis inhibitory activity from the fruit of Alpinia galanga. Molecules 2017, 22, 2279. [Google Scholar] [CrossRef]
- Morikawa, T.; Manse, Y.; Koda, M.; Chaipech, S.; Pongpiriyadacha, Y.; Muraoka, O.; Ninomiya, K. Two new aromatic glycosides, elengiosides A and B, from the flowers of Mimusops elengi. J. Nat. Med. 2018, 72, 542–550. [Google Scholar] [CrossRef]
- Tanabe, G.; Manse, Y.; Ogawa, T.; Sonoda, N.; Marumoto, S.; Ishikawa, F.; Ninomiya, K.; Chaipech, S.; Pongpiriyadacha, Y.; Muraoka, O.; et al. Total synthesis of γ-alkylidenebutenolides, potent melanogenesis inhibitors from Thai medicinal plant Melodorum fruticosum. J. Org. Chem. 2018, 83, 8250–8264. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Akaki, J.; Pongpiriyadacha, Y.; Yoshikawa, M.; Ninomiya, K.; Muraoka, O. Simultaneous quantitative determination of polyphenol constituents in Salacia species from different regions by LC-MS. Jpn. J. Food Chem. Saf. 2018, 25, 130–138. [Google Scholar] [CrossRef]
- Kobayashi, M.; Akaki, J.; Yamaguchi, Y.; Yamasaki, H.; Ninomiya, K.; Pongpiriyadacha, Y.; Yoshikawa, M.; Muraoka, O.; Morikawa, T. Salacia chinensis stem extract and its thiosugar sulfonium constituent, neokotalanol, improves HbA1c in ob/ob mice. J. Nat. Med. 2019, 73, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Luo, F.; Manse, Y.; Sugita, H.; Saeki, S.; Chaipech, S.; Pongpiriyadacha, Y.; Muraoka, O.; Ninomiya, K. Geranylated coumarins from Thai medicinal plant Mammea siamensis with testosterone 5α-reductase inhibitory activity. Front. Chem. 2020, 20, 199. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Sugita, H.; Muraki, K.; Saeki, S.; Chaipech, S.; Pongpiriyadacha, Y.; Muraoka, O.; Morikawa, T. Anti-proliferative activities of coumarins from the Thai medicinal plant Mammea siamensis (Miq.) T. Anders. against human digestive tract carcinoma cell lines. Fitoterapia 2021, 148, 104780. [Google Scholar] [CrossRef]
- Morikawa, T.; Ninomiya, K.; Tanabe, G.; Matsuda, H.; Yoshikawa, M.; Muraoka, O. A review of antidiabetic active thiosugar sulfoniums, salacinol and neokotalanol, from plants of the genus Salacia. J. Nat. Med. 2021, 75, 449–466. [Google Scholar] [CrossRef]
- Morikawa, T.; Okugawa, S.; Manse, Y.; Muraoka, O.; Yoshikawa, M.; Ninomiya, K. Quantitative determination of principal aporphine and benzylisoquinoline alkaloids due to blooming state in lotus flower (flower buds of Nelumbo nucifera) and their hyaluronidase inhibitory activity. Nat. Prod. Commun. 2019, 14, 1934578X19857834. [Google Scholar] [CrossRef]
- Morikawa, T.; Xu, F.; Matsuda, H.; Yoshikawa, M. Structures of novel Norstilbene dimer, Longusone A, and three new stilbene dimers, longusols A, B, and C, with antiallergic and radical scavenging activities from Egyptian natural medicine Cyperus longus. Chem. Pharm. Bull. 2010, 58, 1379–1385. [Google Scholar] [CrossRef]
- Matsuda, H.; Tewtrakul, S.; Morikawa, T.; Nakamura, A.; Yoshikawa, M. Anti-allergic principles from Thai zedoary: Structural requirements of curcuminoids for inhibition of degranulation and effect on the release of TNF-α and IL-4 in RBL-2H3 cells. Bioorg. Med. Chem. 2004, 12, 5891–5898. [Google Scholar] [CrossRef]
- Girish, K.S.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007, 80, 1921–1943. [Google Scholar] [CrossRef]
- Kakegawa, H.; Matsumoto, H.; Satoh, T. Inhibitory effects of some natural products on the activation of hyaluronidase and their anti-allergic actions. Chem. Pharm. Bull. 1992, 40, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Watahiki, M.; Tanaka, Y.; Miyase, T.; Yoshizaki, F. Hyaluronidase inhibitors from Takuran, Lycopus lucidus. Chem. Pharm. Bull. 2010, 58, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Subramanyam, R.M.; Rao, G.; Subba, N.V. Structure of mesuaferrone-A, a new biflavanone from the stamens of Mesua ferrea Linn. Indian J. Chem. Sect. B 1978, 16, 167–168. [Google Scholar]
- Subramanyam, R.M.; Srimannarayana, G.; Subba-Rao, N.V.; Bala, K.R.; Seshadri, T.R. Structure of mesuaferrone-B a new biflavanone from the stamens of Mesua ferrea Linn. Tetrahedron Lett. 1976, 17, 4509–4512. [Google Scholar] [CrossRef]
- Jayasinghe, U.L.B.; Balasooriya, B.A.I.S.; Bandara, A.G.D.; Fujimoto, Y. Glycosides from Grewia damine and Filicium decipiens. Nat. Prod. Res. 2004, 18, 499–502. [Google Scholar] [CrossRef]
- Kato, T.; Morita, Y. C-Glycosylflavones with acetyl substitution from Rumex acetosa L. Chem. Pharm. Bull. 1990, 38, 2277–2280. [Google Scholar] [CrossRef][Green Version]
- Lin, C.N.; Kuo, S.H.; Chung, M.I.; Ko, F.N.; Teng, C.T. A new flavone C-glycoside and antiplatelet and vasorelaxing flavones from Gentiana arisanensis. J. Nat. Prod. 1997, 60, 851–853. [Google Scholar] [CrossRef]
- Zhang, Z.; ElSohly, H.N.; Li, X.C.; Khan, S.I.; Sheldon, E.; Broedel, S.E.J.; Raulli, R.E.; Cihlar, R.L.; Burandt, C.; Walker, L.A. Phenolic compounds from Nymphaea odorata. J. Nat. Prod. 2003, 66, 548–550. [Google Scholar] [CrossRef]
- Fukunaga, T.; Nishiya, K.; Kajikawa, I.; Watanabe, Y.; Suzuki, N.; Takeya, K.; Itokawa, H. Chemical studies on the constituents of Hyphear tanakae Hosokawa from different host trees. Chem. Pharm. Bull. 1988, 36, 1180–1184. [Google Scholar] [CrossRef]
- Yang, X.D.; Xu, L.Z.; Yang, S.L. Xanthones from the stems of Securidaca inappendiculata. Phytochemistry 2001, 58, 1245–1249. [Google Scholar] [CrossRef]
- Mohammad, A.; Mumtaz, A.; Habib, A.; Itrat, A.; Ajmal, K.; Iqbal, C.; Muhammad, R.S. Urease inhibitors from Hypericum oblongifolium WALL. J. Enzym. Inhib. Med. Chem. 2010, 25, 296–299. [Google Scholar] [CrossRef]
- Wenkert, E.; Vernon-Baddeley, G.; Burfitt, I.R.; Moreno, L.N. Carbon-13 nuclear magnetic resonance spectroscopy of naturally-occurring substances-LVII triterpenes related to lupane and hopane. Magn. Reson. Chem. 1978, 11, 337–343. [Google Scholar] [CrossRef]
- Pohjala, L.; Alakurtti, S.; Ahola, T.; Yli-Kauhaluoma, J.; Tammela, P. Betulin-derived compounds as inhibitors of alphavirus replication. J. Nat. Prod. 2009, 72, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Acebey-Castellon, I.L.; Voutquenne-Nazabadioko, L.; Thi-Mai, H.D.; Roseau, N.; Bouthagane, N.; Muhammad, D.; Debar, E.L.M.; Gangloff, S.C.; Litaudon, M.; Sevenet, T.; et al. Triterpenoid saponins from Symplocos lancifolia. J. Nat. Prod. 2011, 74, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Detsi, A.; Majdalani, M.; Kontogiorgis, C.A.; Hadjipavlou-Litina, D.; Kefalas, P. Natural and synthetic 2′-hydroxy-chalcones and aurones: Synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorg. Med. Chem. 2009, 17, 8073–8085. [Google Scholar] [CrossRef] [PubMed]
- Maatooq, G.T.; El-Sharkawy, S.H.; Afifi, M.S.; Rosazza, J.P. C-p-Hydroxybenzoylglycoflavones from Citrullus colocynthis. Phytochemistry 1997, 44, 187–190. [Google Scholar] [CrossRef]
- Wang, M.; Simon, J.E.; Aviles, I.F.; He, K.; Zheng, Q.; Tadmor, Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2003, 51, 601–608. [Google Scholar] [CrossRef]
- Morikawa, T.; Nakanishi, Y.; Inoue, N.; Manse, Y.; Matsuura, H.; Hamasaki, S.; Yoshikawa, M.; Muraoka, O.; Ninomiya, K. Acylated iridoid glycosides with hyaluronidase inhibitory activity from the rhizomes of Picrorhiza kurroa Royle ex Benth. Phytochemistry 2020, 169, 112185. [Google Scholar] [CrossRef]
- Morikawa, T.; Hachiman, I.; Ninomiya, K.; Hata, H.; Sugawara, K.; Muraoka, O.; Matsuda, H. Degranulation inhibitors from the arils of Myristica fragrans in antigen-stimulated rat basophilic leukemia cells. J. Nat. Med. 2018, 72, 464–473. [Google Scholar] [CrossRef]
- Platts-Mills, T.A.E. The role of immunoglobulin E in allergy and asthma. Am. J. Respir. Crit. Care Med. 2001, 164, S1–S5. [Google Scholar] [CrossRef]
- Matsubara, M.; Masaki, S.; Ohmori, K.; Karasawa, A.; Hasegawa, K. Differential regulation of IL-4 expression and degranulation by anti-allergic olopatadine in rat basophilic leukemia (RBL-2H3) cells. Biochem. Pharmacol. 2004, 67, 1315–1326. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Ueda, K.; Managi, H.; Yoshikawa, M. Structural requirements of flavonoids for inhibition of antigen-induced degranulation, TNF-α and IL-4 production. Bioorg. Med. Chem. 2002, 10, 3123–3128. [Google Scholar] [CrossRef]
- Tanaka, Y.; Takagaki, Y.; Nishimune, T. Effects of metal elements on β-hexosaminidase release from rat basophilic leukemia cells (RBL-2H3). Chem. Pharm. Bull. 1991, 39, 2072–2076. [Google Scholar] [CrossRef][Green Version]
- Matsuda, H.; Morikawa, T.; Managi, H.; Yoshikawa, M. Antiallergic principles from Alpinia galanga: Structural requirements of phenylpropanoids for inhibition of degranulation and release of TNF-α and IL-4 in RBL-2H3 cells. Bioorg. Med. Chem. Lett. 2003, 13, 3197–3202. [Google Scholar] [CrossRef]
- Matsuda, H.; Sugimoto, S.; Morikawa, T.; Matsuhira, K.; Mizuguchi, E.; Nakamura, S.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XX. Inhibition of antigen-induced degranulation in RBL-2H3 cells from the seeds of Psoralea corylifolia. Chem. Pharm. Bull. 2007, 55, 106–110. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Xie, H.; Yoshikawa, M. Antiallergic phenanthrenes and stilbenes from the tubers of Gymnadenia conopsea. Planta Med. 2004, 70, 847–855. [Google Scholar] [CrossRef]
- Matsuda, H.; Tewtrakul, S.; Morikawa, T.; Yoshikawa, M. Anti-allergic activity of stilbenes from Korean rhubarb (Rheum undulatum L.): Structure requirements for inhibition of antigen-induced degranulation and their effects on the release of TNF-α and IL-4 production from RBL-2H3 cells. Bioorg. Med. Chem. 2004, 12, 4871–4876. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Tao, J.; Ueda, K.; Yoshikawa, M. Bioactive constituents of Chinese natural medicines. VII. inhibitors of degranulation in RBL-2H3 cells and absolute stereostructures of three new diarylheptanoid glycosides from the bark of Myrica rubra. Chem. Pharm. Bull. 2002, 50, 208–215. [Google Scholar] [CrossRef]
- Morikawa, T.; Tao, J.; Ueda, K.; Matsuda, H.; Yoshikawa, M. Medicinal foodstuffs. XXXI. Structures of new aromatic constituents and inhibitors of degranulation in RBL-2H3 cells from a Japanese folk medicine, the stem of Acer nikoence. Chem. Pharm. Bull. 2003, 51, 62–67. [Google Scholar] [CrossRef]
- Morikawa, T.; Matsuda, H.; Sakamoto, Y.; Ueda, K.; Yoshikawa, M. New farnesane-type sesquiterpenes, hedychiols A and B 8,9-diacetate, and inhibitors of degranulation in RBL-2H3 cells from the rhizomes of Hedychium coronarium. Chem. Pharm. Bull. 2002, 50, 1045–1049. [Google Scholar] [CrossRef]
- Morikawa, T.; Matsuda, H.; Toguchida, I.; Ueda, K.; Yoshikawa, M. Absolute stereostructures of three new sesquiterpenes from the fruit of Alpinia oxyphylla with inhibitory effects on nitric oxide production and degranulation in RBL-2H3 cells. J. Nat. Prod. 2002, 65, 1468–1474. [Google Scholar] [CrossRef]
- Tao, J.; Morikawa, T.; Ando, S.; Matsuda, S.; Matsuda, H.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XI. Inhibitors on NO production and degranulation in RBL-2H3 from Rubia yunnanensis: Structures of rubianosides II, III, and IV, rubianol-g, and rubianthraquinone. Chem. Pharm. Bull. 2003, 51, 654–662. [Google Scholar] [CrossRef]
- Morikawa, T.; Nakamura, S.; Kato, Y.; Muraoka, O.; Matsuda, H.; Yoshikawa, M. Bioactive saponins and glycosides. XXVIII. New triterpene saponins, foliatheasaponins I, II, III, IV, and V, from tencha (the leaves of Camellia sinensis). Chem. Pharm. Bull. 2007, 55, 293–298. [Google Scholar] [CrossRef]
- Sun, B.; Morikawa, T.; Matsuda, H.; Tewtrakul, S.; Wu, L.J.; Harima, S.; Yoshikawa, M. Structures of new β-carboline-type alkaloids with antiallergic effects from Stellaria dichotoma. J. Nat. Prod. 2004, 67, 1464–1469. [Google Scholar] [CrossRef]
- Morikawa, T.; Sun, B.; Matsuda, H.; Wu, L.J.; Harima, S.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XIV. New glycosides of β-carboline-type alkaloid, neolignan, and phenylpropanoid from Stellaria dichotoma L. var. lanceolata and their antiallergic activities. Chem. Pharm. Bull. 2004, 52, 1194–1199. [Google Scholar] [CrossRef]
| Inhibition (%) | |||||
|---|---|---|---|---|---|
| 0 μg/mL | 125 μg/mL | 250 μg/mL | 500 μg/mL | 1000 μg/mL | |
| MeOH extract | 0.0 ± 8.1 | 5.1 ± 6.8 | 10.7 ± 5.6 | 24.2 ± 6.4 | 52.1 ± 4.5 b |
| EtOAc-soluble fraction | 0.0 ± 3.3 | 19.6 ± 7.7 a | 27.2 ± 5.1 b | 52.8 ± 3.8 b | 72.0 ± 3.7 b |
| MeOH-eluted fraction | 0.0 ± 8.4 | 16.6 ± 4.8 | 44.9 ± 5.1 b | 61.9 ± 4.7 b | 79.6 ± 1.4 b |
| H2O-eluted fraction | 0.0 ± 10.0 | −5.7 ± 8.4 | 11.7 ± 7.7 | 12.1 ± 7.0 | 6.9 ± 7.4 |
| Inhibition (%) | IC50 | |||||
| 0 μM | 12.5 μM | 25 μM | 50 μM | 100 μM | (μM) | |
| mesuaferrone-A (1) | 0.0 ± 8.1 | 10.2 ± 14.6 | 26.5 ± 7.3 | 48.8 ± 6.8 b | 71.1 ± 1.4 b | 51.1 |
| mesuaferrone-B (2) | 0.0 ± 4.3 | 7.3 ± 1.9 | 23.6 ± 2.5 b | 46.6 ± 0.9 b | 54.5 ± 1.4 b | 54.7 |
| Inhibition (%) | IC50 | |||||
| 0 μM | 32.5 μM | 75 μM | 150 μM | 300 μM | (μM) | |
| naringenin (1a) | 0.0 ± 8.8 | −0.4 ± 5.3 | −8.8 ± 2.0 | 0.4 ± 2.2 | 20.5 ± 1.7 | — |
| apigenin (3) | 0.0 ± 6.2 | 15.8 ± 7.7 | 27.5 ± 8.1 | 32.9 ± 8.2 | 38.3 ± 6.4 b | — |
| luteolin (4) | 0.0 ± 4.6 | 5.7 ± 2.8 | 8.9 ± 3.8 | 10.5 ± 3.5 | 12.3 ± 3.4 | — |
| vitexin (5) | 0.0 ± 8.3 | 0.4 ± 8.2 | −7.0 ± 7.9 | −11.7 ± 3.8 | −10.9 ± 5.2 | — |
| orientin (6) | 0.0 ± 9.3 | 4.9 ± 5.9 | 5.9 ± 3.2 | 9.0 ± 2.9 | 13.2 ± 2.6 | — |
| saponaretin (7) | 0.0 ± 3.5 | 1.1 ± 2.0 | 4.8 ± 3.1 | 4.6 ± 3.0 | 2.2 ± 2.4 | — |
| homoorientin (8) | 0.0 ± 7.9 | −7.1 ± 8.5 | −2.9 ± 7.6 | −5.0 ± 4.0 | 1.7 ± 5.9 | — |
| apigenin 7-O-Rut (9) | 0.0 ± 7.6 | 4.4 ± 4.5 | 3.8 ± 2.1 | 2.5 ± 3.1 | 1.3 ± 2.4 | — |
| quercetin (10) | 0.0 ± 3.7 | −1.0 ± 1.4 | −4.3 ± 5.2 | −4.3 ± 3.8 | 0.7 ± 4.5 | — |
| kaempferol 3-O-Rha (11) | 0.0 ± 1.2 | 0.5 ± 2.5 | −4.7 ± 3.6 | −1.2 ± 3.7 | 6.3 ± 3.1 | — |
| quercetin 3-O-Rha (12) | 0.0 ± 4.3 | 1.4 ± 4.2 | 4.0 ± 2.3 | 5.9 ± 3.3 | 13.2 ± 2.6 | — |
| 1,7-dihydroxyxthantone (13) | 0.0 ± 3.0 | 3.3 ± 3.0 | 0.4 ± 1.4 | −1.8 ± 3.6 | 5.4 ± 3.9 | — |
| 1,3,7-trihydroxyxthantone (14) | 0.0 ± 7.7 | 7.8 ± 2.7 | 4.7 ± 1.8 | 11.1 ± 2.7 | 18.4 ± 1.9 | — |
| lupeol (15) | 0.0 ± 1.1 | 10.3 ± 3.2 | 9.4 ± 2.8 | 0.8 ± 2.2 | −0.4 ± 6.2 | — |
| betulinaldehyde (16) | 0.0 ± 3.6 | −3.9 ± 2.1 | 0.2 ± 1.9 | 1.0 ± 1.9 | 5.3 ± 5.6 | — |
| ursolic acid (17) | 0.0 ± 1.3 | 0.7 ± 1.5 | 0.5 ± 0.7 | 2.4 ± 1.5 | −5.7 ± 2.8 | — |
| trans-cinnamic acid (18) | 0.0 ± 1.4 | 2.4 ± 0.9 | −1.7 ± 4.7 | −4.0 ± 5.1 | −1.2 ± 5.0 | — |
| p-hydroxybenzoic acid (19) | 0.0 ± 5.0 | −2.8 ± 2.2 | −0.7 ± 1.4 | 2.6 ± 2.7 | −0.2 ± 4.3 | — |
| protocatechuic acid (20) | 0.0 ± 2.5 | 1.0 ± 2.5 | −2.1 ± 1.5 | 4.0 ± 2.3 | 5.6 ± 4.2 | — |
| vanillic acid (21) | 0.0 ± 3.8 | 4.0 ± 1.4 | 2.3 ± 3.1 | −0.7 ± 3.3 | 5.7 ± 3.2 | — |
| protocatechuic aldehyde (22) | 0.0 ± 1.0 | 0.7 ± 1.8 | 4.1 ± 1.1 | 4.7 ± 3.0 | 1.5 ± 0.8 | — |
| gallic acid (23) | 0.0 ± 1.4 | −0.6 ± 1.6 | −0.3 ± 3.1 | 3.6 ± 3.5 | 4.4 ± 4.2 | — |
| disodium cromoglycate [48] | 0.0 ± 2.0 | 4.0 ± 2.4 | 14.4 ± 0.4 a | 39.0 ± 4.9 b | 69.1 ± 2.2 b | 64.8 |
| ketotifen fumarate [48] | 0.0 ± 6.1 | 11.9 ± 1.9 | 26.7 ± 4.9 | 36.4 ± 2.9 b | 54.6 ± 2.5 b | 76.5 |
| Inhibition (%) | IC50 | |||||
| 0 μM | 3 μM | 10 μM | 30 μM | 100 μM | (μM) | |
| mesuaferrone-A (1) | 0.0 ± 7.1 | 24.9 ± 6.6 a | 24.8 ± 1.6 a | 37.8 ± 5.6 b | 86.0 ± 7.8 b | 49.4 |
| mesuaferrone-B (2) | 0.0 ± 9.5 | 13.8 ± 5.3 | 4.2 ± 4.4 | 5.6 ± 6.2 | 113.0 ± 10.1 b | 49.2 |
| Inhibition (%) | IC50 | |||||
| 0 μM | 30 μM | 100 μM | 300 μM | 1000 μM | (μM) | |
| tranilast [49] | 0.0 ± 1.7 | 8.2 ± 1.8 | 22.4 ± 2.5 a | 56.9 ± 3.4 b | 75.0 ± 0.6 b | 282 |
| ketotifen fumarate [49] | 0.0 ± 1.8 | 7.7 ± 1.5 | 27.6 ± 2.2 a | 80.7 ± 1.8 b | 100.7 ± 1.1 b | 158 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manse, Y.; Sakamoto, Y.; Miyachi, T.; Nire, M.; Hashimoto, Y.; Chaipech, S.; Pongpiriyadacha, Y.; Morikawa, T. Antiallergic Properties of Biflavonoids Isolated from the Flowers of Mesua ferrea Linn. Separations 2022, 9, 127. https://doi.org/10.3390/separations9050127
Manse Y, Sakamoto Y, Miyachi T, Nire M, Hashimoto Y, Chaipech S, Pongpiriyadacha Y, Morikawa T. Antiallergic Properties of Biflavonoids Isolated from the Flowers of Mesua ferrea Linn. Separations. 2022; 9(5):127. https://doi.org/10.3390/separations9050127
Chicago/Turabian StyleManse, Yoshiaki, Yusuke Sakamoto, Taiki Miyachi, Mitsuyo Nire, Yoshinori Hashimoto, Saowanee Chaipech, Yutana Pongpiriyadacha, and Toshio Morikawa. 2022. "Antiallergic Properties of Biflavonoids Isolated from the Flowers of Mesua ferrea Linn." Separations 9, no. 5: 127. https://doi.org/10.3390/separations9050127
APA StyleManse, Y., Sakamoto, Y., Miyachi, T., Nire, M., Hashimoto, Y., Chaipech, S., Pongpiriyadacha, Y., & Morikawa, T. (2022). Antiallergic Properties of Biflavonoids Isolated from the Flowers of Mesua ferrea Linn. Separations, 9(5), 127. https://doi.org/10.3390/separations9050127






