Anti-Osteoporotic Effect of Viscozyme-Assisted Polysaccharide Extracts from Portulaca oleracea L. on H2O2-Treated MC3T3-E1 Cells and Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polysaccharides Extracted from POL
2.2. Survival Rate and ROS Detection
2.3. Hoechst 33342 and Acridine Orange/Ethidium Bromide Staining
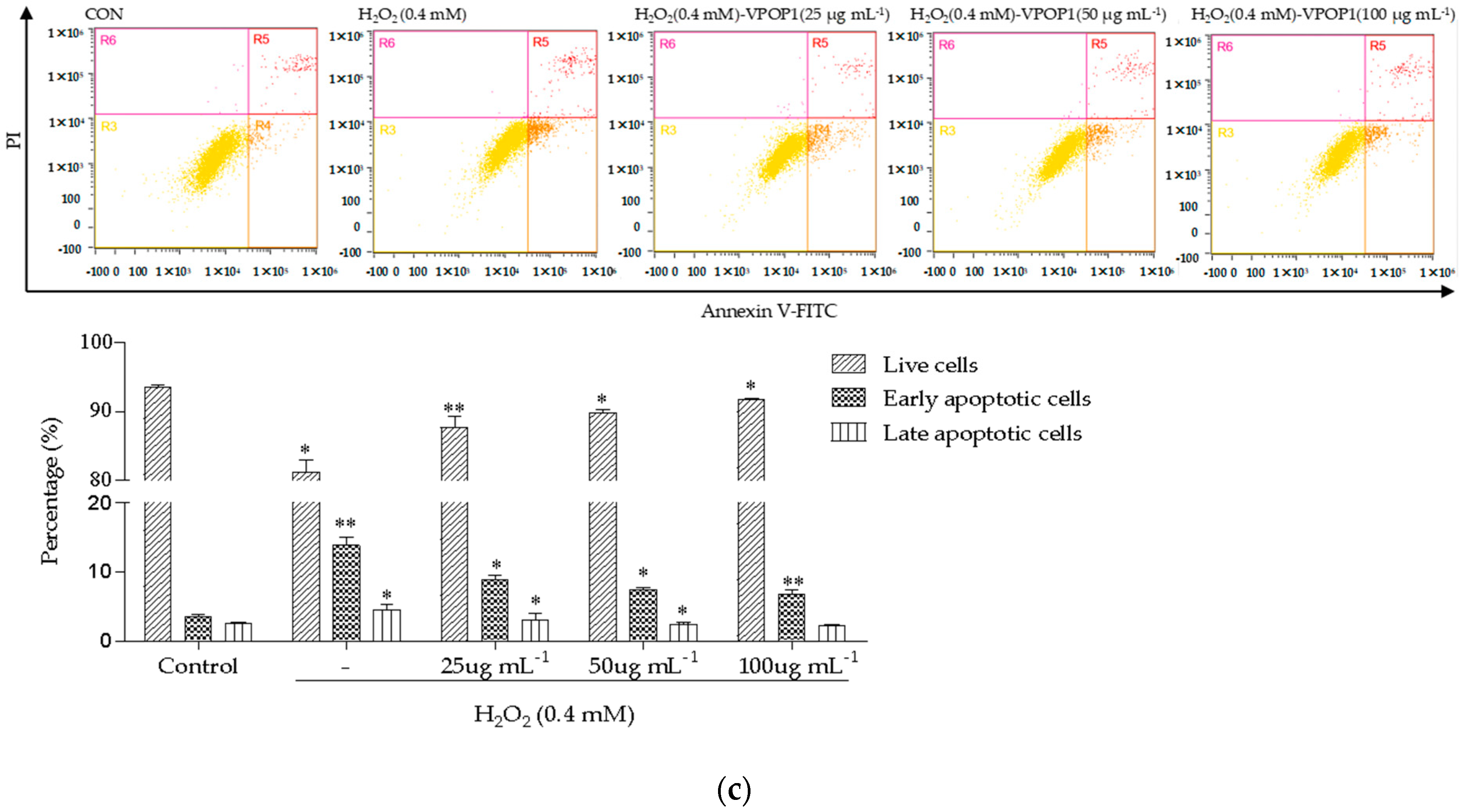
2.4. Flow Cytometry Analysis
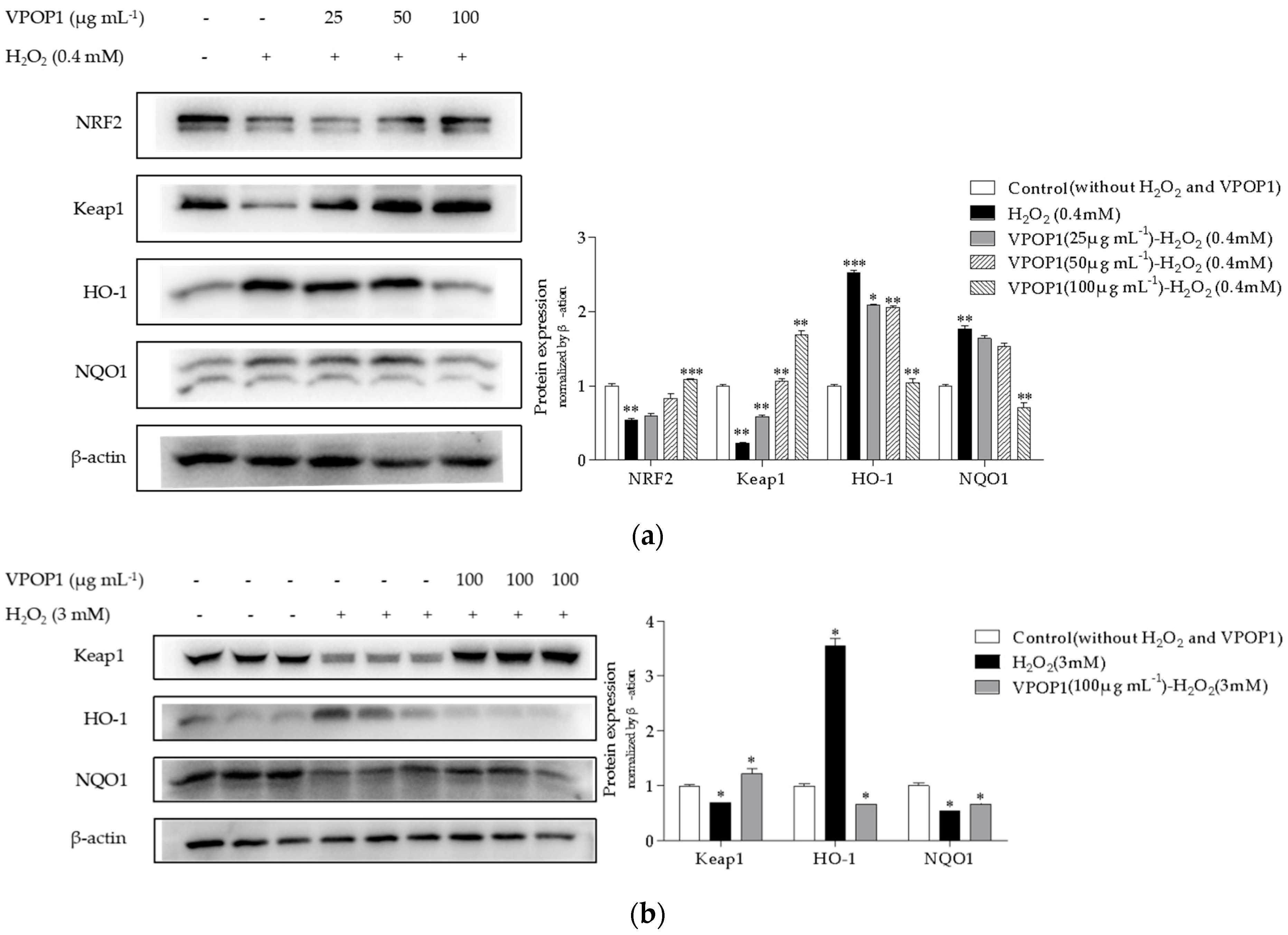
2.5. Western Blot Analysis
2.6. Analysis of Zebrafish Embryos
2.7. Molecular Weight of POPs
2.8. Monosaccharide Composition of POPs
2.9. Statistical Analysis
3. Results
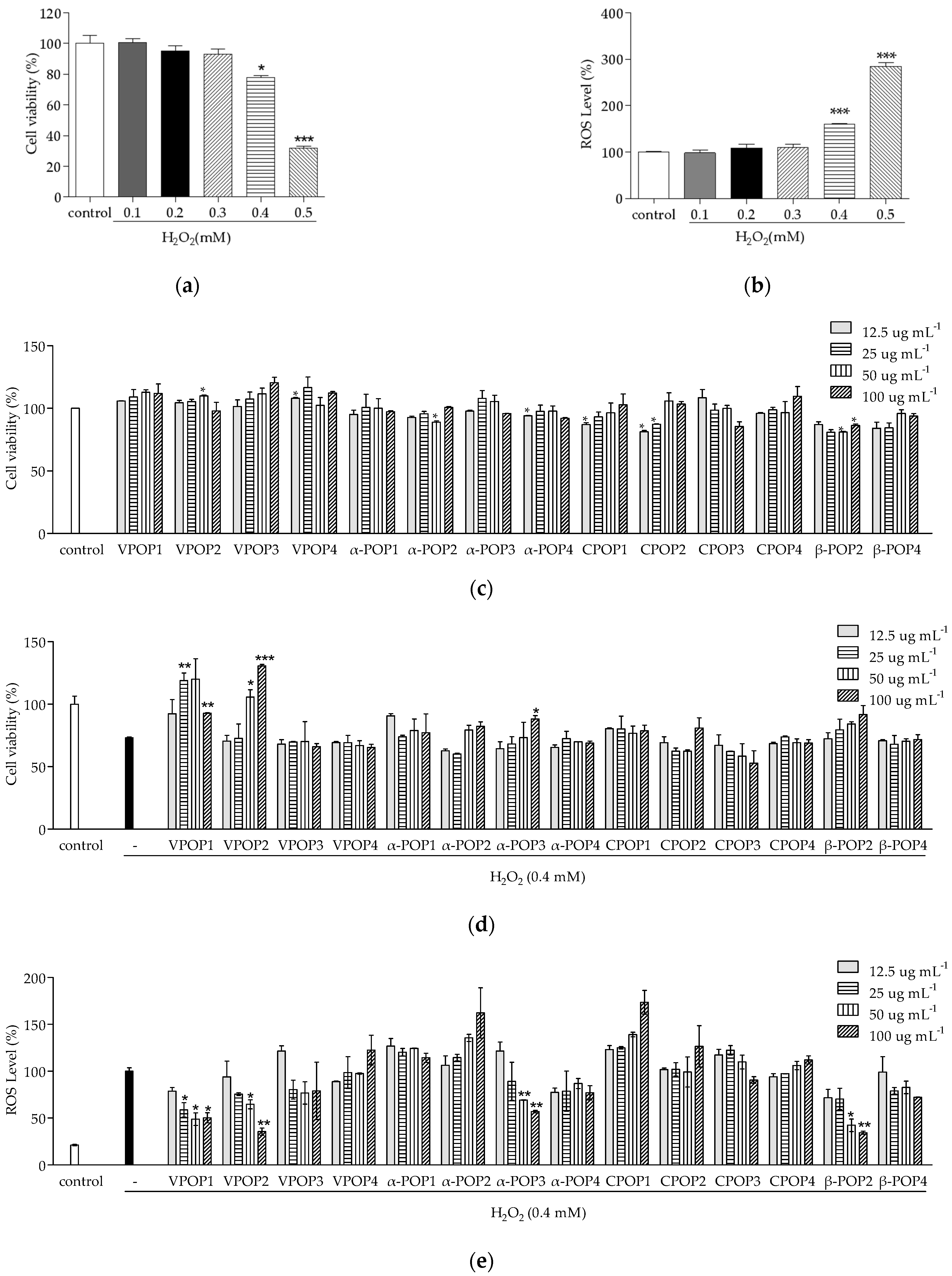
3.1. Separation and Protective Effect of POPs
3.2. Apoptotic Morphology of MC3T3 Cells
3.3. VPOP1 against Oxidative Stress via the Nrf2/Keap1 Pathway
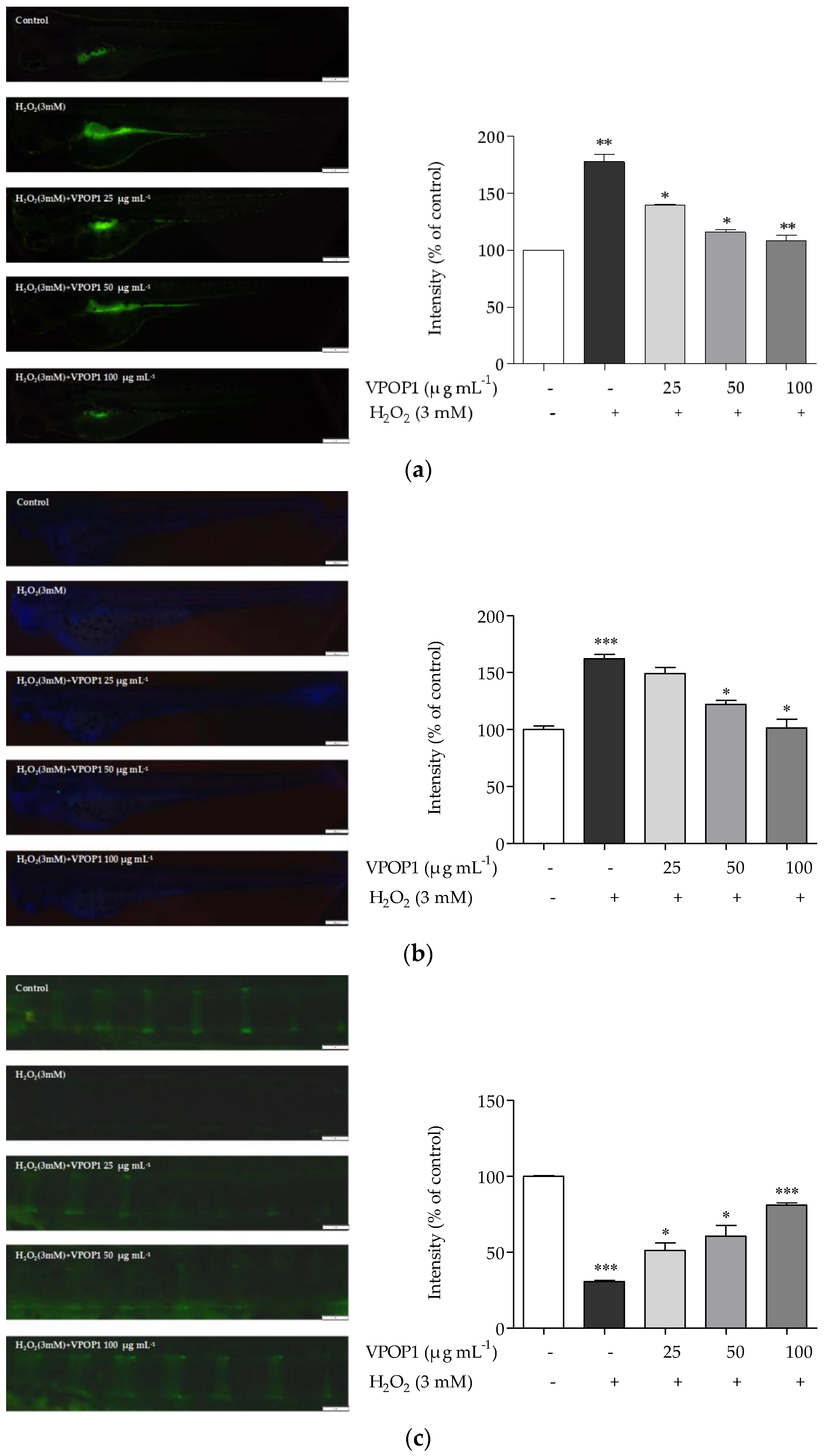
3.4. Protective Effect of VPOP1 against H2O2-Treated Zebrafish Embryonic (72 hpf) and Larvae (9 dpf)
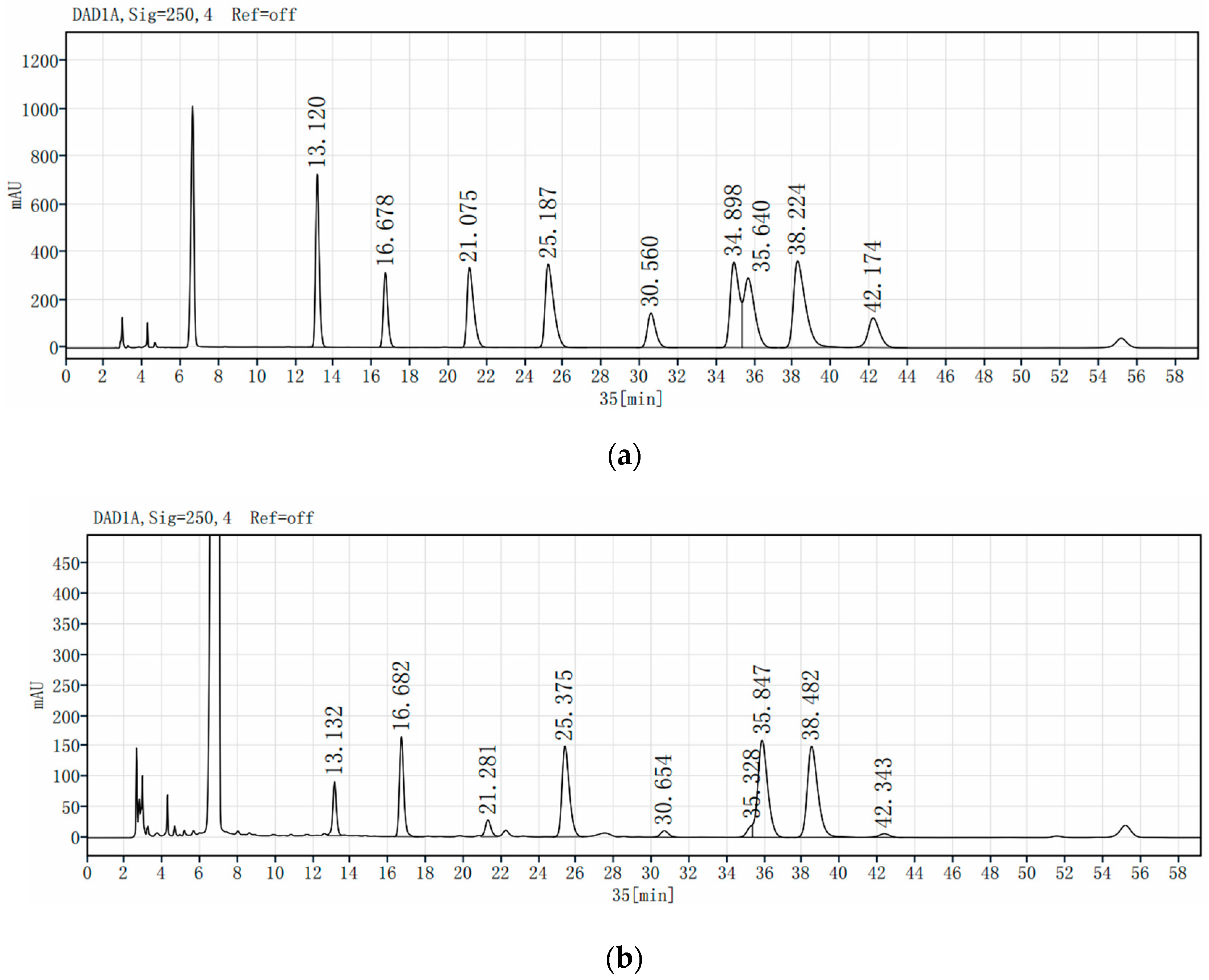
3.5. Chemical Composition of VPOP1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef] [Green Version]
- Brandi, M.L. Microarchitecture, the key to bone quality. Rheumatology 2009, 48 (Suppl. S4), iv3–iv8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Gao, Z.; Chen, Y.; Guan, M.X. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell 2017, 8, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.C.; Wu, Y.T.; Yu, T.H.; Wei, Y.H. Mitochondria in mesenchymal stem cell biology and cell therapy: From cellular differentiation to mitochondrial transfer. Semin. Cell Dev. Biol. 2016, 52, 119–131. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic. Biol. Med. 2016, 100, 81–85. [Google Scholar] [CrossRef]
- El-Bahr, S.M. Biochemistry of free radicals and oxidative stress. Biochemistry 2013, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Monaghan, P.; Metcalfe, N.B.; Torres, R. Oxidative stress as a mediator of life history trade-offs: Mechanisms, measurements and interpretation. Ecol. Lett. 2009, 12, 75–92. [Google Scholar] [CrossRef]
- Kondo, H.; Yumoto, K.; Alwood, J.S.; Mojarrab, R.; Wang, A.; Almeida, E.A.; Searby, N.D.; Limoli, C.L.; Globus, R.K. Oxidative stress and gamma radiation-induced cancellous bone loss with musculoskeletal disuse. J. Appl. Physiol. 2010, 108, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Khajuria, D.K.; Razdan, R.; Mahapatra, D.R. Drugs for the management of osteoporosis: A review. Rev. Bras. Reumatol. 2011, 51, 365–382. [Google Scholar] [PubMed]
- Zhang, N.D.; Han, T.; Huang, B.K.; Rahman, K.; Jiang, Y.P.; Xu, H.T.; Qin, L.P.; Xin, H.L.; Zhang, Q.Y.; Li, Y.M. Traditional Chinesemedicine formulas for the treatment of osteoporosis: Implication for antiosteoporotic drug discovery. J. Ethnopharmacol. 2016, 189, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Xin, H.L.; Rahman, K.; Wang, S.J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. BioMed Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.S.; Kim, J.S.; Lee, Y.J.; Kang, D.G.; Lee, H.S. Anti-TNF-alpha activity of Portulaca oleracea in vascular endothelial cells. Int. J. Mol. Sci. 2012, 13, 5628–5644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iranshahy, M.; Javadi, B.; Iranshahi, M.; Jahanbakhsh, S.P.; Mahyari, S.; Hassani, F.V.; Karimi, G. A review of traditional uses, phytochemistry and pharmacology of Portulaca oleracea L. J. Ethnopharmacol. 2017, 205, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Tao, H.; Peng, Y.; Wang, S.; Zhong, Z.; El-Seedi, H.; Dragan, S.; Zengin, G.; Cheang, W.S.; Wang, Y.; et al. The anti-inflammatory potential of Portulaca oleracea L. (purslane) extract by partial suppression on NF-κB and MAPK activation. Food Chem. 2019, 290, 239–245. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wang, Z.; Xu, S.Y. Optimization of the aqueous enzymatic extraction of rapeseed oil and protein hydrolysates. J. Am. Oil. Chem. Soc. 2007, 84, 97–105. [Google Scholar] [CrossRef]
- Mushtaq, M.; Sultana, B.; Akram, S.; Anwar, F.; Adnan, A.; Rizvi, S.S.H. Enzyme-assisted supercritical fluid extraction: An alternative and green technology for non-extractable polyphenols. Anal. Bioanal. Chem. 2017, 409, 3645–3655. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Fleita, D.; El-Sayed, M.; Rifaat, D.J.L. Evaluation of the antioxidant activity of enzymatically-hydrolyzed sulfated polysaccharides extracted from red algae; Pterocladia capillacea. LWT-Food Sci. Technol. 2015, 63, 1236–1244. [Google Scholar] [CrossRef]
- Fernandes, P.; Carvalho, F. Chapter 19—Microbial Enzymes for the Food Industry. In Biotechnology of Microbial Enzymes; Brahmachari, G., Ed.; Academic Press: New York, NY, USA, 2017; pp. 513–544. [Google Scholar]
- Wang, L.; Liu, F.; Li, T.; Liu, D.; Xu, Y.; Yang, Y. Enzyme Assisted Extraction, Purification and Structure Analysis of the Polysaccharides from Naked Pumpkin Seeds. Appl. Sci. 2018, 8, 1866. [Google Scholar] [CrossRef] [Green Version]
- Talley, K.; Alexov, E.J. On the pH-optimum of activity and stability of proteins. Proteins 2010, 78, 2699–2706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zha, X.-Q.; Xiao, J.-J.; Zhang, H.-N.; Wang, J.-H.; Pan, L.-H.; Yang, X.-F.; Luo, J.-P.J. Polysaccharides in Laminaria japonica (LP): Extraction, physicochemical properties and their hypolipidemic activities in diet-induced mouse model of atherosclerosis. Food Chem. 2012, 134, 244–252. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.J.; Ahn, G. Step gradient alcohol precipitation for the purification of low molecular weight fucoidan from Sargassum siliquastrum and its UVB protective effects. Int. J. Biol. Macromol. 2020, 163, 26–35. [Google Scholar] [CrossRef]
- Mosmann, T.J. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A.J. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Dai, J.; Wu, Y.; Chen, S.W.; Zhu, S.; Yin, H.P.; Wang, M.; Tang, J. Sugar compositional determination of polysaccharides from Dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydr. Polym. 2010, 82, 629–635. [Google Scholar] [CrossRef]
- Yang, Y.H.; Li, B.; Zheng, X.F.; Chen, J.W.; Chen, K.; Jiang, S.D.; Jiang, L.S. Oxidative damage to osteoblasts can be alleviated by early autophagy through the endoplasmic reticulum stress pathway—Implications for the treatment of osteoporosis. Free Radic. Biol. Med. 2014, 77, 10–20. [Google Scholar] [CrossRef]
- Li, H.; Li, D.; Ma, Z.; Qian, Z.; Kang, X.; Jin, X.; Li, F.; Wang, X.; Chen, Q.; Sun, H.; et al. Defective autophagy in osteoblasts induces endoplasmic reticulum stress and causes remarkable bone loss. Autophagy 2018, 14, 1726–1741. [Google Scholar] [CrossRef] [Green Version]
- Gardner, A.M.; Xu, F.H.; Fady, C.; Jacoby, F.J.; Duffey, D.C.; Tu, Y.; Lichtenstein, A. Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic. Biol. Med. 1997, 22, 73–83. [Google Scholar] [CrossRef]
- Yang, Y.; Su, Y.; Wang, D.; Chen, Y.; Wu, T.; Li, G.; Sun, X.; Cui, L.J. Tanshinol attenuates the deleterious effects of oxidative stress on osteoblastic differentiation via Wnt/FoxO3a signaling. Oxid. Med. Cell Longev. 2013, 2013, 351895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sykiotis, G.P.; Bohmann, D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci. Signal. 2010, 3, re3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibáñez, L.; Ferrándiz, M.L.; Brines, R.; Guede, D.; Cuadrado, A.; Alcaraz, M.J. Effects of Nrf2 deficiency on bone microarchitecture in an experimental model of osteoporosis. Oxid. Med. Cell Longev. 2014, 2014, 726590. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Jeong, H.G. Ginsenoside Rb1 protects against 6-hydroxydopamine-induced oxidative stress by increasing heme oxygenase-1 expression through an estrogen receptor-related PI3K/Akt/Nrf2-dependent pathway in human dopaminergic cells. Toxicol. Appl. Pharmacol. 2010, 242, 18–28. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox. Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.H.; Song, W.; Cressatti, M.; Zukor, H.; Wang, E.; Schipper, H.M. Heme oxygenase-1 modulates microRNA expression in cultured astroglia: Implications for chronic brain disorders. Glia 2015, 63, 1270–1284. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.-L.; Kim, G.H.; Kang, M.-C.; Jeon, Y.-J. Protective effects of extracts from six local strains of Pyropia yezoensis against oxidative damage in vitro and in zebrafish model. ALGAE 2020, 35, 189–200. [Google Scholar] [CrossRef]
- Miki, M.; Tamai, H.; Mino, M.; Yamamoto, Y.; Niki, E. Free-radical chain oxidation of rat red blood cells by molecular oxygen and its inhibition by alpha-tocopherol. Arch. Biochem. Biophys. 1987, 258, 373–380. [Google Scholar] [CrossRef]
- Du, S.J.; Frenkel, V.; Kindschi, G.; Zohar, Y. Visualizing normal and defective bone development in zebrafish embryos using the fluorescent chromophore calcein. Dev. Biol. 2001, 238, 239–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Goff, H.D. Fractionation of polysaccharides by gradient non-solvent precipitation: A review. Trends Food Sci. Technol. 2018, 81, 108–115. [Google Scholar] [CrossRef]
- Li, Y.-P.; Yao, L.-H.; Wu, G.-J.; Pi, X.-F.; Gong, Y.-C.; Ye, R.-S.; Wang, C.-X.J. Antioxidant activities of novel small-molecule polysaccharide fractions purified from Portulaca oleracea L. Food Sci. Biotechnol. 2014, 23, 2045–2052. [Google Scholar] [CrossRef]

| Mw | Mn | Composition (%) | PDI (Mw/Mn) |
|---|---|---|---|
| 62,526 | 40,342 | 27.6988 | 1.5499 |
| 7655 | 7363 | 40.0236 | 1.0397 |
| 3644 | 3420 | 10.2537 | 1.0655 |
| 1533 | 1525 | 0.6003 | 1.0052 |
| 492 | 373 | 21.4236 | 1.3190 |
| Monosaccharide | A | A′ | m/(umol∙L−1) | R1/2 |
|---|---|---|---|---|
| Mannose | 9489.649 | 1195.765 | 42.670 | 1 |
| Rhamnose | 4989.372 | 2618.684 | 46.830 | 0.219 |
| Galacturonic acid | 10811.697 | 4052.162 | 35.590 | 0.403 |
| Galactose | 11670.812 | 5856.281 | 57.780 | 0.185 |
| Arabinose | 15805.226 | 5884.442 | 61.870 | 0.233 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Hu, X.; Zhou, D.; Li, X.; Tao, X.; Yang, D.; Zheng, F.; Dai, Y.; Yue, H. Anti-Osteoporotic Effect of Viscozyme-Assisted Polysaccharide Extracts from Portulaca oleracea L. on H2O2-Treated MC3T3-E1 Cells and Zebrafish. Separations 2022, 9, 128. https://doi.org/10.3390/separations9050128
Fu Y, Hu X, Zhou D, Li X, Tao X, Yang D, Zheng F, Dai Y, Yue H. Anti-Osteoporotic Effect of Viscozyme-Assisted Polysaccharide Extracts from Portulaca oleracea L. on H2O2-Treated MC3T3-E1 Cells and Zebrafish. Separations. 2022; 9(5):128. https://doi.org/10.3390/separations9050128
Chicago/Turabian StyleFu, Yunhua, Xuan Hu, Dongyue Zhou, Xue Li, Xingyu Tao, Di Yang, Fei Zheng, Yulin Dai, and Hao Yue. 2022. "Anti-Osteoporotic Effect of Viscozyme-Assisted Polysaccharide Extracts from Portulaca oleracea L. on H2O2-Treated MC3T3-E1 Cells and Zebrafish" Separations 9, no. 5: 128. https://doi.org/10.3390/separations9050128
APA StyleFu, Y., Hu, X., Zhou, D., Li, X., Tao, X., Yang, D., Zheng, F., Dai, Y., & Yue, H. (2022). Anti-Osteoporotic Effect of Viscozyme-Assisted Polysaccharide Extracts from Portulaca oleracea L. on H2O2-Treated MC3T3-E1 Cells and Zebrafish. Separations, 9(5), 128. https://doi.org/10.3390/separations9050128